Abstract
Background
Nonasthmatic eosinophilic bronchitis (EB) has emerged as a useful tool to study the structural and inflammatory mechanisms of airway hyperresponsiveness (AHR) in asthma. We have previously shown that vascular remodeling and reticular basement membrane (RBM) thickening are present in EB. However, it is not known whether other features of structural remodeling including increased airway smooth muscle (ASM) mass, matrix deposition, and glandular hyperplasia are also present in EB.
Objectives
We sought to determine whether structural remodeling occurs in EB and is associated with AHR and airflow limitation.
Methods
Forty-two patients with asthma, 21 patients with EB, and 19 healthy volunteers were recruited. ASM area, RBM thickness, collagen 3 deposition, glandular area, mast cells, and granulocytes were assessed in bronchial biopsy samples.
Results
Nonasthmatic eosinophilic bronchitis and asthma were associated with a significant increase in ASM mass and RBM thickness compared with healthy subjects. In contrast, we did not observe any significant differences in collagen 3 deposition in the lamina propria and ASM or the % area of glands in the lamina propria. Univariate analysis demonstrated that mast cell numbers in the ASM were the only feature of remodeling associated with AHR (β = −0.51; P = .004). Stepwise linear regression revealed that a combination of mast cell numbers in the ASM (β = −0.43) and disease duration (β = −0.25; model-adjusted R2 = 0.26; P = .027) best modeled AHR.
Conclusion
Mast cell localization to the ASM bundle, but not structural remodeling of the airway wall, is associated with AHR in asthma.
Keywords: Asthma, nonasthmatic eosinophilic bronchitis, airway hyperresponsiveness, mast cell, remodeling
Asthma is a common airway disease that accounts for significant healthcare cost.1 It is characterized by variable airflow limitation, airway inflammation, and airway hyperresponsiveness (AHR). Structural and cellular changes within the airway wall in asthma, notably increased airway smooth muscle (ASM) mass,2 vascular remodeling,3 thickening of the reticular basement membrane (RBM), and fibroblast numbers in the lamina propria,4 have been shown to correlate with airflow limitation. Furthermore, cellular infiltration of the airway wall in asthma is related to decline in lung function.5 The association of structural change in the airway wall in asthma with AHR is much more tenuous. A number of reports have drawn conflicting conclusions about the association of AHR with epithelial desquamation/loss of tight junctions,6-8 RBM thickening,9-13 vascular remodeling,2,14 and ASM mass.2,15
Nonasthmatic eosinophilic bronchitis (EB) has emerged as a powerful disease control model to study the mechanisms of AHR in asthma outside the confounding influence of eosinophilic airway inflammation.16 EB is a common cause of chronic cough, accounting for approximately 10% of referral to a specialist cough clinic17 and is characterized by eosinophilic airway inflammation. However, in contrast with asthma, there is an absence of variable airflow obstruction and AHR.18 We have previously shown that EB is characterized by RBM thickening19 and vascular remodeling3 to a similar degree as patients with asthma and eosinophilic airway inflammation. In contrast, mast cell infiltration of the ASM bundle was a defining feature of the asthma phenotype being absent in subjects with EB and healthy subjects.20 Importantly, studies examining the natural history of EB have demonstrated that fixed airflow obstruction may occur,21 which would again support the notion that structural remodeling of the airway wall may occur in EB.
We therefore hypothesized that (1) structural remodeling of the airway wall does occur in EB, (2) structural remodeling of the airway wall is dissociated from AHR in asthma, and (3) mast cell localization to the ASM bundle is a key determinant of AHR in asthma.
RESULTS
Clinical characteristics of patients and healthy subjects are shown in Table I.
TABLE I. Baseline clinical characteristics.
| Asthma (n = 42) |
|||||
|---|---|---|---|---|---|
| Control (n = 19) | Eosinophilic bronchitis (n = 21) | GINA 1 (n = 17) | GINA 2-4 (n = 15) | Refractory (n = 10) | |
| Age (y) | 36.4 (3.2) | 49.8 (2.6)§ | 45.4 (3.9) | 52.9 (4.4)§ | 47.1 (2.1)‡ |
| Sex (M:F) | 8:11 | 10:11 | 7:10 | 9:6 | 4:6 |
| Atopy (%) | 44.4 | 53.3 | 75 | 70 | 50# |
| Disease duration (y) | NA | 5.0 (1.3) | 12.0 (2.5) | 15.9 (3.9)∥ | 18.0 (5.1)‡∥ |
| Inhaled BDP (μg/24 h) | NA | 200 (84) | 0 | 1067 (122)¶ | 1404 (179)‡¶ |
| Oral prednisolone (number; dose) | NA | 0/21; 0 | 0/10; 0 | 0/15; 0 | 8/10; 12.2 (3.2) |
| Postbronchodilator FEV1 (L) | 3.0 (0.16) | 3.8 (0.26) | 2.9 (0.28) | 2.8 (0.2)∥ | 2.5 (0.25)‡∥ |
| Postbronchodilator FEV1 % | 105.5 (2.6) | 99.6 (2.9) | 96.3 (4.0) | 94.6 (4.6) | 80.7 (6.5)‡§∥ |
| FEV1/FVC postbronchodilator | 85.0 (2.7) | 81.4 (1.2) | 75.4 (2.8) | 76.5 (2.0) | 70.2 (2.8)‡§∥ |
| PC20 (mg/mL)* | >16 | >16 | 0.62 (0.25-1.5) | 0.44 (0.17-1.1) | 0.35 (0.02-5.5)‡ |
| Induced sputum | |||||
| Eosinophils (%)* | 0.33 (0.25-0.43) | 6.9 (3.9-12)§ | 2.0 (0.7-5.2)§ | 1.7 (0.66-4.6)§ | 6.9 (1.2-38.5)‡§ |
| Neutrophils (%) | 52.9 (10.4) | 53.4 (5.5) | 45.6 (7.3) | 55.0 (8.3) | 44.9 (10.0) |
| Lamina propria | |||||
| Mast cells/mm2† | 15.9 (10.1-21.7) | 28.1 (16.0-35.6) | 19.5 (15.7-25.3) | 19.0 (11.2-28.0) | 17.0 (13.2-29.8)‡ |
| Eosinophils/mm2† | 2.3 (1.1-5.4) | 31.7 (18.3-57.7)§ | 20.9 (7.8-38.8)§ | 11.3 (4.9-19.7) | 28.1 (6.6-31.9)‡§ |
| Neutrophils/mm2† | 7.7 (2.1-10.3) | 35.5 (16.8-46)§ | 15.4 (10.6-27.2) | 19.5 (5.4-40.7) | 15.6 (3.2-22.3)‡ |
M, Male; F female; FVC, forced vital capacity; NA, not applicable.
Data expressed as means (SEMs), *geometric mean (95% CI), fmedian (interquartile range).
Beclomethasone dipropionate (BDP) equivalents (BDP/24 h): fluticasone 2:1, budesonide 1.25:1, mometasone 1.25:1.
P < .05 1-way ANOVA ; normal vs EB/GINA 1, GINA 2-4, refractory asthma.
P < .05 vs control (Bonferroni/Dunn correction for multiple comparisons).
P < .05 vs EB (Bonferroni /Dunn correction for multiple comparisons).
P < .05 vs EB and GINA 1 asthma (Bonferroni /Dunn correction for multiple comparisons).
P < .0001 χ2 test.
Airway structural remodeling is present in both eosinophilic bronchitis and asthma
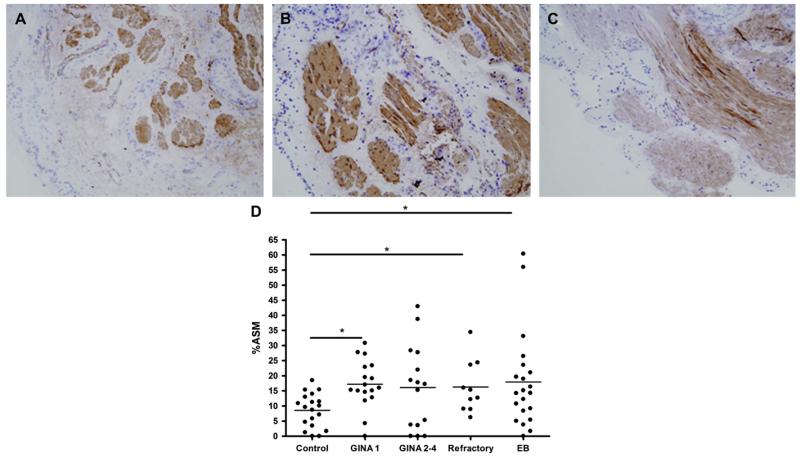
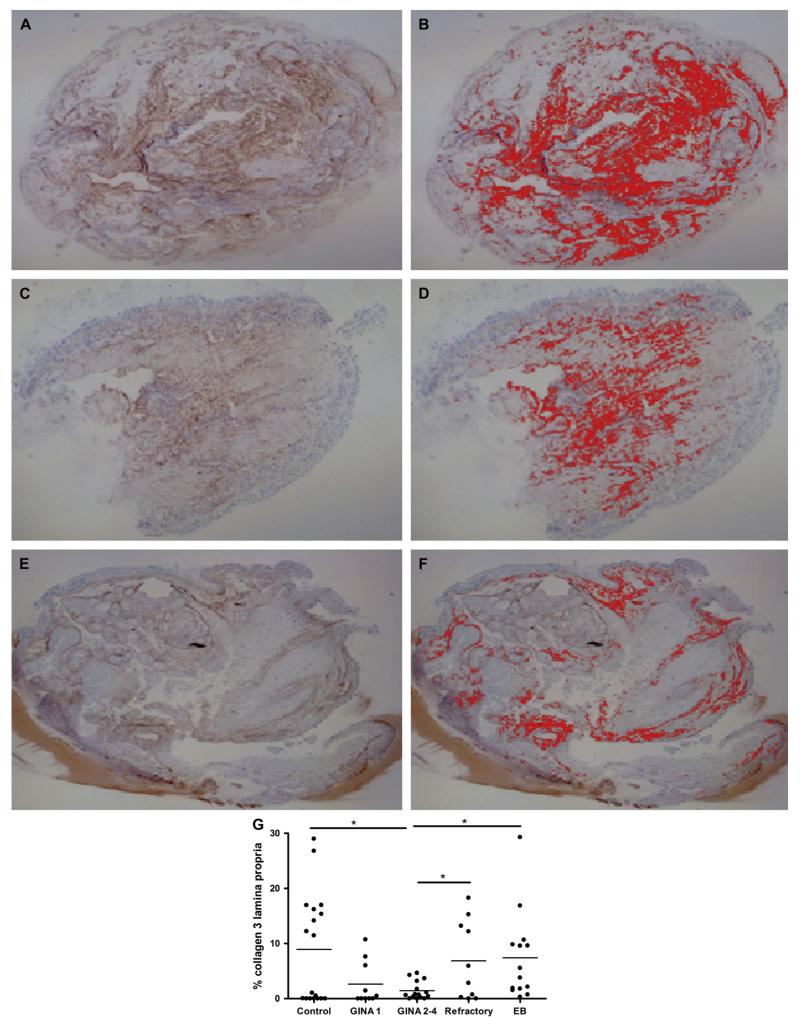
Nonasthmatic eosinophilic bronchitis and asthma were associated with a significant increase in ASM mass and RBM thickness compared with healthy subjects (Table II; Fig 1). In contrast, we did not observe any significant differences in lamina propria or ASM collagen 3 deposition. We observed a trend for increased percentage glands in the lamina propria in refractory asthma (P = .08; Table II; Fig 2).
TABLE II. Airway structural changes in asthma and EB.
| Asthma (n = 42) |
|||||
|---|---|---|---|---|---|
| Control (n = 19) | Eosinophilic bronchitis (n = 21) | GINA 1 (n = 17) | GINA 2-4 (n = 15) | Refractory (n = 10) | |
| RBM thickness (μm) | 6.6 (0.4) | 11.1 (0.96)† | 9.5 (0.77)† | 8.9 (1.1) | 11.0 (0.7)*† |
| Percent ASM | 8.5 (1.3) | 17.9 (3.4)† | 17.2 (1.9)† | 16.1 (3.6) | 16.3 (2.8)*† |
| Percent glands | 1.6 (0.61) | 3.1 (1.4) | 0.82 (0.3) | 2.4 (1.5) | 4.9 (1.6) |
| Percent collagen 3 lamina propria | 8.9 (2.3)‡ | 7.4 (2.1)† | 2.6 (1.3) | 1.4 (0.4) | 6.9 (2.3)*‡ |
| Collagen 3 SQS lamina propria | 2.02 (0.2) | 2.36 (0.15) | 1.85 (0.27) | 1.68 (0.16)§ | 2.23 (0.30) |
| Collagen 3 SQS ASM (mean score) | 0.56 (0.1) | 0.68 (0.08) | 0.58 (0.14) | 0.43 (0.11) | 0.68 (0.13) |
| Collagen 3 SQS ASM (number positive) | 7/18 | 6/14 | 5/10 | 7/15 | 7/10 |
| Mast cells/mm2 ASM | 3.1 (0.63) | 1.03 (0.41) | 8.8 (2.3)†§ | 11.7 (2.3)†§ | 15.6 (2.6)*†§ |
SQS, Semiquantitative score.
Data expressed as means (SEMs).
P < .05 1-way ANOVA; normal vs EB/GINA 1, GINA 2-4, refractory asthma.
P < .05 vs control (Mann-Whitney tests/t tests for intergroup comparisons).
P < .05 vs GINA 2-4 asthma (Mann-Whitney tests/t tests for intergroup comparisons).
P < .05 vs EB (Mann-Whitney tests/t tests for intergroup comparisons).
FIG 1. Increased ASM area in asthma and EB.
Representative photomicrographs of smooth muscle actin–immunostained mucosal biopsies (×100 magnification) in healthy subject (A), EB (B), and refractory asthma (C). D, Dot plot of percentage ASM in subjects with asthma with severity defined according to GINA, subjects with EB, and healthy subjects.
FIG 2. Reduced collagen 3 deposition in the lamina propria in moderate asthma.
Representative photomicrographs of collagen 3 staining (left) and corresponding thresholded collagen 3 (right) in the lamina propria in mucosal biopsies in healthy subject (A and B), EB (C and D), and asthma (E and F) (×25 magnification). G, Dot plot of percent collagen 3 expression in the lamina propria in subjects with asthma with severity defined according to GINA, subjects with EB, and healthy controls.
Mast cell localization to the ASM bundle is present in asthma and with increasing asthma severity
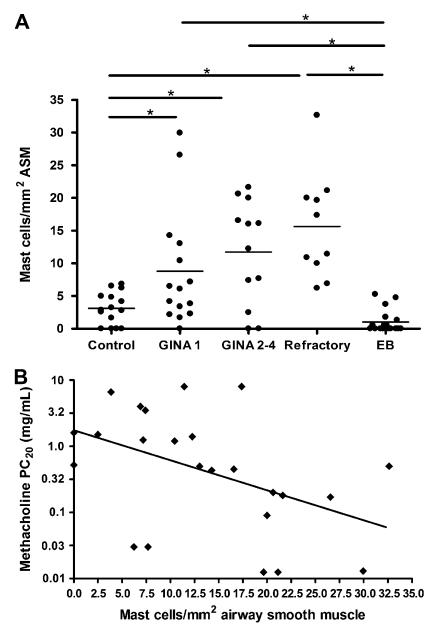
The mean (SEM) of mast cells per millimeter ASM was significantly increased in asthma 11.6 (1.4) compared with patients with EB 1.03 (0.41) and healthy subjects 3.1 (0.63; P <.0001; Table II and Fig 3, A). The mean (SEM) of mast cells per millimeter ASM was significantly increased in asthma independent of severity GINA 1 (8.8 [2.3]), GINA 2 to 4 (11.7 [2.3]), and refractory asthma (15.6 [2.6]) compared with patients with EB and healthy subjects (P < .0001, ANOVA; P < .05, intergroup comparisons;Fig 3, A). We did not observe localization of neutrophils to the ASM bundle in subjects with asthma, subjects with EB, or healthy subjects. In contrast, eosinophils were present in the ASM bundle in low numbers in refractory asthma (2.4 [0.84]/mm2), in contrast with subjects with GINA 1 to 4 asthma, subjects with EB, and healthy subjects, who had no evidence of ASM eosinophilia.
FIG 3. Mast cell numbers in the ASM in asthma correlate with AHR.
A, Dot plot of mast cell numbers (horizontal bar, mean) in the ASM in controls, subjects with asthma with severity defined according to GINA, subjects with refractory asthma, and patients with EB. B, Correlation of mast cell numbers in the ASM (x-axis) and methacholine PC20 in asthma (y-axis).
Mast cell localization to the ASM bundle and not structural remodeling correlates with AHR in asthma
Univariate analysis demonstrated that mast cell number in the ASM was the only feature of remodeling that was associated with log PC20 (β = −0.51; P = .004; Table III and Fig 3, B). Stepwise linear regression revealed that a combination of mast cell numbers in the ASM (β = −0.43) and disease duration (β = −0.25; model-adjusted R2 = 0.26; P = .027) best modeled log PC20.
TABLE III. Univariate and multivariate correlation of airway structure and function in asthma.
| Postbronchodilator FEV1 % | Bronchodilator response FEV1 (%) |
Methacholine PC20 |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis |
Univariate analysis |
(mg/mL) Univariate analysis |
||||||
| 3β | P value | β | P value | ∞ β | β | P value | ∞ β | |
| RBM thickness (μm) | 0.22 | .18 | −0.14 | .40 | x | 0.16 | .34 | x |
| Percent ASM | 0.20 | .22 | −0.53 | .74 | x | 0.16 | .93 | x |
| Percent glands | −0.26 | .10 | 0.10 | .55 | x | 0.12 | .50 | x |
| Percent collagen 3 lamina propria | 0.18 | .92 | −0.072 | .69 | x | 0.05 | .81 | x |
| Lamina propria eosinophil cells/mm2 | −0.09 | .60 | 0.32 | .048 | 0.31; P = .013 | 0.32 | .06 | x |
| Lamina propria neutrophil cells/mm2 | 0.20 | .20 | −0.064 | .70 | x | 0.13 | .45 | x |
| Mast cells/mm2 ASM | −0.03 | .89 | −0.024 | .89 | x | −0.51 | .004 | − 0.43 P < .0001 |
β, Standardized regression coefficient; ∞, stepwise linear regression model–adjusted standardized regression coefficient.
Significant values are in boldface and italics.
Eosinophilic airway inflammation is associated with thickening of the RBM in asthma and EB
Univariate analysis revealed that RBM thickening in the pooled asthma and EB cohort correlated with log sputum eosinophil percentage (β = 0.29; P = .03) but not lamina propria eosinophilia (β = 0.18; P = .20). Stepwise linear regression revealed that this association was independent of age of onset, disease duration, and the beclomethasone dipropionate/24 hour dose of inhaled corticosteroid (β = 0.36; model-adjusted R2 = 0.1; P = .04).
DISCUSSION
We have demonstrated for the first time that structural remodeling of the airway wall, notably increased ASM mass, occurs to similar degrees in EB and asthma. In addition, we did not observe differences in glandular area or collagen 3 deposition between EB and asthma, and we confirm that RBM thickening is a feature of both conditions. This suggests that all of these features of the remodeling process in asthma can be dissociated from AHR. In contrast, mast cell numbers were increased in the ASM bundle in asthma only, independent of disease severity and treatment, and were shown to correlate with the degree of AHR. Finally, eosinophilic inflammation in the airway wall and in induced sputum was related to RBM thickening in asthma and EB.
Our data add further support to the growing body of evidence that indicates a key pathophysiological role for mast cells in the ASM bundle in asthma (see review31) and strengthens our previously reported association of mast cell infiltration of the ASM bundle and AHR in asthma.21 Furthermore, in keeping with a recent report,32 we have demonstrated that mast cell localization to the ASM bundle was present independent of disease severity and treatment. We observed a nonsignificant trend for increased mast cells in the ASM bundle in severe disease. This would suggest that this aspect of the remodeling process may not be modulated by inhaled or oral corticosteroids.
In addition to the relationship between AHR and mast cell localization to the ASM bundle, AHR was also associated with disease duration. Indeed, the combination of these 2 factors best modeled AHR. In subjects with the greatest disease duration, disease onset often occurred in childhood. It is recognized that AHR may precede the development of asthma symptoms in children and persist into adulthood.33,34 Thus, whether the association between disease duration and AHR reflects increased AHR in early-onset disease or the disease burden over timeremains to be determined. Importantly, the multivariate regression coefficient between mast cells in the ASM, disease duration, and AHR in asthma indicated that a substantial proportion of the variance in the model was a result of other factors. AHR is a consequence of complex interactions between a number of factors, and further work is required to define their relative contribution.
We did not find any association between structural remodeling (increased ASM mass, glandular hyperplasia, RBM thickening, collagen 3 deposition, and glandular hyperplasia) and AHR in asthma. Furthermore, the presence or structural remodeling of these airway compartments to a similar degree in EB strengthens our view that structural remodeling of the proximal airway wall can be dissociated from AHR. In keeping with this observation, we have previously demonstrated that vascular remodeling of the proximal airway wall and expression of VEGF3 are present to similar degrees in asthma and EB, again supporting the notion that compartmental remodeling of the airway wall can be dissociated from AHR.
Importantly, bronchial biopsies enable the detailed assessment of the structural components of the proximal airway but cannot determine whether these features of remodeling are associated with changes in the airway geometry. Imaging studies of the airway wall provide global measures of airway geometry. Niimi et al35 have demonstrated that thickening of the right bronchus segment 1 was inversely associated with airway reactivity (the slope of the methacholine–airway resistance dose response curve), suggesting that global remodeling of the airway wall may be protective against AHR. In contrast, Boulet et al36 have demonstrated that thickening of the airway wall in asthma is associated with increased airway responsiveness in patients with fixed airflow obstruction but not in patients with near-normal lung function. The apparent discordance between these imaging studies may lie in the fact that airway reactivity and AHR may be linked to different mechanical properties of the airway wall. Further studies linking measures of static airway geometry by imaging and mucosal biopsies in the same patients are required to define further the association of structure and function.
We did not find an association between RBM thickening, matrix deposition, and increased ASM mass with airflow limitation in asthma. In contrast, glandular hyperplasia was significantly associated with airflow limitation in asthma. We and others have demonstrated that vascular remodeling, increased expression of sputum VEGF,3 and remodeling of the right bronchus segment 1 are associated with postbronchodilator lung function in asthma.37-39 Importantly, our subjects with severe refractory asthma did not have fixed airflow obstruction, and this may explain the lack of association between structural remodeling and postbronchodilator lung function reported in other studies.4
We have confirmed that eosinophilic airway inflammation is independently associated with thickening of the RBM in both asthma and EB. This would support the growing body of evidence from animal models of asthma40 and from immunopathological studies of eosinophilic versus noneosinophilic asthma41 that have linked this feature of the remodeling process to eosinophilic airway inflammation.
One potential limitation of the current study is the cross-sectional design and the need to confirm our findings in longitudinal studies of airway wall remodeling. However, we are confident that our findings are robust. We used nonstereologic measures of ASM mass and quantified collagen 3 deposition as a proxy for total matrix deposition in the lamina propria and ASM. However, the measurements of ASM area correlated well with the ASM volume fraction measured by using the stereologic Cavalieri method.42 Furthermore, the measurement of collagen 3 using a validated and automated repeatable thresholding procedure correlated well with a previous study that demonstrated similar collagen 3, collagen 1, and total collagen deposition in the lamina propria in subjects with asthma across the spectrum of severity and healthy subjects, using an automated thresholding procedure.12 Interestingly, in keeping with our findings, this study demonstrated a similar trend for reduced collagen 3 deposition in moderate asthma.
In conclusion, we have shown for the first time that remodeling of the airway wall is similar in asthma and EB and dissociated from AHR. In contrast, mast cell localization to the ASM bundle was associated with AHR in asthma independent of disease severity, treatment, and airway wall structure. Further studies are required to establish how global airway wall thickening and compartment remodeling of the airway wall differentially alter the micromechanical properties of the airway wall in asthma and translate into disordered airway function.
METHODS
Clinical characterization
Asthma was defined by 1 or more of the following objective criteria: significant bronchodilator reversibility of >200 mL, PC20 <8 mg/mL, or a peak flow amplitude percent mean over a period of 2 weeks of more than 20%. Asthma severity was classified using the current GINA guidelines based on the GINA treatment stepsE1—GINA 1 to 2 (n = 17), intermittent to mild persistent asthma; GINA 3 to 4 (n = 15), moderate to severe persistent asthma—and the American Thoracic Society criteria for refractory asthma (n = 10).E2 Normal subjects had no history of respiratory disease and normal spirometry and methacholine responsiveness. EB was defined by the American College of Chest Physicians Criteria.E3 In brief, patients had a history of chronic cough without symptoms of variable airflow obstruction, a sputum eosinophil count >3%, and normal lower airway responsiveness to methacholine (PC20 >16 mg/mL). All subjects were nonsmokers with a smoking history of less than 10 pack-years.
Spirometric tests were performed by using a dry bellows spirometer (Vitalograph, Buckingham, UK) with FEV1 recorded as the best of successive readings within 100 mL. Allergen skin prick tests were performed to Dermatophagoides pteronyssinus, cat fur, dog, grass pollen, and Aspergillus fumigatus solutions with normal saline and histamine controls (Bencard, Sussex, UK). A positive response to an allergen on the skin prick tests was recorded by the presence of a wheal of >2 mm more than the negative control.
Bronchoscopy was undertaken by using an Olympus fiber optic bronchoscope (Olympus Co, Tokyo, Japan) in accordance with British Thoracic Society guidelines.E4 Bronchial mucosal biopsy specimens were taken from the right middle and lower lobe carinae.
Mucosal biopsies were immediately transferred into ice-cooled acetone containing the protease inhibitors iodoacetamide (20 mmol/L) and phenylmethylsulfonyl fluoride (2 mmol/L) for fixation, stored at 20°C for 24 hours, and then processed into the water soluble resin glycol methacrylate (Polysciences, Northampton, UK) for embedding.E5
Image analysis and quantitative morphometry
Morphometry was assessed by computer-assisted image analysis on hematoxylin-stained sections and α-smooth muscle actin–stained sections for ASM analysis. Total biopsy area was determined in at least 2 noncontiguous tissue sections from the same biopsy, and values are expressed in square millimeters. The area of the lamina propria was derived by subtracting the ASM area, glandular area, epithelial area, and area of vessels and lymphatics from the total biopsy area
Measurement of ASM area
We used the Cavalieri method to estimate the volume of ASM in 8 patients with EB.E6 In brief, a single biopsy was cut into 28 sections 2 μm apart, giving a reference volume of 56 μm, and sections were counterstained with Mayers hematoxylin. The reference volume was then interrogated at 5 systematically uniform random sections (SURSs) by generating a random number between 1 and 11.2 and subsequently adding 11.2 iteratively 4 times to the original random number. A point counting system was used to determine the total area of the section and the ASM area for the 5 SURSs. The top right quadrant of each cross was used as the 0-dimensional reference point. Point counts were converted into section areas by multiplying the total number of points counted Σp, by the area per test point a(p).
The Cavalieri volume was then estimated by multiplying the distance between sections t by their total cross-sectional area:
Using this method, the mean (SEM) volume fraction of ASM was 0.23 (0.05) mm3/mm3.
We found an excellent correlation between this volume fraction and the area of ASM measured by using image analysis planimetry in the individual SURSs (r values = 0.85; P = .01).
We also found an excellent correlation between ASM area estimated by using α-smooth muscle actin staining and hematoxylin staining in the SURSs (r = 0.97; P < .0001).
We therefore assumed that α-smooth muscle actin staining of ASM was a reasonable estimate of ASM mass for the purpose of this study and used the ASM area in α-smooth muscle actin–stained sections as an estimate of ASM mass. This is in line with previous reports.E7,E8
Measurement of collagen 3 deposition. Intensity of matrix deposition in the lamina propria of collagen 3 was assessed qualitatively using a semiquantitative score (SQS; 0-3) as previously described and in the ASM as either present (1) or absent (0) by 2 blind investigators (V.M., S.S.).E7 The mean of the 2 investigators’ scores was taken as the final score.
For quantitative assessment of matrix deposition in the lamina propria, a thresholding technique was developed on the basis of the hue, saturation, and intensity (HSI) of collagen 3 staining. The HSI color system was defined by a scale of 0 to 255 for HSI. Initially the background noise was defined by assessing the HSI of collagen 3 deposition in the lamina propria in 4 patients with a lamina propria SQS of 3, 2, and 1, respectively. Sections were acquired at ×25 magnification, white balance corrected, and pixels of representative matrix staining selected until high-intensity matrix staining in the lamina propria was appropriately thresholded. Areas of matrix staining in glands and the ASM were manually removed to give the area of matrix in the lamina propria. A minimum of 2 noncontiguous sections were thresholded for each patient. The median (interquartile range) of lower and upper limit of HSI were then defined from the 12 validation patients. The lower quartile of hue (upper limit, 10; lower limit, 0), maximum saturation (upper limit, 255; lower limit, 33) and median intensity (upper limit, 169; lower limit, 94) were then selected as the final threshold to give a threshold that appropriately captured highly saturated red light. The final threshold was then applied to the isotype control slides of each of the 12 validation patients and the percent area of matrix in the lamina propria in the isotypes defined. We then calculated the mean percent isotype staining and the set the limit of noise with the final threshold as the mean + 2SD of the final percentage. The mean + 2SD image noise was 0.88%.
All biopsies were subsequently thresholded using the final threshold, and the noise percent was subtracted from each biopsy. The mean percent area of collagen 3 in the lamina propria on 2 sections at least 20 μm apart was taken as the final percent area of collagen 3 in the lamina propria.
We tested the final threshold by comparing the semiquantitative matrix score with the thresholded percent area of matrix in the lamina propria. We found that there was an excellent correlation (r values = 0.83; P <.0001) and that the regression line crossed the x-axis at a SQS value of 1.01, suggesting that our final validated threshold identified matrix staining corresponding to a SQS of ≤1 as background noise (Fig E1).
FIG E1.

Correlation between SQS (x-axis) and thresholded collagen 3 deposition (y-axis) in the lamina propria. The regression line crosses the x-axis at 1, suggesting that the threshold appropriately excluded weak or absent staining assessed visually as noise.
Repeatability
There was excellent intraobserver and interobserver agreement for the quantitative thresholded matrix area (r values = 1 and 0.99), respectively, and for the qualitative matrix score (r values = 0.74 and 0.83). The between-observer repeatability of collagen 3 scores within the ASM was assessed using the Cohen κ statistic as a binary outcome measure and was found to be moderate (κ = 0.45) and moderate within observer (κ 5 0.47).
Measurement of RBM thickening
RBM thickness was measured by a single observer at ×400 magnification at 50 points orientated perpendicular to the epithelium separated by 20 μm as previously described and validated by Sullivan et al.E9 The mean of these measurements was derived and quoted as the final RBM thickness.
The interobserver variability was determined by measuring RBM thickness by 2 observers (S.S., C.B.) in n = 12 subjects, and good agreement was found (r = 0.79).
Determination of mast cell numbers in the ASM
We determined mast cell infiltration in the ASM in 2 sections at least 10 μm apart.
The minimum area of ASM used to enumerate mast cells was 0.1 mm2 as previously described.E10 Tryptase-positive cells within the ASM bundle were expressed in square millimeters of ASM, and the mean of the 2 sections was used to determine the final count.
Fifteen healthy subjects, 37 subjects with asthma (15 GINA 1, 12 GINA 2 to 4, and 10 refractory) and 18 patients with EB had sufficient ASM for mast cell quantification.
Quantification of submuscosal cell counts
The area of the lamina propria was derived by subtracting the area of glands, ASM, epithelium, lymphatics, and vessels from the total biopsy area. Nucleated immunostained cells staining for major basic protein (eosinophils), elastase (neutrophils), and tryptase (mast cells) were enumerated in the subepithelium and expressed in square millimeters of the lamina propria. The mean cell count in 2 sections at least 20 μm apart was used to determine the final count.
Key messages.
Structural remodeling of the airway wall is dissociated from AHR in asthma.
Mast cell infiltration of the ASM is independently associated with AHR in asthma.
Eosinophilic airway inflammation is associated with RBM thickening in both asthma and EB.
Acknowledgments
We thank Dr Sarah Bolton and Dr Martyn Foster at AstraZeneca, Charnwood, UK, for their help in devising the thresholding protocol and access to microscopy equipment and software.
Supported by Asthma UK, the Department of Health Clinician Scientist award, and a Wellcome Senior Clinical Fellowship (C.B.).
Abbreviations used
- AHR
Airway hyperresponsiveness
- ASM
Airway smooth muscle
- EB
Nonasthmatic eosinophilic bronchitis
- GINA
Global Initiative for Asthma
- RBM
Reticular basement membrane
- UK
United Kingdom
Footnotes
Disclosure of potential conflict of interest: I. Pavord is on the speakers’ bureau for GlaxoSmithKline and AstraZeneca and has received research support from Glaxo-SmithKline. C. Brightling is on the speakers’ bureau for AstraZeneca, GlaxoSmith-Kline, and MSD; has received research support from GlaxoSmithKline, AstraZeneca, and Medimmune; and is on the scientific board at Medimmune. The rest of the authors have declared that they have no conflict of interest.
REFERENCES
- 1.Barnes PJ, Jonsson B, Klim JB. The costs of asthma. Eur Respir J. 1996;9:636–42. doi: 10.1183/09031936.96.09040636. [DOI] [PubMed] [Google Scholar]
- 2.Pepe C, Foley S, Shannon J, Lemiere C, Olivenstein R, Ernst P, et al. Differences in airway remodeling between subjects with severe and moderate asthma. J Allergy Clin Immunol. 2005;116:544–9. doi: 10.1016/j.jaci.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui S, Sutcliffe A, Shikotra A, Woodman L, Doe C, McKenna S, et al. Vascular remodeling is a feature of asthma and nonasthmatic eosinophilic bronchitis. J Allergy Clin. Immunol. 2007;120:813–9. doi: 10.1016/j.jaci.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 4.Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003;167:1360–8. doi: 10.1164/rccm.200209-1030OC. [DOI] [PubMed] [Google Scholar]
- 5.van Rensen EL, Sont JK, Evertse CE, Willems LN, Mauad T, Hiemstra PS, et al. Bronchial CD8 cell infiltrate and lung function decline in asthma. Am J Respir Crit Care Med. 2005;172:837–41. doi: 10.1164/rccm.200504-619OC. [DOI] [PubMed] [Google Scholar]
- 6.Jeffery PK, Wardlaw AJ, Nelson FC, Collins JV, Kay AB. Bronchial biopsies in asthma: an ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis. 1989;140:1745–53. doi: 10.1164/ajrccm/140.6.1745. [DOI] [PubMed] [Google Scholar]
- 7.Ohashi Y, Motojima S, Fukuda T, Makino S. Airway hyperresponsiveness, increased intracellular spaces of bronchial epithelium, and increased infiltration of eosinophils and lymphocytes in bronchial mucosa in asthma. Am Rev Respir Dis. 1992;145:1469–76. doi: 10.1164/ajrccm/145.6.1469. [DOI] [PubMed] [Google Scholar]
- 8.Lozewicz S, Wells C, Gomez E, Ferguson H, Richman P, Devalia J, et al. Morpho-logical integrity of the bronchial epithelium in mild asthma. Thorax. 1990;45:12–5. doi: 10.1136/thx.45.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulet LP, Laviolette M, Turcotte H, Cartier A, Dugas M, Malo JL, et al. Bronchial subepithelial fibrosis correlates with airway responsiveness to methacholine. Chest. 1997;112:45–52. doi: 10.1378/chest.112.1.45. [DOI] [PubMed] [Google Scholar]
- 10.Ward C, Pais M, Bish R, Reid D, Feltis B, Johns D, et al. Airway inflammation, basement membrane thickening and bronchial hyperresponsiveness in asthma. Thorax. 2002;57:309–16. doi: 10.1136/thorax.57.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chetta A, Foresi A, Del Donno M, Consigli GF, Bertorelli G, Pesci A, et al. Bronchial responsiveness to distilled water and methacholine and its relationship to inflammation and remodeling of the airways in asthma. Am J Respir Crit Care Med. 1996;153:910–7. doi: 10.1164/ajrccm.153.3.8630572. [DOI] [PubMed] [Google Scholar]
- 12.Chu HW, Halliday JL, Martin RJ, Leung DY, Szefler SJ, Wenzel SE. Collagen deposition in large airways may not differentiate severe asthma from milder forms of the disease. Am J Respir Crit Care Med. 1998;158:1936–44. doi: 10.1164/ajrccm.158.6.9712073. [DOI] [PubMed] [Google Scholar]
- 13.Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160:1001–8. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- 14.Hoshino M, Nakamura Y, Hamid QA. Gene expression of vascular endothelial growth factor and its receptors and angiogenesis in bronchial asthma. J Allergy Clin Immunol. 2001;107:1034–8. doi: 10.1067/mai.2001.115626. [DOI] [PubMed] [Google Scholar]
- 15.Woodruff PG, Dolganov GM, Ferrando RE, Donnelly S, Hays SR, Solberg OD, et al. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. Am J Respir Crit Care Med. 2004;169:1001–6. doi: 10.1164/rccm.200311-1529OC. [DOI] [PubMed] [Google Scholar]
- 16.Brightling CE, Pavord ID. Eosinophilic bronchitis: what is it and why is it important? Clin Exp Allergy. 2000;30:4–6. doi: 10.1046/j.1365-2222.2000.00740.x. [DOI] [PubMed] [Google Scholar]
- 17.Brightling CE, Ward R, Goh KL, Wardlaw AJ, Pavord ID. Eosinophilic bronchitis is an important cause of chronic cough. Am J Respir Crit Care Med. 1999;160:406–10. doi: 10.1164/ajrccm.160.2.9810100. [DOI] [PubMed] [Google Scholar]
- 18.Brightling CE. Chronic cough due to nonasthmatic eosinophilic bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:116S–21S. doi: 10.1378/chest.129.1_suppl.116S. [DOI] [PubMed] [Google Scholar]
- 19.Brightling CE, Symon FA, Birring SS, Bradding P, Wardlaw AJ, Pavord ID. Comparison of airway immunopathology of eosinophilic bronchitis and asthma. Thorax. 2003;58:528–32. doi: 10.1136/thorax.58.6.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 21.Park SW, Lee YM, Jang AS, Lee JH, Hwangbo Y, Kim DJ, et al. Development of chronic airway obstruction in patients with eosinophilic bronchitis: a prospective follow-up study. Chest. 2004;125:1998–2004. doi: 10.1378/chest.125.6.1998. [DOI] [PubMed] [Google Scholar]
- 22.Global Initiative for Asthma [Accessed December 29, 2007];Global Initiative for Asthma Guidelines. 2007 http://www.ginasthma.com/Guidelineitem.asp??l152&l251&intId560
- 23.American Thoracic Society Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162:2341–51. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 24.Morgan AJ, Symon FA, Berry MA, Pavord ID, Corrigan CJ, Wardlaw AJ. IL-4-expressing bronchoalveolar T cells from asthmatic and healthy subjects preferentially express CCR 3 and CCR 4. J Allergy Clin Immunol. 2005;116:594–600. doi: 10.1016/j.jaci.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 25.Morgan AJ, Guillen C, Symon FA, Huynh TT, Berry MA, Entwisle JJ, et al. Expression of CXCR6 and its ligand CXCL16 in the lung in health and disease. Clin Exp Allergy. 2005;35:1572–80. doi: 10.1111/j.1365-2222.2005.02383.x. [DOI] [PubMed] [Google Scholar]
- 26.Juniper EF, Cockcroft DW, Hargreave FE. Histamine and methacholine inhalation tests: a laboratory tidal breathing protocol. 2nd ed. Astra Draco AB; Lund, Sweden: 1994. [Google Scholar]
- 27.Pavord ID, Pizzichini MM, Pizzichini E, Hargreave FE. The use of induced sputum to investigate airway inflammation. Thorax. 1997;52:498–501. doi: 10.1136/thx.52.6.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.British Thoracic Society British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax. 2001;56(suppl):i1–21. doi: 10.1136/thorax.56.suppl_1.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Britten KM, Howarth PH, Roche WR. Immunohistochemistry on resin sections: a comparison of resin embedding techniques for small mucosal biopsies. Biotech Histochem. 1993;68:271–80. doi: 10.3109/10520299309105629. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan P, Stephens D, Ansari T, Costello J, Jeffery P. Variation in the measurements of basement membrane thickness and inflammatory cell number in bronchial biopsies. Eur Respir J. 1998;12:811–5. doi: 10.1183/09031936.98.12040811. [DOI] [PubMed] [Google Scholar]
- 31.Siddiqui S, Hollins F, Saha S, Brightling CE. Inflammatory cell microlocalisation and airway dysfunction: cause and effect? Eur Respir J. 2007;30:1043–56. doi: 10.1183/09031936.00162506. [DOI] [PubMed] [Google Scholar]
- 32.Saha S, Berry M, Parker D, Siddiqui S, Morgan A, May R, et al. Increased sputum and bronchial biopsy (IL)-13 expression in severe asthma. J Allergy Clin Immunol. 2008;121:685–91. doi: 10.1016/j.jaci.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obase Y, Shimoda T, Kawano T, Saeki S, Tomari S, Izaki K. Bronchial hyperresponsiveness and airway inflammation in adolescents with asymptomatic childhood asthma. Allergy. 2003;58:213–20. doi: 10.1034/j.1398-9995.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- 34.Rasmussen F, Taylor DR, Flannery EM, Cowan JO, Greene JM, Herbison GP, et al. Outcome in adulthood of asymptomatic airway hyperresponsiveness in childhood: a longitudinal population study. Pediatr Pulmonol. 2002;34:164–71. doi: 10.1002/ppul.10155. [DOI] [PubMed] [Google Scholar]
- 35.Niimi A, Matsumoto H, Takemura M, Ueda T, Chin K, Mishima M. Relationship of airway wall thickness to airway sensitivity and airway reactivity in asthma. Am J Respir Crit Care Med. 2003;168:983–8. doi: 10.1164/rccm.200211-1268OC. [DOI] [PubMed] [Google Scholar]
- 36.Boulet L, Belanger M, Carrier G. Airway responsiveness and bronchial-wall thickness in asthma with or without fixed airflow obstruction. Am J Respir Crit Care Med. 1995;152:865–71. doi: 10.1164/ajrccm.152.3.7663797. [DOI] [PubMed] [Google Scholar]
- 37.Niimi A, Matsumoto H, Amitani R, Nakano Y, Mishima M, Minakuchi M, et al. Airway wall thickness in asthma assessed by computed tomography: relation to clinical indices. Am J Respir Crit Care Med. 2000;162:1518–23. doi: 10.1164/ajrccm.162.4.9909044. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto H, Niimi A, Takemura M, Ueda T, Minakuchi M, Tabuena R, et al. Relationship of airway wall thickening to an imbalance between matrix metalloproteinase-9 and its inhibitor in asthma. Thorax. 2005;60:277–81. doi: 10.1136/thx.2004.028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gono H, Fujimoto K, Kawakami S, Kubo K. Evaluation of airway wall thickness and air trapping by HRCT in asymptomatic asthma. Eur Respir J. 2003;22:965–71. doi: 10.1183/09031936.03.00085302. [DOI] [PubMed] [Google Scholar]
- 40.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–9. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 41.Berry M, Morgan A, Shaw DE, Parker D, Green R, Brightling C, et al. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007;62:1043–9. doi: 10.1136/thx.2006.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–63. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
METHODS REFERENCES
- E1.Global Initiative for Asthma [Accessed December 29, 2007];Global Initiative for Asthma Guidelines. 2007 http://www.ginasthma.com/Guidelineitem.asp??l152&l251&intId560
- E2.American Thoracic Society Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162:2341–51. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- E3.Brightling CE. Chronic cough due to nonasthmatic eosinophilic bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:116S–21S. doi: 10.1378/chest.129.1_suppl.116S. [DOI] [PubMed] [Google Scholar]
- E4.British Thoracic Society British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax. 2001;56(suppl):i1–21. doi: 10.1136/thorax.56.suppl_1.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E5.Britten KM, Howarth PH, Roche WR. Immunohistochemistry on resin sections: a comparison of resin embedding techniques for small mucosal biopsies. Biotech Histochem. 1993;68:271–80. doi: 10.3109/10520299309105629. [DOI] [PubMed] [Google Scholar]
- E6.Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–63. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- E7.Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003;167:1360–8. doi: 10.1164/rccm.200209-1030OC. [DOI] [PubMed] [Google Scholar]
- E8.Trian T, Benard G, Begueret H, Rossignol R, Girodet P-O, Ghosh D, et al. Bronchial smooth muscle remodeling involves calcium-dependant enhanced mitochondrial biogenesis in asthma. J Exp Med. 2007;204:3173–81. doi: 10.1084/jem.20070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E9.Sullivan P, Stephens D, Ansari T, Costello J, Jeffery P. Variation in the measurements of basement membrane thickness and inflammatory cell number in bronchial biopsies. Eur Respir J. 1998;12:811–5. doi: 10.1183/09031936.98.12040811. [DOI] [PubMed] [Google Scholar]
- E10.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]