Abstract
Asthma is a major cause of morbidity and mortality worldwide. It is characterized by airway dysfunction and inflammation. A key determinant of the asthma phenotype is infiltration of airway smooth muscle bundles by activated mast cells. We hypothesized that interactions between these cells promotes airway smooth muscle differentiation into a more contractile phenotype. In vitro coculture of human airway smooth muscle cells with β-tryptase, or mast cells with or without IgE/anti-IgE activation, increased airway smooth muscle-derived TGF-β1 secretion, α-smooth muscle actin expression and agonist-provoked contraction. This promotion to a more contractile phenotype was inhibited by both the serine protease inhibitor leupeptin and TGF-β1 neutralization, suggesting that the observed airway smooth muscle differentiation was driven by the autocrine release of TGF-β1 in response to activation by mast cell β-tryptase. Importantly, in vivo we found that in bronchial mucosal biopsies from asthmatics the intensity of α-smooth muscle actin expression was strongly related to the number of mast cells within or adjacent to an airway smooth muscle bundle. These findings suggest that mast cell localization in the airway smooth muscle bundle promotes airway smooth muscle cell differentiation into a more contractile phenotype, thus contributing to the disordered airway physiology that characterizes asthma.
Asthma is a common disease and remains a significant cause of morbidity and mortality worldwide. It affects 10% of children and 5% of adults, and its prevalence continues to rise (1). It is characterized by the presence of variable airflow obstruction, airway hyperresponsiveness (AHR),3 and an airway inflammatory response characterized by eosinophilic airway inflammation, Th2 cytokine expression and reticular basement membrane thickening, features that have been implicated in the development of the disordered airway physiology (2, 3). We have demonstrated that many of the immunopathological features of asthma are also observed in the airways of patients with eosinophilic bronchitis (4–6), a condition that presents with a cortico-steroid-responsive chronic cough, but which in contrast to asthma is not associated with AHR or airflow obstruction (7). The striking difference we found in the pathology of these two diseases was the infiltration of asthmatic airway smooth muscle (ASM) by mast cells, suggesting that this is a major determinant of the disordered airway physiology observed in asthma (4).
Notably, the mast cells in the ASM bundle are in an activated state as evidenced by increased IL-4 and -13 expression (8). Mast cells have the capacity to secrete many autacoids, cytokines, and proteases, which have the ability to induce ASM proliferation and contraction (9). However, the mechanisms that drive the development of disordered airway physiology in asthma as a consequence of mast cell-ASM interactions have not been fully elucidated. Indeed, to date whether mast cells promote ASM differentiation to a more contractile phenotype is unknown.
Here we demonstrate that TGF-β1 secretion by ASM was increased by mast cell β-tryptase. This in turn up-regulates ASM contractile protein expression in an autocrine manner; and increases spontaneous and agonist-provoked contraction. Critically, there was a strong relationship between mast cell localization and α-smooth muscle actin (α-SMA) expression intensity in ASM bundles in biopsies from asthmatics supporting the view that mast cells drive ASM differentiation toward a more contractile phenotype.
Materials and Methods
Subjects
Subjects were recruited from Glenfield Hospital, Leicester, U.K. Subjects with asthma had a consistent history and had objective evidence of asthma, as indicated by one or more of the following: 1) methacholine AHR (PC20FEV1 < 8 mg/ml); 2) >15% improvement in FEV1 15 min after administration of 200 μg of inhaled salbutamol; or 3) >20% of maximum within-day amplitude from twice daily peak expiratory flow measurements over 14 days. Severity of asthma was defined based on Global Initiative Network for Asthma treatment steps (10). Subjects with asthma and controls with normal lung function underwent bronchoscopy and bronchial biopsy (11). Additional non-asthmatic subjects were undergoing lung resection. The study was approved by the Leicestershire Ethics Committees and all patients gave their written informed consent.
Immunohistochemical assessment of proximal airway
Bronchial biopsies from subjects with asthma were fixed in acetone and embedded in glycomethacrylate as described previously (12). Sections (2 μm) were cut and stained sequentially using mAbs against α-SMA (DakoCytomation), tryptase for mast cells (DakoCytomation) and appropriate isotype controls (DakoCytomation).
Morphometry was assessed using a computerized image analysis system. In brief, the ASM bundle was identified at ×200 magnification and its area determined [mean (SEM) area 0.072 mm2]. The threshold (hue, saturation, and intensity) required to identify high intensity α-SMA staining within the bundle was then identified by visually selecting high intensity α-SMA stained pixels. The percentage of the ASM bundle area that was occupied by high intensity α-SMA staining was then determined. The corresponding sequential section stained for tryptase was then used to determine the number of mast cells within the bundle (×400 magnification) and within a 30-μm perimeter; determined by a line drawn perpendicular from the perimeter of the bundle to neighboring mast cells. The total number of mast cells within the bundle and perimeter were corrected for bundle area and the area of the bundle plus the perimeter, respectively, and expressed as /mm2.
Mast cell and ASM isolation and culture
Human lung mast cells (HLMC) were obtained from non-asthmatic lung (n = 18) obtained at lung resection surgery using immunomagnetic affinity purification. Cells were cultured in DMEM, 10% FBS supplemented with stem cell factor (100 ng/ml), IL-10 (10 ng/ml), and IL-6 (50 ng/ml) (R&D Systems). The human mast cell line (HMC)-1 cells were a generous gift from Dr. J. Butterfield (Mayo Clinic, Rochester, MN). HMC-1 cells were maintained in Iscove’s modified DMEM (13).
Pure ASM bundles were isolated from bronchial biopsies obtained from fiberoptic bronchoscopy and additional airways isolated from lung resection were dissected free of surrounding tissue. Primary ASM was cultured in DMEM with Glutamax-1 supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml amphotericin, 100 μM non-essential amino acids, and 1 mM sodium pyruvate as previously described (14). ASM passage 2–6 was used for experiments.
α-SMA expression
Flow cytometry
ASM were fixed with 4% paraformaldehyde; resuspended, and permeabilized in PBS/0.5% BSA and 0.1% saponin (1 × 106 cells/ml) and stained with α-SMA FITC direct conjugate or appropriate isotype control (DakoCytomation). α-SMA expression was assessed by flow cytometry (FACScan, CellQuest software; BD Biosciences) and quantified as fold difference in geometric mean fluorescent intensity (GMFI) compared with isotype control or the proportion of positive cells.
Immunofluorescence
ASM were labeled with α-SMA mAb FITC direct conjugate and compared with an appropriate isotype control (Dako-Cytomation). ASM and HLMC cocultures were counterstained with tryptase biotin (Chemicon) indirectly conjugated with streptavidin Texas red (Vector Laboratories) and compared with an appropriate isotype control (DakoCytomation).
Real-time RT-PCR
Total RNA was isolated using TRIzol (Invitrogen). The concentration and purity of each sample were determined by analyzing spectrophotometric absorption at 260/280 nm. Real-time RT-PCR was performed using a single tube full velocity SYBR green kit (Stratagene). The internal normalizer gene used was β-actin and was conducted with β-actin forward (TTCAACTCCATCATGAAGTGTGACGTG), and β-actin reverse (CTA AGTCATAGTCCGCCTAGAAGCATT) primers, while amplification of α-SMA was conducted with α-SMA forward (CTGTTCCAGCCATCCT TCAT), and α-SMA reverse (CCGTGATCTCCTTCTGCATT) primers (15). Amplification was evaluated using 10-fold serial dilutions of positive controls and calculated from the slopes of log input amounts plotted vs Ct values. The efficiency of the α-SMA primer was confirmed to be high (>95%) and comparable (less than 2% difference with respect to β-actin).
Assessment of ASM contraction by collagen gel analysis
Collagen gels (299 μl of collagen (Inamed Biomaterials), 37 μl of 10X DMEM (Invitrogen), 20 μl of sodium bicarbonate (Invitrogen) were impregnated with 0.125 × 106 ASM cells resuspended in 144 μl of serum-free medium with stimulus as required. The gels were added to 24-well plates (PBS/0.5% BSA) and left to polymerize at 37°C for 90 min. The gels were then detached and suspended in 500 μl of serum-free medium with stimulus as required and incubated for 48 h. Histamine (Sigma-Aldrich) was added to appropriate wells to a final concentration of 100 μM, with photographs taken at 0.25, 0.5, 1, 2, 4, and 8 h. The surface area of each gel was measured at each time point using ImageJ (http://rsb.info.nih.gov/ij) by a blinded observer. The intersubject variability in the assessment of gel size was assessed between five observers and demonstrated excellent agreement (intraclass correlation = 0.99).
Study Protocol
Modulation of α-SMA expression by mast cells and their products
ASM was serum deprived for 72 h in ITS medium (DMEM with Glutamax-1 supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml amphotericin, 100 μM non-essential amino acids, and 1 mM sodium pyruvate and 1% insulin-transferrin-selenium) and cocultured for 1, 3, or 7 days with 1) whole HLMC, 2) HLMC sensitized with 2.5 μg/ml IgE (Calbiochem) for 1 h, 3) sensitized HLMC activated with 1:1000 anti-IgE (Sigma-Aldrich), 4) HLMC or HMC-1 lysates, and 5) isolated 0.5 μg/ml human lung tryptase (Europa Bioproducts) a concentration equivalent to the tryptase concentration found in a 1:4 mast cell to ASM ratio (16). HLMC or lysates were cocultured with ASM at a 1:4 mast cell/ASM ratio to reflect the ratio that is found in vivo (4). Experiments were performed in the presence and absence of the serine protease inhibitor leupeptin (Sigma-Aldrich) and lactoferrin (Sigma-Aldrich), a member of the transferrin class of proteins, at appropriate concentrations to inhibit the activity of the tryptase (17, 18). After 1, 3, or 7 days, α-SMA protein and mRNA expression were examined by flow cytometry, immunofluorescence, and quantitative PCR.
Cell-free supernatants from cocultures of ASM with β-tryptase, HLMC or HLMC lysates with and without inhibitors were stored at −80°C for later analysis of TGF-β1 and TGF-β2 expression by ELISA (R&D Systems) with a limit of detection of 31.25 pg/ml for both assays.
Modulation of α-SMA expression by TGF-β
Recombinant TGF-β1 or TGF-β2 (0.25, 2, or 10 ng/ml) (R&D Systems) was added to ASM that had been serum deprived for 72 h. After 1, 3, or 7 days, α-SMA protein expression and ASM contractile activity was examined by flow cytometry and collagen gel analysis.
TGF-β1 (2 ng/ml) was pre-incubated with or without the TGF-β1 neutralizing Ab or isotype control (R&D Systems) for 30 min at 37°C before addition to the ASM that had been serum deprived for 72 h. The concentration of TGF-β1 and the neutralizing Ab used was based on the TGF-β1 content measured in ASM and tryptase culture supernatants. α-SMA protein expression and ASM contractile activity were examined by flow cytometry and collagen gel analysis.
PAR-2 expression by ASM
Surface and intracellular protein expression by ASM of PAR-2 was assessed by flow cytometry as described above using an indirectly FITC-labeled PAR-2 mouse mAb (R&D Systems) and appropriate isotype control (DakoCytomation).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 4. Data are presented as mean (± SEM). Paired and unpaired data were analyzed by using paired and unpaired t tests, respectively. Comparison across groups was assessed using ANOVA and Tukey’s multiple comparison test was used for between group comparisons. Differences were considered significant when p ≤ 0.05.
Results
Clinical populations
We recruited 12 subjects with asthma and 29 non-asthmatic controls. Eleven of the controls had chronic obstructive pulmonary disease (COPD) and 18 had normal lung function. The clinical characteristics of the subjects are as described in Table I.
Table I.
Clinical characteristics mean (SEM)a
| Asthma (GINAa treatment step I = 4, II–IV = 5, V = 3) |
COPD | Controls | |
|---|---|---|---|
| Number | 12 | 11 | 18 |
| Gender male/female | 5/7 | 7/4 | 13/5 |
| Age (yrs) | 47 (6) | 65 (12) | 60 (13) |
| FEV1 (liters) | 2.6 (0.3) | 1.68 (0.12) | 2.5 (0.11) |
| FEV1% predicted | 82.4 (9.8)% | 61 (3) | 85 (4) |
| FEV1/FVC (%) | 71.9 (5.3) | 59 (3) | 79 (2) |
GINA, Global Initiative for Asthma.
Increased ASM α-SMA expression in vivo localized with mast cells
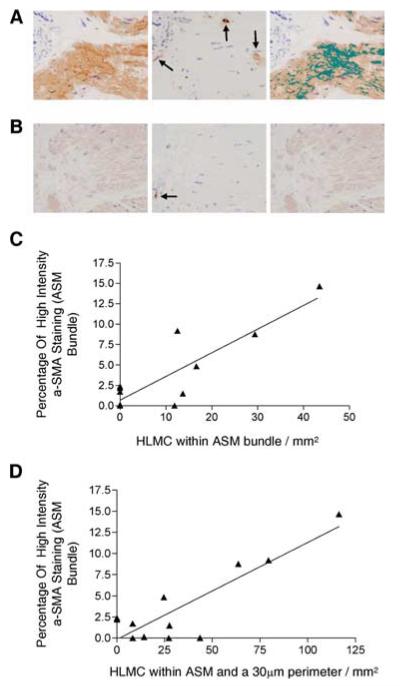
In bronchial biopsies from asthmatic subjects, mast cells were identified in and adjacent to ASM bundles (n = 12). Representative photomicrographs of sequential sections illustrating the association between the location of mast cells and the intensity of α-SMA are as shown in Fig. 1A. The mean (SEM) number of mast cells within the ASM bundle was 10.6 (4.05)/mm2 and 34.4 (10.3)/mm2 in the bundle and perimeter. There was a strong correlation between the percentage of high intensity α-SMA staining and the number of mast cells in the ASM bundle alone (r = 0.87; p = 0.0003; Fig. 1B) and together with the number of mast cells adjacent to the bundle (r = 0.87; p = 0.0002; Fig. 1C).
FIGURE 1.
Increased ASM α-SMA expression in vivo localized with mast cells. A, Photomicrographs of a bronchial biopsy from an asthmatic illustrating ASM bundles stained for α-SMA (left), sequential tryptase stained section revealing one mast cell within the ASM bundle and two in the 30-μm perimeter of the bundle (middle), and high intensity thresholded α-SMA-stained bundle covering 8.76% of the total ASM bundle area (right) (×400). B, Photomicrograph of another asthmatic subject again illustrating α-SMA-stained ASM bundle (left), sequential tryptase-stained section revealing one mast cell in the 30-μm perimeter and none within the ASM bundle (middle), and high intensity thresholded α-SMA-stained ASM bundle covering 0% of the total ASM bundle area (right) (×400). C, Correlation between the percentage of high intensity α-SMA staining and the number of mast cells in the ASM bundle alone (r = 0.87; p = 0.0003) and D, together with the number of mast cells in the 30 μm perimeter of the ASM bundle (r = 0.87; p = 0.0002).
Mast cells and β-tryptase increase ASM α-SMA expression
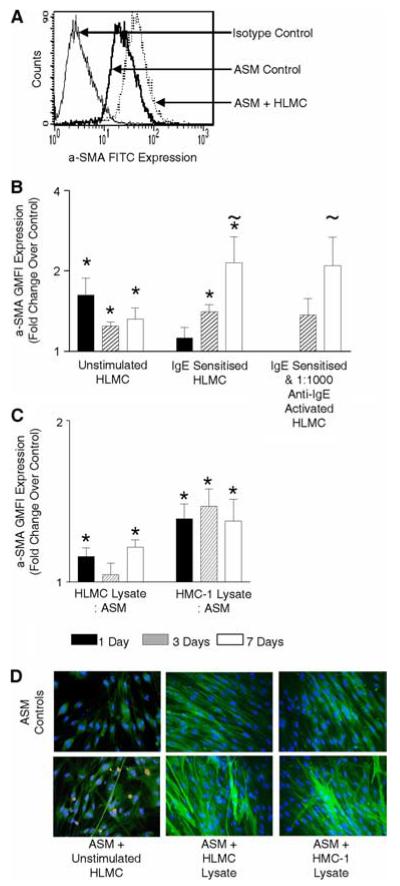
The mean (SEM) proportion of cells that expressed α-SMA by flow cytometry was consistently very high as illustrated in the representative histogram (Fig. 2A) and was not different between those subjects with 97.6 (0.7)% and without asthma 91.6 (2.2)% ( p = 0.23). In coculture we therefore chose to examine the change in the GMFI of ASM α-SMA (Fig. 2A).
FIGURE 2.
Mast cells increase ASM α-SMA expression. A, Example flow cytometry histogram of ASM stained with isotype control or α-SMA mAb FITC direct conjugate cocultured with or without unstimulated HLMC (ratio 1:4, HLMC/ASM) for 7 days. Isotype controls for both conditions were overlapping, therefore only one is shown for illustration. B and C, Bar charts illustrating the fold change over control of α-SMA GMFI expression by ASM as assessed by flow cytometry after coculture with HLMC (ratio 1:4, HLMC/ASM) unstimulated or IgE sensitized (2.5 μg/ml) (n = 9 ASM donors, n = 9 HLMC) or sensitized and anti-IgE activated (1:1000) (n = 5 ASM donors, n = 7 HLMC) (B) or HLMC (n = 8 ASM donors, n = 7 HLMC) or HMC-1 lysates (n = 16 ASM donors) at a ratio of 1:4 mast cell lysate to ASM for 1, 3, or 7 days (C). D, Example immunofluorescence images from three experiments of ASM cocultured with unstimulated HLMC, HLMC, or HMC-1 lysates for 7 days (ratio 1:4, mast cell/ASM) bottom panels and the corresponding controls in the top panels. Cell nuclei were labeled with 4′,6-diamidino-2-phenylindole (DAPI), ASM were labeled with α-SMA mAb FITC direct conjugate, ASM and HLMC cocultures were counterstained with tryptase biotin indirectly conjugated with streptavidin Texas red. Changes in ASM α-SMA expression are illustrated by an increase in the intensity of α-SMA mAb FITC fluorescence. *, p < 0.05 per time point; ~, p < 0.05 across all time points.
Mast cells or their lysates in coculture with ASM increased ASM α-SMA expression markedly over 1, 3, and 7 days assessed by flow cytometry (Fig. 2, A–C). The increase in ASM α-SMA expression in response to coculture with mast cells was not different between ASM cocultured with unstimulated or IgE/anti-IgE stimulated HLMCs (Fig. 2B; ANOVA p > 0.05 for all time points). The mast cell mediated up-regulation of ASM α-SMA actin expression was also not significantly different between those ASM donors with or without asthma or between those controls with or without COPD (unpublished data). Our flow cytometry findings were confirmed by immunofluorescence (Fig. 2D).
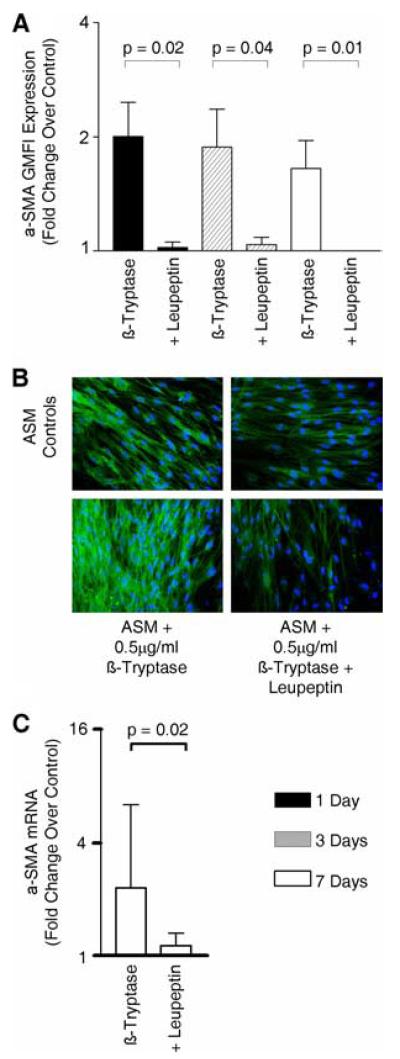
Similar effects on ASM differentiation were observed with the mast cell-derived serine protease β-tryptase. Human lung β-tryptase (0.5 μg/ml) increased α-SMA expression assessed by flow cytometry (Fig. 3A), immunofluorescence (Fig. 3B), and mRNA expression (Fig. 3C).
FIGURE 3.
β-Tryptase increases ASM α-SMA expression. A, Bar chart illustrating the fold change over control of α-SMA GMFI expression by ASM as assessed by flow cytometry after culture with β-tryptase (0.5 μg/ml) with or without leupeptin (10.6 μg/ml) for 1, 3, and 7 days (n = 4 ASM donors). B, Example immunofluorescence images from three experiments of ASM cultured with β-tryptase with or without leupeptin for 7 days (bottom panels) and the corresponding controls (top panels). Changes in ASM α-SMA expression are illustrated by an increase in the intensity of α-SMA mAb FITC fluorescence. C, Changes in α-SMA mRNA expression by ASM cultured with β-tryptase with or without leupeptin for 7 days as assessed by quantitative PCR (n = 3 ASM donors).
Both the β-tryptase-mediated increase in protein and mRNA α-SMA was significantly inhibited by the serine protease inhibitor leupeptin (10.6 μg/ml) (Fig. 3). Similarly, the increase in α-SMA protein following coculture with HLMC or HMC-1 lysates was significantly inhibited by leupeptin (10.6 μg/ml) (Fig. 4). This suggests that the mast cell mediated up-regulation in ASM α-SMA expression was mediated by tryptase.
FIGURE 4.
Mast cells mediate increased ASM α-SMA expression via β-tryptase. Bar charts illustrating the fold change over control of α-SMA GMFI expression by ASM as assessed by flow cytometry after culture with HLMC (n = 3 ASM donors, n = 3 HLMC) (A), HLMC or HMC-1 lysates (ratio 1 mast cell to 4 ASM; n = 4 ASM donors) for 1 or 3 days, with or without leupeptin (10.6 μg/ml) (B).
Enhanced ASM contractility in response to β-tryptase
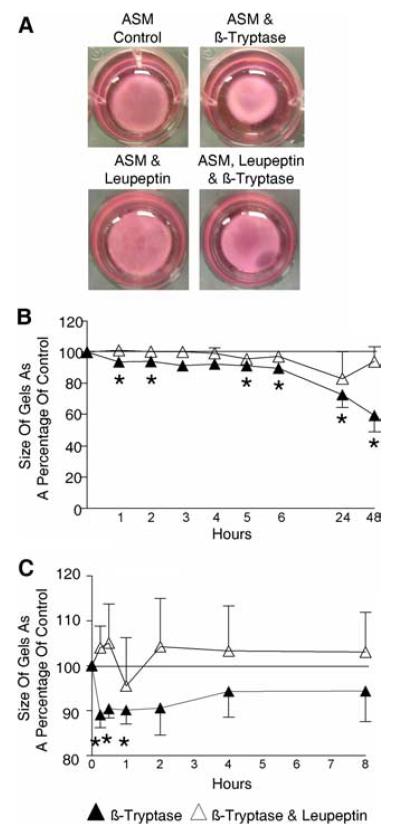
We assessed the functional consequence of the β-tryptase-mediated increased α-SMA expression by ASM using a gel contraction assay. The size of the gel impregnated with β-tryptase primed ASM was reduced after 48 h compared with control (mean difference [95% CI] 59.3 [31.5–87.1]%; p = 0.01; n = 5), an effect inhibited by leupeptin (Fig. 5, A and B). In addition, the contractile response of β-tryptase-primed ASM to histamine was increased compared with control (after 1 h mean difference [95% CI] 90.2 [81.5–98.9]%; p = 0.03; n = 5). This increased contractility was inhibited by leupeptin (Fig. 5C).
FIGURE 5.
Enhanced ASM contractility in response to β-tryptase. Example photographs (A) and time course of contraction of collagen gels impregnated with ASM with or without β-tryptase (0.32 μg/ml) with or without leupeptin (6.7 μg/ml) for 48 h (n = 5 ASM donors) (B), followed by subsequent histamine (100 μM) stimulation to the same gels compared with appropriate control analyzed using ImageJ (C). *, p < 0.05.
TGF-β1 and TGF-β2 increase ASM α-SMA expression and contractility
The TGF-β1 concentration was increased in cell-free supernatants from ASM cocultured for 1 and 3 days with HLMC (day 3: geometric mean [95% CI] 5.52 [0.55–55.59] ng/ml/106 cells; n = 3; p = 0.03) or HLMC lysates (day 3: 6.65 [0.46–95.54] ng/ml/106 cells; n = 3; p = 0.04) or β-tryptase (day 3: 9.62 [5.72–16.14] ng/ml/106 cells; n = 3; p =<0.01) corrected for basal release by ASM and mast cells (Fig. 6A). The increases in TGF-β1 concentration in cell-free supernatants from ASM and HLMC or β-tryptase cocultures were significantly inhibited by the application of leupeptin or lactoferrin (Fig. 6B).
FIGURE 6.
Mast cells mediate increased TGF-β1 expression via β-tryptase. Bar charts illustrating the increase in TGF-β1 production by ASM as assessed by ELISA after culture with HLMC, HLMC lysates or β-tryptase (ratio 1:4 mast cell/ASM or 0.5 μg/ml β-tryptase; n = 3) for 1 or 3 days (A), with or without leupeptin (10.6 μg/ml) or lactoferrin (80.6 ng/ml) (B). *, p < 0.05.
There was a small nonsignificant increase in the TGF-β2 concentration in cell-free supernatants from ASM cocultured for 1 and 3 days with HLMC (day 3: 0.93 [0.1–8.58] ng/ml/106 cells, n = 3) or HLMC lysates (day 3: 0 [0–0] ng/ml/106 cells, n = 3) or β-tryptase (day 3: 1.57 [0.23–10.86] ng/ml/106 cells, n = 3) corrected for basal release by ASM and mast cells.
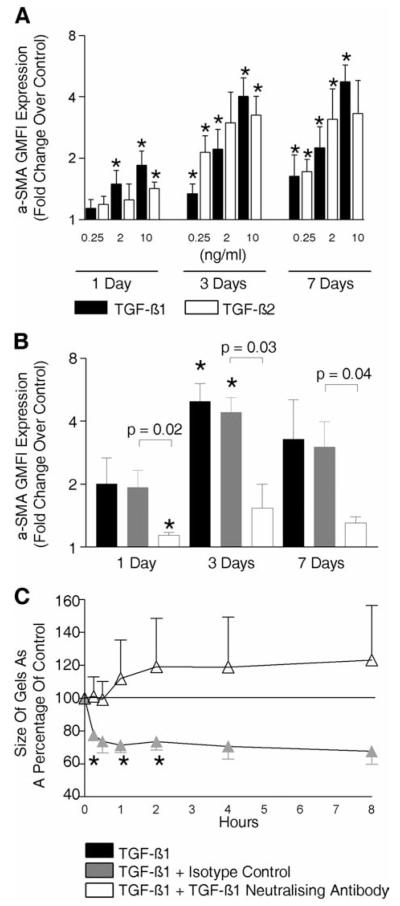
The expression of α-SMA in ASM significantly increased as a result of stimulation with both recombinant TGF-β1 and TGF-β2 (0.25–10 ng/ml), after 1, 3, and 7 days ( p < 0.05) (Fig. 7A), stimulation with TGF-β2 was only significantly greater than TGF-β1 at 3 days with 0.25 ng/ml. TGF-β1 (10 ng/ml) mediated up-regulation of ASM α-SMA after 7 days in those with (geometric mean [95% CI] 7.0 [1.2–41.3] fold increase; p = 0.04) or without asthma (4.9 [1.7–13.8] fold increase; p = 0.04), but there was no difference between groups (mean difference [95% CI] asthma vs non-asthma 1.44 [0.4–5.4] fold; p = 0.49; n = 3). The increase in α-SMA expression mediated by TGF-β1 (2 ng/ml) was significantly inhibited by TGF-β1-neutralizing Ab (Fig. 7B). TGF-β1 also increased the contractile response of ASM to hista-mine (after 1 h mean difference [95% CI] 74 [51.7–91.1]%; p = 0.02; n = 3), which was inhibited by the TGF-β1-neutralizing Ab (Fig. 7C).
FIGURE 7.
TGF-β1 and TGF-β2 increase ASM α-SMA expression and contractility. Bar chart illustrating the fold change over control of α-SMA GMFI expression by ASM assessed by flow cytometry after culture with TGF-β1 or TGF-β2 (0.25, 2, or 10 ng/ml) (n = 9 ASM donors) (A) or TGF-β1 (2 ng/ml) with or without TGF-β1 neutralizing Ab or appropriate isotype control for 1, 3, or 7 days (n = 3 ASM donors) (B). C, Time course after 100 μM/ml histamine stimulation of collagen gel contraction in gels previously impregnated with TGF-β1 (2 ng/ml) in the presence or absence of TGF-β1 neutralizing Ab or appropriate isotype control for 48 h (n = 3 ASM donors). *, p < 0.05.
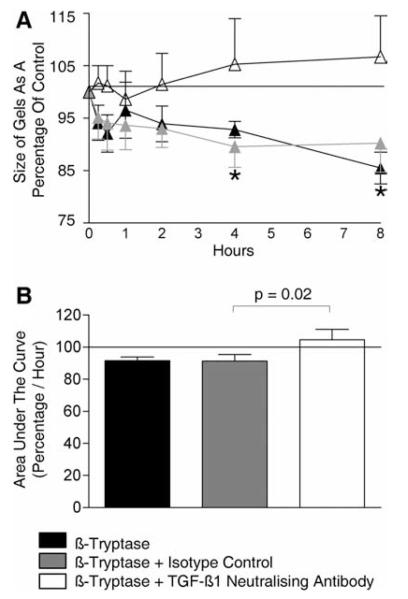
The neutralization of TGF-β1 inhibited β-tryptase-mediated increased ASM contraction
The increase in ASM contraction in response to histamine mediated by β-tryptase priming was markedly inhibited by TGF-β1 neutralization compared with isotype control (Fig. 8). This suggests that the β-tryptase induced up-regulation in ASM α-SMA may be attenuated by the inhibition of the TGF-β1 released by the ASM in response to β-tryptase stimulation.
FIGURE 8.
The neutralization of TGF-β1 inhibited β-tryptase-mediated increased ASM contraction. Time course (A) and area under the curve of the time course (B), after histamine (100 μM/ml) stimulation of collagen gel contraction in gels previously impregnated with β-tryptase (0.32 μg/ml) in the presence or absence of TGF-β1-neutralizing Ab or appropriate isotype control for 48 h (n = 4 ASM donors). *, p < 0.05.
PAR-2 expression by ASM
The proportion of primary cultured ASM that expressed PAR-2 on their cell surface was (mean ± SEM) 7.6 ± 0.99%; p = 0.02 (n = 3) and the PAR-2 expression by permeabilized ASM was 24.53 ± 4.7%; p = 0.03 (n = 3).
Discussion
This is the first report to demonstrate that mast cells promote ASM differentiation to a more contractile phenotype. Since mast cell localization to the ASM bundle is a key feature of the immunopathology of asthma, we have identified a novel mechanism whereby mast cell-ASM interactions may aggravate the disordered airway physiology that characterizes asthma.
Mast cells increased ASM α-SMA mRNA and protein expression. This was mediated by β-tryptase and was attenuated by the serine protease inhibitor leupeptin. We confirmed that mast cell-derived β-tryptase increased ASM TGF-β1 secretion (19), which itself increased α-SMA expression (20). The mast cell-derived β-tryptase mediated increases in TGF-β1 production were inhibited by leupeptin and lactoferrin. We confirmed that ASM expressed the β-tryptase receptor; PAR-2, but whether β-tryptase exerts its effects in ASM via this receptor requires further study. Importantly the increased ASM α-SMA expression by β-tryptase was inhibited by TGF-β1 neutralization suggesting that its effects were predominately exerted via the autocrine activation of ASM by TGF-β1. However, we cannot exclude the possibility that some of the β-tryptase-mediated effects on ASM may have been direct or via other important mediators and therefore TGF-β independent. We are confident that our in vitro findings are relevant in asthma as in vivo we found that in bronchial mucosal biopsies from asthmatics, the intensity of α-SMA expression was strongly related to the number of mast cells within or adjacent to an ASM bundle. Although we present here the first evidence of HLMCs driving ASM differentiation, the importance of mast cell-mesenchymal interactions is not restricted to the airway. Current evidence suggests that colocalization of mast cells with pericryptal fibroblasts in the lamina propria of the duodenum is important in maintaining normal villous morphology (21) and coculture of dermal fibroblasts and HMC-1 cells result in fibroblast differentiation (22).
Mast cell microlocalization to the ASM bundle is a feature of asthma across severities. Mast cell number correlates positively with the degree of AHR (4) and with the bronchoconstrictor response to a deep inspiration (23) supporting the view that mast cell-ASM cell interactions are central in the development of the disordered physiology in asthma. Importantly, there is evidence that mast cells infiltrating the ASM bundle are activated with increased expression of Th2 cytokines IL-4 and IL-13 (8). Post mortem studies of fatal and non-fatal asthma have demonstrated that there was a marked increase in mast cell degranulation in the ASM bundle in both the large and small airways (24) and that increased numbers of mast cells are associated with increased ASM shortening in fatal asthma (25). In contrast, there is a paucity of other inflammatory cells in the ASM bundle in asthma (as reviewed in Ref. 26). Our data suggest that mast cell β-tryptase is central in the development of ASM dysfunction, which is supported by early clinical trials of tryptase inhibitors in sheep models of asthma (27) and in human disease (28, 29). However, the link between mast cell-ASM interactions and disordered airway physiology has been questioned by the inconsistent effect of anti-IgE therapy on AHR (30–33). Importantly, our findings indicate that mast cell-derived β-tryptase from HLMCs can promote ASM differentiation independently of IgE. We have therefore identified a novel IgE-independent mast cell-mediated mechanism that may be an important driver in the development of AHR in asthma.
Smooth muscle contraction is under the control of a well-described multistep process (34). Specifically, ASM contraction occurs due to myosin-actin cross-bridging following phosphorylation of smooth muscle myosin L chain by myosin L chain kinase, which in turn is activated by cytoplasmic Ca2+ bound to calmodulin. Although α-SMA is a key contractile protein involved in ASM contraction, this process can be modulated by the content and activation of several proteins and kinases. We restricted our investigations to α-SMA expression as there is a considerable body of evidence that there is close relationship between the α-SMA content and mesenchymal cell contraction (35). Fibroblasts from an OVA-challenged mouse model of asthma demonstrated augmented gel contraction and expressed more α-SMA than fibroblasts from control animals (36). In α-SMA null mice, there is diminished vascular tone (37) and reduced contractile force by bladder smooth muscle (38). In humans, there was a very strong correlation between α-SMA expression and contraction of bone marrow stroma-derived mesenchymal stem cells (39). We are therefore confident that the changes we observed in the up-regulation of α-SMA expression by ASM in response to coculture with mast cells is critical in the transition of the ASM to a more contractile phenotype.
One possible criticism of our study is that our in vitro findings are limited to primary cells rather than ex vivo bronchial rings or in vivo whole animal studies. To date there is not a good animal model of asthma to study mast cell-ASM interactions (26). Contraction studies using bronchial rings have the advantage over gel contraction assays in that the ASM bundle and the microenvironment are intact but are limited in their ability to examine interactions between cell types. However, in support of our findings sensitized bronchial rings primed with β-tryptase demonstrate increased contractility to histamine (40). Critically, the mechanisms proposed by our ASM-mast cell cocultures are strengthened by two lines of evidence. First, we observed a remarkably strong correlation between the intensity of α-SMA expression and the number of mast cells within an ASM bundle; second, improvements in lung function in asthmatics following treatment with tryptase inhibitors are encouraging (28, 29).
In conclusion, mast cell localization to the ASM bundle facilitates interactions between these cell types and leads to an altered ASM phenotype with increased α-SMA expression and contraction. This ASM differentiation to a more contractile phenotype mediated by mast cell-derived β-tryptase presents a novel target to improve the disordered airway physiology that is the hallmark of asthma.
Footnotes
Disclosures The authors have no financial conflict of interest.
Funding was received from Asthma UK, DoH Clinician Scientist Award (to C.B.) and Wellcome Senior Clinical Fellowship (to C.B.).
Abbreviations used in this paper: AHR, airway hyper-responsiveness; ASM, airway smooth muscle; α-SMA, α-smooth muscle actin; COPD, chronic obstructive pulmonary disease; GMFI, geometric mean fluorescent intensity; HLMC, human lung mast cell; HMC-1, human mastocytoma cell line.
References
- 1.Hartert TV, Peebles RS., Jr Epidemiology of asthma: the year in review. Curr. Opin. Pulm. Med. 2000;6:4–9. doi: 10.1097/00063198-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Kay AB. Pathology of mild, severe, and fatal asthma. Am. J. Respir. Crit Care Med. 1996;154:S66–S69. doi: 10.1164/ajrccm/154.2_Pt_2.S66. [DOI] [PubMed] [Google Scholar]
- 3.Wardlaw AJ, Brightling C, Green R, Woltmann G, Pavord I. Eosinophils in asthma and other allergic diseases. Br. Med. Bull. 2000;56:985–1003. doi: 10.1258/0007142001903490. [DOI] [PubMed] [Google Scholar]
- 4.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N. Engl. J. Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 5.Brightling CE, Symon FA, Birring SS, Bradding P, Pavord ID, Wardlaw AJ. TH2 cytokine expression in bronchoalveolar lavage fluid T lymphocytes and bronchial submucosa is a feature of asthma and eosinophilic bronchitis. J. Allergy Clin. Immunol. 2002;110:899–905. doi: 10.1067/mai.2002.129698. [DOI] [PubMed] [Google Scholar]
- 6.Brightling CE, Symon FA, Birring SS, Bradding P, Wardlaw AJ, Pavord ID. Comparison of airway immunopathology of eosinophilic bronchitis and asthma. Thorax. 2003;58:528–532. doi: 10.1136/thorax.58.6.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brightling CE, Ward R, Goh KL, Wardlaw AJ, Pavord ID. Eosinophilic bronchitis is an important cause of chronic cough. Am. J. Respir. Crit Care Med. 1999;160:406–410. doi: 10.1164/ajrccm.160.2.9810100. [DOI] [PubMed] [Google Scholar]
- 8.Brightling CE, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID, Bradding P. Interleukin-4 and -13 expression is co-localized to mast cells within the airway smooth muscle in asthma. Clin. Exp. Allergy. 2003;33:1711–1716. doi: 10.1111/j.1365-2222.2003.01827.x. [DOI] [PubMed] [Google Scholar]
- 9.Bradding P, Holgate ST. The mast cell as a source of cytokines in asthma. Ann. NY Acad. Sci. 1996;796:272–281. doi: 10.1111/j.1749-6632.1996.tb32589.x. [DOI] [PubMed] [Google Scholar]
- 10.Global Initiative for Asthma Guidelines. 2008 Available from: http://www.ginasthma.com.
- 11.British Thoracic Society Bronchoscopy Guidelines Committee, a Subcommittee of Standards of Care Committee of British Thoracic Society British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax. 2001;56(Suppl. 1):i1–i21. doi: 10.1136/thorax.56.suppl_1.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Britten KM, Howarth PH, Roche WR. Immunohistochemistry on resin sections: a comparison of resin embedding techniques for small mucosal biopsies. Biotech. Histochem. 1993;68:271–280. doi: 10.3109/10520299309105629. [DOI] [PubMed] [Google Scholar]
- 13.Duffy SM, Leyland ML, Conley EC, Bradding P. Voltage-dependent and calcium-activated ion channels in the human mast cell line HMC-1. J. Leukocyte Biol. 2001;70:233–240. [PubMed] [Google Scholar]
- 14.Brightling CE, Ammit AJ, Kaur D, Black JL, Wardlaw AJ, Hughes JM, Bradding P. The CXCL10/CXCR3 axis mediates human lung mast cell migration to asthmatic airway smooth muscle. Am. J. Respir. Crit Care Med. 2005;171:1103–1108. doi: 10.1164/rccm.200409-1220OC. [DOI] [PubMed] [Google Scholar]
- 15.Meyer-Ter-Vehn T, Gebhardt S, Sebald W, Buttmann M, Grehn F, Schlunck G, Knaus P. p38 inhibitors prevent TGF-β-induced myofibroblast transdifferentiation in human tenon fibroblasts. Invest. Ophthalmol. Vis. Sci. 2006;47:1500–1509. doi: 10.1167/iovs.05-0361. [DOI] [PubMed] [Google Scholar]
- 16.Patella V, Marino I, Lamparter B, Arbustini E, Adt M, Marone G. Human heart mast cells: isolation, purification, ultrastructure, and immunologic characterization. J. Immunol. 1995;154:2855–2865. [PubMed] [Google Scholar]
- 17.He SH, Chen P, Chen HQ. Modulation of enzymatic activity of human mast cell tryptase and chymase by protease inhibitors. Acta Pharmacol. Sin. 2003;24:923–929. [PubMed] [Google Scholar]
- 18.Elrod KC, Moore WR, Abraham WM, Tanaka RD. Lactoferrin, a potent tryptase inhibitor, abolishes late-phase airway responses in allergic sheep. Am. J. Respir. Crit Care Med. 1997;156:375–381. doi: 10.1164/ajrccm.156.2.9607012. [DOI] [PubMed] [Google Scholar]
- 19.Berger P, Girodet PO, Begueret H, Ousova O, Perng DW, Marthan R, Walls AF, Tunon de Lara JM. Tryptase-stimulated human airway smooth muscle cells induce cytokine synthesis and mast cell chemotaxis. FASEB J. 2003;17:2139–2141. doi: 10.1096/fj.03-0041fje. [DOI] [PubMed] [Google Scholar]
- 20.Goldsmith AM, Bentley JK, Zhou L, Jia Y, Bitar KN, Fingar DC, Hershenson MB. Transforming growth factor-β induces airway smooth muscle hypertrophy. Am. J. Respir. Cell Mol. Biol. 2006;34:247–254. doi: 10.1165/rcmb.2005-0166OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crivellato E, Finato N, Isola M, Pandolfi M, Ribatti D, Beltrami CA. Number of pericryptal fibroblasts correlates with density of distinct mast cell phenotypes in the crypt lamina propria of human duodenum: implications for the homeostasis of villous architecture. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2006;288:593–600. doi: 10.1002/ar.a.20325. [DOI] [PubMed] [Google Scholar]
- 22.Gailit J, Marchese MJ, Kew RR, Gruber BL. The differentiation and function of myofibroblasts is regulated by mast cell mediators. J. Invest. Dermatol. 2001;117:1113–1119. doi: 10.1046/j.1523-1747.2001.15211.x. [DOI] [PubMed] [Google Scholar]
- 23.Slats AM, Janssen K, van Schadewijk A, van der Plas DT, Schot R, van den Aardweg JG, de Jongste JC, Hiemstra PS, Mauad T, Rabe KF, Sterk PJ. Bronchial inflammation and airway responses to deep inspiration in asthma and chronic obstructive pulmonary disease. Am. J. Respir. Crit Care Med. 2007;176:121–128. doi: 10.1164/rccm.200612-1814OC. [DOI] [PubMed] [Google Scholar]
- 24.Carroll NG, Mutavdzic S, James AL. Distribution and degranulation of airway mast cells in normal and asthmatic subjects. Eur. Respir. J. 2002;19:879–885. doi: 10.1183/09031936.02.00275802. [DOI] [PubMed] [Google Scholar]
- 25.Chen FH, Samson KT, Miura K, Ueno K, Odajima Y, Shougo T, Yoshitsugu Y, Shioda S. Airway remodeling: a comparison between fatal and nonfatal asthma. J. Asthma. 2004;41:631–638. doi: 10.1081/jas-200026405. [DOI] [PubMed] [Google Scholar]
- 26.Siddiqui S, Hollins F, Saha S, Brightling CE. Inflammatory cell microlocalisation and airway dysfunction: cause and effect? Eur. Respir. J. 2007;30:1043–1056. doi: 10.1183/09031936.00162506. [DOI] [PubMed] [Google Scholar]
- 27.Costanzo MJ, Yabut SC, Almond HR, Jr., Andrade-Gordon P, Corcoran TW, De Garavilla L, Kauffman JA, Abraham WM, Recacha R, Chattopadhyay D, Maryanoff BE. Potent, small-molecule inhibitors of human mast cell tryptase: antiasthmatic action of a dipeptide-based transition-state analogue containing a benzothiazole ketone. J. Med. Chem. 2003;46:3865–3876. doi: 10.1021/jm030050p. [DOI] [PubMed] [Google Scholar]
- 28.Krishna MT, Chauhan A, Little L, Sampson K, Hawksworth R, Mant T, Djukanovic R, Lee T, Holgate S. Inhibition of mast cell tryptase by inhaled APC 366 attenuates allergen-induced late-phase airway obstruction in asthma. J. Allergy Clin. Immunol. 2001;107:1039–1045. doi: 10.1067/mai.2001.115631. [DOI] [PubMed] [Google Scholar]
- 29.Cairns JA. Inhibitors of mast cell tryptase β as therapeutics for the treatment of asthma and inflammatory disorders. Pulm. Pharmacol. Ther. 2005;18:55–66. doi: 10.1016/j.pupt.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 30.Boulet LP, Chapman KR, Cote J, Kalra S, Bhagat R, Swystun VA, Laviolette M, Cleland LD, Deschesnes F, Su JQ, et al. Inhibitory effects of an anti-IgE antibody E25 on allergen-induced early asthmatic response. Am. J. Respir. Crit Care Med. 1997;155:1835–1840. doi: 10.1164/ajrccm.155.6.9196083. [DOI] [PubMed] [Google Scholar]
- 31.Djukanovic R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, Bao W, Fowler-Taylor A, Matthews J, Busse WW, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am. J. Respir. Crit Care Med. 2004;170:583–593. doi: 10.1164/rccm.200312-1651OC. [DOI] [PubMed] [Google Scholar]
- 32.Fahy JV, Fleming HE, Wong HH, Liu JT, Su JQ, Reimann J, Fick RB, Jr., Boushey HA. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am. J. Respir. Crit. Care Med. 1997;155:1828–1834. doi: 10.1164/ajrccm.155.6.9196082. [DOI] [PubMed] [Google Scholar]
- 33.Noga O, Hanf G, Kunkel G. Immunological and clinical changes in allergic asthmatics following treatment with omalizumab. Int. Arch. Allergy Immunol. 2003;131:46–52. doi: 10.1159/000070434. [DOI] [PubMed] [Google Scholar]
- 34.Gunst SJ, Tang DD. The contractile apparatus and mechanical properties of airway smooth muscle. Eur. Respir. J. 2000;15:600–616. doi: 10.1034/j.1399-3003.2000.15.29.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Zohar R, McCulloch CA. Multiple roles of α-smooth muscle actin in mechanotransduction. Exp. Cell Res. 2006;312:205–214. doi: 10.1016/j.yexcr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Sugiura H, Liu X, Duan F, Kawasaki S, Togo S, Kamio K, Wang XQ, Mao L, Ahn Y, Ertl RF, et al. Cultured lung fibroblasts from ovalbumin-challenged “asthmatic” mice differ functionally from normal. Am. J. Respir. Cell Mol. Biol. 2007;37:424–430. doi: 10.1165/rcmb.2007-0089OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schildmeyer LA, Braun R, Taffet G, Debiasi M, Burns AE, Bradley A, Schwartz RJ. Impaired vascular contractility and blood pressure homeostasis in the smooth muscle α-actin null mouse. FASEB J. 2000;14:2213–2220. doi: 10.1096/fj.99-0927com. [DOI] [PubMed] [Google Scholar]
- 38.Zimmerman RA, Tomasek JJ, McRae J, Haaksma CJ, Schwartz RJ, Lin HK, Cowan RL, Jones AN, Kropp BP. Decreased expression of smooth muscle α-actin results in decreased contractile function of the mouse bladder. J. Urol. 2004;172:1667–1672. doi: 10.1097/01.ju.0000139874.48574.1b. [DOI] [PubMed] [Google Scholar]
- 39.Kinner B, Zaleskas JM, Spector M. Regulation of smooth muscle actin expression and contraction in adult human mesenchymal stem cells. Exp. Cell Res. 2002;278:72–83. doi: 10.1006/excr.2002.5561. [DOI] [PubMed] [Google Scholar]
- 40.Johnson PR, Ammit AJ, Carlin SM, Armour CL, Caughey GH, Black JL. Mast cell tryptase potentiates histamine-induced contraction in human sensitized bronchus. Eur. Respir. J. 1997;10:38–43. doi: 10.1183/09031936.97.10010038. [DOI] [PubMed] [Google Scholar]