Abstract
Background
Airway smooth muscle hyperplasia is a feature of asthma, and increases with disease severity. CCR3-mediated recruitment of airway smooth muscle progenitors towards the airway smooth muscle bundle has been proposed as one possible mechanism involved in airway smooth muscle hyperplasia. Mast cells are microlocalized to the airway smooth muscle bundle and whether mast cells influence CCR3-mediated migration is uncertain.
Methods
We examined the expression of CCR3 by primary cultures of airway smooth muscle cells from asthmatics and nonasthmatics. CCR3 function was examined using intracellular calcium measurements, chemotaxis, wound healing, cell proliferation and survival assays. We investigated the recovery and function of both recombinant and airway smooth muscle-derived CCL11 (eotaxin) after co-culture with β-tryptase and human lung mast cells.
Results
Airway smooth muscle expressed CCR3. Airway smooth muscle CCR3 activation by CCL11 mediated intracellular calcium elevation, concentration-dependent migration and wound healing, but had no effect on proliferation or survival. Co-culture with β-tryptase or mast cells degraded recombinant and airway smooth muscle-derived CCL11, and β-tryptase inhibited CCL11-mediated airway smooth muscle migration.
Conclusions
CCL11 mediates airway smooth muscle migration. However co-culture with β-tryptase or mast cells degraded recombinant and airway smooth muscle-derived CCL11 and inhibited CCL11-mediated airway smooth muscle migration. Therefore these findings cast doubt on the importance of the CCL11/CCR3 axis in the development of airway smooth muscle hyperplasia in asthma.
Keywords: airway smooth muscle, asthma, CCL11, CCR3, mast cells
Asthma is characterized by typical symptoms, airway hyperresponsiveness (AHR) and variable airflow obstruction, which can become fixed in severe disease. In addition there is associated airway inflammation together with features of tissue repair known as remodelling (1). The airway inflammation in asthma is typically eosinophilic with increased expression of Th2 cytokines. Importantly the number of mast cells localized within the airway smooth muscle (ASM) bundle is increased in asthma and is related to the degree of AHR (2-5).
Airway remodelling in asthma encompasses several structural changes in the airway wall including reticular lamina and basement membrane thickening, an increased number of subepithelial myofibroblasts and increased ASM mass (6). This latter feature is due to a combination of both ASM hyperplasia (7) and hypertrophy, which increases with disease severity and is associated with fixed airflow obstruction (6, 8).
The cause of ASM hyperplasia in asthma is unknown and is often attributed to increased proliferation. Indeed ASM proliferation is increased in ex vivo asthmatic ASM (9, 10), but several reports have been unable to demonstrate increased ASM proliferation in vivo (7, 8, 11). An alternative explanation is that ASM progenitors either located within the airway wall or derived from peripheral blood fibroblast progenitors (fibrocytes) (12), migrate to the ASM bundle and differentiate into ASM. In support of this view myofibroblasts expressing fibrocyte markers have been identified following ovalbumin (OVA) challenge in a mouse model of asthma and after allergen challenge in human disease (13). We have demonstrated that mast cell and ASM-derived CCL19, a CCR7 ligand, mediates ASM migration (14). The CCR3 ligand CCL11 (eotaxin) is released by ASM (15, 16) and in bronchial biopsies the intensity of expression increases with disease severity (6) suggesting that CCR3-mediated ASM migration may be important in severe asthma.
We hypothesized that the CCR3/CCL11 axis mediates ASM migration. To test our hypothesis we examined ASM CCR3 expression, function and its modulation by mast cells.
Materials and methods
Subjects
Asthmatic subjects and nonasthmatic controls were recruited from Leicester, UK. Subjects with asthma had a consistent history and had objective evidence of asthma, as indicated by one or more of the following: (i) methacholine airway hyperresponsiveness (PC20FEV1 < 8 mg/ml); (ii) greater than 15% improvement in FEV1 15 min after administration of 200 μg of inhaled salbutamol; or (iii) greater than 20% of maximum within-day amplitude from twice daily peak expiratory flow measurements over 14 days. The study was approved by the Leicestershire Ethics Committees and all patients gave their written informed consent.
ASM and mast cell isolation and culture
Pure ASM bundles in bronchial biopsies obtained from fibreoptic bronchoscopy (n = 20, 18 asthmatic subjects, 2 nonasthmatic) and additional airways isolated from lung resection (n = 23) were dissected free of surrounding tissue. Primary ASM was cultured and characterized as previously described (4). Clinical characteristics of the asthmatic and nonasthmatic subjects from which primary ASM was derived are as shown in Table 1.
Table 1.
Subject characteristics of airway smooth muscle donors [mean (SEM)]
| Nonasthmatic | Asthmatic | |
|---|---|---|
| Number | 25 | 18 |
| Male (n) | 18 | 11 |
| Age (years) | 62 (3) | 53 (4) |
| FEV1 | 2.2 (0.1) | 2.2 (0.2) |
| FEV1% predicted | 76 (5) | 74 (6) |
| FEV1/FVC | 74 (4) | 65 (3) |
Human lung mast cells (HLMC) were isolated and cultured from nonasthmatic lung (n = 12) as previously described (17).
CCR3 expression
Flow cytometry
ASM were stained with CCR3 monoclonal antibody (mAb) or appropriate isotype control (R&D Systems, Abingdon, Oxfordshire, UK), indirectly labelled with R-Phycoerythrin (RPE), then analysed using single colour flow cytometry on a FACScan (BD, Oxford, UK).
Immunofluorescence
ASM were grown to confluence on chamber slides and serum deprived for 24 h. The cells were labelled with CCR3 mAb or appropriate isotype control, and indirectly labelled with RPE. Cells were counterstained with 4′,6′-diamidino-2 phenylindole (DAPI, Sigma, Gillingham, UK).
Functional assessment of ASM CCR3
Calcium imaging
Changes in cytosolic Ca2+ concentration ([Ca2+]i) in ASM cells in response to CCL11 (100 ng/ml) were measured by ratiometric imaging on FURA-2-loaded cells using Openlab software (Improvision, Coventry, UK). This was converted to [Ca2+]i using a calibration kit (Invitrogen Molecular Probes, Paisley, UK).
Chemotaxis assay
We used a validated chemotaxis assay (14). In brief ASM cells were seeded onto 8-rectangular well plates coated with 10 μg/ml fibronectin at a density of 0.25 × 106 cells/well, allowed to adhere overnight, then serum deprived in ITS media for 24 h prior to experimentation. Cells were removed by scraping between the top of the well and a line predrawn across the width of the well, on the underside of the plate, 22 mm from the bottom of the well. Cell debris was removed by washing with ITS media. Blotting paper (25 mm × 6 mm; Sigma) was then placed along the upper edge of the well, secured in place using silicon grease. Recombinant CCL11 (12.5–300 ng; R&D) or ITS control media, plus β-tryptase (0.5–1.25 μg, Europa Bioproducts, Ely, Cambridgeshire, UK) where appropriate, was impregnated onto blotting paper from which it diffused into the media. The number of cells that moved towards the resultant concentration gradient of CCL11 were enumerated after 6h by a blinded observer. Where appropriate experiments were performed in the presence of CCR3 blocking antibody (8 μg/ml; Millenium, Cambridge, MA, USA) or isotype control (8 μg/ml, Dako, Ely).
Wound healing assay
ASM cells were seeded as per the chemotaxis assay. Wounds were introduced using a sterile 200 μl pipette tip. The number of cells that moved into the wound in the presence of ITS control media and vehicle/CCL11 (25–200 ng/ml) in the presence or absence of CCR3 blocking antibody or isotype control (10 μg/ml, R&D) over 6 h were counted by a blinded observer (14).
Cell metabolic activity
ASM cell metabolic activity was assessed using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H tetrazolium inner salt (MTS) assay, according to manufacturer’s instructions (Promega, Southampton, UK), following incubation with 12.5–100 ng/ml CCL11 in 10% FBS media or ITS media for 24 h.
Proliferation and survival
ASM proliferation was assessed by cell counts following incubation with 100 ng/ml CCL11 in 10% FBS media or ITS media for 24 h and using the CellTrace CFSE Cell Proliferation Kit according to manufacturer’s instructions (Invitrogen Molecular Probes). Cells treated with 50 μg/ml mito-mycin C (Sigma) for 3 h to mitotically arrest cells at the parent population, prior to readdition of 10% FBS media, were cultured in parallel to cells exposed to 10% FBS media ± 100 ng/ml CCL11 for 72 h.
The percentage of apoptotic ASM cells exposed to 10% FBS media ± 100 ng/ml CCL11 for 24 h was identified by DAPI staining of cell nuclei, and by staining with FITC-conjugated Annexin V (1 μl/200 μl binding buffer, BD Bioscience) ± propidium iodide (PI, 0.5 μg/ml, BD Bioscience), prior to analysis on a FACScan (BD).
Effect of β-tryptase and HLMC on recombinant and ASM-derived CCL11
Airway smooth muscle cells were grown to confluence and growth arrested in ITS media for 24 h. These ASM cultures were co-cultured at 37 °C with or without β-tryptase (0.5 μg/ml, equivalent to the amount present in 5 × 104 mast cells) (18) and/or leupeptin (20 nM/ml) for 3 days; or for 1, 3 and 7 days with: (i) HLMC lysates (in a ratio of HLMC: ASM, 1: 2–1: 16), (ii) whole unstimulated HLMC, (iii) HLMC sensitized with human myeloma immunoglobulin E (IgE) (2.5 μg/ml) (Calbiochem-Novabiochem, Nottingham, UK) and (iv) sensitized with IgE and activated with goat polyclonal anti-human IgE (1: 1000 dilution) (Sigma). The ratio of HLMC:ASM for the whole cell co-cultures was 1:4.
HLMC lysates and unstimulated whole cells (ratio of HLMC : ASM, 1 : 4) were incubated with CCL11 (50 pg/ml) for 2 h at 37 °C with various concentrations of leupeptin (0–80 nM/ml).
The concentration of recovered CCL11 for all of the above conditions was measured using a commercial ELISA (BD Pharmingen, Cowley, Oxfordshire, UK) with a limit of detection of 6.25 pg/ml and inter and intra-assay variation <5%.
To assess the effect of HLMC on CCL11 mRNA expression by ASM, supernatants from IgE/anti-IgE activated HLMC (10 × 106 cells pooled from three donors) were incubated with ASM from asthmatic (n = 3) and nonasthmatic donors (n = 3) for 6 and 24 h. The proportion of HLMC: ASM cells was 1: 4. RNA expression levels of CCL11 extracted from the ASM was examined using the Human Genome U133A probe array (GeneChip, Affymetrix, Santa Clara, CA, USA). RNA was prepared and analysed as described (19). Hybridized biotinylated cRNA was stained with streptavidin-phycoerythrin (Molecular Probes, Eugene, OR, USA), scanned with a HP Gene Array Scanner, and data analysed using the GeneChip Analysis Suite 4.0 (Affymetrix) as described (19).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 4 (GraphPad software, San Diego, CA, USA). Data are presented as mean ± SEM. Data was analysed by anova across groups and t-tests between groups with Tukey’s correction for multiple comparisons. Differences were considered significant when P < 0.05.
Results
CCR3 expression by ASM
Flow cytometry and immunofluorescence
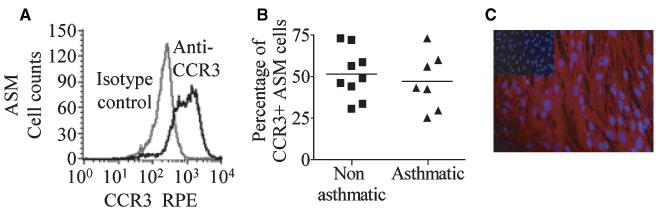
The proportion of primary cultured ASM cells that expressed CCR3 on their cell surface was 50 ± 4% (n = 16) (Fig. 1A). CCR3 expression by permeabilized ASM was 79 ± 4% (n = 6). There was no difference in expression between ASM derived from asthmatics or nonasthmatic controls (52 ± 5%, n = 9, vs 47 ± 6%, n = 7, respectively; P = 0.59, Fig. 1B) and CCR3 expression was not upregulated through activation by CCL11 (data not shown). CCR3 expression by ASM was also confirmed by immunofluorescence (Fig. 1C).
Figure 1.
CCR3 expressed by ASM. The example fluorescent histograms represent populations of (A) primary cultured ASM cells that were CCR3+ (black line) plotted with the corresponding isotype control (grey line), (B) percentage of CCR3+ ASM cells from asthmatic donors vs nonasthmatic controls, (C) CCR3 expression by ASM was confirmed by immunofluorescence (nuclei stained blue, CCR3 red; isotype control shown as insert).
ASM CCR3 functional response to CCL11 activation
Calcium imaging
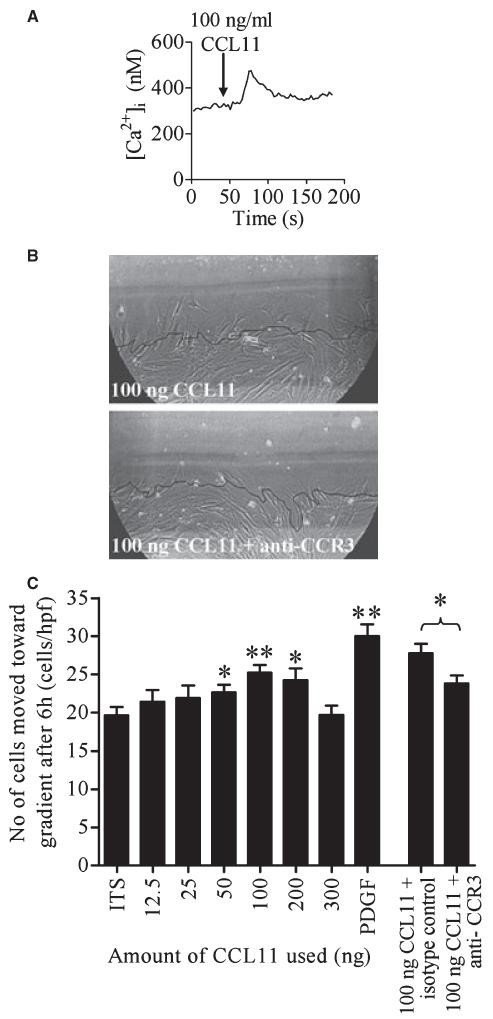
Following ASM activation by CCL11, 58% of cells responded with a transient mean increase in [Ca2+]i of 244 ± 38 nM (n = 26 individual cells, from three nonasthmatic donors, Fig. 2A).
Figure 2.
ASM CCR3 is functional. (A) Addition of 100 ng/ml CCL11 (indicated by arrow) stimulated [Ca2+]i elevation in ASM cells. (B) Representative pictures of ASM chemotaxis after 6 h in one high-powered field (hpf) towards 100 ng CCL11 or 100 ng CCL11 + anti-CCR3. Black line = position of cells at 0 h. (C) Concentration-dependent chemotaxis of ASM towards CCL11, PDGF (10 ng) is a positive control. Data are presented as mean SEM for four donors, comparisons made to ITS alone. *P < 0.05, **P < 0.01.
Chemotaxis
CCL11 mediated dose-dependent ASM (n = 4, P = 0.01) migration (Fig. 2B,C). The number of ASM cells per high-powered field that migrated to CCL11 (100 ng on blot) was blocked using a CCR3 blocking antibody (23.8 ± 1.1 vs 27.8 ± 1.2; P = 0.02; n = 4, Fig. 2B). This directional movement was predominately due to chemotaxis rather than chemokinesis, as addition of CCL11 directly to the media did not result in significantly more ASM movement than ITS media alone (n = 5; data not shown). ASM from nonasthmatic donors was used for chemotaxis assays.
Wound healing
Wound healing was promoted by 10 ng/ml PDGF (35.4 ± 2.0 cells/hpf vs 27.1 ± 1.1 in control; P < 0.001; n = 4). Recombinant CCL11 promoted wound healing in a concentration-dependent manner, but was only significant for CCL11 100 ng/ml (32.6 ± 1.1 cells/hpf vs 27.1 ± 1.1 in control; P = 0.001; n = 4). Anti-CCR3 did not significantly inhibit wound healing by recombinant CCL11 (data not shown), but did significantly reduce the wound healing response (mean difference 6.0 ± 2.4 cells/hpf; P = 0.015; n = 5) in the presence of ITS media alone, suggesting that the endogenous release of CCL11 is important in mediating wound repair. ASM from nonasthmatic donors was used for wound healing assays.
Metabolic activity
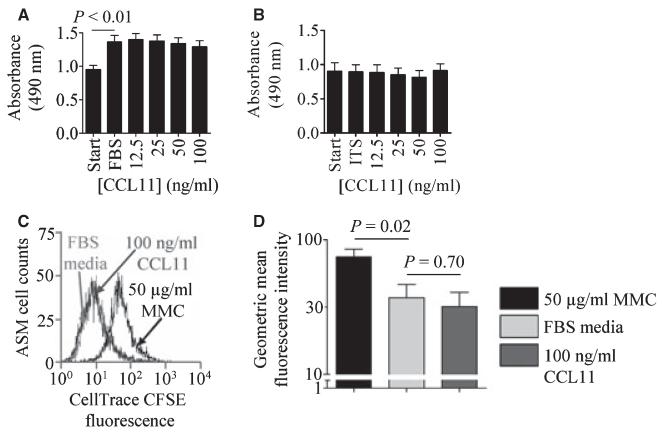
The absorbance by formazan seen at 490 nm in the MTS assay was increased in ASM in media supplemented with FBS compared to ASM cells in serum free media (mean difference 0.39 ± 0.07 absorbance units, P < 0.01, n = 10, Fig. 3A). Recombinant CCL11 had no effect on the MTS assay in the presence of FBS media (n = 10, Fig. 3A) or ITS media (n = 6, Fig. 3B). No difference in the effect of CCL11 was observed between ASM cells derived from nonasthmatic vs asthmatic subjects.
Figure 3.
CCL11 has no effect on ASM cell metabolic activity and proliferation. ASM cell metabolic activity in (A) serum free media vs FBS media CCL11 (data are presented as mean SEM for 10 donors) and (B) serum free media vs ITS media CCL11 (data are presented as mean SEM for 6 donors). (C) Histogram illustrating CFSE fluorescence in ASM cells incubated with 50 μg/ml MMC, black line, or FBS media 100 ng/ml CCL11 for 72 h, light grey and dark grey lines respectively, (D) ASM cell proliferation was observed after 72 h (n = 7), but was unaffected by incubation with CCL11 (n = 6). Data are presented as geometric mean ± SEM.
Proliferation
By assessing cell numbers in the presence of FBS media (n = 8) or ITS media (n = 6) we saw no effect of 100 ng/ml CCL11 after 24 h, with no difference between nonasthmatic or asthmatic subjects (data not shown). Using the Cell Trace CFSE Cell Proliferation assay cell proliferation was seen after 72 h in the presence of 10% FBS media compared to MMC treated cells (mean decrease in fluorescence intensity 37.7 ± 4.0, P < 0.05, n = 7) however this was unaffected by incubation with 100 ng/ml CCL11 (n = 6) (Fig. 3C,D) with no difference observed between ASM cells derived from nonasthmatic vs asthmatic subjects.
Survival
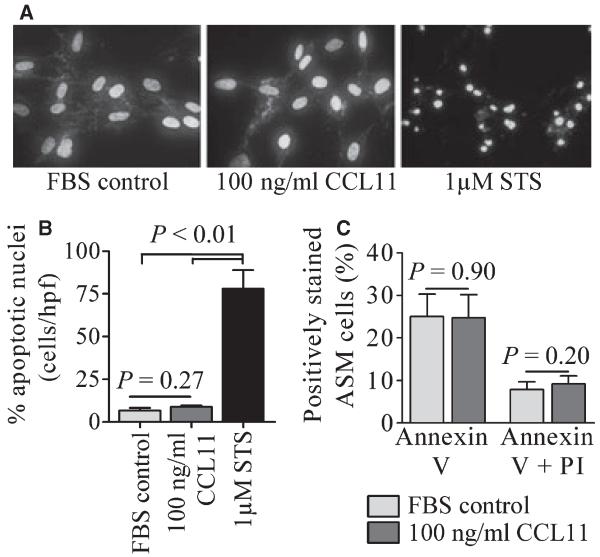
The percentage of ASM nuclei showing nuclear condensation and fragmentation characteristic of apoptosis, detected by DAPI staining, was unaffected by incubation with 100 ng/ml CCL11 for 24 h (control, 6.6 ± 1.6% vs 100 ng/ml CCL11, 8.7 ± 1.0, n = 8). In marked contrast in the presence of staurosporine (STS, 1 μM, 20 h), a positive control, 77.9 ± 10.9% of ASM cells showed nuclear morphology characteristic of cells undergoing apoptosis (n = 8) (Fig. 4A,B).
Figure 4.
CCL11 has no effect on ASM survival. (A) representative photographs showing DAPI staining of nuclear morphology under control conditions, following incubation with: 100 ng/ml CCL11 for 24 h, both showing normal nuclear morphology, or STS as a positive control, (B) percentage of apoptotic cells in each condition, n = 8 donors, (C) detection of annexin V+/PI− and annexin V+/PI+ ASM cells using two colour flow cytometry, n = 9 donors. Data are presented as mean ± SEM. Statistical differences were assessed using unpaired t-tests.
The above data was confirmed using annexin V/PI staining of ASM cells. The percentage of annexin V+/PI− [early apoptotic, (20)] ASM cells was unaffected following incubation with 100 ng/ml CCL11 for 24 h (control, 25.0 ± 5.3% vs 100 ng/ml CCL11, 24.7 ± 5.5% cells, n = 9), the same is seen for annexin V+/PI+ [late apoptotic/necrotic (20)] ASM cells (control, 7.8 ± 1.8% vs 100 ng/ml CCL11, 9.2 ± 1.9% cells, n = 9) (Fig. 4C).
Using both DAPI and annexin V/PI staining no difference in the effect of CCL11 was observed between cells derived from asthmatic subjects compared to non-asthmatic controls (data not shown).
Modulation of recombinant and ASM-derived CCL11 by tryptase and mast cells
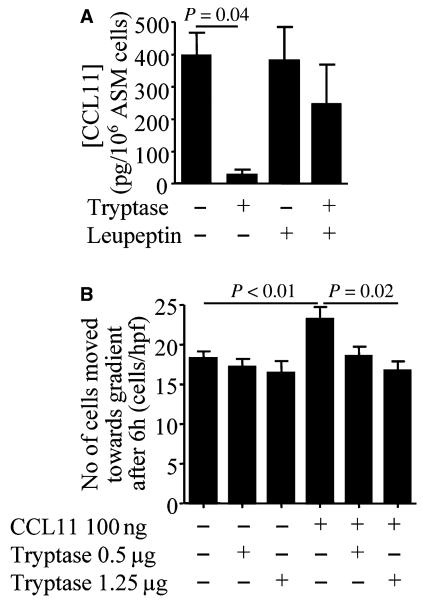
The concentration of CCL11 in ASM supernatant cultured for 3 days (400 ± 69 pg/106 cells) was attenuated by the presence of β-tryptase [27 ± 17 pg/106 cells; mean difference (95% CI) 373 (32–713); P = 0.04] (Fig. 5A). This effect was attenuated by leupeptin (Fig. 5A).
Figure 5.
β-tryptase degrades CCL11 and inhibits CCL11-mediated ASM migration. (A) The concentration of CCL11 (pg/106 cells) in supernatants from ASM cultures for 3 days alone, with β-tryptase and/or leupeptin (20 nM) (n = 3). (B) Chemotaxis of ASM towards CCL11 (100 ng on blot) was inhibited by β-tryptase (n = 4). Data are presented as mean ± SEM.
Chemotaxis assays confirmed that chemotaxis was promoted by 100 ng CCL11 (23.2 ± 1.5 cells/hpf vs 18.3 ± 0.8 in control; P < 0.01) and β-tryptase reduced the migratory response significantly (mean difference 6.5 ± 2.8 cells/hpf; P = 0.02; n = 4) (Fig. 5B). β-tryptase did not significantly inhibit chemotaxis in the absence of CCL11.
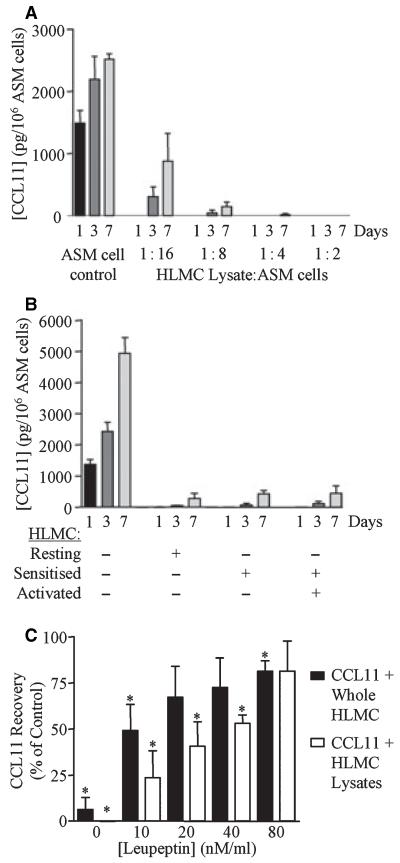
Co-culture of ASM with either HLMC lysates, unstimulated or stimulated whole HLMC reduced markedly the CCL11 concentration in the co-culture supernatant compared to ASM culture alone (Fig. 6A,B). The percentage of recombinant CCL11 recovery compared to control was also markedly reduced following incubation with HLMC lysates and whole HLMC. The addition of leupeptin inhibited this reduction in CCL11 concentration in a concentration-dependent manner (Fig. 6C).
Figure 6.
Recombinant and ASM-derived CCL11 degraded in co-culture with HLMC. (A) The concentration of CCL11 in day 1, 3, and 7 ASM culture supernatants alone and co-cultured with HLMC lysates in the proportion of HLMC: ASM 1 : 2-1 : 16 (n = 3; P < 0.001), (B) co-cultured with whole HLMC after sensitization and/or activation (n = 6, P < 0.001) and (C) the % recovery of recombinant CCL11 after incubation with whole HLMC and lysates for 2 h ± increasing concentrations of leupeptin. *P < 0.05 paired t-test. Data are presented as mean ± SEM for three donors.
Quantified by gene array analysis, CCL11 mRNA was present in all ASM donors (75 ± 15% of GAPDH mRNA, asthmatic, n = 3 vs 24 ± 6%, nonasthmatic, n = 3, P = 0.04), but was neither up nor down-regulated in co-culture with the HLMC supernatants (data not shown).
Discussion
We found that ASM cells express CCR3 and that CCL11 mediates ASM migration. Importantly we found that β-tryptase, and stimulated or unstimulated HLMC degrade recombinant and ASM-derived CCL11 and that β-tryptase inhibited CCL11-mediated ASM migration. In addition HLMC supernatants did not up- or down-regulate CCL11 mRNA expression. We found that the CCR3/CCL11 axis has the potential to mediate ASM migration and repair in asthma, but mast cell localization to the ASM-bundle is a feature of the disease and it is therefore likely that mast cells will have a profound effect on local CCL11 concentrations. Therefore, taken together our findings question the biological importance of ASM-derived CCL11 in ASM hyperplasia in asthma.
CCR3 is expressed preferentially, but not exclusively, by Th2-lymphocytes (21), basophils (22) and mast cells (17, 23). The number of CCR3+ cells is increased in bronchial biopsies in asthma (24) and CCR3 is considered an important potential therapeutic target in asthma and other allergic diseases (25). There is increasing recognition that structural cells can express functional chemokine receptors. Indeed bronchial epithelial cells (26), vascular (27) and, in one report, ASM expresses CCR3 (28). Here we confirm that ASM from both asthmatics and nonasthmatics equally express functional CCR3. However we found that CCL11 had no effect on ASM proliferation. There is a paucity of data on the effect of chemokine receptor activation on ASM proliferation with a single report that CCR7 activation did not affect proliferation (14). In contrast there are several studies of chemokine receptor activation modulating vascular smooth muscle proliferation with CCR2 (29), CXCR6 (30) and CX3CR1 (31) activation increasing proliferation and CCR3 having no effect (32). In addition we report for the first time that ASM metabolic activity and survival was not affected by CCR3 activation. In keeping with an earlier (28) report we found that CCL11 mediated increased [Ca2+]i, concentration-dependent migration and extended these findings to support a role in wound healing. The time course for the both assays was 6 h, which is not sufficient time to observe proliferation by cell counts. Therefore proliferation alone cannot explain our observations. Thus CCR3 has the potential to play a role in ASM repair and hyperplasia in asthma.
If CCR3 expression by ASM is important in asthma then the CCR3 ligands should be differentially expressed in health and disease. Primary ASM from asthmatics and nonasthmatics express CCL11, but it is contentious whether the expression is increased in disease with one report supporting (33) and two refuting that CCL11 release is increased in asthma (4, 16) and here we found increased CCL11 mRNA expression in primary ASM from asthmatics compared to nonasthmatics. However perhaps more importantly, CCL11 expression in bronchial biopsies from asthmatics increases with worsening severity of disease (6). Even though CCL11 is expressed by ASM paradoxically there is a marked paucity of eosinophils in the ASM bundle. One possible explanation for this apparent anomaly is that mast cells are microlocalized within the ASM bundle and that mast cell products degrade CCL11.This view is supported by one study suggesting that β-tryptase inactivates CCL11 (34). Here we have extended this earlier observation by examining the effect of mast cell-ASM co-culture upon CCL11 production and recovery. We confirm that β-tryptase, and for the first time show that mast cells degrade recombinant and ASM-derived CCL11. These effects were blocked by leupeptin. Importantly CCL11 mRNA expression was not affected by co-culture confirming that the reduction in CCL11 concentration in ASM supernatants is unlikely to be due to reduced synthesis. β-tryptase attenuated markedly CCL11-mediated ASM migration. These findings support the view that mast cells can modulate the functional consequences of CCL11 released by ASM.
Our observations question the role of the CCL11/CCR3 axis in the recruitment of ASM and its progenitors towards the ASM bundle in asthma. However, the control of the migration of mesenchymal cells within the airway is likely to be under the influence of a variety of mediators, which in turn are themselves controlled in terms of their synthesis, release and degradation. The concept that ASM hyperplasia may be a consequence of fibrocytes trafficking to the airway remains plausible and recent evidence supports a role for CCR7, in mediating ASM migration towards the ASM bundle (14). In contrast to CCL11, CCL19 a ligand for CCR7 is not degraded by mast cells but is expressed and released by ASM and mast cells. In addition recombinant and mast cell-derived CCL19 mediated ASM migration. Future studies that examine the role of other chemokine receptors involved in ASM migration need to consider the complexity of cellular interactions and the microenvironment relevant to the airway compartment to be studied.
One criticism of our study is that the HLMC were derived from lung resection tissue and not asthmatic subjects. Currently we are limited by our inability to isolate sufficient mast cells from the asthmatic airway. However, we feel that using HLMC that are sensitized and IgE/anti-IgE activated is likely to be reflective of asthmatic mast cells. The proportion of mast cells within the ASM-bundle is unknown, but we have estimated the ratio of mast cells : ASM cells based on our experience with the assessment of bronchial biopsies and we are confident that the range we have chosen captures the proportions of these cells in the asthmatic airway.
In conclusion, we have found that ASM express CCR3. The CCR3/CCL11 axis mediated ASM migration. Critically, recombinant and ASM-derived CCL11 was inactivated by β-tryptase and co-culture with mast cells. Mast cells are microlocalized to the ASM-bundle in asthma. Therefore our findings question the importance of ASM-expressed CCR3 in the development of ASM hyperplasia in asthma.
Acknowledgments
We are grateful to Millenium for kindly providing the CCR3 mAb. This work was supported by Asthma UK, a DoH Clinician Scientist Award and a Wellcome Trust Senior Clinical Fellowship.
Abbreviations
- AHR
airway hyperresponsiveness
- ASM
airway smooth muscle
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- DAPI
4′,6′-diamidino-2 phenylindole
- FEV1
forced expiratory volume in one second
- FITC
fluorescein isothiocyanate
- HLMC
human lung mast cell
- hpf
high-powered field
- MMC
mitomycin C
- MTS
(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- OVA
ovalbumin
- PI
propidium iodide
- RPE
R-pycoerythrin
- STS
staurosporine
References
- 1.Wardlaw AJ, Brightling CE, Green R, Woltmann G, Bradding P, Pavord ID. New insights into the relationship between airway inflammation and asthma. Clin Sci (Lond) 2002;103:201–211. doi: 10.1042/cs1030201. [DOI] [PubMed] [Google Scholar]
- 2.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-Cell Infiltration of Airway Smooth Muscle in Asthma. N Engl J Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 3.Berger P, Girodet PO, Begueret H, Ousova O, Perng DW, Marthan R, et al. Tryptase-stimulated human airway smooth muscle cells induce cytokine synthesis and mast cell chemotaxis. FASEB J. 2003;17:2139–2141. doi: 10.1096/fj.03-0041fje. [DOI] [PubMed] [Google Scholar]
- 4.Brightling CE, Ammit AJ, Kaur D, Black JL, Wardlaw AJ, Hughes JM, et al. The CXCL10/CXCR3 axis mediates human lung mast cell migration to asthmatic airway smooth muscle. Am J Respir Crit Care Med. 2005;171:1103–1108. doi: 10.1164/rccm.200409-1220OC. [DOI] [PubMed] [Google Scholar]
- 5.El-Shazly A, Berger P, Girodet PO, Ousova O, Fayon M, Vernejoux JM, et al. Fraktalkine produced by airway smooth muscle cells contributes to mast cell recruitment in asthma. J Immunol. 2006;176:1860–1868. doi: 10.4049/jimmunol.176.3.1860. [DOI] [PubMed] [Google Scholar]
- 6.Pepe C, Foley S, Shannon J, Lemiere C, Olivenstein R, Ernst P, et al. Differences in airway remodeling between subjects with severe and moderate asthma. J Allergy Clin Immunol. 2005;116:544–549. doi: 10.1016/j.jaci.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Woodruff PG, Dolganov GM, Ferrando RE, Donnelly S, Hays SR, Solberg OD, et al. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. Am J Respir Crit Care Med. 2004;169:1001–1006. doi: 10.1164/rccm.200311-1529OC. [DOI] [PubMed] [Google Scholar]
- 8.Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003;167:1360–1368. doi: 10.1164/rccm.200209-1030OC. [DOI] [PubMed] [Google Scholar]
- 9.Johnson P, Roth M, Tamm M, Hughes M, Ge Q, King G, et al. Airway smooth muscle cell proliferation is increased in asthma. Am J Respir Crit Care Med. 2001;164:474–477. doi: 10.1164/ajrccm.164.3.2010109. [DOI] [PubMed] [Google Scholar]
- 10.Trian T, Benard G, Begueret H, Rossignol R, Girodet PO, Ghosh D, et al. Bronchial smooth muscle remodeling involves calcium-dependent enhanced mitochondrial biogenesis in asthma. J Exp Med. 2007;204:3173–3181. doi: 10.1084/jem.20070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Begueret H, Berger P, Vernejoux JM, Dubuisson L, Marthan R, Tunon-de-Lara JM. Inflammation of bronchial smooth muscle in allergic asthma. Thorax. 2007;62:8–15. doi: 10.1136/thx.2006.062141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 14.Kaur D, Saunders R, Berger P, Siddiqui S, Woodman L, Wardlaw A, et al. Airway smooth muscle and mast cell-derived CCL19 mediate airway smooth muscle migration in asthma. Am J Respir Crit Care Med. 2006;174:1179–1188. doi: 10.1164/rccm.200603-394OC. [DOI] [PubMed] [Google Scholar]
- 15.Hirst SJ, Hallsworth MP, Peng Q, Lee TH. Selective induction of eotaxin release by interleukin-13 or interleukin-4 in human airway smooth muscle cells is synergistic with interleukin-1beta and is mediated by the interleukin-4 receptor alpha-chain. Am J Respir Crit Care Med. 2002;165:1161–1171. doi: 10.1164/ajrccm.165.8.2107158. [DOI] [PubMed] [Google Scholar]
- 16.Sutcliffe A, Kaur D, Page S, Woodman L, Armour CL, Baraket M, et al. Mast cell migration to Th2 stimulated airway smooth muscle from asthmatics. Thorax. 2006;61:657–662. doi: 10.1136/thx.2005.056770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brightling CE, Kaur D, Berger P, Morgan AJ, Wardlaw AJ, Bradding P. Differential expression of CCR3 and CXCR3 by human lung and bone marrow-derived mast cells: implications for tissue mast cell migration. J Leukoc Biol. 2005;77:759–766. doi: 10.1189/jlb.0904511. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz LB, Lewis RA, Austen KF. Tryptase from human pulmonary mast cells. Purification and characterization. J Biol Chem. 1981;256:11939–11943. [PubMed] [Google Scholar]
- 19.Bradding P, Okayama Y, Kambe N, Saito H. Ion channel gene expression in human lung, skin, and cord blood-derived mast cells. J Leukoc Biol. 2003;73:614–620. doi: 10.1189/jlb.1202602. [DOI] [PubMed] [Google Scholar]
- 20.Vermes I, Haanen C, Steffensnakken H, Reutelingsperger C. A novel assay for apoptosis-flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein-labeled annexin-V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 21.Morgan AJ, Symon FA, Berry MA, Pavord ID, Corrigan CJ, Wardlaw AJ. IL-4-expressing bronchoalveolar T cells from asthmatic and healthy subjects preferentially express CCR3 and CCR4. J Allergy Clin Immunol. 2005;116:594–600. doi: 10.1016/j.jaci.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 22.Uguccioni M, Mackay CR, Ochensberger B, Loetscher P, Rhis S, LaRosa GJ, et al. High expression of the chemokine receptor CCR3 in human blood basophils. Role in activation by eotaxin, MCP-4, and other chemokines. J Clin Invest. 1997;100:1137–1143. doi: 10.1172/JCI119624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romagnani P, De Paulis A, Beltrame C, Annunziato F, Dente V, Maggi E, et al. Tryptase-chymase double-positive human mast cells express the eotaxin receptor CCR3 and are attracted by CCR3-binding chemokines. Am J Pathol. 1999;155:1195–1204. doi: 10.1016/S0002-9440(10)65222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ying S, Meng Q, Zeibecoglou K, Robinson DS, Macfarlane A, Humbert M, et al. Eosinophil chemotactic chemokines (eotaxin, eotaxin-2, RAN-TES, monocyte chemoattractant protein-3 (MCP-3), and MCP-4), and C-C chemokine receptor 3 expression in bronchial biopsies from atopic and nonatopic (intrinsic) asthmatics. J Immunol. 1999;163:6321–6329. [PubMed] [Google Scholar]
- 25.De Lucca G. Recent developments in CCR3 antagonists. Curr Opin Drug Discov Devel. 2006;9:516–524. [PubMed] [Google Scholar]
- 26.Stellato C, Brummet ME, Plitt JR, Shahabuddin S, Baroody FM, Liu MC, et al. Cutting Edge: Expression of the C-C chemokine receptor CCR3 in human airway epithelial cells. J Immunol. 2001;166:1457–1461. doi: 10.4049/jimmunol.166.3.1457. [DOI] [PubMed] [Google Scholar]
- 27.Haley KJ, Lilly CM, Yang JH, Feng Y, Kennedy SP, Turi TG, et al. Over-expression of eotaxin and the CCR3 receptor in human atherosclerosis: using genomic technology to identify a potential novel pathway of vascular inflammation. Circulation. 2000;102:2185–2189. doi: 10.1161/01.cir.102.18.2185. [DOI] [PubMed] [Google Scholar]
- 28.Joubert P, Lajoie-Kadoch S, Labonte I, Gounni AS, Maghni K, Wellemans V, et al. CCR3 expression and function in asthmatic airway smooth muscle cells. J Immunol. 2005;175:2702–2708. doi: 10.4049/jimmunol.175.4.2702. [DOI] [PubMed] [Google Scholar]
- 29.Roque M, Kim WJH, Gazdoin M, Malik A, Reis ED, Fallon JT, et al. CCR2 Deficiency decreases intimal hyperplasia after arterial injury. Arterioscler Thromb Vasc Biol. 2002;22:554–559. doi: 10.1161/hq0402.105720. [DOI] [PubMed] [Google Scholar]
- 30.Chandrasekar B, Bysani S, Mummidi S. CXCL16 signals via Gi, phosphatidyl-inositol 3-kinase, Akt, I{kappa}B kinase, and nuclear factor-{kappa}B and induces cell-cell adhesion and aortic smooth muscle cell proliferation. J Biol Chem. 2004;279:3188–3196. doi: 10.1074/jbc.M311660200. [DOI] [PubMed] [Google Scholar]
- 31.Chandrasekar B, Mummidi S, Perla RP, Bysani S, Dulin NO, Liu L, et al. Fractalkine (CX3CL1) stimulated by nuclear factor kappaB (NF-kappaB)-dependent inflammatory signals induces aortic smooth muscle cell proliferation through an autocrine pathway. Biochem J. 2003;373:547–558. doi: 10.1042/BJ20030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kodali RB, Kim WJH, Galaria II, Miller C, Schecter AD, Lira SA, et al. CCL11 (Eotaxin) induces CCR3-dependent smooth muscle cell migration. Arterioscler Thromb Vasc Biol. 2004;24:1211–1216. doi: 10.1161/01.ATV.0000131654.90788.f5. [DOI] [PubMed] [Google Scholar]
- 33.Chan V, Burgess JK, Ratoff JC, O’Connor BJ, Greenough A, Lee TH, et al. Extracellular matrix regulates enhanced eotaxin expression in asthmatic airway smooth muscle cells. Am J Respir Crit Care Med. 2006;174:379–385. doi: 10.1164/rccm.200509-1420OC. [DOI] [PubMed] [Google Scholar]
- 34.Pang L, Nie M, Corbett L, Sutcliffe A, Knox AJ. Mast cell beta-tryptase selectively cleaves eotaxin and RANTES and abrogates their eosinophil chemotactic activities. J Immunol. 2006;176:3788–3795. doi: 10.4049/jimmunol.176.6.3788. [DOI] [PubMed] [Google Scholar]