Abstract
The microlocalization of mast cells within specific tissue compartments is thought to be critical for the pathophysiology of many diverse diseases. This is particularly evident in asthma where they localize to the airway smooth muscle (ASM) bundles. Mast cells are recruited to the ASM by numerous chemoattractants and adhere through CADM1, but the functional consequences of this are unknown. In this study, we show that human ASM maintains human lung mast cell (HLMC) survival in vitro and induces rapid HLMC proliferation. This required cell-cell contact and occurred through a cooperative interaction between membrane-bound stem cell factor (SCF) expressed on ASM, soluble IL-6, and CADM1 expressed on HLMC. There was a physical interaction in HLMC between CADM1 and the SCF receptor (CD117), suggesting that CADM1-dependent adhesion facilitates the interaction of membrane-bound SCF with its receptor. HLMC-ASM coculture also enhanced constitutive HLMC degranulation, revealing a novel smooth muscle-driven allergen-independent mechanism of chronic mast cell activation. Targeting these interactions in asthma might offer a new strategy for the treatment of this common disease.
Asthma is a major cause of morbidity and mortality worldwide and its prevalence is increasing (1, 2). It is characterized by the presence of variable airflow obstruction, airway hyperresponsiveness (AHR),5 and chronic airway inflammation (3). Mast cells play an important role in the pathophysiology of asthma through their chronic activation by IgE (4), allergen, and other diverse nonimmunological stimuli (5). This results in the secretion of a plethora of bronchospastic and proinflammatory autacoid mediators, proteases, and cytokines (5). Mast cells are present in the lamina propria of normal human airways (6) but in asthma they re-locate to three key structures: the airway epithelium (6), the airway submucosal glands (7), and the airway smooth muscle (ASM) (8-14). This anatomical relocation places activated mast cells deep within these dysfunctional airway elements, potentially facilitating an intimate functional relationship.
The presence of mast cells within the asthmatic ASM bundles is of particular interest because this is not evident in eosinophilic bronchitis (EB) (9). EB is a common cause of cough and is characterized by the presence of a sputum eosinophilia occurring in the absence of variable airflow obstruction and AHR (15). A detailed comparison of the immunopathology of asthma and EB revealed an identical pathology in terms of mucosal inflammation, Th2 cytokine expression, epithelial integrity, and sub-basement membrane collagen deposition (9, 16-18). This suggests that many of the immunopathological features previously attributed to causing asthma may not be so important for the development of airflow obstruction, AHR, and remodeling as previously suggested. The striking difference between the pathology of asthma and EB resides within the ASM bundles, which contain numerous mast cells in patients with asthma but virtually none in the patients with EB (9) or normal subjects (9-14). This mast cell myositis is evident across asthma phenotypes (13). ASM mast cell density correlates with the severity of AHR (9), and the mast cells within the ASM bundles in asthma demonstrate ultrastructural features of activation (14). Taken together these studies suggest that infiltration of ASM by mast cells is of great functional relevance and is one of the critical determinants of the asthmatic phenotype.
The specific recruitment of mast cells to the ASM in asthma raises many important questions, in particular, do the cells interact, and if so, what are the functional consequences for airway function? Studies examining whole cell interactions in vitro are sparse. However, human lung mast cells (HLMC) adhere to human ASM cells, in part, via an Ig superfamily member known as CADM1 (19) (previously known as TSLC1, IGSF4, SgIGSF, SynCAM, Necl2, RA175), suggesting that specific cellular cross-talk occurs. In addition, potential mechanisms of mast cell recruitment by the ASM have been identified, particularly the CXCR3/CXCL10 axis (20).
In addition to mast cell recruitment and adhesion, it is likely that the HLMC hyperplasia present within asthmatic ASM depends upon the ability of the ASM to maintain HLMC survival and could also result in part from the proliferation of resident HLMC. It is well established that primary differentiated HLMC have the potential to proliferate (21, 22) and ASM is known to produce the mast cell growth and survival factors stem cell factor (SCF) and IL-6 (23, 24). In this study, we show for the first time that ASM not only maintains HLMC survival in vitro, but a direct interaction between the two cell types leads to the rapid proliferation of HLMC through a cooperative SCF-, IL-6-, and CADM1-dependent mechanism. This is coupled with enhanced constitutive HLMC activation. Targeting this interaction in the ASM compartment in asthma might offer a new strategy for the treatment of this increasingly common disease.
Materials and Methods
Reagents and cytokines
Antibiotic/antimycotic solution, CFSE (Cell Trace CFSE Cell Proliferation Kit), DMEM, HEPES, HBSS without Ca2+ and Mg2+, and nonessential amino acid solution were all purchased from Invitrogen Life Technologies. The 4′,6-diamidino-2-phenylindole (DAPI), heat-inactivated FCS, ITS plus 3 liquid medium supplement and sodium pyruvate were obtained from Sigma-Aldrich.
Recombinant human IL-6, IL-10, and SCF were obtained from R&D Systems. Accutase enzyme cell detachment medium was purchased from Insight Biotechnology. Transwell 0.4 μm inserts were purchased from Becton Dickinson Labware. Histamine and S-adenosyl-l-methyl-[3H]methionine were purchased from Amersham Life Science; rat kidney histamine methyl transferase was a generous gift from Dr. S. Harper (Astra Charnwood, Loughborough, U.K.). RIPA buffer containing protease inhibitors and protein A/G beads were purchased from Santa Cruz Biotechnology.
Antibodies
The following Abs were purchased from R&D Systems: anti-human IL-6 mAb (clone 6708), chicken IgY isotype control, goat anti-human SCF, IgG1 isotype control and secondary NorthernLights, PE (RPE). Anti-human CD117-RPE mAb (clone 104D2) and isotype IgG2a-FITC were obtained from DAKO. Anti-human myosin mAb (clone, SMMS-1), anti-human α-smooth muscle actin-FITC mAb (clone, 1A4), and goat IgG control were purchased from Sigma-Aldrich. Anti-human CD117 (clone, YB5B8), Annexin V, IgG1-RPE, and propidium iodide (PI) were purchased from BD Pharmingen. Human myeloma IgE was obtained from Calbiochem-Novabiochem. Allophycocyanin goat anti-mouse secondary and sheep anti-human IgG dynabeads were obtained from Invitrogen. Chicken anti-CADM1 mAbs 3E1 and 9D2 were generated as described previously (25). Histamine and S-adenosyl-l-methyl-[3H]methionine were purchased from Amersham Life Science; rat kidney histamine methyl transferase was a generous gift from Dr. S. Harper (Astra Charnwood, Loughborough, U.K.).
HLMC isolation and culture
The study was approved by the Leicestershire Research Ethics Committee, and all tissue donors gave written informed consent. HLMC were obtained from macroscopically normal lung obtained at surgery for carcinoma by positive selection using anti-CD117-coated immunomagnetic Dynabeads as described previously (26). Final mast cell purity was >99% and viability was >98%. Immediately following purification, HLMC were cultured in DMEM/Glutamax/HEPES containing antibiotic/antimycotic solution, non-essential amino acids, 10% FCS, and cytokines (100 ng/ml SCF, 50 ng/ml IL-6, and 10 ng/ml IL-10) as described previously (22).
Human ASM isolation and culture
Pure ASM bundles in airways isolated from lung resection and bronchial biopsy tissue were dissected free of surrounding tissue as described previously (20). The muscle bundles were cultured in DMEM supplemented with 10% FCS, 4 mM l-glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin, and 0.25 μg/ml amphotericin. ASM characteristics were determined by immunofluorescence and light microscopy with α-smooth muscle actin-FITC direct conjugate and myosin indirectly conjugated with FITC.
ASM/HLMC coculture assay
HLMCs taken from culture 3 days following isolation were washed with FCS-free media and seeded onto confluent ASM that had been FCS-deprived for 3 days in 6-well plates in DMEM media containing 1% ITS plus 3 liquid media supplement, 1% antibiotic/antimycotic, 1% nonessential amino acids, and 1% sodium pyruvate. Abs (anti-human SCF [1 μg/ml]; anti-human IL-6 [0.6 μg/ml]; anti-CADM1 9D2 [10 μg/ml], isotype controls for anti-human SCF [goat IgG] and IL-6 [mouse IgG1] and for anti-CADM1 [chicken IgY]) were included when required alone or in combination with 0.4 μM Transwell inserts to prevent cell contact between the two cell types. Because analysis of bronchial biopsies reveals a mean mast cell density in asthmatic ASM bundles of ~4 ASM cell:1 HLMC, HLMC were seeded onto confluent FCS-starved ASM at a 1:4 ratio (equivalent to 4 × 104 HLMC/well). Cell number during the culture period was assessed using Kimura staining, which readily differentiates red metachromatic mast cells from unlabeled ASM cells. HLMC monoculture controls were established in parallel and included mast cells in FCS-free and cytokine-free media, mast cells with the cytokines SCF (100 ng/ml) and IL-6 (50 ng/ml) but no FCS, and mast cells with SCF, IL-6, and 10% FCS.
Dye quantification of HLMC cell proliferation
CSFE (5 μM) was prepared from 5 mM stock using PBS (1 × 106 cells/ml), and the required concentration of cells was then resuspended in the appropriate volume of diluted CFSE (~1 × 106 cells/ml). The cells were incubated for 15 min at 37°C, 5% CO2 before centrifugation and resuspended in fresh media. The cells were then incubated for a further 30 min at 37°C, 5% CO2 to ensure complete modification of the probe. The cells were washed once more before plating out or analyzing by flow cytometry as required. For culture, the cells were plated out onto confluent FCS-starved ASM in T75 flasks and incubated for either 5 or 10 days before harvesting and counterstaining with CD117-PE and analysis using BD FACSCanto flow cytometer.
Quantification of HLMC death by flow cytometry
HLMC were seeded at a density of 0.5 × 106 cells per T75 flask in either FCS free and cytokine free ITS media or in the presence of 10% FCS and exogenous cytokine (IL-6 and SCF) media. ASM cells had been grown to confluence in T75 flasks and FCS deprived for 3 days in ITS media before coculturing with HLMC (at a ratio of 1:4 HLMC:ASM). The HLMC were then incubated at 37°C over 10 days either in the presence or absence of ASM cells in ITS or just in the presence of FCS and cytokines. After days 1, 3, 7, and 10, the cell supernatant from the cultures were spun down and kept and the cells were washed with HBSS, harvested with Accutase, and pooled with their respective supernatant pellets and washes that may have detached the cells during the experiment. In brief, for HLMC alone the percentage of apoptotic or necrotic cells was assessed by suspending the HLMC cells in 1× Annexin binding buffer containing FITC-conjugated Annexin V (1 μl/200 μl binding buffer) ± PI (0.5 μg/ml), before analysis on the BD FACSCanto flow cytometer. Three-color flow cytometry was conducted with HLMC cocultured with ASM cells. The cocultured cultures were stained with mAb against CD117 (clone:YB5.B8) and then with allophycocyanin secondary (to identify the HLMC) and then stained as above with Annexin and PI. Appropriate isotype controls were used.
Morphological detection of HLMC apoptosis
Nuclear morphology of HLMC was assessed by DAPI staining. For these experiments, HLMC were seeded at a density of 1 × 104 cells/well into 8-well chamber slides in either FCS-free and cytokine-free ITS media or in the presence of 10% FCS and exogenous cytokine IL-6 (50 ng/ml), SCF (100 ng/ml) media. ASM cells had been grown to confluence in 8-well chamber slides and FCS deprived for 3 days in ITS media before coculturing with HLMC (at a ratio of 1:4 HLMC:ASM). The HLMC were then incubated at 37°C over 10 days either in the presence or absence of ASM cells in ITS or in the presence of FCS and cytokines. ASM cells cocultured with HLMC were stained with directly conjugated mAb against RPE-CD117 (clone 104D2) and indirectly labeled with secondary Northen-Lights RPE secondary. The cells were then counterstained with DAPI and mounted with photo bleach retardant mounting medium. CD117 was used to identify the HLMC in the presence of ASM cells. HLMC cultured alone were harvested and cytospun down onto slides and stained with DAPI alone and mounted. For each HLMC mono- or coculture (n = 3), six random high-powered fields were examined for morphologic features of apoptosis such as nuclear condensation and fragmentation.
Immunofluorescence and confocal microscopy
ASM were grown to confluence on chamber slides and FCS deprived for 3 days and then 5 × 104 HLMC were added to the cells for 3 days. The cells were fixed with methanol, blocked with 3% BSA, and labeled with conjugated mAb against RPE-CD117 (clone 104D2) and indirectly labeled with secondary NorthenLights RPE secondary. The cells were also labeled with chicken anti-human CADM1 IgY mAb (3E1) indirectly labeled with FITC-labeled goat anti-IgY polyclonal. Cells were counterstained with DAPI and mounted with photo bleach-retardant mounting medium. Appropriate isotype controls were performed. The cells were either studied by a standard fluorescent microscope or visualized using an Olympus Optical FV500 scanning laser confocal IX70 inverted microscope. Images were captured using an oil immersion ×60 objective. Digital images and data files of immunofluorescence intensity transectioning specimens in the xy plane and x,y,z plane were recorded using Flowview software (FV300 Olympus). Images were rendered using paintshop pro 8.
SCF expression
Flow cytometry
Confluent ASM cells in T25 flasks were FCS deprived for 24 h, washed with HBSS, and harvested using Accutase. ASM were stained with goat anti-human SCF, indirectly labeled with polyclonal rabbit anti-goat FITC secondary, and compared with appropriate goat isotype control. Analysis was performed using single-color flow cytometry on the BD FACSCanto flow cytometer.
Immunofluorescence
ASM were grown to confluence on chamber slides and FCS deprived for 24 h. The cells were labeled with goat anti-human SCF, indirectly labeled with polyclonal rabbit anti-goat FITC secondary, and compared with appropriate goat isotype control. Cells were counter-stained with DAPI and mounted.
ELISA analysis of supernatant content
SCF and IL-6 were measured by ELISA (R&D Systems) according to the manufacturer’s instructions.
Mast cell activation for histamine release
HLMC were activated using IgE and anti-IgE stimulation. HLMC were harvested and sensitized with human myeloma IgE (2.4 μg/ml) for 1 h before washing and resuspending at 37°C in fresh medium containing goat anti-human IgE (1/1000 dilution). After 30-min incubation, supernatant was collected and the cell pellet was lysed in sterile de-ionized water. Histamine was measured by sensitive radioenzymatic assay based on the conversion of histamine to methylhistamine in the presence of the enzyme histamine-N-methyltransferase as previously described (26).
Immunoprecipitation of CADM1 and Western blotting
A total of 8 × 107 HMC-1 cells were lysed in 4 ml ice-cold RIPA buffer containing protease inhibitors. The insoluble debris was removed by centrifugation at 10,000 g for 10 min. The cell lysate (4 ml) was then incubated with 80 μl protein A/G beads (Santa Cruz Biotechnology) overnight at 4°C with rotation to remove any proteins that adhere nonspecifically. The beads were pelleted by centrifugation and the 4 ml “cleaned” cell lysate was transferred into new vials. Chicken anti-CADM1 (3E1) was added to the cell lysate at 10 μg/ml and the sample was incubated at 4°C overnight with rotation. After overnight incubation with anti-CADM1, 2 ml cell lysate was incubated with 20 μl PBS control and the remaining 2 ml cell lysate was incubated with 20 μl goat anti-chicken IgY (final concentration 10 μg/ml). These samples were incubated for 4 h at 4°C. Protein A/G beads were then added to all samples (20 μl beads/ml sample) and incubated at 4°C overnight with rotation. The samples were then centrifuged, and the supernatant diluted at 1/100 in sample loading buffer. The beads were washed four times with PBS, and beads in each sample then resuspended in 200 μl sample loading buffer. All samples were boiled for 10 min and stored at −20°C before running on a 10% SDS gel. After transfer to polyvinylidene difluoride membrane, the membrane was probed for CD117 using mouse IgG1 anti-CD117 Ab (0.2 μg/ml) and visualized by goat anti-mouse IgG-HRP.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 4 (GraphPad). Data are presented as mean (±SEM). Paired and unpaired data were analyzed by using paired and unpaired t tests, respectively. Comparison across groups was assessed using ANOVA and Tukey’s multiple comparison test where appropriate. Differences were considered significant when p values were less than 0.05.
Results
ASM supports the survival and proliferation of HLMC
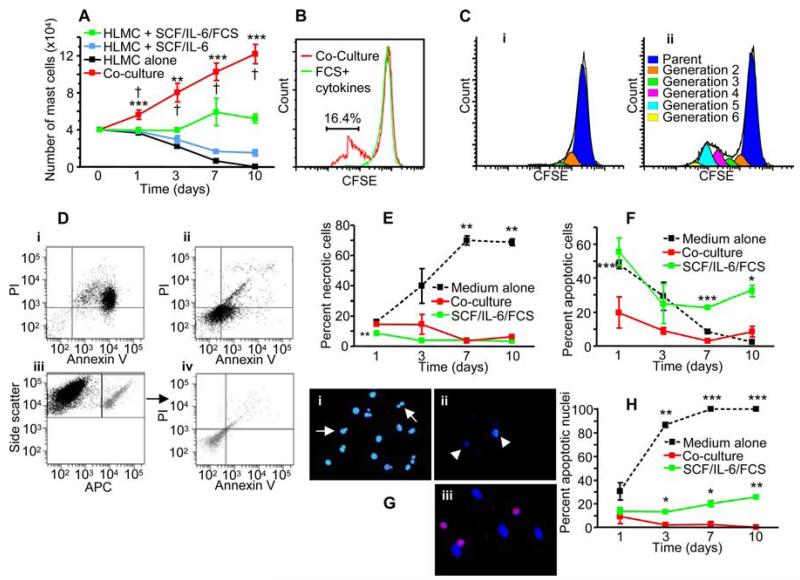
When HLMC (4 × 104) were incubated in culture medium alone, their numbers diminished to zero by day 10 (Fig. 1A). In the presence of IL-6 and SCF, but no FCS, 1.5 ± 0.35 × 104 HLMC survived by day 10 (Fig. 1A), while SCF/IL-6 plus 10% FCS sustained survival with minor proliferation evident by day 10 (5.2 ± 0.67 × 104; Fig. 1A). Remarkably, when HLMC were cocultured with asthmatic or nonasthmatic ASM (4:1 ASM:HLMC ratio) in the absence of FCS or exogenous cytokines, not only did they survive, but there was rapid proliferation evident from day 1 and, at day 10, the number of mast cells had increased to 12.2 ± 1.0 × 104 (n = 14, p < 0.001; Fig. 1A). The HLMC number from days 1–10 in ASM coculture was significantly greater than each of the other conditions (Fig. 1A). This survival and proliferative effect of the ASM did not differ between asthmatic or nonasthmatic ASM cells (p = 0.34).
FIGURE 1.
HLMCs survive and proliferate in coculture with ASM in the absence of FCS and exogenous cytokines. A, Numbers of metachromatic mast cells present over 10 days of culture in culture medium alone (black line), SCF (100 ng/ml) and IL-6 (50 ng/ml) (blue line), medium plus SCF/IL-6/FCS (green line), and coculture with ASM in the absence of SCF/IL-6/FCS (red line); n = 14 experiments using 8 HLMC donors and 14 ASM donors. **, p < 0.01; and ***, p < 0.001 by ANOVA. †, p < 0.05 by Tukey’s multiple comparison test comparing HLMC-ASM coculture to each of the other conditions. B, HLMC were loaded with CFSE before culturing in SCF/IL-6/FCS (green line) or coculture with ASM in the absence of SCF/IL-6/FCS (red line) for 10 days, and then analyzed using flow cytometry. Cocultured HLMC exhibit a subpopulation with significantly reduced fluorescence beyond the control HLMC population, indicating the presence of a proliferating pool of cells (p = 0.046 by paired t test). The histogram is representative of three experiments using three HLMC donors and three ASM donors. C, Analysis of CSFE staining using Modfit LT software revealed the presence of a reduced number of generations following 10 days in monoculture (i) compared with ASM-coculture (ii). Representative of three experiments. D–F, The percentage of dead/apoptotic cells over 10 days of culture. D, Representative dot plots for FITC-Annexin V/PI dual-color flow cytometry of HLMC cultured for 7 days (i) alone in the absence of SCF/IL-6/FCS and (ii) alone with SCF/IL-6/FCS. Three color flow cytometry of 7-day HLMC labeled with CD117-allophycocyanin cocultured with ASM in the absence SCF/IL-6/FCS (iii), and gated CD117 cells analyzed for Annexin V and PI (iv). E, Percentage of dead cells revealed by PI staining using flow cytometry. F, Percentage of apoptotic cells revealed by Annexin V staining using flow cytometry. G, Representative micrographs at day 7 showing DAPI staining on HLMC (i) in the presence of SCF/IL-6/FCS, (ii) in absence of SCF/IL-6/FCS, or (iii) in coculture with ASM (stained with DAPI and overlaid with anti-CD117 [red]). In i and ii, note nuclear blebbing (arrows) and fragmentation (arrowheads). H, Quantification of apoptosis identified by nuclear morphology. Fig. 1E, F, and H: *, p < 0.05; **, p < 0.01; and ***, p < 0.001 comparing HLMC monoculture in the presence or absence of SCF/IL-6/FCS to HLMC-ASM coculture. Paired t test; n = 3 HLMC and ASM donors.
To confirm the results obtained by cell counting, the proliferation of HLMC in ASM coculture was quantified using HLMC that were preloaded with the fluorescent marker CFSE. This stable dye is not passed between cells upon adhesion and is split equally upon cell mitosis, allowing detection of new generations using flow cytometry. Loaded HLMC were cocultured with ASM for 10 days or cultured alone in SCF/IL-6/FCS-supplemented medium (positive control). The proportion of HLMC that proliferated in coculture was significantly greater than the control (mean difference [95% confidence interval] 16.4% [0.7-32.2%]; p = 0.046; n = 3; Fig. 1B). The fluorescence histograms were also analyzed using Modfit LT software, which accurately approximates generation peaks. Cocultured HLMC were found to contain more generations following 10 days of culture when compared with the control HLMC (Fig. 1C).
HLMC apoptosis is attenuated in ASM coculture
The extent of cell death in the various culture conditions was analyzed using a combination of PI and Annexin V staining, with DAPI staining of the nuclei. PI and Annexin V analysis of cell death was assessed using flow cytometry in combination with CD117-allophycocyanin to identify the HLMC population (Fig. 1D). Staining with PI demonstrated that HLMC cell death in both the ASM coculture and the SCF/IL-6/FCS-supplemented monoculture was relatively low and stable (Fig. 1E). In contrast, HLMC in the cytokine-free monoculture demonstrated accelerated cell death (Fig. 1E).
Annexin V staining on HLMC increased consistently in the SCF/IL-6/FCS-supplemented control compared with the ASM coculture suggesting a decreased rate of apoptosis in coculture (p < 0.001 at day 7; Fig. 1F). This was further supported by analysis of nuclear fragmentation using DAPI staining (Fig. 1, G and H). By day 3, there was a significant increase in the percentage of apoptotic HLMC nuclei in the SCF/IL-6/FCS-supplemented control compared with the HLMC in ASM coculture (p = 0.011). There was some discrepancy in the number of apoptotic HLMC analyzed by Annexin V vs DAPI in the SCF/IL-6/FCS-supplemented monoculture. This may be accounted for by the degranulation of HLMC following resuspension in 100 ng/ml SCF (27). Nevertheless, the data demonstrate consistently greater cell death within the SCF/IL-6/FCS-supplemented HLMC monoculture compared with the HLMC in ASM coculture.
ASM-induced HLMC proliferation is dependent on SCF-, IL-6-, and CADM1-dependent adhesion
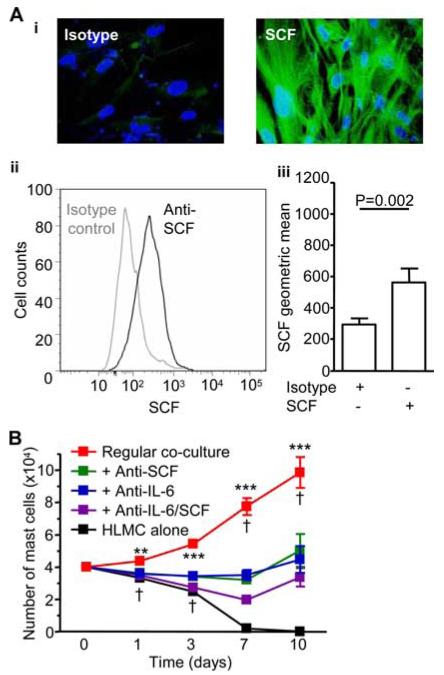
SCF is an essential mast cell growth factor (28-30), IL-6 promotes cord blood mast cell progenitor growth (31) and prevents HLMC apoptosis (32), and both cytokines are produced by ASM (23, 24). IL-6 concentrations in the HLMC-ASM coculture-conditioned medium at day 3 were 12.2 ± 0.67 ng/ml, which is above the optimal 1 ng/ml concentration of IL-6 required for the development of human mast cells from peripheral blood progenitors (30). SCF concentrations in this medium were only 0.29 ± 0.06 ng/ml, which exert only a weak survival capacity on cultured human mast cells (30). However, SCF also exists in a membrane-bound form generated by alternative mRNA splicing (33). Membrane-bound SCF was highly expressed as demonstrated by both flow cytometry and immunofluorescent staining in ASM from both asthmatics and nonasthmatics (Fig. 2A). Addition of neutralizing Abs targeting SCF or IL-6 inhibited HLMC proliferation in ASM coculture to a similar degree, and using both Abs together had no significant additive effect (Fig. 2B). With cytokine neutralization, cell number at day 10 remained similar to that present at day 0, suggesting that while both IL-6 and SCF play important roles in mediating HLMC proliferation, a further factor was supporting their survival.
FIGURE 2.
HLMC proliferation in coculture with ASM is dependent on SCF and IL-6. A, Human ASM expresses membrane-bound SCF, (i) positive immunofluorescence for SCF (green) in ASM monolayers. Representative histogram of SCF expression on ASM by flow cytometry (ii) and geometric mean analysis of ASM demonstrating surface SCF expression (iii), n = 8 experiments. B, Effect of cytokine neutralization. HLMC were cultured in media alone (black line) or cocultured with ASM in the absence SCF/IL-6/FCS (red line), and in the presence of the neutralizing Abs to IL-6 (blue line), SCF (green line), or both (purple line), n = 5 experiments using three HLMC donors and five ASM donors. **, p < 0.01; ***, p < 0.001 by one-way ANOVA. †, p < 0.05 by Tukey’s multiple comparison test comparing cocultured HLMC to addition of neutralizing Abs singularly and in combination.
To address whether ASM-induced HLMC survival and proliferation requires cell-cell contact or soluble factors only, HLMC were cultured in FCS-free ASM-conditioned medium generated over 3 days. The soluble mediators within the media maintained HLMC survival for 7 days but no proliferation was evident (Fig. 3A). By day 10, HLMC numbers began to decline, indicating a possible depletion of survival factors. Addition of SCF- or IL-6-neutralizing Abs or both in combination to this culture reduced the number of surviving HLMC further (Fig. 3A).
FIGURE 3.
HLMC proliferation in coculture with ASM is dependent on cell-cell contact and CADM1. A, In the presence of ASM-conditioned media, HLMC survived for 7 days but did not proliferate (red line). This survival effect was attenuated equally by neutralizing Abs to IL-6 (blue line) and SCF (green line), with a nonsignificant additive effect evident with both SCF and IL-6 neutralization (purple line), n = 6 experiments using three HLMC donors and six ASM donors. **, p < 0.01; and ***, p < 0.001 by one-way ANOVA. †, p < 0.05 by Tukey’s multiple comparison test comparing HLMC cultured in ASM-conditioned to addition of combined SCF and IL-6 neutralizing Abs. B, Separation of HLMC from ASM cells using a 0.4 μM Transwell insert inhibits their proliferation and survival. HLMC were cultured in media alone (black solid line), exogenous SCF/IL-6/FCS (light green solid line solid line), or in the presence of ASM without SCF/IL-6/FCS (red solid line). HLMC were also cocultured with ASM with separation by a 0.4 μM Transwell insert (T) to block physical contact between the two cell types in the presence and absence of isotype control Ab (red and gray dashed line respectively), neutralizing anti-IL6 Ab (blue dashed line), neutralizing anti-SCF Ab (green dashed line), or both anti-IL-6 and -SCF (purple dashed line), n = 5 experiments using three HLMC donors and five ASM donors. ***, p < 0.001 by one-way ANOVA. †, p < 0.05 by Tukey’s multiple comparison test comparing cocultured HLMC to the addition of Transwell inserts in the presence and absence of neutralizing Abs. C, In keeping with the Transwell experiments, addition of an adhesion-blocking Ab to CADM1 also prevented HLMC proliferation in coculture with ASM, suggesting that CADM1-dependent adhesion is an important component of the ASM-induced HLMC proliferation pathway, n = 5 experiments using three HLMC donors and five ASM donors. **, p < 0.01 by paired t test comparing isotype control cocultured HLMC with the anti-CADM1 Ab. D, HLMC-ASM coculture in the presence of neutralizing Abs to SCF, IL-6, and CADM1 revealed a minor additional effect over neutralization of each in isolation suggesting a cooperative effect between the three molecules, n = 6 experiments using three HLMC donors and six ASM donors. *, p < 0.05; **, p < 0.01; and ***, p < 0.001 by paired t test comparing isotype control cocultured HLMC with anti-SCF/IL-6/CADM1-treated cells.
We then examined ASM-dependent HLMC survival and proliferation using the Transwell system. ASM cells were grown to confluence before a 0.4 μM Transwell membrane was inserted and the HLMC then added (4:1 ASM:HLMC ratio as previously). Use of the Transwell inserts significantly reduced survival and proliferation of the HLMC compared with the coculture without inserts (Fig. 3B), comparable to the effects seen in ASM-conditioned media. Addition of IL-6- and SCF-neutralizing Abs individually and in combination reduced this survival further but this was not significant when allowing for multiple comparisons (Fig. 3B). These data confirm the importance of cell-cell contact for HLMC survival and proliferation.
HLMC adhere to ASM in part through CADM1, an adhesion molecule highly expressed on HLMC but not ASM (19). Interestingly, the adhesion-blocking anti-CADM1 mAb 9D2 (19) significantly reduced the proliferation of HLMC in ASM coculture compared with the isotype control (p < 0.01 at days 7 and 10; Fig. 3C), indicating that adhesion via CADM1 is also a critical factor for the induction of HLMC proliferation by ASM. Inhibiting CADM1, IL-6, and SCF together (Fig. 3D) reduced the survival and proliferation of HLMC further than that seen with the individual Abs (p = 0.007 at day 10 by one-way ANOVA). Nevertheless, this inhibition was not complete and 56.9 ± 25.5% of HLMC remained viable in the culture after 10 days, indicating that either blockade of these molecules was not complete or a further factor(s) may be important. There was no evidence of any physical or functional cross-reaction between the anti-SCF, anti-IL-6, or 9D2 anti-CADM1 Abs. Taken together, the marked effects of inhibiting each of SCF, IL-6, and CADM1 in isolation, but relatively small additive effects seen when inhibiting them together, suggests the presence of a cooperative interaction between these three molecules.
CD117 and CADM1 colocalize and physically interact in HLMC
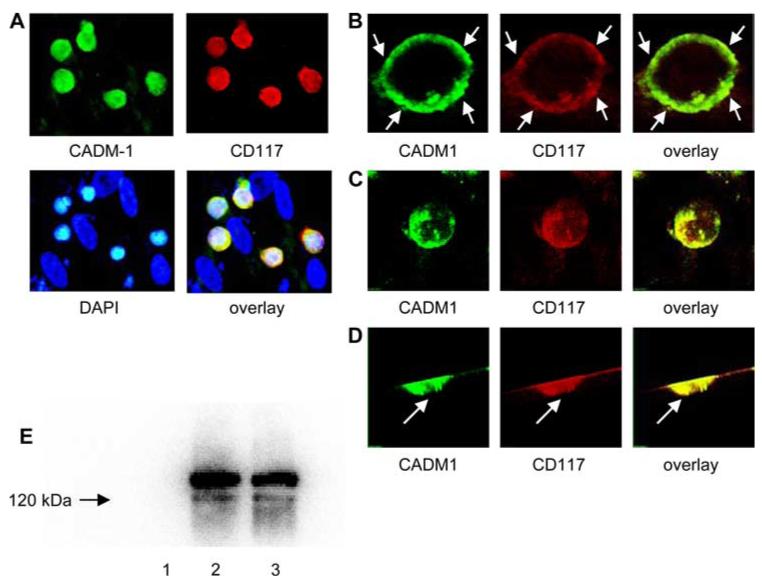
Our data suggest that there is a cooperative interaction between SCF and CADM1. It is possible, therefore, that CADM1-dependent adhesion facilitates the interaction of membrane-bound SCF on human ASM with its receptor CD117 (c-kit) on HLMC. Confocal immunofluorescence demonstrated strong colocalization of CD117 and CADM1 in the HLMC plasma membrane (Fig. 4A-D). There was evidence of immunofluorescent hotspots for both CADM1 and CD117, which were 100% colocalized and present at points of adhesion with ASM (Fig. 4). Interestingly, immunoprecipitation of CADM1 resulted in the coimmunoprecipitation of CD117, indicating the presence of a physical interaction (Fig. 4E).
FIGURE 4.
CD117 colocalizes with CADM1. A, HLMC were cocultured with ASM cells over 3 days and stained with anti-human RPE-CD117 (104D2, red), anti-CADM1 (3E1, green), and DAPI (blue cell nuclei). The cells negative for CD117 and CADM1 are the ASM cells (×400 magnification). B, Representative micrographs of confocal laser scanning microscopic sections of HLMC cocultured on confluent ASM cells for 3 days. Diffuse plasma membrane staining was punctuated by hotspots of staining (arrows). Notably the cell surface is the most intensely fluorescent and CD117 and CADM1 are colocalized in the HLMC plasma membrane. C, HLMC XYZ plane images were captured and a 3D image cropping series animation was produced of CADM1 and CD117 staining. The displays of the measured 3D image stack are shown for CADM1, CD117, and the overlay, demonstrating strong colocalization. D, The 3D image was then rotated and demonstrated that there were hot spots of colocalized staining present at the point of contact with the ASM (arrow). E, Lane 2, CADM1 was immunoprecipitated from the HMC-1 human mast cell line using the 3E1 anti-CADM1 Ab, run on an SDS gel, transferred to a polyvinylidene difluoride membrane, and then probed for CD117. CD117 was consistently present as a characteristic doublet of 145 and 122 kDa (representative of three experiments). Lane 1, Negative control i.p. with anti-chicken IgY omitted. Lane 3, Positive control for CD117 present in HMC-1 cell lysate.
HLMC in coculture with ASM demonstrate increased constitutive histamine release and decreased histamine content
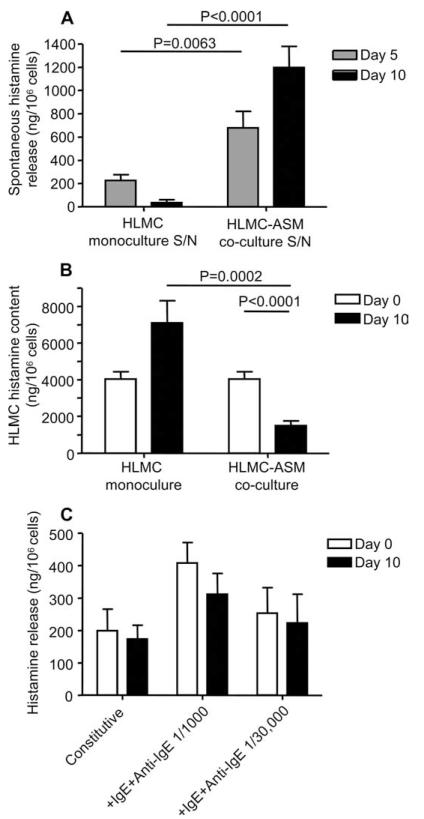
HLMC within the ASM bundles of patients with asthma demonstrate morphological features of activation with loss of granule contents (14), so-called piecemeal degranulation. We, therefore, collected coculture supernatants and harvested the cells for analysis of total histamine content and activation in the presence of IgE/anti-IgE. These were compared with fresh HLMC at day 0 and parallel HLMC cultured in SCF, IL-6, and FCS.
HLMC cocultured with ASM released significantly more histamine into the culture supernatant over 10 days (1198 ± 182 ng/106 cells present at day 10) when compared with HLMC cultured alone with SCF/IL-6/FCS (35 ± 24 ng/106 cells present at day 10, p < 0.001; Fig. 5A). This suggests that HLMC in coculture with ASM is activated. In keeping with the increased histamine content in the coculture supernatant, the histamine content of HLMC in the coculture was approximately half that of the control cells and the cells at baseline (1491 ± 253 ng/106 cells in coculture, 7101 ± 1214 ng/106 cells in SCF/IL-6/FCS, and 4029 ± 398 ng/106 cells at baseline, both p < 0.001; Fig. 5B). Although the 10-day cocultured HLMC contained less histamine than the day 0 controls, when they were activated with IgE/anti-IgE for 30 min, the total amount of histamine released was similar to that at day 0 (Fig. 5C). Thus, HLMC are constitutively activated while in coculture with ASM, and maintain their responsiveness to stimulation through the high affinity IgE receptor.
FIGURE 5.
HLMC in coculture with ASM demonstrate increased constitutive histamine release and decreased histamine content. A, Histamine concentrations were measured in the culture supernatant and corrected for the number of cells present at the appropriate time point. Increased constitutive histamine release was evident in the HLMC-ASM coculture at both days 5 and 10 compared with the SCF/IL-6/FCS-supplemented HLMC monoculture. B, In keeping with increased constitutive release, histamine content corrected for cell number was significantly reduced in the coculture compared with both the monoculture control and the original cells analyzed at day 0. C, Total histamine release corrected for cell number in response to IgE-dependent activation for 30 min was similar in HLMC at day 0 compared with cells after 10 days in coculture.
Discussion
We show for the first time that human ASM supports the survival and proliferation of HLMC, effects mediated through a cooperative interaction between ASM membrane-bound SCF, HLMC membrane-expressed CADM1, and soluble IL-6. Furthermore, HLMC in ASM coculture demonstrate enhanced constitutive activation as measured by the release of histamine.
Human ASM was predicted to maintain HLMC survival because it produces SCF, an essential mast cell growth factor (23). However, the rapid proliferation of HLMC was remarkable, particularly as they have long been considered to represent terminally differentiated cells. Nevertheless, HLMC will survive and proliferate slowly over many weeks in culture with SCF, IL-6, and IL-10 (21, 22), but this is in marked contrast to the rapid rate of HLMC proliferation in the ASM coculture. High-resolution dye tracking confirmed the results observed by metachromatic counting, while DAPI and Annexin V staining indicated a decreased rate of HLMC apoptosis in the ASM coculture compared with the HLMC SCF/IL-6/FCS-supplemented monoculture. The increased HLMC numbers evident in ASM coculture compared with the cytokine monoculture is, therefore, due to both proliferation and protection from apoptosis.
Cell-cell contact was an essential requirement for HLMC proliferation in coculture with HLMC. The effect of cell separation by a Transwell membrane was largely mimicked by an adhesion-blocking Ab to CADM1, a Ca2+-independent adhesion molecule known to mediate, in part, the adhesion of HLMC to ASM (19), and the adhesion of mouse mast cells to fibroblasts (34) and nerves (25). Homophilic CADM1 interactions in epithelial cells promote its tumor suppressor activity, but its heterophilic ligation in HLMC clearly promotes cell proliferation. A potential mechanism that would explain this is a specific interaction with the SCF receptor CD117 (c-kit). Several lines of evidence support this hypothesis. Firstly, the effects of blocking CD117 and CADM1 were only marginally additive, suggesting a cooperative interaction. The SCF expressed by ASM was predominantly membrane-bound, and we also observed strong colocalization of CD117 and CADM1 in the HLMC plasma membrane. Furthermore, and perhaps most importantly, CD117 coimmunoprecipitated with CADM1, indicating a physical interaction. Interestingly in mouse mast cells, CD117-dependent signaling through PI3K has been shown to be important for CADM1-dependent adhesion (35), although this does not appear to apply to humans (19). Finally, there is a precedent for the interaction of CADM1-like molecules with other tyrosine kinase receptors. For example, CADM1 binds a membrane guanylate kinase family member, Pals2, which binds to Lin-7. Lin-7 is required for the correct membrane localization of an epidermal growth factor receptor homologue in Caenorhabditis elegans (36). Thus, the ability of CADM1 to mediate HLMC adhesion to ASM, its direct physical interaction with CD117, and its apparent cooperative role in CD117-dependent signaling suggests that CADM1 plays a fundamental role in many CD117-dependent mast cell processes. We, therefore, envisage a model whereby CADM1-dependent adhesion and CADM1-dependent CD117 membrane localization facilitates the interaction of CD117 with membrane-bound SCF on ASM and other stromal cells.
IL-6 was also an important mediator of the HLMC proliferative response in the coculture environment. The concentrations present in the coculture supernatant were above the optimal concentration required to promote cultured human mast cell proliferation (30). The source of this IL-6 might be ASM but could also arise from HLMC, which release this cytokine in large quantities (32, 37). IL-6 appeared to cooperate with SCF and CADM1 as its contribution was similar to these molecules and the effects of its blockade were barely additive. Determining how IL-6 interacts with SCF and CADM1 requires further investigation.
Human gut fibroblasts maintain gut mast cell survival but do not induce their proliferation. This did not require cell-cell contact and was not mediated via SCF (38), which is surprising as SCF- and CADM1-dependent adhesion mediates mouse mast cell adhesion to fibroblasts and promotes their survival (34, 35). The gut fibroblast survival activity had a mass between 10 and 100 kDA, suggesting that IL-6, which was not measured, may have been partially responsible. In contrast, coculture of human gut mast cells with HUVECs not only promoted mast cell survival but also induced their proliferation (39). However the proliferative effect was much slower than that seen in our experiments with ASM and the mechanism was distinct. Although gut mast cell-HUVEC coculture required adhesion for proliferation, the adhesive process was mediated via membrane-bound SCF and the Ig superfamily molecule VCAM-1 expressed on HUVEC (39). Neither of these is involved in the adhesion of HLMC to ASM (19). Furthermore, blocking SCF in coculture with HUVECs killed all mast cells, whereas in our experiments, proliferation was attenuated but the cells remained viable. Human bronchial epithelial cells also maintain the survival of human cord blood-derived mast cells through a SCF-dependent mechanism but over 4 days do not induce proliferation (40). Determining whether HLMC interact with airway epithelial cells, endothelial cells, and fibroblasts via CADM1 will be of great interest.
Not only do HLMC proliferate in coculture with ASM, but they are also activated. There was increased basal histamine release during the coculture period, which was mirrored by a decrease in HLMC histamine content. This is particularly interesting because mast cells in the asthmatic ASM bundle are activated as shown by the presence of piecemeal degranulation (14). Our data, therefore, suggests that this can occur as a result of the interaction of mast cells with ASM independently of allergen and/or monomeric IgE (4). This might explain why anti-IgE therapy is able to inhibit airway inflammation but have no effect on AHR (41). The mechanisms behind this require investigation but could be mediated in part through the interaction of HLMC CD117 with membrane-bound SCF expressed by ASM. It will also be important to investigate the role of CADM1 in this activation process. Of further importance, these activated, histamine-depleted HLMC are still able to release equivalent amounts of histamine following IgE-dependent activation, indicating that they can still respond to allergen inhalation. These findings are in contrast to those with human airway epithelium, which actually inhibits constitutive and IgE-dependent mast cell activation (40, 42), further highlighting the potential importance of the ASM-HLMC interaction.
In summary, we have demonstrated that human ASM supports HLMC survival and proliferation which may contribute to the mast cell myositis evident in asthma. Furthermore, this interaction activates mast cells, which supports the view that they aggravate smooth muscle contractile dysfunction in asthma. Perturbing the SCF-CADM1-IL-6-dependent pathway through which these effects are achieved may offer a new approach to asthma therapy.
Footnotes
This work was supported in part by a Department of Health/Medical Research Council Clinician Scientist Fellowship and Wellcome Senior Clinical Fellowship for C.E.B., and a PhD studentship cofunded by University of Leicester and Cambridge Antibody Technology for F.H.
Abbreviations used in this paper: AHR, airway hyperresponsiveness; ASM, airway smooth muscle; HLMC, human lung mast cell; EB, eosinophilic bronchitis; SCF, stem cell factor; PI, propidium iodide; DAPI, 4′,6-diamidino-2-phenylindole.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, Williams H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 2.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 3.Wardlaw AJ, Brightling CE, Green R, Woltmann G, Bradding P, Pavord ID. New insights into the relationship between airway inflammation and asthma. Clin. Sci. 2002;103:201–211. doi: 10.1042/cs1030201. [DOI] [PubMed] [Google Scholar]
- 4.Cruse G, Kaur D, Yang W, Duffy SM, Brightling CE, Bradding P. Activation of human lung mast cells by monomeric immunoglobulin E. Eur. Respir. J. 2005;25:858–863. doi: 10.1183/09031936.05.00091704. [DOI] [PubMed] [Google Scholar]
- 5.Bradding P, Walls AF, Holgate ST. The role of the mast cell in the pathophysiology of asthma. J. Allergy Clin. Immunol. 2006;117:1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 6.Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, Mueller R, Heusser CH, Howarth PH, Holgate ST. Interleukin-4, -5, and -6 and tumor necrosis factor-α in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am. J. Respir. Cell Mol. Biol. 1994;10:471–480. doi: 10.1165/ajrcmb.10.5.8179909. [DOI] [PubMed] [Google Scholar]
- 7.Carroll NG, Mutavdzic S, James AL. Increased mast cells and neutrophils in submucosal mucous glands and mucus plugging in patients with asthma. Thorax. 2002;57:677–682. doi: 10.1136/thorax.57.8.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll NG, Mutavdzic S, James AL. Distribution and degranulation of airway mast cells in normal and asthmatic subjects. Eur. Respir. J. 2002;19:879–885. doi: 10.1183/09031936.02.00275802. [DOI] [PubMed] [Google Scholar]
- 9.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast cell infiltration of airway smooth muscle in asthma. N. Engl. J. Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 10.Berger P, Girodet PO, Begueret H, Ousova O, Perng DW, Marthan R, Walls AF, Tunon de Lara JM. Tryptase-stimulated human airway smooth muscle cells induce cytokine synthesis and mast cell chemotaxis. FASEB J. 2003;17:2139–2141. doi: 10.1096/fj.03-0041fje. [DOI] [PubMed] [Google Scholar]
- 11.Amin K, Janson C, Boman G, Venge P. The extracellular deposition of mast cell products is increased in hypertrophic airways smooth muscles in allergic asthma but not in nonallergic asthma. Allergy. 2005;60:1241–1247. doi: 10.1111/j.1398-9995.2005.00823.x. [DOI] [PubMed] [Google Scholar]
- 12.El-Shazly A, Berger P, Girodet PO, Ousova O, Fayon M, Vernejoux JM, Marthan R, Tunon-de-Lara JM. Fraktalkine produced by airway smooth muscle cells contributes to mast cell recruitment in asthma. J. Immunol. 2006;176:1860–1868. doi: 10.4049/jimmunol.176.3.1860. [DOI] [PubMed] [Google Scholar]
- 13.Berry MA, Morgan A, Shaw DE, Parker D, Green RH, Brightling CE, Bradding P, Wardlaw AJ, Pavord ID. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007;62:1043–1049. doi: 10.1136/thx.2006.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Begueret H, Berger P, Vernejoux JM, Dubuisson L, Marthan R, Tunon-De-Lara JM. Inflammation of bronchial smooth muscle in allergic asthma. Thorax. 2007;62:8–15. doi: 10.1136/thx.2006.062141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brightling CE, Ward R, Goh KL, Wardlaw AJ, Pavord ID. Eosinophilic bronchitis is an important cause of chronic cough. Am. J. Respir. Crit. Care Med. 1999;160:406–410. doi: 10.1164/ajrccm.160.2.9810100. [DOI] [PubMed] [Google Scholar]
- 16.Brightling CE, Ward R, Woltmann G, Bradding P, Sheller JR, Dworski R, Pavord ID. Induced sputum inflammatory mediator concentrations in eosinophilic bronchitis and asthma. Am. J. Respir. Crit. Care Med. 2000;162:878–882. doi: 10.1164/ajrccm.162.3.9909064. [DOI] [PubMed] [Google Scholar]
- 17.Brightling CE, Symon FA, Birring SS, Bradding P, Wardlaw AJ, Pavord ID. Comparison of airway immunopathology of eosinophilic bronchitis and asthma. Thorax. 2003;58:528–532. doi: 10.1136/thorax.58.6.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brightling CE, Symon FA, Birring SS, Bradding P, Pavord ID, Wardlaw AJ. TH2 cytokine expression in bronchoalveolar lavage fluid T lymphocytes and bronchial submucosa is a feature of asthma and eosinophilic bronchitis. J. Allergy Clin. Immunol. 2002;110:899–905. doi: 10.1067/mai.2002.129698. [DOI] [PubMed] [Google Scholar]
- 19.Yang W, Kaur D, Okayama Y, Ito A, Wardlaw AJ, Brightling CE, Bradding P. Human lung mast cells adhere to human airway smooth muscle, in part, via tumor suppressor in lung cancer-1. J. Immunol. 2006;176:1238–1243. doi: 10.4049/jimmunol.176.2.1238. [DOI] [PubMed] [Google Scholar]
- 20.Brightling CE, Ammit AJ, Kaur D, Black JL, Wardlaw AJ, Hughes JM, Bradding P. The CXCL10/CXCR3 axis mediates human lung mast cell migration to asthmatic airway smooth muscle. Am. J. Respir. Crit. Care Med. 2005;171:1103–1108. doi: 10.1164/rccm.200409-1220OC. [DOI] [PubMed] [Google Scholar]
- 21.Cruse G, Duffy SM, Brightling CE, Bradding P. Functional KCa3.1 K+ channels are required for human lung mast cell migration. Thorax. 2006;61:880–885. doi: 10.1136/thx.2006.060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duffy SM, Lawley WJ, Kaur D, Yang W, Bradding P. Inhibition of human mast cell proliferation and survival by tamoxifen in association with ion channel modulation. J. Allergy Clin. Immunol. 2003;112:970–977. doi: 10.1016/j.jaci.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Kassel O, Schmidlin F, Duvernelle C, Gasser B, Massard G, Frossard N. Human bronchial smooth muscle cells in culture produce stem cell factor. Eur. Respir. J. 1999;13:951–954. doi: 10.1034/j.1399-3003.1999.13e04.x. [DOI] [PubMed] [Google Scholar]
- 24.Elias JA, Wu Y, Zheng T, Panettieri R. Cytokine- and virus-stimulated airway smooth muscle cells produce IL-11 and other IL-6-type cytokines. Am. J. Physiol. 1997;273:L648–L655. doi: 10.1152/ajplung.1997.273.3.L648. [DOI] [PubMed] [Google Scholar]
- 25.Furuno T, Ito A, Koma Y, Watabe K, Yokozaki H, Bienenstock J, Nakanishi M, Kitamura Y. The SgIGSF/SynCAM mast cell adhesion molecule promotes interaction with nerves. J. Immunol. 2005;174:6934–6942. doi: 10.4049/jimmunol.174.11.6934. [DOI] [PubMed] [Google Scholar]
- 26.Sanmugalingam D, Wardlaw AJ, Bradding P. Adhesion of human lung mast cells to bronchial epithelium: evidence for a novel carbohydrate-mediated mechanism. J. Leukocyte Biol. 2000;68:38–46. [PubMed] [Google Scholar]
- 27.Demo SD, Masuda E, Rossi AB, Throndset BT, Gerard AL, Chan EH, Armstrong RJ, Fox BP, Lorens JB, Payan DG, et al. Quantitative measurement of mast cell degranulation using a novel flow cytometric annexin-V binding assay. Cytometry. 1999;36:340–348. doi: 10.1002/(sici)1097-0320(19990801)36:4<340::aid-cyto9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 28.Valent P, Spanblochl E, Sperr WR, Sillaber C, Zsebo KM, Agis H, Strobl H, Geissler K, Bettelheim P, Lechner K. Induction of differentiation of human mast cells from bone marrow and peripheral blood mononuclear cells by recombinant human stem cell factor/kit-ligand in long-term culture. Blood. 1992;80:2237–2245. [PubMed] [Google Scholar]
- 29.Irani AM, Nilsson G, Miettinen U, Craig SS, Ashman LK, Ishizaka T, Zsebo KM, Schwartz LB. Recombinant human stem cell factor stimulates differentiation of mast cells from dispersed human fetal liver cells. Blood. 1992;80:3009–3021. [PubMed] [Google Scholar]
- 30.Yanagida M, Fukamachi H, Ohgami K, Kuwaki T, Ishii H, Uzumaki H, Amano K, Tokiwa T, Mitsui H, Saito H, et al. Effects of T-helper 2-type cytokines, interleukin-3 (IL-3), IL-4, IL-5, and IL-6 on the survival of cultured human mast cells. Blood. 1995;86:3705–3714. [PubMed] [Google Scholar]
- 31.Igarashi Y, Kurosawa M, Ishikawa O, Miyachi Y, Saito H, Ebisawa M, Iikura Y, Yanagida M, Uzumaki H, Nakahata T. Characteristics of histamine release from cultured human mast cells. Clin. Exp. Allergy. 1996;26:597–602. [PubMed] [Google Scholar]
- 32.Oskeritzian CA, Zhao W, Pozez AL, Cohen NM, Grimes M, Schwartz LB. Neutralizing endogenous IL-6 renders mast cells of the MCT type from lung, but not the MCTC type from skin and lung, susceptible to human recombinant IL-4-induced apoptosis. J. Immunol. 2004;172:593–600. doi: 10.4049/jimmunol.172.1.593. [DOI] [PubMed] [Google Scholar]
- 33.Flanagan JG, Chan DC, Leder P. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell. 1991;64:1025–1035. doi: 10.1016/0092-8674(91)90326-t. [DOI] [PubMed] [Google Scholar]
- 34.Ito A, Jippo T, Wakayama T, Morii E, Koma Y, Onda H, Nojima H, Iseki S, Kitamura Y. SgIGSF: a new mast-cell adhesion molecule used for attachment to fibroblasts and transcriptionally regulated by MITF. Blood. 2003;101:2601–2608. doi: 10.1182/blood-2002-07-2265. [DOI] [PubMed] [Google Scholar]
- 35.Koma Y, Ito A, Watabe K, Hirata T, Mizuki M, Yokozaki H, Kitamura T, Kanakura Y, Kitamura Y. Distinct role for c-kit receptor tyrosine kinase and SgIGSF adhesion molecule in attachment of mast cells to fibroblasts. Lab. Invest. 2005;85:426–435. doi: 10.1038/labinvest.3700231. [DOI] [PubMed] [Google Scholar]
- 36.Shingai T, Ikeda W, Kakunaga S, Morimoto K, Takekuni K, Itoh S, Satoh K, Takeuchi M, Imai T, Monden M, Takai Y. Implications of nectin-like molecule-2/IGSF4/RA175/SgIGSF/TSLC1/SynCAM1 in cell-cell adhesion and transmembrane protein localization in epithelial cells. J. Biol. Chem. 2003;278:35421–35427. doi: 10.1074/jbc.M305387200. [DOI] [PubMed] [Google Scholar]
- 37.Cruse G, Cockerill S, Bradding P. IgE alone promotes human lung mast cell survival through the autocrine production of IL-6. BMC Immunol. 2008;9:2. doi: 10.1186/1471-2172-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sellge G, Lorentz A, Gebhardt T, Levi-Schaffer F, Bektas H, Manns MP, Schuppan D, Bischoff SC. Human intestinal fibroblasts prevent apoptosis in human intestinal mast cells by a mechanism independent of stem cell factor, IL-3, IL-4, and nerve growth factor. J. Immunol. 2004;172:260–267. doi: 10.4049/jimmunol.172.1.260. [DOI] [PubMed] [Google Scholar]
- 39.Mierke CT, Ballmaier M, Werner U, Manns MP, Welte K, Bischoff SC. Human endothelial cells regulate survival and proliferation of human mast cells. J. Exp. Med. 2000;192:801–811. doi: 10.1084/jem.192.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsieh FH, Sharma P, Gibbons A, Goggans T, Erzurum SC, Haque SJ. Human airway epithelial cell determinants of survival and functional phenotype for primary human mast cells. Proc. Natl. Acad. Sci. USA. 2005;102:14380–14385. doi: 10.1073/pnas.0503948102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Djukanovic R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, Bao W, Fowler-Taylor A, Matthews J, Busse WW, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am. J. Respir. Crit. Care Med. 2004;170:583–593. doi: 10.1164/rccm.200312-1651OC. [DOI] [PubMed] [Google Scholar]
- 42.Yang W, Wardlaw AJ, Bradding P. Attenuation of human lung mast cell degranulation by bronchial epithelium. Allergy. 2006;61:569–575. doi: 10.1111/j.1398-9995.2006.01041.x. [DOI] [PubMed] [Google Scholar]