Summary
IL-13 is a T-helper type 2 cytokine. Animal models have implicated IL-13 as a critical cytokine in the development of asthma and chronic obstructive pulmonary disease (COPD). In vitro IL-13 exerts important effects on both structural and inflammatory cells within the airway and has the capacity to drive the clinical features of airways disease. In asthma, this view is strongly supported by associations with IL-13 genetic polymorphisms and increased mRNA and protein expression in blood, sputum and bronchial submucosa. In particular, IL-13 up-regulation is associated with severe disease. Current evidence in COPD is conflicting, with some reports supporting and others refuting a role for IL-13. Early clinical trials of anti-IL-13 therapies in asthma have shown promise, and the results of further efficacy studies are eagerly awaited.
Introduction
IL-13, a T-helper type 2 (Th2) cytokine, was first described in 1993, identified by molecular cloning in activated human T lymphocytes [1]. In the same year, IL-13 was reported to direct cells towards the Th2 pathway, with induction of B cell production of IgE [2], and its gene position was mapped in close proximity to IL-4 on chromosome 5q 23–31 [1].
Straddling the new millennium, a cluster of reports from murine models of asthma and chronic obstructive pulmonary disease (COPD) positioned IL-13 as critical in the immuonpathogenesis of obstructive airways disease [3-5]. The view that IL-13 is pivotal in asthma was further supported by associations with genetic polymorphisms, increased expression in disease and the biological effects it exerts on airway inflammatory and structural cells. The role of IL-13 in COPD is more contentious, with the initial enthusiasm in animal models dampened by conflicting reports in human disease. The interest in anti-IL-13 strategies in asthma has led to considerable investment in the development of novel biological and small molecule approaches to modulate IL-13. These are beginning to enter early-phase studies. Therefore, we shall shortly have a greater understanding of the role of IL-13 in airways disease. This review will summarize the biology of IL-13, the current evidence positioning its role in asthma and COPD and will explore the potential effects of its inhibition on clinical outcomes in asthma.
Interleukin-13 signalling
Several cell types have been reported as sources of IL-13. In particular, T cells, mast cells and eosinophils are the predominant source of IL-13 in asthma, with a contribution from the macrophage in COPD [1, 6-8]. Other inflammatory cells and structural cells have the capacity to produce IL-13 in airways disease. The crystal structures of the IL-4/IL-13 receptor system have been described recently [9]. IL-13 exerts its effects predominantly via a dimeric receptor comprising of IL-4Rα and IL-13Rα1 (IL-4RII). IL-13 binds IL-13Rα1 with a low affinity and then IL-4Rα binds to form a high-affinity cytokine-binding heterodimer. IL-13Rα1 is expressed by airway epithelium, fibroblasts, smooth muscle and most leucocytes including mast cells within the airway, except T lymphocytes [10-14]. Binding of IL-13 to this receptor activates the tyrosine kinases Jak 1, Jak 3 and Tyk 2. These kinases phosphorylate tyrosine residues on the IL-4α receptor, which in turn leads to recruitment and subsequent phosphorlyation of signal transducer and activator of transcription 6 (STAT6). STAT6 dimerizes and translocates to the nucleus and modulates gene expression [15]. In addition to IL-13 and its cognate receptor, this signalling pathway presents potential novel targets to modulate the IL-13 axis.
IL-13Rα2 binds IL-13 exclusively and with high affinity. This receptor lacks a signalling motif and exists in soluble and membrane-bound forms. These characteristics led to the view that coupling to this receptor disallows binding of the IL-13 protein with IL-13Rα1, and therefore IL-13Rα2 acts as a ‘decoy’ receptor. Recently, the functional purpose of the IL-13Rα2 subunit has gathered much speculation. In vitro studies with human airway fibroblasts suggest that activation of the IL-13Rα2 subunit may attenuate the actions of IL-13 and -4 [16]. In support of this view, comparison of the effects of lung-targeted transgenic IL-13 in mice with wild-type and null Rα2 loci demonstrates that IL-13Rα2 is a selective and powerful inhibitor of IL-13-induced responses [17]. However, in the bleomycin model of lung fibrosis, a controversial role for the IL-13Rα2 subunit was proposed, which suggested that activation of this receptor led to induction of TGF-β and the development of lung fibrosis [18].
Evidence of a critical role for interleukin-13 in the pathogenesis of asthma
Animal models
A considerable weight of evidence supporting a role for IL-13 in airways disease is derived from animal models. In 1998, Grunig and colleagues first reported that in a murine model of allergic asthma, selective neutralization of IL-13 led to reversal of airway hyperresponsiveness (AHR) and inflammation. In addition, they found that administration of IL-13 conferred an asthma-like phenotype to non-immunized T cell-deficient mice by an IL-4Rα-dependent pathway [3]. Similarly, Wills-Karp et al. [4] found that the addition of IL-13 to non-immunized mice was sufficient to induce the pathophysiological features of asthma independent of IgE and eosinophils.
Subsequent murine studies suggested that IL-13 may exacerbate airway responsiveness via direct effects on epithelial cells [19] and airway smooth muscle [20]. Mice lacking STAT6 were protected from all pulmonary effects of IL-13. Reconstitution of STAT6 only in epithelial cells was sufficient for IL-13-induced AHR and mucus production in the absence of inflammation, fibrosis or other lung pathology, highlighting the importance of the effects of IL-13 on epithelial cells. IL-13 also exerts direct effects on airway smooth muscle, leading to increased force of contraction as a consequence of augmentation of G protein-coupled receptor-associated calcium signalling [20].
Polymorphisms of interleukin-13 and its receptor
A recent report re-examined the published asthma genetic studies [21], including candidate gene studies and positional cloning, up to the end of December 2007. Two of the four genes with the highest number of positive association reports were IL-4R and IL-13. Indeed, polymorphisms within the IL-13 gene have been associated with various aspects of the asthma phenotype. Analysis of an adult Dutch population demonstrated that the −1111 promoter region is strongly associated with asthma disease, AHR and atopy [22]. In addition, polymorphisms within the IL-13 gene have been identified to predict asthma [23] and higher serum IL-13 levels [24]. Recombinant IL-13 protein of one of these variants (glutamine substitution for arginine at position 110 on the mature protein-Arg110Gln) has demonstrated greater biological activity, implying that genetic variations in the IL-13 gene influence the asthma phenotype [23]. The IL-13 gene locus is associated with atopy and allergy in the broader sense, with four single nucleotide polymorphisms (SNPs) associated with a variant in the IL-13 gene (Arg130Gln polymorphism), resulting in elevated IgE in three separate populations of children [25].
Interleukin-13 expression in asthma
Blood
As described above, peripheral blood T cells, eosinophils and basophils, but not neutrophils [26], are important sources of IL-13 [1, 6-8, 27, 28]. The IL-13 concentration in peripheral blood is increased in asthma across disease severity in a stable state [29, 30] and is up-regulated at exacerbations [31]. The potential functional importance of this increased IL-13 expression is underscored by comparisons between asthma and non-asthmatic eosinophilic bronchitis (EB). EB is a common cause of chronic cough [32] and can be distinguished from asthma by the absence of variable airflow obstruction and AHR. Therefore, differences between these conditions may provide important clues as to the pathogenesis of the disordered airway physiology in asthma. Both conditions share many immunopathological features, with the notable exception of increased mast cell infiltration of the airway smooth muscle bundle in asthma [33-36]. Interestingly, following stimulation ex vivo T cells from asthmatics demonstrate increased IL-13 expression compared with subjects with EB or controls [37, 38]. This suggests that for the same stimulus, cells derived from asthmatics have a greater capacity to release IL-13. Similarly, T cells from atopic individuals upon stimulation with grass pollen and house dust mite showed elevated IL-5 and -13 production compared with non-atopic controls [39, 40].
Sputum
Induced sputum provides a non-invasive assessment of airway inflammation and allows for the measurement of inflammatory cells and important pro-inflammatory cytokines. The use of the mucolytic dithiothreitol aids cell dispersion to produce a more reliable sputum cytospin, but can affect the measurement of cytokines. For this reason, the IL-13 measurement in sputum has been dogged by technical problems. This has been overcome using a number of approaches including sputum dialysis [41]. Again, important differences have been observed between asthma and EB, with increased concentrations in asthma [41] and in particular in severe disease [35]. The IL-13 concentration in sputum was related to asthma control [35]. This increased IL-13 expression in asthma is confirmed by examination of sputum cytokine mRNA expression [42] and by ex vivo stimulation of sputum T cells [43].
Bronchoalveolar lavage Fluid
Following a segmental allergen challenge, bronchoalveolar lavage (BAL) IL-13 is increased in asthma [44, 45], is associated predominantly with the late asthma phase and is correlated with eosinophil numbers [45]. Ex vivo BAL T cells express IL-13 mRNA [46] and its expression in CD4+ and CD8+ cells was inversely related to forced expiratory volume in 1 s% predicted [47]. In addition, BAL-derived macrophages are a potential source of IL-13 with significant amounts of IL-13 mRNA found within BAL enriched for alveolar macrophages, which correlated with BAL eosinophils [48]. Importantly, in severe asthma, the proportion of macrophages staining for IL-13 was increased. In nocturnal asthma, the number of BAL cells expressing IL-13 mRNA was increased [49]. This expression was only in part attenuated by dexamethasone. BAL-derived alveolar macrophages from these subjects demonstrated overexpression of glucocorticoid receptor (GR)-β (a receptor complex that competes with the active GR-α receptor), but reduced expression when treated with IL-13 neutralizing antibodies [49]. This provides a possible explanation for the reduced GR affinity seen when IL-13 is incubated with peripheral blood monocytes [50].
Bronchial mucosa
Several studies have consistently reported up-regulated IL-13 expression in bronchial biopsies [41, 45, 51-55]. Increased IL-13 mRNA expression was first described within the submucosa in bronchial biopsies from a small population (n = 9) of stable asthmatics compared with healthy control. IL-4 mRNA expression was also assessed and the number of IL-13+ mRNA cells was significantly higher compared with IL-4+ mRNA cells. All cells that expressed IL-4+ mRNA co-expressed IL-13 mRNA concurrently. In contrast, only 60% of IL-13+ mRNA cells co-expressed IL-4+ mRNA. Ninety percent of the cells expressing IL-13+ mRNA were characterized as CD3 T lymphocytes [51]. IL-13 mRNA [52] and protein [35] were increased in moderate to severe disease. Surprisingly, IL-13 expression within the airway is not closely related to atopy, but there was an association with eosinophilic inflammation [35, 41]. IL-13 protein expression has been quantified using immunohistochemistry in large airway biopsy specimens from subjects with corticosteroid-naïve asthma, EB and healthy controls. Increased inflammatory cells expressing IL-13 were found within the submucosa of the asthma group in comparison with the controls and EB. Although mRNA expression is usually associated with T cells, over 80% of cells expressing IL-13 protein were eosinophils, with 8% of cells being identified as mast cells [41]. This apparent anomaly may be a consequence of T cell secreting its synthesized IL-13 rapidly, whereas IL-13 may be stored by eosinophils and mast cells. Mast cells are an important source of IL-13 in the lung [6] and in asthma mast cells within the airway smooth muscle bundle, express IL-13 [55].
Evidence of a role for interleukin-13 in the pathogenesis of chronic obstructive pulmonary disease
Animal models
In addition to asthma, murine models have highlighted a role of IL-13 in COPD. Zheng et al. [5] reported that IL-13 overexpression in the adult murine lung induced emphysema, mucus metaplasia, inflammation and fibrosis. These effects were mediated by matrix metalloproteinase (MMP) and cathepsin-based proteolytic pathways and were reversed by the addition of MMP or cysteine proteinase antagonists. Potentially, IL-13 regulation in emphysema may also be related to up-regulation of IL-18. Transgenic IL-18 murine models resulted in increased IL-13 coupled with pulmonary inflammation and structural changes reflective of emphysema [56].
Recently, murine models have been used to extend previous concepts that chronic lung disease is a result of an innate immune response to low-grade infection [7]. Mice infected with Sendai virus demonstrated development of mucus metaplasia and AHR mimicking features of asthma and COPD despite clearance of the virus. In the acute phase, CD4+ T lymphocytes were the predominant source of IL-13 but in the chronic phase, macrophages stimulated by invariant natural killer cells became the most significant source. These findings provide new insights into a novel iNKT-macrophage mediated IL-13 overproduction, leading to chronic lung disease, and expand the possible role of IL-13 in the onset and chronicity of airways disease.
Polymorphisms of interleukin-13 and its receptor
In COPD compared with smokers and never smokers, there was an association with the changes of cytosine to thymine at −1055 within a promoter region associated with IL-13 [57]. Smoking has an influence on specific SNPs, with subjects with extensive smoking exposure possessing the −1112 C/T allele developing worsening airflow obstruction [58]. Importantly, these polymorphisms (−1055 and −1112) are the same and are also known as rs1800925. Therefore, in COPD IL-13 polymorphisms have been associated with disease, although the strength of this association is not as compelling as for asthma.
Interleukin-13 expression in chronic obstructive pulmonary disease
Increased IL-13 expression is a consistent feature of asthma in peripheral blood, sputum, BAL and bronchial biopsies. In contrast, the data for COPD are conflicting. In peripheral blood, IL-13 has been reported to be increased [59], but this finding has not been replicated in another study [60]. In sputum, IL-13 concentration was not increased across severities compared with smoking and non-smoking controls [53]. In ex vivo BAL T cells from COPD subjects IL-13 mRNA was expressed [47]. However, IL-13 protein quantification within BAL has not been determined. Intriguingly, BAL IL-13 mRNA expression from asymptomatic smokers was decreased compared with healthy never smokers [61], again questioning the role of IL-13 in smoking-related COPD. In the bronchial submucosa in smokers with chronic bronchitis, IL-13 expression was increased [62], whereas in another study, the IL-13 expression was not different between subjects with COPD, smoking and non-smoking controls [53]. Contrary to murine models, IL-13 mRNA and protein expression was decreased in severe emphysema [63], but was increased in another group of COPD subjects and related to mucus cell metaplasia [7]. The role of IL-13 in COPD is therefore uncertain. Current evidence perhaps suggests that IL-13 is important in the development of some aspects of the COPD phenotype, but not others.
Effects of interleukin-13 on airway inflammatory and structural cells
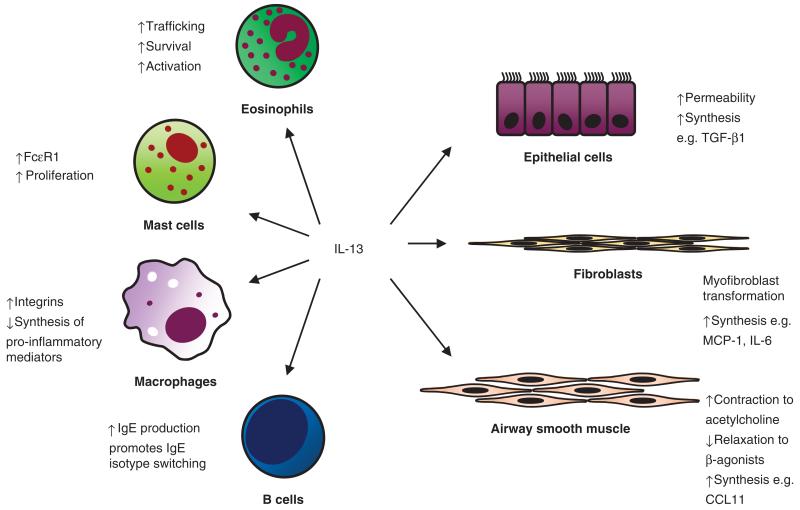
IL-13 exerts effects on both inflammatory and structural cells implicated in the pathogenesis of asthma summarized in Fig. 1.
Fig. 1.
Summary of the effects of IL-13 on important structural and inflammatory cells (see text for details).
Recent evidence from clinical trials of anti-IL-5 has implicated eosinophils as pivotal in the pathogenesis of severe exacerbations in refractory asthma [64, 65]. IL-13 plays a role in the development and persistence of eosinophilic airway inflammation. Trafficking of eosinophils from the blood compartment to target tissue is mediated in part by IL-13. Adhesion of eosinophils to the endothelium is promoted by IL-13 through the up-regulation of P-selectin, suggesting that IL-13 is implicated in the first stages of transmigration of peripheral blood eosinophils to tissue [66]. IL-13 also augments eosinophil survival and activation [67, 68]. IL-13 is important in allergic inflammation. Together with IL-4, IL-13 drives B cell isotype switching [2] and up-regulation of mast cell FcεR1 expression [14]. IL-13-primed mast cells demonstrate increased proliferation and activation following IgE/anti-IgE activation. These effects were inhibited by a specific IL-13 blocking antibody [14]. In monocytes and macrophages, IL-13 enhances the expression of integrins [69], but inhibits the production of pro-inflammatory mediators including prostaglandins [70] and reactive oxygen species [71]. This inhibition is in contrast to the pro-inflammatory effects exerted on other inflammatory cells and is possibly mediated by suppression of nuclear factor κ B [72].
In addition to inflammatory cells, IL-13 exerts important effects on structural cells. The airway epithelium presents a physical barrier and provides a critical interface between the environment and the underlying structural and inflammatory cells. IL-13 modulates epithelial barrier function, increasing epithelial permeability as measured by mannitol influx and down-regulating proteins associated with maintaining a tight junction within these barriers [73]. In addition, IL-13 drives epithelial cells into a hypersecretory phase, contributing to increased airway inflammation [74]. Indirectly, IL-13 promotes myofibroblast activation via release of TGF-β 2 from epithelial cells, which in turn influences myofibroblasts into releasing cytokines, chemokines and α-actin smooth muscle [75]. Both IL-13 and 4 induce granulocyte macrophage colony stimulating factor and IL-8 release from ex vivo epithelial cell lines in the presence or absence of Der p 1 [11]. Mesenchymal cell differentiation and function is modulated by IL-13 with induction of fibroblast to myofibroblast transformation [76], augmented by the synergistic effect of TGF-β [75]. These effects may, in part, contribute to airway remodelling. The release of many important mediators that participate in airway inflammation and inflammatory cell recruitment including β-1 integrin, vascular adhesion molecule 1, monocyte chemoattractant protein 1 and IL-6 is promoted by IL-13 [77]. Perhaps one of the most interesting effects of IL-13 is its modulation of airway smooth muscle. IL-13 has been implicated in playing a key role in airway smooth muscle contraction and the potential development of AHR. In vitro IL-13, but not IL-4, has been shown to attenuate airway smooth muscle relaxation to β-agonists [13] and augment contractility to acetylcholine [78]. Airway smooth muscle is also an important source of mediators. Following stimulation with IL-13, airway smooth muscle releases increased CCL11 and other chemokines. This IL-13-mediated effect is augmented in combination with TNF [79], IL-9 [80] and IL-4 with IL-1β [81]. Importantly, IL-13-induced release of CCL11 from airway smooth muscle is increased with airway smooth muscle derived from subjects with asthma compared with healthy controls [82]. Interestingly, conditioned medium from IL-13-stimulated airway smooth muscle is chemotactic for mast cells [81], suggesting that airway smooth muscle-derived chemokines may be important for the selective recruitment of mast cells to the airway smooth muscle bundle [81, 83].
Therefore, IL-13 has the capacity to influence key aspects of the asthma paradigm including allergic inflammation, persistence of eosinophilic airway inflammation, airway remodelling and the development of AHR. These potentially broad-spectrum effects have made IL-13 an attractive target for drug development.
Clinical studies of treatments targeted towards the interleukin-13 axis in asthma
Early studies with a soluble recombinant human IL-4 receptor [Altrakincept, Immunex (Amgen), Thousand Oaks, CA, USA] in patients with mild-to-moderate asthma showed some efficacy in maintaining asthma control when inhaled corticosteroids were being withdrawn [84], but this effect was not subsequently confirmed and development was stopped. Two recent placebo-controlled allergen challenge studies showed that an IL-4 variant (pitrakinra) administered subcutaneously or nebulized can inhibit the binding of IL-4 and -13 to the IL-4Rα subunit. Pitrakinra reduced the allergen-induced late-phase response and the need for rescue medication in asthmatic patients [85]. Trials are now underway using an inhaled preparation [86]. Similarly, a humanized monoclonal antibody IMA-638 inhibited both the early and the late allergen challenge response, but did not affect allergen-induced hyperresponsiveness to methacholine [87]. Intriguingly, although preclinical data support the view that IL-13 is critical in the development of AHR, to date, studies that have included allergen-induced AHR have failed to show an effect on this outcome. Whether anti-IL-13 strategies have an impact on ‘wild-type’ AHR needs to be addressed. Several other monoclonal antibodies against IL-13 or IL-4Rα have completed early safety trials in humans, including CAT-354 [88] and AMG 317 [89], and are undergoing clinical trials for asthma. To date, there are no studies of anti-IL-13 therapies in COPD.
Future implications
We are now entering a new therapeutic age for airways disease. Over the next 2–3 years, findings from clinical trials will define the role of anti-IL-13 strategies. In parallel, the positioning of other novel therapies including those directed towards other cytokines, chemokine receptors, immunomodulators [90] and thermoplasty [91] will provide us with a choice, particularly in those with severe asthma. It is unlikely that these treatments will suit all patients, and therefore the recognition of asthma and COPD as heterogeneous conditions will become increasingly important [92]. The application of current and the development of novel outcome measures and biomarkers will be needed to ensure that the most appropriate treatment or combinations of treatments are selected for patients.
The wealth of data implicating IL-13 as a pivotal cytokine in the pathogenesis of asthma presents a compelling case to predict the likely success of anti-IL-13 in the clinic. In the not too distant future, we shall either be able to use a new therapy for our patients with asthma or we will need to revise the asthma paradigm.
Acknowledgements
Conflict of interest: C. B. has received consultancy fees from AstraZeneca, MedImmune, Roche and Glaxo SmithKline, research funding from AstraZeneca, MedImmune and GlaxoSmithKline and is principal investigator for a commercially sponsored study of an anti-IL-13 therapy; S. K. and F. H. have no conflict of interest.
References
- 1.Minty A, Chalon P, Derocq JM, et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362:248–50. doi: 10.1038/362248a0. [DOI] [PubMed] [Google Scholar]
- 2.Punnonen J, Aversa G, Cocks BG, et al. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci USA. 1993;90:3730–4. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grunig G, Warnock M, Wakil AE, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–3. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wills-Karp M, Luyimbazi J, Xu X, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 5.Zheng T, Zhu Z, Wang Z, et al. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest. 2000;106:1081–93. doi: 10.1172/JCI10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaffe JS, Raible DG, Post TJ, et al. Human lung mast cell activation leads to IL-13 mRNA expression and protein release. Am J Respir Cell Mol Biol. 1996;15:473–81. doi: 10.1165/ajrcmb.15.4.8879181. [DOI] [PubMed] [Google Scholar]
- 7.Kim EY, Battaile JT, Patel AC, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14:633–40. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmid-Grendelmeier P, Altznauer F, Fischer B, et al. Eosinophils express functional IL-13 in eosinophilic inflammatory diseases. J Immunol. 2002;169:1021–7. doi: 10.4049/jimmunol.169.2.1021. [DOI] [PubMed] [Google Scholar]
- 9.LaPorte SL, Juo ZS, Vaclavikova J, et al. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell. 2008;132:259–72. doi: 10.1016/j.cell.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang IM, Lin H, Goldman SJ, Kobayashi M. STAT-1 is activated by IL-4 and IL-13 in multiple cell types. Mol Immunol. 2004;41:873–84. doi: 10.1016/j.molimm.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 11.Lordan JL, Bucchieri F, Richter A, et al. Cooperative effects of Th2 cytokines and allergen on normal and asthmatic bronchial epithelial cells. J Immunol. 2002;169:407–14. doi: 10.4049/jimmunol.169.1.407. [DOI] [PubMed] [Google Scholar]
- 12.Murata T, Husain SR, Mohri H, Puri RK. Two different IL-13 receptor chains are expressed in normal human skin fibroblasts, and IL-4 and IL-13 mediate signal transduction through a common pathway. Int Immunol. 1998;10:1103–10. doi: 10.1093/intimm/10.8.1103. [DOI] [PubMed] [Google Scholar]
- 13.Laporte JC, Moore PE, Baraldo S, et al. Direct effects of interleukin-13 on signaling pathways for physiological responses in cultured human airway smooth muscle cells. Am J Respir Crit Care Med. 2001;164:141–8. doi: 10.1164/ajrccm.164.1.2008060. [DOI] [PubMed] [Google Scholar]
- 14.Kaur D, Hollins F, Woodman L, et al. Mast cells express IL-13Ralpha1: IL-13 promotes human lung mast cell proliferation and FcepsilonRI expression. Allergy. 2006;61:1047–53. doi: 10.1111/j.1398-9995.2006.01139.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Malabarba MG, Nagy ZS, Kirken RA. Interleukin 4 regulates phosphorylation of serine 756 in the transactivation domain of Stat6. Roles for multiple phosphorylation sites and Stat6 function. J Biol Chem. 2004;279:25196–203. doi: 10.1074/jbc.M313668200. [DOI] [PubMed] [Google Scholar]
- 16.Andrews AL, Nasir T, Bucchieri F, Holloway JW, Holgate ST, Davies DE. IL-13 receptor alpha 2: a regulator of IL-13 and IL-4 signal transduction in primary human fibroblasts. J Allergy Clin Immunol. 2006;118:858–65. doi: 10.1016/j.jaci.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 17.Zheng T, Liu W, Oh SY, et al. IL-13 receptor alpha2 selectively inhibits IL-13-induced responses in the murine lung. J Immunol. 2008;180:522–9. doi: 10.4049/jimmunol.180.1.522. [DOI] [PubMed] [Google Scholar]
- 18.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 19.Kuperman DA, Huang X, Koth LL, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–9. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 20.Tliba O, Deshpande D, Chen H, et al. IL-13 enhances agonist-evoked calcium signals and contractile responses in airway smooth muscle. Br J Pharmacol. 2003;140:1159–62. doi: 10.1038/sj.bjp.0705558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vercelli D. Advances in asthma and allergy genetics in 2007. J Allergy Clin Immunol. 2008;122:267–71. doi: 10.1016/j.jaci.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Howard TD, Whittaker PA, Zaiman AL, et al. Identification and association of polymorphisms in the interleukin-13 gene with asthma and atopy in a Dutch population. Am J Respir Cell Mol Biol. 2001;25:377–84. doi: 10.1165/ajrcmb.25.3.4483. [DOI] [PubMed] [Google Scholar]
- 23.van der Pouw Kraan TC, van Veen A, Boeije LC, et al. An IL-13 promoter polymorphism associated with increased risk of allergic asthma. Genes Immun. 1999;1:61–5. doi: 10.1038/sj.gene.6363630. [DOI] [PubMed] [Google Scholar]
- 24.Heinzmann A, Mao XQ, Akaiwa M, et al. Genetic variants of IL-13 signalling and human asthma and atopy. Hum Mol Genet. 2000;9:549–59. doi: 10.1093/hmg/9.4.549. [DOI] [PubMed] [Google Scholar]
- 25.Graves PE, Kabesch M, Halonen M, et al. A cluster of seven tightly linked polymorphisms in the IL-13 gene is associated with total serum IgE levels in three populations of white children. J Allergy Clin Immunol. 2000;105:506–13. doi: 10.1067/mai.2000.104940. [DOI] [PubMed] [Google Scholar]
- 26.Reglier H, Arce-Vicioso M, Fay M, Gougerot-Pocidalo MA, Chollet-Martin S. Lack of IL-10 and IL-13 production by human polymorphonuclear neutrophils. Cytokine. 1998;10:192–8. doi: 10.1006/cyto.1997.0272. [DOI] [PubMed] [Google Scholar]
- 27.Gibbs BF, Haas H, Falcone FH, et al. Purified human peripheral blood basophils release interleukin-13 and preformed interleukin-4 following immunological activation. Eur J Immunol. 1996;26:2493–8. doi: 10.1002/eji.1830261033. [DOI] [PubMed] [Google Scholar]
- 28.Ochensberger B, Daepp GC, Rihs S, Dahinden CA. Human blood basophils produce interleukin-13 in response to IgE-receptor-dependent and -independent activation. Blood. 1996;88:3028–37. [PubMed] [Google Scholar]
- 29.Wong CK, Ho CY, Ko FW, et al. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-gamma, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin Exp Immunol. 2001;125:177–83. doi: 10.1046/j.1365-2249.2001.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silvestri M, Bontempelli M, Giacomelli M, et al. High serum levels of tumour necrosis factor-alpha and interleukin-8 in severe asthm: markers of systemic inflammation? Clin Exp Allergy. 2006;36:1373–81. doi: 10.1111/j.1365-2222.2006.02502.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee YC, Lee KH, Lee HB, Rhee YK. Serum levels of interleukins (IL)-4, IL-5, IL-13, and interferon-gamma in acute asthma. J Asthma. 2001;38:665–71. doi: 10.1081/jas-100107544. [DOI] [PubMed] [Google Scholar]
- 32.Brightling CE, Ward R, Goh KL, Wardlaw AJ, Pavord ID. Eosinophilic bronchitis is an important cause of chronic cough. Am J Respir Crit Care Med. 1999;160:406–10. doi: 10.1164/ajrccm.160.2.9810100. [DOI] [PubMed] [Google Scholar]
- 33.Berger P, Girodet PO, Begueret H, et al. Tryptase-stimulated human airway smooth muscle cells induce cytokine synthesis and mast cell chemotaxis. FASEB J. 2003;17:2139–41. doi: 10.1096/fj.03-0041fje. [DOI] [PubMed] [Google Scholar]
- 34.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 35.Saha SK, Berry MA, Parker D, et al. Increased sputum and bronchial biopsy IL-13 expression in severe asthma. J Allergy Clin Immunol. 2008;121:685–91. doi: 10.1016/j.jaci.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siddiqui S, Mistry V, Doe C, et al. Airway hyperresponsiveness is dissociated from airway wall structural remodeling. J Allergy Clin Immunol. 2008;122:335–41. doi: 10.1016/j.jaci.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park SW, Jangm HK, An MH, et al. Interleukin-13 and interleukin-5 in induced sputum of eosinophilic bronchitis: comparison with asthma. Chest. 2005;128:1921–7. doi: 10.1378/chest.128.4.1921. [DOI] [PubMed] [Google Scholar]
- 38.Siddiqui S, Cruse G, McKenna S, et al. IL-13 expression by blood T cells and not eosinophils is increased in asthma compared to non-asthmatic eosinphilic bronchitis. BMC Pulm Med. 2009;9:34. doi: 10.1186/1471-2466-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Till S, Durham S, Dickason R, et al. IL-13 production by allergen-stimulated T cells is increased in allergic disease and associated with IL-5 but not IFN-gamma expression. Immunology. 1997;91:53–7. doi: 10.1046/j.1365-2567.1997.00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashimoto T, Akiyama K, Kawaguchi H, et al. Correlation of allergen-induced IL-5 and IL-13 production by peripheral blood T cells of asthma patients. Int Arch Allergy Immunol. 2004;134(Suppl. 1):7–11. doi: 10.1159/000077786. [DOI] [PubMed] [Google Scholar]
- 41.Berry MA, Parker D, Neale N, et al. Sputum and bronchial submucosal IL-13 expression in asthma and eosinophilic bronchitis. J Allergy Clin Immunol. 2004;114:1106–9. doi: 10.1016/j.jaci.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 42.Truyen E, Coteur L, Dilissen E, et al. Evaluation of airway inflammation by quantitative Th1/Th2 cytokine mRNA measurement in sputum of asthma patients. Thorax. 2006;61:202–8. doi: 10.1136/thx.2005.052399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boniface S, Koscher V, Mamessier E, et al. Assessment of T lymphocyte cytokine production in induced sputum from asthmatics: a flow cytometry study. Clin Exp Allergy. 2003;33:1238–43. doi: 10.1046/j.1365-2222.2003.01762.x. [DOI] [PubMed] [Google Scholar]
- 44.Huang SK, Xiao HQ, Kleine-Tebbe J, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688–94. [PubMed] [Google Scholar]
- 45.Kroegel C, Julius P, Matthys H, Virchow JC, Luttmann W. Endobronchial secretion of interleukin-13 following local allergen challenge in atopic asthma: relationship to interleukin-4 and eosinophil counts. Eur Respir J. 1996;9:899–904. doi: 10.1183/09031936.96.09050899. [DOI] [PubMed] [Google Scholar]
- 46.Bodey KJ, Semper AE, Redington AE, et al. Cytokine profiles of BAL T cells and T-cell clones obtained from human asthmatic airways after local allergen challenge. Allergy. 1999;54:1083–93. doi: 10.1034/j.1398-9995.1999.00889.x. [DOI] [PubMed] [Google Scholar]
- 47.Barcelo B, Pons J, Fuster A, et al. Intracellular cytokine profile of T lymphocytes in patients with chronic obstructive pulmonary disease. Clin Exp Immunol. 2006;145:474–9. doi: 10.1111/j.1365-2249.2006.03167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prieto J, Lensmar C, Roquet A, et al. Increased interleukin-13 mRNA expression in bronchoalveolar lavage cells of atopic patients with mild asthma after repeated low-dose allergen provocations. Respir Med. 2000;94:806–14. doi: 10.1053/rmed.2000.0826. [DOI] [PubMed] [Google Scholar]
- 49.Kraft M, Hamid Q, Chrousos GP, Martin RJ, Leung DY. Decreased steroid responsiveness at night in nocturnal asthma. Is the macrophage responsible? Am J Respir Crit Care Med. 2001;163:1219–25. doi: 10.1164/ajrccm.163.5.2002058. [DOI] [PubMed] [Google Scholar]
- 50.Spahn JD, Szefler SJ, Surs W, Doherty DE, Nimmagadda SR, Leung DY. A novel action of IL-13: induction of diminished monocyte glucocorticoid receptor-binding affinity. J Immunol. 1996;157:2654–9. [PubMed] [Google Scholar]
- 51.Kotsimbos TC, Ernst P, Hamid QA. Interleukin-13 and interleukin-4 are coexpressed in atopic asthma. Proc Assoc Am Phys. 1996;108:368–73. [PubMed] [Google Scholar]
- 52.Naseer T, Minshall EM, Leung DY, et al. Expression of IL-12 and IL-13 mRNA in asthma and their modulation in response to steroid therapy. Am J Respir Crit Care Med. 1997;155:845–51. doi: 10.1164/ajrccm.155.3.9117015. [DOI] [PubMed] [Google Scholar]
- 53.Saha S, Mistry V, Siva R, et al. Induced sputum and bronchial mucosal expression of interleukin-13 is not increased in chronic obstructive pulmonary disease. Allergy. 2008;63:1239–43. doi: 10.1111/j.1398-9995.2008.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Humbert M, Durham SR, Kimmitt P, et al. Elevated expression of messenger ribonucleic acid encoding IL-13 in the bronchial mucosa of atopic and nonatopic subjects with asthma. J Allergy Clin Immunol. 1997;99:657–65. doi: 10.1016/s0091-6749(97)70028-9. [DOI] [PubMed] [Google Scholar]
- 55.Brightling CE, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID, Bradding P. Interleukin-4 and -13 expression is co-localized to mast cells within the airway smooth muscle in asthma. Clin Exp Allergy. 2003;33:1711–6. doi: 10.1111/j.1365-2222.2003.01827.x. [DOI] [PubMed] [Google Scholar]
- 56.Hoshino T, Kato S, Oka N, et al. Pulmonary inflammation and emphysema: role of the cytokines IL-18 and IL-13. Am J Respir Crit Care Med. 2007;176:49–62. doi: 10.1164/rccm.200603-316OC. [DOI] [PubMed] [Google Scholar]
- 57.van der Pouw Kraan TC, Kucukaycan M, Bakker AM, et al. Chronic obstructive pulmonary disease is associated with the −1055 IL-13 promoter polymorphism. Genes Immun. 2002;3:436–9. doi: 10.1038/sj.gene.6363896. [DOI] [PubMed] [Google Scholar]
- 58.Sadeghnejad A, Meyers DA, Bottai M, Sterling DA, Bleecker ER, Ohar JA. IL13 promoter polymorphism 1112C/T modulates the adverse effect of tobacco smoking on lung function. Am J Respir Crit Care Med. 2007;176:748–52. doi: 10.1164/rccm.200704-543OC. [DOI] [PubMed] [Google Scholar]
- 59.Lee JS, Rosengart MR, Kondragunta V, et al. Inverse association of plasma IL-13 and inflammatory chemokines with lung function impairment in stable COPD: a cross-sectional cohort study. Respir Res. 2007;8:64. doi: 10.1186/1465-9921-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Imaoka H, Hoshino T, Takei S, et al. Interleukin-18 production and pulmonary function in COPD. Eur Respir J. 2008;31:287–97. doi: 10.1183/09031936.00019207. [DOI] [PubMed] [Google Scholar]
- 61.Meuronen A, Majuri ML, Alenius H, et al. Decreased cytokine and chemokine mRNA expression in bronchoalveolar lavage in asymptomatic smoking subjects. Respiration. 2008;75:450–8. doi: 10.1159/000114855. [DOI] [PubMed] [Google Scholar]
- 62.Miotto D, Ruggieri MP, Boschetto P, et al. Interleukin-13 and -4 expression in the central airways of smokers with chronic bronchitis. Eur Respir J. 2003;22:602–8. doi: 10.1183/09031936.03.00046402. [DOI] [PubMed] [Google Scholar]
- 63.Boutten A, Bonay M, Laribe S, et al. Decreased expression of interleukin 13 in human lung emphysema. Thorax. 2004;59:850–4. doi: 10.1136/thx.2004.025247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–84. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nair P, Pizzichini MM, Kjarsgaard M, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–93. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 66.Woltmann G, McNulty CA, Dewson G, Symon FA, Wardlaw AJ. Interleukin-13 induces PSGL-1/P-selectin-dependent adhesion of eosinophils, but not neutrophils, to human umbilical vein endothelial cells under flow. Blood. 2000;95:3146–52. [PubMed] [Google Scholar]
- 67.Horie S, Okubo Y, Hossain M, et al. Interleukin-13 but not interleukin-4 prolongs eosinophil survival and induces eosinophil chemotaxis. Intern Med. 1997;36:179–85. doi: 10.2169/internalmedicine.36.179. [DOI] [PubMed] [Google Scholar]
- 68.Luttmann W, Knoechel B, Foerster M, Matthys H, Virchow JC, Jr, Kroegel C. Activation of human eosinophils by IL-13. Induction of CD69 surface antigen, its relationship to messenger RNA expression, and promotion of cellular viability. J Immunol. 1996;157:1678–83. [PubMed] [Google Scholar]
- 69.Zurawski G, de Vries JE. Interleukin 13 elicits a subset of the activities of its close relative interleukin 4. Stem Cells. 1994;12:169–74. doi: 10.1002/stem.5530120204. [DOI] [PubMed] [Google Scholar]
- 70.Endo T, Ogushi F, Sone S. LPS-dependent cyclooxygenase-2 induction in human monocytes is down-regulated by IL-13, but not by IFN-gamma. J Immunol. 1996;156:2240–6. [PubMed] [Google Scholar]
- 71.Sozzani P, Cambon C, Vita N, et al. Interleukin-13 inhibits protein kinase C-triggered respiratory burst in human monocytes. Role of calcium and cyclic AMP. J Biol Chem. 1995;270:5084–8. doi: 10.1074/jbc.270.10.5084. [DOI] [PubMed] [Google Scholar]
- 72.Lentsch AB, Shanley TP, Sarma V, Ward PA. In vivo suppression of NF-kappa B and preservation of I kappa B alpha by interleukin-10 and interleukin-13. J Clin Invest. 1997;100:2443–8. doi: 10.1172/JCI119786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ahdieh M, Vandenbos T, Youakim A. Lung epithelial barrier function and wound healing are decreased by IL-4 and IL-13 and enhanced by IFN-gamma. Am J Physiol Cell Physiol. 2001;281:C2029–38. doi: 10.1152/ajpcell.2001.281.6.C2029. [DOI] [PubMed] [Google Scholar]
- 74.Danahay H, Atherton H, Jones G, Bridges RJ, Poll CT. Interleukin-13 induces a hypersecretory ion transport phenotype in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L226–36. doi: 10.1152/ajplung.00311.2001. [DOI] [PubMed] [Google Scholar]
- 75.Richter A, Puddicombe SM, Lordan JL, et al. The contribution of interleukin (IL)-4 and IL-13 to the epithelial–mesenchymal trophic unit in asthma. Am J Respir Cell Mol Biol. 2001;25:385–91. doi: 10.1165/ajrcmb.25.3.4437. [DOI] [PubMed] [Google Scholar]
- 76.Hashimoto S, Gon Y, Takeshita I, Maruoka S, Horie T. IL-4 and IL-13 induce myofibroblastic phenotype of human lung fibroblasts through c-Jun NH2-terminal kinase-dependent pathway. J Allergy Clin Immunol. 2001;107:1001–8. doi: 10.1067/mai.2001.114702. [DOI] [PubMed] [Google Scholar]
- 77.Doucet C, Brouty-Boye D, Pottin-Clemenceau C, Jasmin C, Canonica GW, Azzarone B. IL-4 and IL-13 specifically increase adhesion molecule and inflammatory cytokine expression in human lung fibroblasts. Int Immunol. 1998;10:1421–33. doi: 10.1093/intimm/10.10.1421. [DOI] [PubMed] [Google Scholar]
- 78.Grunstein MM, Hakonarson H, Leiter J, et al. IL-13-dependent autocrine signaling mediates altered responsiveness of IgE-sensitized airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2002;282:L520–8. doi: 10.1152/ajplung.00343.2001. [DOI] [PubMed] [Google Scholar]
- 79.Moore PE, Church TL, Chism DD, Panettieri RA, Jr, Shore SA. IL-13 and IL-4 cause eotaxin release in human airway smooth muscle cells: a role for ERK. Am J Physiol Lung Cell Mol Physiol. 2002;282:L847–53. doi: 10.1152/ajplung.00245.2001. [DOI] [PubMed] [Google Scholar]
- 80.Baraldo S, Faffe DS, Moore PE, et al. Interleukin-9 influences chemokine release in airway smooth muscle: role of ERK. Am J Physiol Lung Cell Mol Physiol. 2003;284:L1093–102. doi: 10.1152/ajplung.00300.2002. [DOI] [PubMed] [Google Scholar]
- 81.Sutcliffe A, Kaur D, Page S, et al. Mast cell migration to Th2 stimulated airway smooth muscle from asthmatics. Thorax. 2006;61:657–62. doi: 10.1136/thx.2005.056770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan V, Burgess JK, Ratoff JC, et al. Extracellular matrix regulates enhanced eotaxin expression in asthmatic airway smooth muscle cells. Am J Respir Crit Care Med. 2006;174:379–85. doi: 10.1164/rccm.200509-1420OC. [DOI] [PubMed] [Google Scholar]
- 83.Brightling CE, Ammit AJ, Kaur D, et al. The CXCL10/CXCR3 axis mediates human lung mast cell migration to asthmatic airway smooth muscle. Am J Respir Crit Care Med. 2005;171:1103–8. doi: 10.1164/rccm.200409-1220OC. [DOI] [PubMed] [Google Scholar]
- 84.Borish LC, Nelson HS, Corren J, et al. Efficacy of soluble IL-4 receptor for the treatment of adults with asthma. J Allergy Clin Immunol. 2001;107:963–70. doi: 10.1067/mai.2001.115624. [DOI] [PubMed] [Google Scholar]
- 85.Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007;370:1422–31. doi: 10.1016/S0140-6736(07)61600-6. [DOI] [PubMed] [Google Scholar]
- 86.Getz EB, Wilbraham D, Lalor C, Longphre M, Fuller R. Pharmacokinetics and local tolerance of an IL-4/IL-13 antagonist inhalation powder in humans. ERS Abstract. 2008 [Google Scholar]
- 87.Gauvreau GM, Boulet LP, Fitzgerald JM, et al. The effects of IMA-638 on allergen induced airway responses in subjects with mild atopic asthma. ERS Abstract. 2008 [Google Scholar]
- 88.Bhowmick B, Singh D, Molfino N, et al. A double-blind, placebo-controlled, study to assess the pharmacokinetics, safety and tolerability of multiple ascending intravenous doses of CAT 354, a recombinant human anti-IL13 antibody, in subjects with moderate asthma. ERS Abstract. 2008 [Google Scholar]
- 89.Banfield C, Vincent M, Kakkar T, et al. Multiple-dose study of AMG317 in adults with asthma: pharmacokinetics and safety. ATS Abstract. 2008 [Google Scholar]
- 90.Adcock IM, Caramori G, Chung KF. New targets for drug development in asthma. Lancet. 2008;372:1073–87. doi: 10.1016/S0140-6736(08)61449-X. [DOI] [PubMed] [Google Scholar]
- 91.Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327–37. doi: 10.1056/NEJMoa064707. [DOI] [PubMed] [Google Scholar]
- 92.Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–24. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]