Abstract
BACKGROUND
Exacerbations of asthma are associated with substantial morbidity and mortality and with considerable use of health care resources. Preventing exacerbations remains an important goal of therapy. There is evidence that eosinophilic inflammation of the airway is associated with the risk of exacerbations.
METHODS
We conducted a randomized, double-blind, placebo-controlled, parallel-group study of 61 subjects who had refractory eosinophilic asthma and a history of recurrent severe exacerbations. Subjects received infusions of either mepolizumab, an anti-interleukin-5 monoclonal antibody (29 subjects), or placebo (32) at monthly intervals for 1 year. The primary outcome measure was the number of severe exacerbations per subject during the 50-week treatment phase. Secondary outcomes included a change in asthma symptoms, scores on the Asthma Quality of Life Questionnaire (AQLQ, in which scores range from 1 to 7, with lower values indicating more severe impairment and a change of 0.5 unit considered to be clinically important), forced expiratory volume in 1 second (FEV1) after use of a bronchodilator, airway hyperresponsiveness, and eosinophil counts in the blood and sputum.
RESULTS
Mepolizumab was associated with significantly fewer severe exacerbations than placebo over the course of 50 weeks (2.0 vs. 3.4 mean exacerbations per subject; relative risk, 0.57; 95% confidence interval [CI], 0.32 to 0.92; P = 0.02) and with a significant improvement in the score on the AQLQ (mean increase from baseline, 0.55 vs. 0.19; mean difference between groups, 0.35; 95% CI, 0.08 to 0.62; P = 0.02). Mepolizumab significantly lowered eosinophil counts in the blood (P<0.001) and sputum (P = 0.002). There were no significant differences between the groups with respect to symptoms, FEV1 after bronchodilator use, or airway hyperresponsiveness. The only serious adverse events reported were hospitalizations for acute severe asthma.
CONCLUSIONS
Mepolizumab therapy reduces exacerbations and improves AQLQ scores in patients with refractory eosinophilic asthma. The results of our study suggest that eosinophils have a role as important effector cells in the pathogenesis of severe exacerbations of asthma in this patient population. (Current Controlled Trials number, ISRCTN75169762.)
Asthma is a complex chronic inflammatory disorder of the bronchial tree. Persons with asthma present with variable symptoms of cough, breathlessness, and wheezing; these episodes may be punctuated by periods of more severe and sustained deterioration in control of symptoms — termed exacerbations — that necessitate emergency treatment. Exacerbations are associated with substantial morbidity and mortality and with considerable health care costs.1
Exacerbations differ from day-to-day symptoms in that they respond poorly to usual inhaled therapy and are more closely linked to increased airway inflammation.2 The link to eosinophilic airway inflammation may be particularly important, since infiltration of the airway mucosa with activated eosinophils is seen in postmortem examinations of patients who have died of acute severe asthma,3 and markers of eosinophilic airway inflammation increase well before the onset of exacerbations that are induced by the withdrawal of corticosteroid treatment.4,5 Moreover, management strategies that control eosinophilic airway inflammation as well as the clinical manifestations of asthma are associated with a reduction in the frequency of exacerbations.6,7
A study of asthma therapy involving mepolizumab, a humanized monoclonal antibody against interleukin-5, offers the prospect of clarifying the role of eosinophils in exacerbations, since mepolizumab is a selective and effective inhibitor of eosinophilic inflammation.8-11 Results of clinical trials of this agent among persons with asthma have been disappointing,9,11 although these studies have focused on outcome measures that are not closely associated with eosinophilic airway inflammation and have included populations that were selected on the basis of clinical and physiological characteristics rather than the presence of eosinophilic airway inflammation.12
We tested the hypothesis that eosinophils are important in the pathogenesis of asthma exacerbations by studying the effect of treatment with mepolizumab for 12 months on the frequency of exacerbations among subjects who had refractory asthma and evidence of eosinophilic airway inflammation despite treatment with high doses of corticosteroids. Secondary aims included assessments of the effects of treatment on airway inflammation, asthma symptoms, asthma-related quality of life, forced expiratory volume in 1 second (FEV1), and, since chronic eosinophilic airway inflammation may be associated with airway remodeling,8 airway structure as assessed with the use of computed tomography (CT).
METHODS
SUBJECTS
All subjects were older than 18 years of age and had a clinical diagnosis of asthma that was supported by one or more of the following criteria: variability in the maximum diurnal peak expiratory flow of more than 20% over the course of 14 days, an increase in FEV1 of more than 15% after inhalation of 200 μg of albuterol, and a 20% reduction in FEV1 in response to a provocative concentration of inhaled methacholine (PC20) of less than 8 mg per milliliter. Subjects were recruited among patients attending a refractory-asthma clinic that provided secondary asthma care for a mixed urban and rural population of 1 million people and tertiary care for 4 million people. Patients who attend this clinic undergo a standardized assessment, which includes a noninvasive assessment of airway inflammation every 2 to 4 months by means of an analysis of induced-sputum specimens. Inclusion criteria were a diagnosis of refractory asthma according to American Thoracic Society criteria,13 a sputum eosinophil percentage of more than 3% on at least one occasion in the previous 2 years despite high-dose corticosteroid treatment, and at least two exacerbations requiring rescue prednisolone treatment in the previous 12 months. Additional criteria for inclusion were stable treatment requirements and an absence of exacerbations for more than 6 weeks before enrollment in the study. Exclusion criteria were current smoking, serologic evidence of a parasitic infection, a serious coexisting illness, the possibility of conception, and poor adherence to treatment.
All subjects provided written informed consent. The study protocol was approved by the local research ethics committee and the United Kingdom Medicines and Healthcare Products Regulatory Agency.
DESIGN OF THE STUDY
The study was a single-center, randomized, double-blind, placebo-controlled, parallel-group clinical trial conducted from April 2006 through August 2008. The funding organization (GlaxoSmithKline) supplied the study drug and placebo but had no role in the accrual or analysis of the data. Representatives of the funding organization contributed to the study design and to the preparation of the manuscript. The academic authors made the decision to submit the manuscript for publication and vouch for the accuracy and integrity of the contents.
The study measurements are described in the Supplementary Appendix, and the protocol is summarized in Figure 1 in the Supplementary Appendix. At a baseline visit, information on demographic characteristics was collected from all subjects, and spirometry was performed before and after use of a bronchodilator. Regular treatment was kept constant from this time until completion of the study. After a 2-week run-in period, baseline PC20 was measured; a day later, the fraction of exhaled nitric oxide (FeNO) was measured, symptoms were assessed, and the Asthma Quality of Life Questionnaire (AQLQ) was administered. Symptoms were assessed with the use of three 100-mm visual-analogue scales — one assessing cough, one assessing breathlessness, and one assessing wheezing — each of which had “no symptoms” at one end and “the worst symptoms ever” at the other end, and with the use of the modified Juniper Asthma Control Questionnaire (JACQ), which assesses daytime and nighttime symptoms and activity limitation on the basis of five questions that are scored on a scale of 0 to 6, with lower numbers representing better control of symptoms.14 Quality of life was assessed with the use of the AQLQ, a questionnaire comprising 32 items, each of which is scored on a scale of 1 to 7, with higher scores indicating better asthma-related quality of life.15 The items are grouped into four domains, and the reported score is the mean of responses across the four domains. The minimal clinically important change in the JACQ and AQLQ scores is 0.5.14
To assess both the responsiveness of symptoms, FeNO, and FEV1 to treatment with oral corticosteroids and the way in which the responsiveness was influenced by mepolizumab therapy, subjects were treated with oral prednisolone for 2 weeks at a dose of 0.5 mg per kilogram of body weight per day, with a maximum dose of 40 mg per day, at the beginning and end of the study. For the subgroup of participants who consented to have a bronchoscopic examination, the procedure was performed before treatment with prednisolone. At visit 3, after completing the 2-week course of prednisolone and before receiving the first study treatment, subjects underwent a further assessment of symptom scores, measurement of FeNO, and spirometry before and after bronchodilator use, as well as CT scanning in the subgroup of patients who provided consent for this assessment.
Subjects were randomly assigned with the use of the minimization method (Table 1) to receive 12 infusions of either 750 mg of mepolizumab delivered intravenously or matched placebo (150 ml of 0.9% saline) at monthly intervals between visits 3 and 14. The criteria used for minimization were the frequency of exacerbations in the previous 12 months, the baseline eosinophil count in the sputum, and the number of subjects taking oral corticosteroids. FeNO, spirometry before and after use of a bronchodilator, and symptom scores were recorded at each visit; the AQLQ was administered at visits 5, 8, 11, and 14; and PC20 was measured the day before visits 8 and 14. The treatment phase ended 2 weeks after visit 14 — that is, 50 weeks after treatment was started. At this time, bronchoscopy was performed in subjects who consented to the procedure, and all subjects were given an additional 2-week course of oral prednisolone. After the course of oral prednisolone was completed, FeNO and symptom scores were assessed, and spirometry and, in the subgroup of patients who provided consent, CT scanning were performed.
Table 1. Baseline Characteristics of Subjects in the Intention-to-Treat Population*.
| Characteristic | Mepolizumab (N = 29) | Placebo (N = 32) | P Value† |
|---|---|---|---|
| Sex (no. of subjects) | 0.80 | ||
| Male | 14 | 18 | |
| Female | 15 | 14 | |
| Age (yr) | 0.34 | ||
| Mean | 48 | 50 | |
| Range | 21–63 | 24–72 | |
| Age at onset of symptoms (yr) | 0.99 | ||
| Mean | 26 | 26 | |
| Range | 2–53 | 2–57 | |
| Body-mass index‡ | 29.4±7.3 | 29.2±5.9 | 0.92 |
| Positive atopic status (% of subjects)§ | 67.9 | 68.8 | 0.78 |
| Total IgE (U/ml)¶ | 177.8±2.47 | 195±2.64 | 0.75 |
| Presence of nasal polyps (% of subjects) | 34.4 | 31.2 | 0.59 |
| Severe exacerbations per subject in previous year (no.)∥ | 5.5 | 5 | 0.71 |
| Previous admission to the intensive care unit for asthma (% of subjects) | 27.5 | 31.3 | 0.78 |
| PC20 (mg/ml)¶ | 0.6±1.24 (N = 16) | 1.1±1.1 (N = 18) | 0.38 |
| FEV1 after bronchodilator use (% of predicted value) | 78.1±20.9 | 77.6±24.1 | 0.93 |
| FEV1:FVC ratio (%) | 72.2±9.6 | 67.7±13.5 | 0.15 |
| Improvement in FEV1 after bronchodilator use (%) | 9.1±14.2 | 7.0±13.1 | 0.57 |
| Eosinophil count in sputum (%)¶∥ | 6.84±0.64 | 5.46±0.75 | 0.60 |
| Eosinophil count in blood (×10−9/liter)¶ | 0.32±0.38 | 0.35±0.30 | 0.57 |
| FeNO (ppb)¶** | 44.4±0.40 | 35.5±0.40 | 0.31 |
| Score on modified Juniper Asthma Control Questionnaire | 1.98±1.07 | 2.38±1.35 | 0.28 |
| Score on Asthma Quality of Life Questionnaire | 4.72±1.26 | 4.84±1.13 | 0.71 |
| Dose of inhaled corticosteroid — beclomethasone dipropionate–equivalent (μg)†† | 0.03 | ||
| Daily dose | 2038 | 1711 | |
| Range | 1000–4000 | 1000–4000 | |
| Use of long-acting beta-agonists (% of subjects) | 92.9 | 90.6 | 0.99 |
| Use of oral prednisolone∥ | |||
| Regular use (% of subjects) | 57.1 | 53.1 | 0.80 |
| Daily maintenance dose (mg) | 0.72 | ||
| Mean | 9 | 10 | |
| Range | 5–20 | 2–40 | |
| Use of montelukast (% of subjects) | 21.4 | 25 | 0.76 |
| Use of methotrexate for asthma (no. of subjects) | 0 | 2 | 0.49 |
Plus–minus values are means ±SD unless otherwise stated. FeNO denotes the fraction of nitric oxide in exhaled air, FEV1 forced expiratory volume in 1 second, FVC forced vital capacity, and PC20 the provocative concentration of inhaled methacholine required to lower the FEV1 by 20%.
P values were calculated with the use of a two-sided independent t-test for variables with a parametric distribution, Fisher’s exact test for comparison of proportions, and the Mann–Whitney U test for comparison of nonparametric variables.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Positive atopic status was defined as a positive skin test for any of four specified aeroallergens (for details, see the Supplementary Appendix).
Values are geometric means ±log10 SD.
This variable was used for stratifying randomization with use of the minimization method, which was performed by an independent clinician.
FeNO was measured at a flow of 50 ml per second (for details, see the Supplementary Appendix).
The doses of all inhaled corticosteroids were converted to the equivalent dose of beclomethasone dipropionate.
Exacerbations during the treatment phase of the study were managed in accordance with standard clinical guidelines.16 Subjects who initiated treatment at home did so with guidance from their personalized management plan. In all cases, subjects were instructed to seek medical advice as soon as possible after starting therapy. Oral prednisolone therapy was prescribed at a dose of 0.5 mg per kilogram per day, with a maximum dose of 40 mg per day. Decisions about whether to use adjunctive therapy such as antibiotics and about the need for hospitalization were made by the study physician or the subject’s general practitioner. For subjects who were assessed by the study team within 72 hours after an exacerbation, assessments included symptom scores, FeNO, peak expiratory flow, and spirometry performed before and after use of a bronchodilator. In addition, sputum samples were obtained for cell counts and microbial analysis.
Because of the expected anti-eosinophil effects of mepolizumab, results of FeNO measurements, sputum analyses, and leukocyte differential counts that were obtained during scheduled and unscheduled visits were not disclosed to investigators. Exacerbations requiring hospitalization were managed by the admitting clinical team, whose members were unaware of the treatment assignments and of the results of the measurements of inflammatory factors.
SAFETY ASSESSMENT
Safety was assessed on the basis of laboratory tests, physical examinations, measurement of vital signs both before and after infusion, and adverse-event reports. Serious adverse events were also reported to GlaxoSmithKline as part of their ongoing collection of data.
STATISTICAL ANALYSIS
The primary outcome measure of the study was the number of severe exacerbations of asthma per subject; exacerbations were defined as periods of deterioration in asthma control in subjects who had been treated with high-dose oral prednisolone for at least 5 days.14 Exacerbations that occurred in the 50 weeks between the completion of the first treatment visit and 2 weeks after the final treatment visit were included in the analysis. A recurrence of asthma symptoms shortly after completion of a course of prednisolone was recorded as a separate exacerbation if baseline control of symptoms had been restored for a period of at least 5 days. Secondary outcome measures were changes in eosinophil values in blood and sputum samples, FeNO, FEV1 (percent of the predicted value) after bronchodilator use, PC20 AQLQ score, symptom scores, CT assessment of airway-wall geometry, and bronchoscopic assessment of eosinophilic airway inflammation.
All subjects who completed at least one treatment visit were included in an intention-to-treat analysis of the primary outcome. In the case of subjects who withdrew from the study, the adjusted number of exacerbations was calculated with the use of the following equation: recorded number of exacerbations + [(visits remaining ÷ total visits) × mean exacerbation frequency in the study group]. Exacerbation frequency was calculated and compared between the study groups with the use of a negative binomial model and verified with the Mann–Whitney U test, as previously described.17 In a study of a similar cohort,6 the mean (±SD) number of exacerbations was 3.2±2.1 per subject per year. Assuming a mean of two exacerbations per subject per year, we needed to include 60 subjects in order to have 80% power to detect a 50% reduction in exacerbation frequency. Secondary-outcome values were log-transformed where appropriate. Between-group and within-group comparisons were made for the mean change between baseline values and the mean or geometric mean of the posttreatment values with the use of unpaired and paired t-tests, respectively, for parametric distributions and the Mann–Whitney U test for nonparametric distributions. Proportions were compared with the use of Fisher’s exact test. Statistical software packages used for various analyses included SPSS, version 13 (SPSS), Stata, version 7 (Stata), and GraphPad Prism, version 4 (GraphPad Software).
RESULTS
ENROLLMENT AND BASELINE CHARACTERISTICS
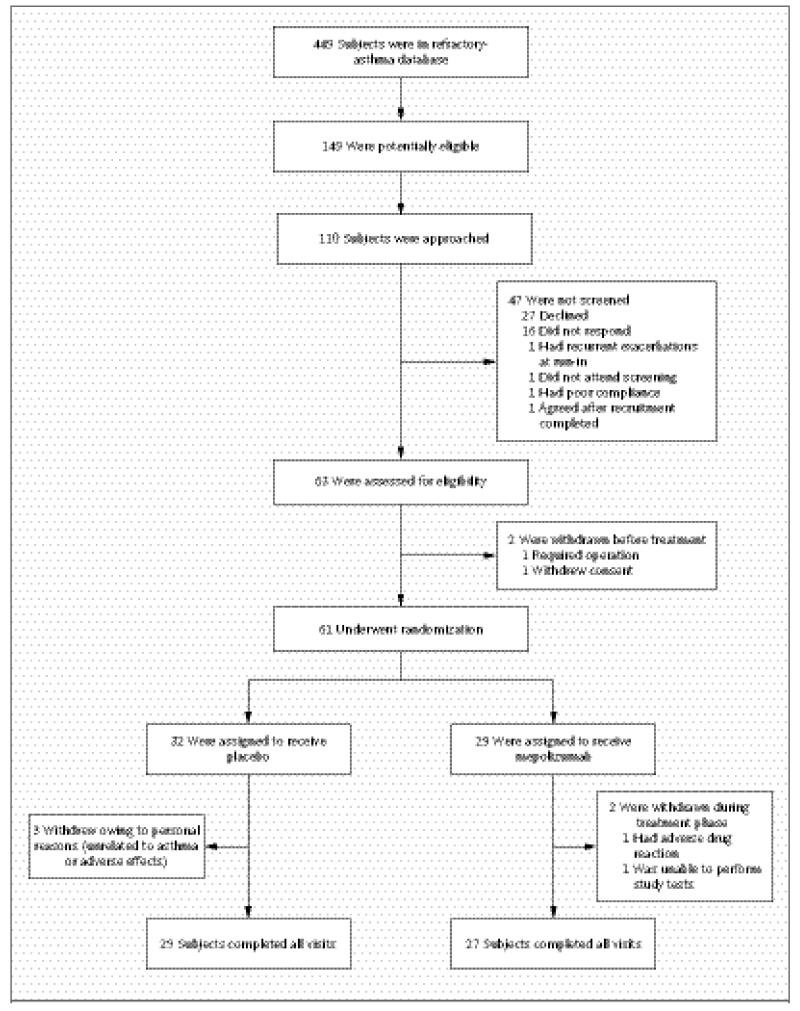
Figure 1 shows the numbers of subjects who were screened, enrolled, and randomly assigned to a study group and who completed the study. A total of 61 of the 63 subjects who were screened started treatment and constituted the modified intention-to-treat population. Thirty-two subjects were randomly assigned to receive placebo. Overall, 94.9% of treatment visits were completed. Subjects who withdrew completed a mean of 4.6 treatment visits (38.3%). Subjects in the two groups were well matched with respect to baseline characteristics (Table 1).
Figure 1. Numbers of Patients Who Were Screened, Enrolled, and Assigned to a Study Group and Who Completed the Study.
All subjects were recruited from a database of patients who were attending our refractory-asthma clinic. Of the 449 persons in the database, 52.3% had a history of sputum eosinophilia of more than 3% on at least one occasion in the previous 2 years, and 63.4% of these patients had been treated with two or more courses of oral corticosteroid therapy in the previous 12 months.
EFFICACY
Frequency of Severe Exacerbations
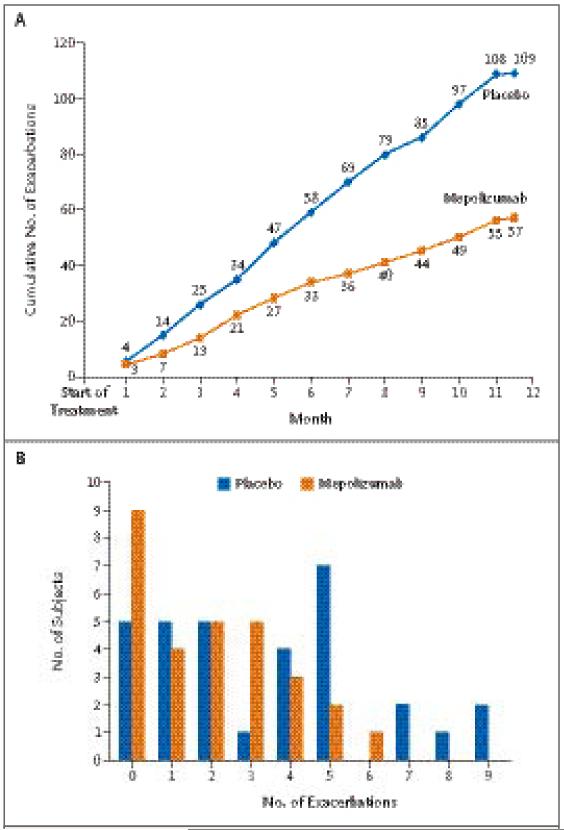
The median treatment period was 348 days in the mepolizumab group and 340 days in the placebo group (P = 0.30). During this period, a total of 57 exacerbations occurred in the group of subjects who were assigned to receive mepolizumab and 109 in the group assigned to receive placebo (Fig. 2A). The mean number of severe exacerbations per subject was 2.0 in the mepolizumab group, as compared with 3.4 in the placebo group (relative risk, 0.57; 95% confidence interval [CI], 0.32 to 0.92; P = 0.02) (Fig. 2A and 2B). The difference in the number of exacerbations remained significant with nonparameteric analysis (P = 0.04). Thirty-one percent of the subjects in the mepolizumab group had no exacerbations during the study period, as compared with 16% in the placebo group (P = 0.23) (Fig. 2B). The mean duration of prednisolone therapy per exacerbation was similar in the two groups (10.9 days in the mepolizumab group and 11.7 days in the placebo group, P = 0.31). There were three hospital admissions for exacerbations of asthma in the mepolizumab group, as compared with 11 admissions in the placebo group (P = 0.07). The total number of days in the hospital was significantly less for the subjects receiving mepolizumab treatment than for those receiving placebo (12 days vs. 48 days, P<0.001).
Figure 2. Severe Exacerbations during the Course of the Study.
Panel A shows the cumulative number of severe exacerbations that occurred in each study group over the course of 50 weeks. Panel B shows the distribution of the number of exacerbations among subjects in each study group during the treatment period of the study. The mean number of exacerbations per subject over the course of the 50-week treatment period was 2.0 in the mepolizumab group, as compared with 3.4 in the placebo group (relative risk, 0.57; 95% confidence interval, 0.32 to 0.92; P=0.02).
Treatment for an exacerbation was initiated by the subject in 20% of the cases, by the primary care physician or a physician at a hospital other than the study site in 25%, and by the study team in 55%. In the 77% of cases in which exacerbations were assessed within 72 hours after the initiation of prednisolone therapy, there were no significant differences between the groups in peak expiratory flow, FEV1 before and after bronchodilator use, symptom scores, or rescue bronchodilator use. Sputum samples were obtained from patients during 61% of the exacerbations. The geometric mean eosinophil percentage in the sputum during an exacerbation was significantly lower in the mepolizumab group than in the placebo group (1.5% vs. 4.4%), with the values differing by a factor of 2.9 (95% CI, 1.4 to 6.1; P = 0.005), but the mean total neutrophil count in the sputum did not differ significantly between the two groups (3846 cells per milligram of sputum in the mepolizumab group and 4122 cells per milligram in the placebo group, P = 0.80). The eosinophil percentage in the sputum was higher than 3% in 59% of the episodes in the placebo group and in 36% of the episodes in the mepolizumab group (P = 0.04).
Inflammatory Markers
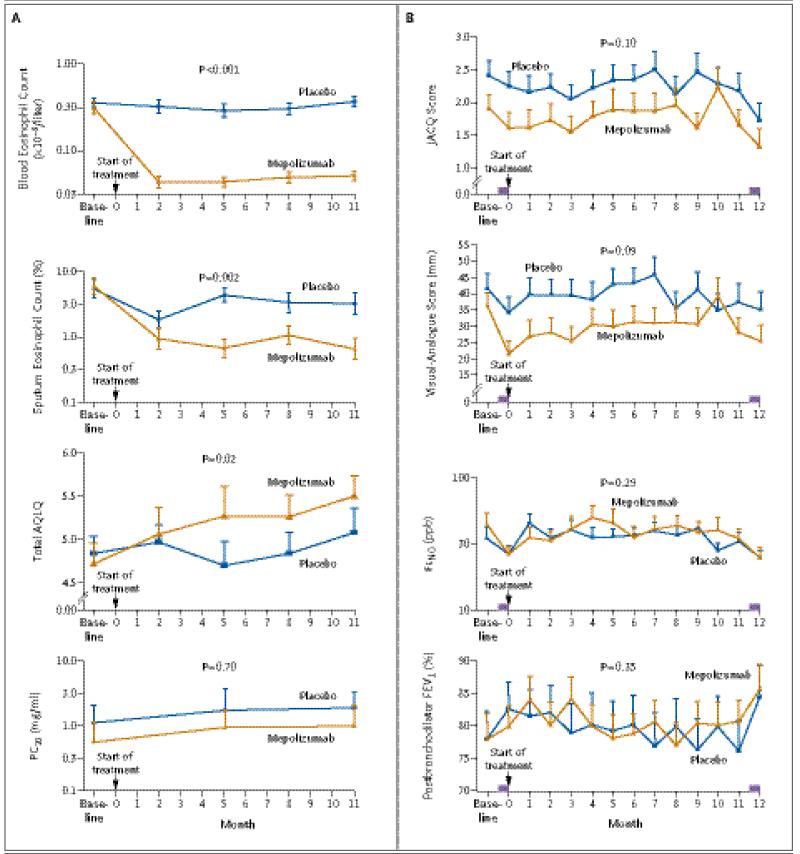
Mepolizumab therapy was associated with significant between-group and within-group reductions in eosinophil counts in both blood and sputum (Fig. 3A). The geometric mean of eosinophil counts in the blood during the treatment phase, as compared with the baseline value, was reduced by a factor of 6.6 in the mepolizumab group and by a factor of 1.1 in the placebo group, with the changes from baseline differing between the groups by a factor of 6.1 (95% CI, 4.1 to 8.9; P<0.001). Sputum induction at 90% of visits resulted in cytospin preparations that could be assessed for eosinophil counts. The geometric mean eosinophil count in the sputum was reduced by a factor of 7.1 in the mepolizumab group and by a factor of 1.9 in the placebo group, with the changes from baseline differing between the groups by a factor of 3.7 (95% CI, 1.6 to 8.4; P = 0.002). There were no significant between-group differences in the change in FeNO (P = 0.29) or the total neutrophil count in the sputum (P = 0.22) (Fig. 3B, and Table 1 in the Supplementary Appendix).
Figure 3. Comparison of Secondary Outcomes between Study Groups.
Panel A shows geometric mean (log10 SE) eosinophil counts in blood and sputum specimens, the provocative concentration of methacholine required to cause a 20% fall in FEV1 (PC20), and mean scores on the Asthma Quality of Life Questionnaire (AQLQ). The AQLQ comprises 32 items, each of which is scored on a scale of 1 to 7, with higher scores indicating better asthma-related quality of life. The items are grouped into four domains, and the reported score is the mean of responses across the four domains. Longitudinal data on PC20 were available for 16 and 18 subjects in the mepolizumab and placebo groups, respectively. Panel B shows mean symptom scores, the forced expiratory volume in 1 second (FEV1) after bronchodilator use, and the geometric mean (log10 SE) fraction of exhaled nitric oxide at an expiratory flow of 50 ml per second (FeNO) before, during, and after the treatment phase of the study. The score on the modified Juniper Asthma Control Questionnaire (JACQ) represents the mean of responses to five questions about daytime and nighttime symptoms and limitation of activities, with each response scored on a scale of 0 to 6; higher scores represent worse symptoms. The mean visual-analogue score represents the total scores divided by 3 for cough, wheezing, and breathlessness, each of which was assessed on a 100-mm scale, with “no symptoms” at one end and “the worst symptoms ever” at the other end. Higher scores indicate worse symptoms. These factors were evaluated before and after administration of 0.5 mg of prednisolone per kilogram per day, with a maximum dose of 40 mg per day, for 14 days, at the beginning and end of the treatment phase. The purple bars represent the 2-week course of prednisolone therapy. P values are for the mean difference between the groups for the change from baseline to the mean or geometric mean of the post-treatment values. Further details are given in Tables 1 and 2 in the Supplementary Appendix. The term ppb denotes parts per billion.
Paired bronchial-biopsy specimens (specimens obtained before and after the study treatment) were available for 14 subjects (of whom 9 were in the mepolizumab group), paired bronchoalveolar-lavage specimens for 11 subjects (8 in the mepolizumab group), and paired bronchial-wash specimens for 10 subjects (7 in the mepolizumab group). Changes in eosinophil counts after infusions of mepolizumab, as compared with changes after placebo infusions, were reduced by a factor of 2.1 (95% CI, 0.6 to 68.1; P = 0.68) in bronchial-biopsy specimens, by a factor of 8.2 (95% CI, 0.9 to 75.4; P = 0.06) in bronchoalveolar-lavage specimens, and by a factor of 16.0 (95% CI, 1.8 to 140; P = 0.02) in bronchial-wash specimens (see Table 1 and Fig. 2 in the Supplementary Appendix).
Other Outcomes
There were no significant differences between the groups in the change from baseline symptom scores, whether they were assessed with the use of visual-analogue scales or JACQ (Fig. 3B, and Table 1 in the Supplementary Appendix). The mean improvement in the AQLQ score was 0.55 in the mepolizumab group, as compared with 0.19 in the placebo group (mean difference between the groups, 0.35; 95% CI, 0.08 to 0.63; P = 0.02) (Fig. 3A). There were no significant between-group differences in changes from baseline values for FEV1 after bronchodilator use or PC20 (Fig. 3B, and Table 1 in the Supplementary Appendix).
There were no significant between-group differences in the changes in FEV1 or symptom scores after prednisolone treatment given at the end of the study period, as compared with prednisolone given at the beginning of the study period (Fig. 3B, and Table 2 in the Supplementary Appendix). Nine subjects who were assigned to the mepolizumab group had more than a 0.5-point decrease in JACQ scores after the 2-week course of prednisolone that was given before the initiation of mepolizumab therapy. These subjects had a similar within-group decrease in JACQ scores after the prednisolone treatment that was given at the end of the study (mean reduction, 1.2 points before mepolizumab therapy and 0.9 points afterward; mean difference, −0.3; 95% CI, −1.0 to 0.4; P = 0.32).
CT scans were obtained before and after the treatment phase of the study in 26 patients in each group. The results of all CT assessments are shown in Figure 3 and Table 1 in the Supplementary Appendix. There was a significant between-group difference in the change from baseline for airway wall area (mean between-group difference, 1.1 mm2 per square meter of body-surface area; 95% CI, 0.2 to 2.1; P = 0.02) and in the change in total area (mean between-group difference, 1.5 mm2 per square meter; 95% CI, 0.2 to 2.8; P = 0.03).
At the completion of the study, subjects were asked to guess their treatment assignment. Forty-five percent of the subjects were unsure of their treatment assignment, 36% guessed correctly, and 19% guessed incorrectly. There was no significant difference between the study groups in the proportions of patients choosing each response (P = 0.42).
SAFETY
Intravenous mepolizumab had an acceptable adverse-event and side-effect profile throughout the 12 months of treatment. The only serious adverse events reported were hospitalizations for acute severe asthma (Table 2). No local effects of infusion were observed. One subject was withdrawn from the study because of a transient maculopapular rash that developed 24 hours after the first infusion of mepolizumab.
Table 2. Reported Adverse Events during the 50-Week Treatment Phase of the Study.
| Event | Mepolizumab (N = 29) | Placebo (N = 32) |
|---|---|---|
| no. of patients (%) | ||
| Serious adverse event | ||
| Hospitalization for asthma | 3 (10) | 11 (34) |
| Adverse event | ||
| Chest pain | 1 (3) | 0 |
| Facial flushing | 2 (7) | 1 (3) |
| Erectile or ejaculatory dysfunction | 2 (7) | 0 |
| Rash | 2 (7) | 4 (12) |
| Pruritus | 2 (7) | 0 |
| Subconjunctival hemorrhage | 0 | 1 (3) |
| Conjunctivitis | 1 (3) | 1 (3) |
| Upper respiratory tract infection | 1 (3) | 4 (12) |
| Shingles | 1 (3) | 0 |
| Fatigue | 2 (7) | 1 (3) |
| Night sweats | 0 | 1 (3) |
| Nasal ulcer | 0 | 1 (3) |
| Nasal polypectomy (elective) | 0 | 1 (3) |
| Musculoskeletal pain | 1 (3) | 2 (6) |
| Gout | 0 | 1 (3) |
| Delayed wound healing | 0 | 1 (3) |
| Paresthesia of the hands | 1(3) | 0 |
| Loss of taste | 0 | 1 (3) |
| Abdominal pain | 0 | 1 (3) |
| Diarrhea | 0 | 1 (3) |
| Syncope | 0 | 1 (3) |
| Dizziness | 1 (3) | 0 |
DISCUSSION
We found that mepolizumab treatment significantly reduced the number of asthma exacerbations that resulted in the prescription of corticosteroid therapy and increased asthma-related quality of life in subjects who had refractory eosinophilic asthma and a history of recurrent exacerbations. There was no significant improvement in symptoms or in FEV1, measures that are commonly used for quantifying asthma control. Treatment effectively lowered eosinophil counts in the blood and sputum and was well tolerated over the course of the 12-month study period.
Previous studies of mepolizumab treatment in patients with less severe asthma have been too short to evaluate the effect of treatment on the frequency of exacerbations, although the largest study to date, like ours, showed a reduction in severe exacerbations, which approached significance.9 The lack of effect of mepolizumab on symptoms, FEV1, and airway responsiveness in our study is also consistent with the results of previous studies.8-11 Treatment had a larger effect on eosinophil numbers in blood and sputum samples than on those in biopsy specimens, findings that are consistent with earlier work,10 although sputum eosinophilia was present in 36% of the exacerbations, despite mepolizumab therapy. Further studies are required to investigate the mechanisms underlying heterogeneity in the biologic response to mepolizumab and the relative resistance of eosinophils in tissue to anti–interleukin-5.
We have previously shown that the main effect of a management strategy that suppresses eosinophilic airway inflammation is a reduction in the frequency of exacerbations and have suggested a causal link between eosinophilic airway inflammation and exacerbations.6 This view is strongly supported by the results of the current study, since mepolizumab is a selective inhibitor of eosinophilic airway inflammation.
Mepolizumab treatment had no effect on asthma symptoms, FeNO, or lung function, although these measures did improve in some subjects after prednisolone treatment, even when the prednisolone was administered after mepolizumab treatment, when eosinophilic airway inflammation was suppressed. This finding suggests that symptoms, FeNO, and lung function can be disassociated from eosinophilic inflammation and are improved with corticosteroid treatment through another mechanism. Modulation of the interaction between airway smooth muscle and infiltrating mast cells18 is a possible explanation for the effect of prednisolone on lung function and symptoms. The absence of an association between the risk of exacerbations and eosinophilic airway inflammation, on the one hand, and lung function and day-to-day clinical manifestations of asthma, on the other, has important implications for the way asthma is managed and assessed in patients with refractory asthma. Our study showed a small but significant improvement in asthma-related quality of life with mepolizumab therapy, perhaps reflecting the value to patients of the prevention of exacerbations.
We found that airway-wall thickness and total wall area, as measured by CT, were reduced in subjects who were treated with mepolizumab as compared with those who were given placebo. The CT scans were obtained after a 2-week course of prednisolone and after administration of bronchodilators, so the findings are unlikely to be confounded by bronchomotor tone and acute airway inflammation. Whether the changes in airway-wall dimensions translate into important long-term clinical effects requires further investigation.
The therapeutic effect that was seen with mepolizumab treatment shows how we can learn more about the pathogenesis of different airway responses by studying selective inhibitors of inflammation. The patients who were included in this study had refractory eosinophilic asthma despite maximum tolerated therapy, which in many cases included regular use of oral corticosteroids. Their asthma resembled the exacerbation-prone phenotype of severe asthma, as described by Moore et al.,19 and the phenotype of asthma with predominant eosinophilic inflammation, which we have described.20 Our results should not be extrapolated beyond the highly selected group of patients we recruited for this study. However, further clinical trials should be performed to establish more clearly the risks and benefits of mepolizumab treatment in a wider population of patients. Many patients with fluctuating respiratory symptoms and eosinophilic airway inflammation do not meet current criteria for a diagnosis of asthma,18,21-23 and we have previously argued that new ways of classifying airway disease are needed to allow proper evaluation of new therapies.24 Investigators planning future trials should be mindful of disease characteristics that suggest a response to therapy and should include patients with airway disease and eosinophilic airway inflammation rather than only those who meet arbitrary physiological criteria.
Supplementary Material
Acknowledgments
Supported by an unrestricted educational grant from Glaxo-SmithKline.
Dr. Brightling reports receiving lecture fees, consulting fees, and grant support from AstraZeneca, GlaxoSmithKline, and MedImmune; Dr. Wardlaw, receiving consulting fees from MedImmune and GlaxoSmithKline, lecture fees from Merck, and grant support from GlaxoSmithKline and AstraZeneca; Dr. Pavord, receiving lecture fees from AstraZeneca, GlaxoSmithKline, and Aerocrine, consulting fees from Merck, GlaxoSmithKline, Wyeth, and AstraZeneca, and grant support from GlaxoSmith-Kline; Dr. Bradding, receiving consulting fees from GlaxoSmith-Kline; and Drs. Sousa and Marshall, being employees of Glaxo-SmithKline and holding equity in that company. No other potential conflict of interest relevant to this article was reported.
We thank Debbie Parker, Natalie Neale, and Katie Roach for their help in the laboratory; Maria Shelley, Sue McKenna, Hilary Pateman, Michelle Bourne, and Amisha Singapuri for their help with assessments of the patients; Anita Raj, Shiron Saha, Mona Bafadhel, Jon Bennett, James Entwisle, and Dean Mawby for their help with preparation of study drugs, bronchoscopy, and CT; and the study participants for their unfailing commitment and enthusiasm.
References
- 1.Global Initiative for Asthma (GINA) [Accessed February 9, 2009];Global strategy for asthma management and prevention. 2007 http://www.ginasthma.org
- 2.FitzGerald JM, Gibson PG. Asthma exacerbations. 4: Prevention. Thorax. 2006;61:992–9. doi: 10.1136/thx.2005.045195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houston JC, De Navasquez S, Trounce JR. A clinical and pathological study of fatal cases of status asthmaticus. Thorax. 1953;8:207–13. doi: 10.1136/thx.8.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jatakanon A, Lim S, Barnes PJ. Changes in sputum eosinophils predict loss of asthma control. Am J Respir Crit Care Med. 2000;161:64–72. doi: 10.1164/ajrccm.161.1.9809100. [DOI] [PubMed] [Google Scholar]
- 5.Deykin A, Lazarus SC, Fahy JV, et al. Sputum eosinophil counts predict asthma control after discontinuation of inhaled corticosteroids. J Allergy Clin Immunol. 2005;115:720–7. doi: 10.1016/j.jaci.2004.12.1129. [DOI] [PubMed] [Google Scholar]
- 6.Green RH, Brightling CE, McKenna S, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360:1715–21. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 7.Jayaram L, Pizzichini MM, Cook RJ, et al. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. Eur Respir J. 2006;27:483–94. doi: 10.1183/09031936.06.00137704. [DOI] [PubMed] [Google Scholar]
- 8.Flood-Page P, Menzies-Gow A, Phipps S, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–36. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flood-Page P, Swenson C, Faiferman I, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. 2007;176:1062–71. doi: 10.1164/rccm.200701-085OC. [DOI] [PubMed] [Google Scholar]
- 10.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167:199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- 11.Leckie MJ, ten Brinke A, Khan J, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144–8. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 12.O’Byrne PM. The demise of anti IL-5 for asthma, or not. Am J Respir Crit Care Med. 2007;176:1059–60. doi: 10.1164/rccm.200708-1264ED. [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162:2341–51. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 14.Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the Asthma Control Questionnaire. Respir Med. 2005;99:553–8. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Juniper EF, Guyatt GH, Ferrie PJ, Griffith LE. Measuring quality of life in asthma. Am Rev Respir Dis. 1993;147:832–8. doi: 10.1164/ajrccm/147.4.832. [DOI] [PubMed] [Google Scholar]
- 16.British Thoracic Society Scottish Intercollegiate Guidelines Network British guideline on the management of asthma. Thorax. 2008;63(Suppl 4):iv1–iv121. doi: 10.1136/thx.2008.097741. [DOI] [PubMed] [Google Scholar]
- 17.Keene ON, Calverley PM, Jones PW, Vestbo J, Anderson JA. Statistical analysis of exacerbation rates in COPD: TRISTAN and ISOLDE revisited. Eur Respir J. 2008;32:17–24. doi: 10.1183/09031936.00161507. [DOI] [PubMed] [Google Scholar]
- 18.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 19.Moore WC, Bleecker ER, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–13. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–24. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siva R, Green RH, Brightling CE, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J. 2007;29:906–13. doi: 10.1183/09031936.00146306. [DOI] [PubMed] [Google Scholar]
- 22.Brightling CE, Ward R, Wardlaw AJ, Pavord ID. Airway inflammation, airway responsiveness and cough before and after inhaled budesonide in patients with eosinophilic bronchitis. Eur Respir J. 2000;15:682–6. doi: 10.1034/j.1399-3003.2000.15d10.x. [DOI] [PubMed] [Google Scholar]
- 23.Brightling CE, Monteiro W, Ward R, et al. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2000;356:1480–5. doi: 10.1016/S0140-6736(00)02872-5. [DOI] [PubMed] [Google Scholar]
- 24.Wardlaw AJ, Silverman M, Siva R, Pavord ID, Green R. Multi-dimensional phenotyping: towards a new taxonomy for airway disease. Clin Exp Allergy. 2005;35:1254–1262. doi: 10.1111/j.1365-2222.2005.02344.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.