Abstract
Yunnan Baiyao (YNBY) is widely used to treat rhexis haemorrhage and ulcer in China. This meta-analysis was conducted to determine the efficacy of YNBY on local haemostasis and antiulcer. Randomized controlled trials were included on condition that assessing the effects of YNBY with/without routine drugs versus the same routine drugs on haemorrhage or ulcer after searching major databases. Data were validated, extracted and synthesized using relative risk (RR) for dichotomous data using random effects models. Fifty-five studies involving 5,150 patients were identified. (1) YNBY alone for haemorrhage (RR = 1.16; 95% CI 1.06 to 1.28) (2) YNBY alone for antiulcer (RR = 1.26; 95% CI 1.03 to 1.53). We found certain effects on ulcerative colitis (RR = 1.22) and skin ulcer (RR = 1.20) in subgroup analysis. (3) YNBY plus routine haemostatic drugs for haemorrhage (RR = 1.23; 95% CI 1.17 to 1.29) with a significant funnel plot asymmetry (Begg’s test, p = 0). (4) YNBY plus routine antiulcer drugs for antiulcer (RR = 1.18; 95% CI 1.05 to 1.33). Treatment effect in the 2nd and 4th group was unstable when RCTs at high risk of bias were excluded. Great heterogeneities and possible publication bias were found among the trials which preclude certain conclusions. The existing data showed that YNBY alone was helpful in treating uterine haemorrhage, ulcerative colitis and skin ulcer. YNBY plus routine antiulcer drugs was more effective in treating ulcerative colitis versus antiulcer drugs alone.
Keywords: Yunnan Baiyao, haemorrhage, ulcer, meta-analysis
Introduction
Haemorrhage is an undesirable event occurring after trauma, surgery or ulcer [1-3]. Current haemostatic drugs for rhexis haemorrhage include antifibrinolytic amino acids (ε-aminocaproic acid and tranexamic acid), aprotinin, desmopressin, and conjugated estrogens etc [4]. Specifically, antifibrinolytics such as ε-aminocaproic acid, tranexamic acid and aprotinin are widely used for rhexis haemorrhage like gynaecological, upper gastrointestinal, urinary tract and oral bleeding but cannot be used for bleeding with a tendency of thrombosis or kidney malfunction [1,2,4-6]. Desmopressin acts through von Willebrand factor and is used for bleeding in patients with coagulation disorders and bleeding caused by trauma or surgery [7,8]. Orally administration of conjugated estrogens is used for gynaecological bleeding, gastrointestinal bleeding and surgical bleeding. It might cause urinary tract bleeding and cannot be used for undiagnosed abnormal genital bleeding [9,10]. For treating upper gastrointestinal ulcer, proton pump inhibitors and histamine H2-receptor antagonists have been widely used. Non-steroidal anti-inflammatory drug (mesalazine) is commonly used for ulcerative colitis. Honey and Vaseline have been typically used for oral, skin and venous ulcer [11-16]. Yunnan Baiyao (YNBY) is a secret herbal medicinal formula developed in 1902 by Qu Huangzhang. According to publications [17-21] and information from the U.S. Food and Drug Administration, (http://www.fda.gov/ohrms/dockets/dailys/02/Sep02/091102/97s-0163-let0634-vol18.pdf), YNBY comprises 40.7% Panax notoginseng (San Qi), 17.3% Saussureae Radix, 13.2% Discorea opposita Thumb, 10.2% Discorea nipponica Makino, 7.3% Erodium stephanianum Willd. Geranium wilfordii Maxim, 6.2% Alpinia officinarum Hance, and 5.1% Bos taurus domesticus, Gmelin Bubalus bubalis L. Yunnan Baiyao has several forms of preparation (powder, capsule, aerosol and tincture) for local application and taking orally. The dosage limitation was 0.25 g to 4 g per day for both local application and taking orally. According to the Traditional Chinese Medicine theory, YNBY can either inhibit bleeding (removing excessive hot blood) or promote normal blood flow in the vessels (removing excessive stagnant dry blood), thereby the use of it can stop bleeding and disperse blood stasis despite the largely unknown exact mechanisms. It has also been shown to regulate immune function and anti-inflammation [19].
YNBY has been widely used by Traditional Chinese Medicine (TCM) practitioners to stop bleeding caused by traumatic injury and surgery, haemoptysis, hematochezia, hemorrhoid haemorrhage, metrorrhagia, metrostaxis and ulcer (ulcerative colitis, peptic ulcer, oral ulcer and skin ulcer) [19,20,22-25] based on the traditional wise in TCM that both ulcer and bleeding share a similar mechanism of abnormal microcirculation with excessive “hot blood (bleeding)” and “dry blood (stasis)” due to hot and cold evil [26].
YNBY can be used in monotherapy or combined with other haemostatic drugs or antiulcer agents. The number of randomized controlled trials (RCTs) which assessed YNBY is continually increasing. It is necessary to assess the effect of YNBY on specific disease. The objective of current systematic review and meta-analysis is to determine the benefits and harms of YNBY has the efficacy on haemorrhage and ulcer and to determine the most suitable indications for YNBY by subgroup analysis.
Material and methods
Data sources and search strategies
We searched (without languages, countries and publication status restrictions) the electronic libraries including PubMed, Embase, CINAHL, Cochrane Library (Issue 6, 2012), CNKI (China National Knowledge Infrastructure), VIP and Sinomed using the terms “Yunnan Baiyao”, “Yunnan Bai Yao”, “Yunnan Paiyao”, “clinical trial”, “clinical observation” as free text terms and “haemorrhage”, “ulcer” as medical subject headings. All databases were searched from inception to July 2012. In addition, we conducted a recursive manual search of reference lists of all identified articles, narrative reviews, and recently published editorials. We also contacted experts for unpublished trials and authors of included primary trials to obtain additional data when needed.
Study selection
The eligibility of included trials was assessed by two reviewers (XZQ and ZH) independently. Disagreement was resolved by discussion including the third person (YB). For inclusion into the meta-analysis, studies had to conform to all the following criteria: 1) trial: randomized controlled trials (RCTs); 2) patients: haemorrhage or ulcer with clear definitions; 3) interventions: YNBY versus routine drugs for haemorrhage and ulcer or YNBY plus drug treatments versus the same drug treatments alone; 4) main outcome: dichotomous or ordinal data assessing the overall responses to interventions. Research articles were excluded if they met one the following criteria: 1) trial: preventive RCTs; 2) patients with both haemorrhage and ulcer; 3) drug: YNBY not from the only legitimate enterprise (Yunnan Baiyao Group Co., Ltd.); 4) interventions: YNBY plus drug treatments versus the third drug treatments; 5) outcome: haemorrhage and ulcer improvement with no clear definitions; 6) data: incomplete data and further information could not be obtained from the original author; 7) duplicated publications.
Outcome assessment
The outcomes assessed were the efficacy of YNBY compared with other haemostatic or antiulcer agents in terms of response to therapy. Dichotomous data included “no improvement” and “improvement”. If ordinal data were given to define the degree of improvement, they were transformed into dichotomous data as “improvement” by combining all the number of patients that shows different degrees of improvement (e.g. in one study investigating the role of Yunnan Baiyao on uterine haemorrhage, the outcome was defined as follows: 2, full recovery, bleeding was stopped within 7 days of treatment; 1, partial improvement, bleeding was stopped between 7 and 14 days after treatment; 0, bleeding continued after 14 days’ treatment or uterine curettage was needed to stop bleeding. The number of patients with a score of 2 or 1 was added together and rescored as improvement.).
Data extraction and quality assessment
All data were extracted independently by two reviewers (XZQ and YB) onto a Microsoft Excel spread sheet (Microsoft Office Excel 2007; Microsoft Corp, Redmond, Washington, USA) as dichotomous outcomes (response or no response to therapy). In addition, the following data were extracted for each trial, where available: authors, year of publication, geographical location of the study, study population, gender, sample size, interventions, dropouts, adverse events, and intention to treat analysis. We also consulted authors of the original studies to get more information if any problem occurred.
The qualities of the included studies were assessed by two reviewers (XZQ and YB) independently using the CONSORT statement [27,28]. For each item of the following: 2-detailed mentioned, 1-simply mentioned, 0-not mentioned. Studies with score > 13 were arbitrarily defined as at low risk of bias, while those with score ≤ 13 were at high risk of bias.
Data synthesis and analysis
Included studies were categorized according to the type of treatment interventions, type of symptoms (haemorrhage or ulcer), and locations of disease (nose, oral cavity, skin, upper digestive tract, lower digestive tract, uterus). Meta-analysis was carried out using Review Manager Software 5.0 (2009, Cochrane Collaboration and Updated Software). We calculated Risk Ratio (RR) and corresponding 95% confidence intervals (CIs) from dichotomous variables using a Mantel-Haenszel random effects model to produce wider confidence intervals and more conservative estimates [29]. We assessed the possibility of publication bias both visually by evaluating a funnel plot (using Review Manager 5.0) for asymmetry which could result from the non publication of trials with negative results and formally with Begg’s test (using Stata 10.0, StataCorp, College Station, Texas, USA) [30,31]. We reanalysed the data excluding studies at high risk of bias to test how robust the results of our review were.
Results
Study selection
As was shown in Figure 1, our search initially generated 244 citations. Of these, 58 potential duplications were found and discarded. 109 studies were discarded because of not meeting the inclusion criteria after reviewing the abstracts. We obtained 77 papers for detail evaluation. After consulting the author, 2 studies were abandoned. 55 of them met the eligibility criteria and were included in this meta-analysis. No additional study was identified by checking the references of relevant papers, and no unpublished relevant studies were obtained.
Figure 1.
Search flow diagram for studies included in the meta-analysis RCT, randomized controlled trial; YNBY, Yunnan Baiyao.
Study characteristics and quality assessment
All included 55 studies were single centre RCT conducted in China, involving 5,150 patients (Tables 2 and 3). There were 41 studies assessing efficacy of YNBY on haemorrhage, of the total, 2 studies on epistaxis, 29 studies on upper gastrointestinal haemorrhage (10 trials involving 667 patients assessed on neonatal upper gastrointestinal haemorrhage), 9 studies on uterine haemorrhage and 1 study on lower gastrointestinal haemorrhage; whilst 14 studies assessing efficacy on ulcer, among which, 2 studies on oral ulcer, 2 studies on skin ulcer, 5 studies on ulcerative colitis and 5 studies on peptic ulcer. In one trial of 150 patients assigned to 3 groups, we chose the YNBY group as experimental group and the group of cimetidine as control group, as this intervention was commonly used in other included trials. The scores of quality assessment ranged from 5 to 24 (Table 4). The mean score was 14.29 with a standard deviation of 3.38. 32 studies were at low risk of bias, and 23 studies were at high risk of bias.
Table 2.
Characteristics of included RCTs (Part A)
| Study | Country of origin | Criteria used to define ulcer | Symptom/region | Aetiology | Number of centers | Age range/mean age | Number of participants (female) | Criteria used to define no response |
|---|---|---|---|---|---|---|---|---|
| Yang Y 2007 | China | - | Hemorrhage/Nose | Except hematologic disease and systemic disease | 1 site | 6-72/30 | 76 (36) | Still bleeding, Mucous membrane erosion |
| Chen XM 2010 | China | - | Hemorrhage/Upper digestive tract | Asphyxia neonatorum, pneumonia of newborn, anoxic ischemic encephalopathy, intracranial hemorrhages and septicemia | 1 site | < 24 h 23, 1-3 d 31, > 3 d 6/mean age not mentioned | experimental group: 30 (12); control group: 30 (17) | Hematemesis aggravated or not improved after 48 h |
| Chu XF 2011 | China | - | Hemorrhage/Upper digestive tract | Duodenal ampulla ulcer, stomach ulcer, portal hypertension, stomach neoplasms, acute gastric mucosal lesion, Mallory-Weiss syndrome | 1 site | experimental group: 43 ± 8 (mean age); control group: 44 ± 8 (mean age) | experimental group: 30 (7); control group: 40 (19) | Still bleeding after 48 h |
| Li L 2009 | China | - | Hemorrhage/Upper digestive tract | Asphyxia neonatorum, meconium aspiration syndrome, intracranial hemorrhages, scleredema and neonatal septicemia combined with stress ulcer | 1 site | experimental group: 1-7 d/4.6 d; control group: 1-7 d/4.8 d | experimental group: 50 (19); control group: 50 (20) | Still bleeding after 48 h |
| Yang QH 2001 | China | - | Hemorrhage/Upper digestive tract | Gastric varices, gastric ulcer, acute gastric mucosal lesion, duodenal ulcer, stress ulcer, subtotal gastrectomy, gastric cancer, leiomyoma of stomach | 1 site | experimental group: 16-78/49.5; control group: 17-76 d/50.5 | experimental group: 55 (14); control group: 54 (15) | Still bleeding after 72 h |
| Chen GH 2006 | China | - | Hemorrhage/Uterus | Physiotherapy (microwave) | 1 site | experimental group: 20-43/30 ± 2.7; control group: 22-41/31 ± 2.5 | experimental group: 30; control group: 24 | Still bleeding after treatment |
| Qu J 2003 | China | - | Hemorrhage/Uterus | Drug abortion | 1 site | Not mentioned | experimental group: 100; control group: 100 | Still bleeding after 14 d or need curettage |
| Wang GR 2010 | China | - | Hemorrhage/Uterus | Physiotherapy (microwave) | 1 site | experimental group: 23-51/37.2 ± 4.78; control group: 24-52/36.7 ± 5.31 | experimental group: 38; control group: 38 | Still bleeding after treatment |
| Chen DH 2011 | China | Meet the diagnostic criteria published by Chinese Medical Association in 2000 | Ulcer/Colon | Ulcerative colitis | 1 site | experimental group: 16-61/41.3; control group: 14-63/44.2 | experimental group: 36 (10); control group: 36 (8) | After the treatment, the clinical symptoms and the auxiliary examination results not improved |
| Deng WM 2010 | China | - | Ulcer/Colon | Chronic ulcerative colitis | 1 site | 19-74/mean age not mentioned | experimental group: 30 (12); control group: 30 (14) | The symptom not improved compared with pretherapy |
| Li HG 2000 | China | - | Ulcer/Stomach and duodenum | Helicobacter pylori | 1 site | experimental group: 20-65/41; control group: 22-55/41.5 | experimental group: 44 (11); control group: 38 (9) | Ulcers not at healing stage or scarring stage after 1 month after treatment |
| Zhou Y 2001 | China | Diagnosed by gastroscope, diameter of the ulcer: 5-20 mm | Ulcer/Stomach and duodenum | Helicobacter pylori | 4 site | experimental group: 21-71/57.4; control group: 17-76/49.8 | experimental group: 147 (52); control group: 45 (18) | Size of ulcer region decreased by less than 50% |
| Yang H 1996 | China | - | Ulcer/Skin | - | 1 site | not mentioned | experimental group: 30; control group: 30 | Size of ulcer region not decreased |
| Yang SJ 2008 | China | - | Ulcer/Skin | Compression | 1 site | 40-92/mean age not mentioned | experimental group: 30; control group: 30 | Ulcer not healing and still has exudate |
| Gong XH 2005 | China | - | Ulcer/Oral cavity | - | 1 site | 15-76/mean age not mentioned | experimental group: 104; control group: 60 | Ulcer not healing after 6 d |
| Tian S 2010 | China | - | Hemorrhage/Nose | - | 1 site | 17-76/mean age not mentioned | 60 (26) | Still bleeding after treatment |
| Bian HZ 2008 | China | - | Hemorrhage/Upper digestive tract | Duodenal ulcer, gastric ulcer, acute gastric mucosal lesion | 1 site | experimental group: 22-78/mean age not mentioned; control group: 24-82/mean age not mentioned | experimental group: 100 (37); control group: 90 (35) | Still bleeding after 7 d |
| Chen YP 2003 | China | - | Hemorrhage/Upper digestive tract | Hypoxic ischemic encephalopathy, amnionic fluid aspiration syndrome, scleredema | 1 site | 10h-16 d/mean age not mentioned | experimental group: 18 (8); control group: 18 | Still bleeding after 48 h |
| Chen YP 2008 | China | - | Hemorrhage/Upper digestive tract | Any factors avoid hematopoietic system disease, swallowed blood syndrome, and coagulation disorder | 1 site | 10h-16 d/mean age not mentioned | experimental group: 46; control group: 44 | Still hematemesis after treatment |
| Chu AL 2010 | China | - | Hemorrhage/Upper digestive tract | Hypoxic ischemic encephalopathy, intracranial hemorrhage, purulent meningitis, septicemia | 1 site | experimental group: < 37 weeks 4, 37-40 weeks 28, > 40 weeks 8; control group: < 37 weeks 2, 37-40 weeks 28, > 40 weeks 6 | experimental group: 40 (8); control group: 36 (8) | Still bleeding after 72 h |
| Dong XF 2009 | China | - | Hemorrhage/Upper digestive tract | Craniocerebral trauma combined with stress ulcer | 1 site | experimental group: 5-75/41; control group: 4-76/42 | experimental group: 60 (22); control group: 58 (25) | Still bleeding after 72 h |
| Dong XJ 2004 | China | - | Hemorrhage/Upper digestive tract | Gastric ulcer, duodenal ulcer, complex ulcer, acute gastric mucosal lesion | 1 site | experimental group: 21-69/39.6; control group: 19-70/38.5 | experimental group: 33 (6); control group: 32 (4) | Still bleeding after 72 h |
| Fan J 2011 | China | - | Hemorrhage/Upper digestive tract | Cerebral hemorrhage account for stress ulcer | 1 site | experimental group: 42.5-78.6; control group: 44.3-80.1 | experimental group: 53 (25); control group: 53 (24) | Still bleeding after 1 w and need other drugs or surgery |
| Ge J 1998 | China | - | Hemorrhage/Upper digestive tract | Gastric ulcer, duodenal ulcer, gastric carcinoma, acute gastric mucosal lesion | 1 site | experimental group: 18-79; control group: 21-65 | experimental group: 86 (18); control group: 36 (9) | Still have dark stools or hematemesis or still bleeding certified by gastroscope after 5 days |
| Ge SG 2001 | China | - | Hemorrhage/Upper digestive tract | Duodenal ulcer, gastric ulcer | 1 site | age range not mentioned/47.8 | 72 (14) | Still bleeding after 72 h |
| He SQ 2011 | China | - | Hemorrhage/Upper digestive tract | Craniocerebral injury (GCS < 8) | 1 site | experimental group: 5-75/41; control group: 4-76/42 | experimental group: 30 (9); control group: 29 (8) | Still bleeding after 72 h |
| Huang HY 2008 | China | - | Hemorrhage/Upper digestive tract | Portal hypertension | 1 site | experimental group: 19-60/46; control group: 19-60/44 | experimental group: 45 (10); control group: 42 (10) | Still bleeding after 72 h |
| Li M 2001 | China | - | Hemorrhage/Upper digestive tract | Asphyxia neonatorum, anoxic ischemic encephalopathy, intracranial hemorrhages and meconium aspiration syndrome | 1 site | experimental group: 1-3 days 28, 4-6 days 6, > 7 days 3; control group: 1-3 days 26, 4-6 days 5, > 7 days 4 | experimental group: 37 (13); control group: 35 (12) | Still bleeding after 48 h |
| Li ML 2010 | China | - | Hemorrhage/Upper digestive tract | Peptic ulcer | 1 site | experimental group: 20-65/42.5 ± 15.5; control group: 18-66/41.5 ± 16.0 | experimental group: 60 (24); control group: 55 (22) | Still bleeding after 72 h |
| Li QM 2007 | China | - | Hemorrhage/Upper digestive tract | Portal hypertension | 1 site | experimental group: 60-80/74.5 ± 7.9; control group: 60-80/73.2 ± 7.1 | experimental group: 43 (13); control group: 40 (12) | Still bleeding after 72 h |
| Li YM 2009 | China | - | Hemorrhage/Upper digestive tract | Gastric ulcer, duodenal ulcer, complex ulcer | 1 site | experimental group: 28-71; control group: 25-69 | experimental group: 40 (5); control group: 40 (6) | Still have dark stools or hematemesis after 5 days, symptom was not improved, or FOB positive |
| Luo Y 2012 | China | - | Hemorrhage/Upper digestive tract | Hypoxic ischemic encephalopathy, scleredema neonatorum, septicemia | 1 site | experimental group: 1-3 days 33, 4-6 days 7, ≥ 7 days 2; control group: 1-3 days 29, 4-6 days 9, ≥ 7 days 3 | experimental group: 42 (22); control group: 41 (23) | Still bleeding after 48 h |
| Pang Y 2004 | China | - | Hemorrhage/Upper digestive tract | Craniocerebral trauma | 1 site | 21-78/38.8 | 98 (24) | Still bleeding after 72 h |
| Shi SY 2011 | China | - | Hemorrhage/Upper digestive tract | Portal hypertension, peptic ulcer, gastric mucosal lesion, stomach neoplasms | 1 site | experimental group: 43-67; control group: 42-65 | experimental group: 30 (13); control group: 30 (16) | Still bleeding after 72 h |
| Sun YM 2003 | China | - | Hemorrhage/Upper digestive tract | Cerebral infarction or cerebral hemorrhage account for stress ulcer | 1 site | experimental group: 40-78/56; control group: 37-80/58 | experimental group: 55 (24); control group: 34 (13) | Still bleeding after 72 h |
| Wang CJ 2009 | China | - | Hemorrhage/Upper digestive tract | Asphyxia neonatorum, pneumonia of newborn, anoxic ischemic encephalopathy, intracranial hemorrhages, cold lesion syndrome, feeding intolerance and meconium aspiration syndrome | 1 site | 40 min-7 d | experimental group: 25; control group: 25 | Still have melena after 48 h |
| Wang WD 1996 | China | - | Hemorrhage/Upper digestive tract | Peptic ulcer, gastric mucosal lesion, stomach neoplasms | 1 site | experimental group: 20-72/37.14 ± 16.65; control group: 22-68/35.25 ± 14.51 | experimental group: 114 (21); control group: 42 (11) | Still bleeding after 5 d |
| Zhang XR 2005 | China | - | Hemorrhage/Upper digestive tract | Asphyxia neonatorum, pneumonia of newborn, anoxic ischemic encephalopathy and septicemia account for stress ulcer | 1 site | experimental group: 1-3 days 25, 3-6 days 4, ≥ 7 days 3; control group: 1-3 days 23, 3-6 days 4, ≥ 7 days 3 | experimental group: 32 (12); control group: 30 (10) | Still bleeding after 48 h |
| Zhong LY 2012 | China | - | Hemorrhage/Upper digestive tract | Peptic ulcer | 1 site | experimental group: 51 (median age); control group: 50 (median age) | experimental group: 45 (22); control group: 40 (19) | Still bleeding after 72 h or rebleeding and need other treatment |
| Zhou Y 2006 | China | - | Hemorrhage/Upper digestive tract | Duodenal ulcer, gastric ulcer, complex ulcer, marginal ulcer | 1 site | experimental group: 15-60/mean age not mentioned; control group: 18-62/mean age not mentioned | experimental group: 54 (6); control group: 35 (5) | Still bleeding after 5 d |
| Zhuang DY 2000 | China | - | Hemorrhage/Upper digestive tract | Hypoxic ischemic encephalopathy, intracranial hemorrhage, purulent meningitis, septicemia | 1 site | experimental group: 32-37 weeks 8, 38-40 weeks 10, > 40 weeks 2; control group: 32-37 weeks 10, 38-40 weeks 7, > 40 weeks 1 | experimental group: 20 (7); control group: 18 (8) | Still bleeding after 72 h |
| Zhang CX 2005 | China | - | Hemorrhage/Lower digestive tract | Colonic polyps, rectal polypus, rectal neoplasms, colon neoplasms, vascular malformation, ulcerative colitis, Crohn disease, colon diverticulum | 1 site | experimental group: 11-76/57 ± 12; control group: 15-78/54 ± 10 | experimental group: 55 (19); control group: 55 (21) | Still bleeding after 72 h |
| Huang LR 2009 | China | - | Hemorrhage/Uterus | Physiotherapy (microwave) | 1 site | experimental group: 26-57/34 ± 6.21; control group: 25-53/33 ± 7.28 | experimental group: 45; control group: 35 | No improvement after treatment |
| Li L 2007 | China | - | Hemorrhage/Uterus | Loop electrosurgical excision procedure | 1 site | experimental group: 21-44/32 ± 6.33; control group: 22-47/33 ± 8.37 | experimental group: 32; control group: 25 | The decrease of volume of bleeding was less than 50% |
| Zhang M 2009 | China | - | Hemorrhage/Uterus | Physiotherapy (microwave) | 1 site | 19-45 | experimental group: 40; control group: 28 | No improvement after treatment |
| Zhao X 2008 | China | - | Hemorrhage/Uterus | Physiotherapy (microwave) | 1 site | 19-43 | experimental group: 80 (80); control group: 32 (32) | Still bleeding after treatment |
| Zhu SZ 2004 | China | - | Hemorrhage/Uterus | Drug abortion | 1 site | 17-35/mean age not mentioned | experimental group: 194; control group: 194 | The volume of bleeding was more than the menstrual blood volume |
| Zhu XP 2010 | China | - | Hemorrhage/Uterus | Physiotherapy (microwave) | 1 site | experimental group: 22-45/32.5; control group: 21-48/34.5 | experimental group: 27 (27); control group: 28 (28) | Size of ulcer region not decreased |
| Huang ZX 2008 | China | - | Ulcer/Stomach and duodenum | Peptic ulcer | 1 site | Not mentioned | experimental group: 80; control group: 60 | The symptom not improved compared with pretherapy and size of ulcer region not decreased |
| Liu M 2010 | China | Diagnosed by gastroscope according to the criteria published by the Ministry of Health of the People’s Republic of China | Ulcer/Stomach and duodenum | Peptic ulcer | 1 site | experimental group: 19-75/36.6; control group: 18-73/34.4 | experimental group: 55 (19); control group: 55 (17) | Size of ulcer region decreased by less than 50% |
| Xu XZ 1994 | China | - | Ulcer/Stomach and duodenum | Peptic ulcer | 1 site | experimental group: 18-76/39.5 ± 13.6; control group: 19-65/34.9 ± 11.5 | experimental group: 69 (25); control group: 69 (27) | Size of ulcer region decreased by less than 50% |
| Chen X 2009 | China | Meet the diagnostic criteria published by Chinese Medical Association in 2001 | Ulcer/Colon | Ulcerative colitis | 1 site | 21-73/38.4 ± 11.0 | totle: 134 (35); experimental group: 69; control group: 65 | After the treatment, the clinical symptoms and the examination results not improved |
| Du KT 2011 | China | Meet the diagnostic criteria published by Chinese Medical Association in 2000 | Ulcer/Colon | Ulcerative colitis | 1 site | 20-57/37.5 | totle: 60 (27); experimental group: 30; control group: 30 | After the treatment, the clinical symptoms and the auxiliary examination results not improved |
| Zhang LL 2010 | China | Meet the diagnostic criteria published by Chinese Medical Association in 2000 | Ulcer/Colon | Ulcerative colitis | 1 site | experimental group: 21-60/37.5 ± 12.48; control group: 20-58/36.0 ± 11.29 | experimental group: 30 (5); control group: 30 (6) | After the treatment, the clinical symptoms and the auxiliary examination results not improved |
| Zhao GM 2011 | China | - | Ulcer/Oral cavity | - | 1 site | 18-60/28.5 | totle: 64 (10); experimental group: 32; control group: 32 | After the treatment, the clinical symptoms not improved |
Table 3.
Characteristics of included RCTs (Part B)
| Study | Intervention in experimental group | Intervention in control group | Duration of therapy | Mean volume (or time) of bleeding (or ulcer) | Methodology | Adverse events |
|---|---|---|---|---|---|---|
| Yang Y 2007 | Attach cotton ball with Yunnan Baiyao to the bleeding region, and Yunnan Baiyao powder 0.5 g t.i.d. through oral administration | Attach cotton ball with Erythromycin Eye Ointment to the bleeding region, and carbazochrome through oral administration | 1 week | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Chen XM 2010 | Cure the primary disease, fasting, anti-infection, nutritional support, blood transfusion if needed, body weight < 1200 g 0.15 g t.i.d., body weight ≥ 1200 g 0.25 g t.i.d. administrated through oral if possible, or through nasal feeding | Cure the primary disease, fasting, anti-infection, nutritional support, blood transfusion if needed, tagamet 0.1 g did through gastric canal, ethamsylate 125 mg i.v. | 2 days | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Chu XF 2011 | Cimetidine, nutritional support, blood transfusion if needed, Yunnan Baiyao 4 g with physiological saline 200 ml p.o. or through gastric canal | Cimetidine, nutritional support, blood transfusion if needed, lyophilizing thrombin powder 1-2 KU p.o. or through gastric canal | 3 days | - | Concealment of allocation and blinding unclear, randomized | 0 in experimental group; 0 in control group |
| Li L 2009 | Cure the primary disease, fasting, blood transfusion if needed, gastric lavage, ethamsylate, vit K1 i.v., Yunnan Baiyao 0.25 g and physiological saline 10 mg t.i.d. through nasal feeding | Cure the primary disease, fasting, blood transfusion if needed, gastric lavage, ethamsylate, vit K1 i.v., cimetidine 0.1 g and physiological saline 10 mg t.i.d. through nasal feeding | ≥ 2 days | - | Concealment of allocation and blinding unclear, randomized by drawing lots | Not mentioned |
| Yang QH 2001 | Cure the primary disease, blood transfusion, Yunnan Baiyao 1 g the first time, then 0.5 g t.i.d., after the fecal occult blood turns negative, 0.25 g t.i.d. for 3 days | Cure the primary disease, blood transfusion, famotidine 20 mg i.v. d.i.d. | 3 days | experimental group: 2.0 ± 0.5 d; control group: 3.0 ± 0.5 d | Concealment of allocation and blinding unclear, randomized | 0 in experimental group; 4 in control group |
| Chen GH 2006 | Press the cotton ball with 3% iodine tincture to the bleeding region for 2-3 min, sterilize with 75% alcohol around the bleeding region, then attach sterile gauze with Yunnan Baiyao 1 g to the bleeding region | Press the cotton ball with 3% iodine tincture to the bleeding region for 2-3 min, sterilize with 75% alcohol around the bleeding region, then attach the gelfoam and 1-2 sterile gauze to the bleeding region, and take the sterile gauze out after 24 h | Not mentioned | - | Randomized by treatment number | Not mentioned |
| Qu J 2003 | The first two days Mifepristone 50 mg at 8:00 and 25 mg at 20:00 through oral administration; the third day Misoprostol 600 ug at 8:00. After the gestational sac eliminated, Yunnan Baiyao 2 capsules t.i.d. through oral administration. | The first two days Mifepristone 50 mg at 8:00 and 25 mg at 20:00 through oral administration; the third day Misoprostol 600 ug at 8:00. After the gestational sac eliminated, vit K4 8 mg t.i.d. through oral administration. | 9 days | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Wang GR 2010 | Attach sterile gauze with Yunnan Baiyao 3-5 g to the bleeding region and change dressings every other day | Attach cotton ball with iodophors to the bleeding region and change dressings every other day | Not mentioned | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Chen DH 2011 | Yunnan Baiyao 1 g with 0.9% physiological saline 100 ml by coloclysis | Sulfasalazine 2 g with hydrocortisone 100 mg by coloclysis s.i.d. | 30 days | - | Concealment of allocation and blinding unclear, randomized | 0 in experimental group; nausea and vomiting 5, flushing and tachycardia; 3 in control group |
| Deng WM 2010 | To make enema with Yunnan Baiyao 4 g and administer 80-150 ml before sleep after defaecation every night 70-80 drop/min, the enema should be reserved for 4 h | To make enema with physiological saline 150 ml and gentamicin 160000 u and administer 80-150 ml before sleep after defaecation every night 70-80 drop/min, the enema should be reserved for 4 h | mild case: 15 days; severe case: 30 days | - | Concealment of allocation and blinding unclear, randomized | 3 in experimental group; 3 in control group |
| Li HG 2000 | Yunnan Baiyao 1 g, Amoxicillin 0.5 g, metronidazole 0.2 g q.i.d. for 14 d | Bismuth Potassium Citrate Tablets 0.12 g, Amoxicillin 0.5 g, metronidazole 0.2 g q.i.d. for 14 d | 2 weeks | - | Concealment of allocation and blinding unclear, randomized | 2 in experimental group; 5 in control group |
| Zhou Y 2001 | Yunnan Baiyao 500 mg q.i.d., ranitidine 150 mg b.i.d. through oral administration | Sucralfate 500 mg q.i.d., ranitidine 150 mg b.i.d. through oral administration | 4 weeks | - | Concealment of allocation and blinding unclear, randomized | 0 in experimental group; 0 in control group |
| Yang H 1996 | Attach sterile gauze with Yunnan Baiyao to the ulcer region and change dressings every other day | Attach sterile gauze with vaseline to the ulcer region and change dressings every other day | 20 days | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Yang SJ 2008 | Clean the ulcer surface with physiological saline, attach the Yunnan Baiyao powder to the ulcer and the thickness of the drug should be 1-2 mm t.i.d. for 2 weeks | Clean the ulcer surface with physiological saline, attach sterile gauze with vaseline to the ulcer region and change dressings every other day for 2 weeks | 2 weeks | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Gong XH 2005 | Attach cotton ball with Yunnan Baiyao powder to the ulcer region for 30 min b.i.d. | 3% hydrogen peroxide solution and physiological saline douche the ulcer region then attach the cotton ball with iodine glycerol to the ulcer region | 1 week | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Tian S 2010 | Microwave 30-40 W, antibiotics, hemostatics, Yunnan Baiyao 0.5 g q.i.d. for 1 week | Microwave 30-40 W, antibiotics, hemostatics | 1 week | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Bian HZ 2008 | Bed rest, blood transfusion, inhibit gastric acid and protect gastric mucosa, hemostatics, norepinephrine or thrombin through oral administration and fasting if severe, Yunnan Baiyao 4 g and physiological saline 60 ml 4-6 times a day | Bed rest, blood transfusion, inhibit gastric acid and protect gastric mucosa, hemostatics, norepinephrine or thrombin through oral administration and fasting if severe | 1 week | - | Concealment of allocation and blinding unclear, randomized | 0 in experimental group; 0 in control group |
| Chen YP 2003 | Fasting, gastrointestinal decompression, cimetidine i.v. Yunnan Baiyao: body weight < 2500 g 0.15 g t.i.d. body weight ≥ 2500 g 0.25 g t.i.d. through oral administration or nasal feeding if necessary, blood transfusion if necessary | Fasting, gastrointestinal decompression, cimetidine i.v., blood transfusion if necessary | ≥ 2 days | - | Concealment of allocation and blinding unclear, randomized | 0 in experimental group; 0 in control group |
| Chen YP 2008 | Fasting, gastrointestinal decompression, cimetidine i.v. Yunnan Baiyao: body weight < 2500 g 0.15 g t.i.d. body weight ≥ 2500 g 0.25 g t.i.d. through oral administration or nasal feeding if necessary | Fasting, gastrointestinal decompression, cimetidine i.v. | 2 days | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Chu AL 2010 | Fluid replacement, plasma transfusion, dicynone, vitamin K1, cimetidine, wash the stomach by norepinephrine 2 mg and physiological saline 100 ml, give 5% Yunnan Baiyao suspension 1-2 ml through stomach tube t.i.d. or q.i.d. | Fluid replacement, plasma transfusion, dicynone, vitamin K1, cimetidine, wash the stomach by norepinephrine 2 mg and physiological saline 100 ml | 3 days | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Dong XF 2009 | Cure the primary disease, omeprazole 40 mg bid i.v., Yunnan Baiyao 4 capsules qid through gastric canal | Cure the primary disease, omeprazole 40 mg bid i.v. | 3 days | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Dong XJ 2004 | Blood transfusion, fluid replacement, omeprazole 80 mg i.v. the first time then 40 mg s.i.d. i.v. for 3-5 d, batroxobin 1 KU b.i.d. i.v. for 3-5 d, Yunnan Baiyao 0.75 g p.o. 4-6 times per day | Blood transfusion, fluid replacement, omeprazole 80 mg i.v. the first time then 40 mg s.i.d. i.v. for 3-5 d, batroxobin 1 KU b.i.d. i.v. for 3-5 d | 5 days | - | Concealment of allocation and blinding unclear, randomized | 0 in experimental group; 0 in control group |
| Fan J 2011 | Cure the primary disease, pantoprazole 40 mg i.v. s.i.d., Yunnan Baiyao 0.5 g t.i.d. through nasal feeding | Cure the primary disease, pantoprazole 40 mg i.v. s.i.d. | 2 weeks | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Ge J 1998 | PPI, H2 receptor blocking pharmacon, ethamsylate, carbenzamine, Yunnan Baiyao powder 4 g with physiological saline 30 ml every 4 hours | PPI, H2 receptor blocking pharmacon, ethamsylate, carbenzamine | 5 days | - | Concealment of allocation and blinding unclear, randomized | 0 in experimental group; 0 in control group |
| Ge SG 2001 | Fluid replacement, blood transfusion, cimetidine 1 g/d i.v., wash the stomach by norepinephrine 8 mg/dl and physiological saline 100 ml every hour for 4-6 h, Yunnan Baiyao 4 g/d for 2-3 d | Fluid replacement, blood transfusion, cimetidine 1 g/d i.v., wash the stomach by norepinephrine 8 mg/dl and physiological saline 100 ml every hour for 4-6 h | 3 days | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| He SQ 2011 | Cure the primary disease, omeprazole 40 mg b.i.d. i.v. for 4 d, Yunnan Baiyao powder 4 g q.i.d. for 4 d through stomach tube | Cure the primary disease, omeprazole 40 mg b.i.d. i.v. for 4 d | 4 days | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Huang HY 2008 | Somatostatin, propranolol dinitrate, protect gastric mucosa, blood transfusion, Yunnan Baiyao 10 pills t.i.d. p.o. | Somatostatin, propranolol dinitrate, protect gastric mucosa, blood transfusion | 3 days | - | Concealment of allocation and blinding unclear, randomized | 5 in experimental group; 5 in control group (these 10 participants can’t be tolerant of propranolol dinitrate) |
| Li M 2001 | Cure the primary disease, fasting, anti-infection, nutritional support, gastrointestinal decompression, blood transfusion, Vit K1 3 mg i.v. for 1-3 d, ethamsylate 62.5-125 mg and carbenzamine 20-30 mg i.v. b.i.d. for 3-5 d, cimetidine 20-30 mg i.v. b.i.d., Yunnan Baiyao 0.06-0.08 g with physiological saline 5 ml through gastric canal for 2 d | Cure the primary disease, fasting, anti-infection, nutritional support, gastrointestinal decompression, blood transfusion, Vit K1 3 mg i.v. for 1-3 d, ethamsylate 62.5-125 mg and carbenzamine 20-30 mg i.v. b.i.d. for 3-5 d, cimetidine 20-30 mg i.v. b.i.d. | 5 days | experimental group: 1.42 ± 0.52 d; control group: 3.21 ± 0.54 d | Concealment of allocation and blinding unclear, randomized | 0 in experimental group; 0 in control group |
| Li ML 2010 | Fluid replacement, anti-infection, blood transfusion if needed, gastrointestinal decompression, general hemostatics, omeprazole 40 mg, physiological saline 100 ml i.v. b.i.d. Yunnan Baiyao powder 0.5 g p.o. or through gastric tube with physiological saline 30 ml t.i.d. | Fluid replacement, anti-infection, blood transfusion if needed, gastrointestinal decompression, general hemostatics, omeprazole 40 mg, physiological saline 100 ml i.v. b.i.d. | ≥ 3 days | experimental group: 2.5 ± 0.8 d; control group: 4.5 ± 1.2 d | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Li QM 2007 | Fasting, fluid replacement, blood transfusion, routine hemostasis, antacid, maintain water-electrolyte balance, nutritional support, cure the primary disease, and Yunnan Baiyao 0.5 g q.i.d. p.o. | Fasting, fluid replacement, blood transfusion, routine hemostasis, antacid, maintain Water-Electrolyte Balance, nutritional support, cure the primary disease | 3 days | experimental group: 18.6 ± 11.4 h; control group: 34.5 ± 16.7 h | Concealment of allocation and blinding unclear, randomized | 0 in experimental group; 0 in control group |
| Li YM 2009 | Bed rest, blood transfusion, fasting for 1-4 days, pantoprazole sodium 40 mg with 0.9% physiological saline 250 ml i.v. b.i.d. for 5 days, Yunnan Baiyao 1 g t.i.d. p.o. for 5 days | Bed rest, blood transfusion, fasting for 1-4 days, pantoprazole sodium 40 mg with 0.9% physiological saline 250 ml i.v. b.i.d. for 5 days | 5 days | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Luo Y 2012 | Cure the primary disease, fasting, Vit K1, etamsylate, cimetidine, gastric lavage and blood transfusion if needed, Yunnan Baiyao 0.083 g q.i.d. through gastric canal or p.o. | Cure the primary disease, fasting, Vit K1, etamsylate, cimetidine, gastric lavage and blood transfusion if needed | 3 days | - | Concealment of allocation and blinding unclear, randomized | 0 in experimental group; 0 in control group |
| Pang Y 2004 | Ranitidine 0.2 g i.v. b.i.d., losec 40 mg i.v. b.i.d., aluminium hydroxide gel 20 mg and Yunnan Baiyao 0.5 g through gastric tube q.i.d., blood transfusion if necessary | Ranitidine 0.2 g i.v. b.i.d., losec 40 mg i.v. b.i.d., aluminium hydroxide gel 20 mg through gastric tube q.i.d., blood transfusion if necessary | ≥ 3 days | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Shi SY 2011 | Bed rest, fasting, nutritional support, routine treatment for the primary disease, blood transfusion if needed, antacid, maintain water-electrolyte balance, Yunnan Baiyao 0.5 g p.o. and thrombin 2000 IU with physiological saline 20 ml every four hours | Bed rest, fasting, nutritional support, routine treatment for the primary disease, blood transfusion if needed, antacid, maintain water-electrolyte balance, thrombin 2000 IU with physiological saline 20 ml every four hours | 3 days | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Sun YM 2003 | Routine treatment for the primary disease, famotidine 20 mg and physiological saline 250 ml i.v. b.i.d. Yunnan Baiyao powder 0.5 g and physiological saline 20 ml through gastric tube q.i.d. for 5 d at most | Routine treatment for the primary disease, cimetidine 400 mg and physiological saline 250 ml i.v. b.i.d. | Drug withdrawal when 3 days after hemostasis | - | Concealment of allocation and blinding unclear, randomized | 0 in experimental group; 0 in control group |
| Wang CJ 2009 | Routine treatment for the primary disease, cimetidine i.v., Vit K1 i.m., gastric lavage with 1.4% sodium bicarbonate or physiological saline, Yunnan Baiyao 0.1 g with warm water 5 ml by nasal feeding | Routine treatment for the primary disease, cimetidine i.v., Vit K1 i.m., gastric lavage with 1.4% sodium bicarbonate or physiological saline | Drug withdrawal after hemostasis | - | Concealment of allocation and blinding unclear, randomized | 1 in experimental group; 8 in control group |
| Wang WD 1996 | Routine treatment for the primary disease including H2-recetor blockers, ethamsylate; Yunnan Baiyao powder 4 g with physiological saline 30 ml every 4 hours a time for 5 days | Routine treatment for the primary disease including H2-recetor blockers, ethamsylate | 5 days | - | Concealment of allocation and blinding unclear, randomized | 0 in experimental group; 0 in control group |
| Zhang XR 2005 | Cure the primary disease, fasting, anti-infection, nutritional support, blood transfusion 10 ml/kg if needed, gastrointestinal decompression, Vit K1 10 mg/d i.v. for 3-5 days, Yunnan Baiyao 50 mg with physiological saline 5 ml through gastric tube | Cure the primary disease, fasting, anti-infection, nutritional support, blood transfusion 10 ml/kg if needed, gastrointestinal decompression, Vit K1 10 mg/d i.v. for 3-5 days | 2 days | experimental group: 1.34 ± 0.64 d; control group: 3.23 ± 0.52 d | Concealment of allocation and blinding unclear, randomized | 0 in experimental group; 0 in control group |
| Zhong LY 2012 | Esomeprazole 40 mg b.i.d. i.v., Yunnan Baiyao powder 4 g with physiological saline 20 ml through gastric canal | Esomeprazole 40 mg b.i.d. i.v. | Not mentioned | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Zhou Y 2006 | Fluid replacement, blood transfusion, fasting, wash the stomach, Losec 40 mg b.i.d. i.v., attach the Yunnan Baiyao 4 g to the bleeding region the first time, then 0.5 g t.i.d. | Fluid replacement, blood transfusion, fasting, wash the stomach, Losec 40 mg b.i.d. i.v. | 5 days | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Zhuang DY 2000 | Cure the primary disease, cimetidine, gastric lavage, Yunnan Baiyao 83 mg through nasal feeding tube t.i.d. | Cure the primary disease, cimetidine, gastric lavage | 3 days | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Zhang CX 2005 | Intravenous nutrition, fasting for 3 days, Yunnan Baiyao 8 g, norepinephrine 12 mg with physiological saline 200 ml by enema every 12 h | Intravenous nutrition, fasting for 3 days, norepinephrine 12 mg with physiological saline 200 ml by enema every 12 h | 3 days | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Huang LR 2009 | Press the cotton ball with iodine tincture and Yunnan Baiyao to the bleeding region for 2-3 min, NSAIDs | Press the cotton ball with iodine tincture to the bleeding region for 2-3 min, NSAIDs | 1 day | - | Concealment of allocation and blinding unclear, randomized | 0 in experimental group; 0 in control group |
| Li L 2007 | Apply Yunnan Baiyao powder 3 g to the bleeding region, norlutin 5 mg every 8-12 h a time p.o., after bleeding stopped, norlutin 3.75 mg till 7 days before the next menstrual onset or 20 days after bleeding stopped | Norlutin 5 mg every 8-12 h a time p.o., after bleeding stopped, norlutin 3.75 mg till 7 days before the next menstrual onset or 20 days after bleeding stopped | 20 days | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Zhang M 2009 | Apply Yunnan Baiyao powder 1 g to the bleeding region s.i.d. for 3 days | Attach sterile gauze with iodophors to the bleeding region s.i.d. for 3 days | 3 days | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Zhao X 2008 | Attach cotton ball with Yunnan Baiyao to the bleeding region and change dressings every day for 7 days, Gong Xuening (Chinese herbal drugs) through oral administration for 7 days | Gong Xuening (Chinese herbal drugs) through oral administration for 7 days | 7 days | experimental group: 8.13 ± 2.41 d; control group: 10.31 ± 3.12 d | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Zhu SZ 2004 | Mifepristone, misoprostol, in 24 h after misoprostol administrated leonurus japonicus houtt. through oral administration, then after misoprostol administration 72 h give Yunnan Baiyao 0.5 g t.i.d. for 5 d | Mifepristone, misoprostol, in 24 h after misoprostol administrated leonurus japonicus houtt. through oral administration | 5 days | experimental group: 8.7 ± 4.6 d; control group: 15.7 ± 5.1 d | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Zhu XP 2010 | Attach cotton ball with iodophors to the ulcer region for 2-3 min, Yunnan Baiyao powder 1 g to the ulcer surface s.i.d, NSAIDs | Attach sterile gauze with iodophors to the ulcer region s.i.d. NSAIDs | 1 day | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Huang ZX 2008 | Yunnan Baiyao 2 pills t.i.d. p.o. and omeprazole 20 mg b.i.d. p.o. | Omeprazole 20 mg b.i.d. p.o. | 4 weeks | - | Concealment of allocation unclear, single blind and randomized | Not mentioned |
| Liu M 2010 | Amoxicillin 0.5 g t.i.d., cimetidine 0.2 g t.i.d. and Yunnan Baiyao 3 g b.i.d. | Losec 20 mg b.i.d., amoxicillin 0.5 g t.i.d. and cimetidine 0.2 g t.i.d. | 6 weeks | - | Concealment of allocation and blinding unclear, randomized, follow up for 2 years | Not mentioned |
| Xu XZ 1994 | Yunnan Baiyao 0.5 g t.i.d. half an hour before meals, ranitidine 0.15 g b.i.d. | Ranitidine 0.15 g b.i.d. | 4 weeks | - | Allocation arranged by registration order, blinding unclear | Slight dizzy, dry mouth, skin rash and constipation in both groups |
| Chen X 2009 | Yunnan Baiyao Enema (Yunnan Baiyao 4-6 g/d, metronidazole 0.4 g, dexamethasone 5-10 mg/d) by coloclysis s.i.d., SASP 4-6 g/d p.o. | Common Enema (metronidazole 0.4 g, dexamethasone 5-10 mg/d) by coloclysis s.i.d., SASP 4-6 g/d p.o. | > 4 weeks | - | Concealment of allocation and blinding unclear, randomized | Asthenia, dizzy, nausea, increasing abdominal pain, skin rash, leukopenia and transaminase increasing |
| Du KT 2011 | Sulfasalazine 1 g q.i.d. p.o., Yunnan Baiyao 0.5 g t.i.d. p.o. | Sulfasalazine 1 g q.i.d. p.o. | 8 weeks | - | Concealment of allocation and blinding unclear, randomized | Not mentioned |
| Zhang LL 2010 | Mesalazine 1.0 g t.i.d. p.o., 0.9% physiological saline 100 ml with Yunnan Baiyao powder 2.0 g by coloclysis s.i.d. at 21 o’clock after defecation and retention enema for 1-2 hours | Mesalazine 1.0 g t.i.d. p.o. | 3 weeks | - | Concealment of allocation and blinding unclear, randomized | 2 in experimental group; 4 in control group. All of these were abdominal discomfort |
| Zhao GM 2011 | Ranitidine 150 mg b.i.d. p.o., Yunnan Baiyao 500 mg t.i.d. 0.5 h before meals | Ranitidine 150 mg b.i.d. p.o. | 30 days | experimental group: 3.56 ± 0.23 d; control group: 7.25 ± 0.56 d | Concealment of allocation and blinding unclear, randomized | Not mentioned |
Table 4.
CONSORT score of included RCTs
| Trials | Standard CONCORT (2010) checklist items | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | ||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||
| A | B | A | B | A | B | A | B | 5 | A | B | A | B | A | B | 9 | 10 | A | B | A | B | A | B | A | B | 15 | 16 | A | B | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | SUM | |
| Yang Y 2007 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 15 |
| Chen XM 2010 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 2 | 0 | 0 | 0 | 16 |
| Chu XF 2011 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 15 |
| Li L 2009 | 0 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 2 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 19 |
| Yang QH 2001 | 0 | 1 | 2 | 0 | 0 | 0 | 2 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 2 | 0 | 2 | 2 | 1 | 1 | 0 | 0 | 0 | 21 |
| Chen GH 2006 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 16 |
| Qu J 2003 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 13 |
| Wang GR 2010 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 10 |
| Chen DH 2011 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 15 |
| Deng WM 2010 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 14 |
| Li HG 2000 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 13 |
| Zhou Y 2001 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 17 |
| Yang H 1996 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 5 |
| Yang SJ 2008 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 13 |
| Gong XH 2005 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 10 |
| Tian S 2010 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 9 |
| Bian HZ 2008 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 10 |
| Chen YP 2003 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 14 |
| Chen YP 2008 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 13 |
| Chu AL 2010 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 9 |
| Dong XF 2009 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 13 |
| Dong XJ 2004 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 13 |
| Fan J 2011 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 17 |
| Ge J 1998 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 14 |
| Ge SG 2001 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 10 |
| He SQ 2011 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 10 |
| Huang HY 2008 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 13 |
| Li M 2001 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 14 |
| Li ML 2010 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 14 |
| Li QM 2007 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 15 |
| Li YM 2009 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 13 |
| Luo Y 2012 | 0 | 1 | 0 | 2 | 0 | 0 | 2 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 20 |
| Pang Y 2004 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 13 |
| Shi SY 2011 | 0 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 17 |
| Sun YM 2003 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 16 |
| Wang CJ 2009 | 0 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 16 |
| Wang WD 1996 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 16 |
| Zhang XR 2005 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 2 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 18 |
| Zhong LY 2012 | 0 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 17 |
| Zhou Y 2006 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 9 |
| Zhuang DY 2000 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 13 |
| Zhang CX 2005 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 14 |
| Huang LR 2009 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 14 |
| Li L 2007 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 16 |
| Zhang M 2009 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 13 |
| Zhao X 2008 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 15 |
| Zhu SZ 2004 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 10 |
| Zhu XP 2010 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 16 |
| Huang ZX 2008 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 13 |
| Liu M 2010 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 15 |
| Xu XZ 1994 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 2 | 1 | 1 | 0 | 0 | 0 | 19 |
| Chen X 2009 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 17 |
| Du KT 2011 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 13 |
| Zhang LL 2010 | 0 | 1 | 2 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 2 | 0 | 2 | 2 | 0 | 2 | 0 | 0 | 0 | 24 |
| Zhao GM 2011 | 0 | 1 | 1 | 2 | 0 | 0 | 2 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 19 |
Efficacy of YNBY
YNBY versus routine haemostatic drugs
Dichotomous data were reported by 8 RCTs, containing 744 patients. There were 332 (89.5%) of 371 patients assigned to YNBY group who responded to therapy, compared with 290 (77.7%) of 373 allocated to routine haemostatic drugs (carbazochrome, famotidine, cimetidine and dicynone) group (RR = 1.16; 95% CI 1.06 to 1.28) (Figure 2), with borderline heterogeneity between studies (I² = 47%). There was no significant funnel plot asymmetry (Begg’s test, p = 0.711). After excluding 2 studies at high risk of bias, the result was relatively stable (RR = 1.18; 95% CI 1.04 to 1.35) (Table 1).
Figure 2.
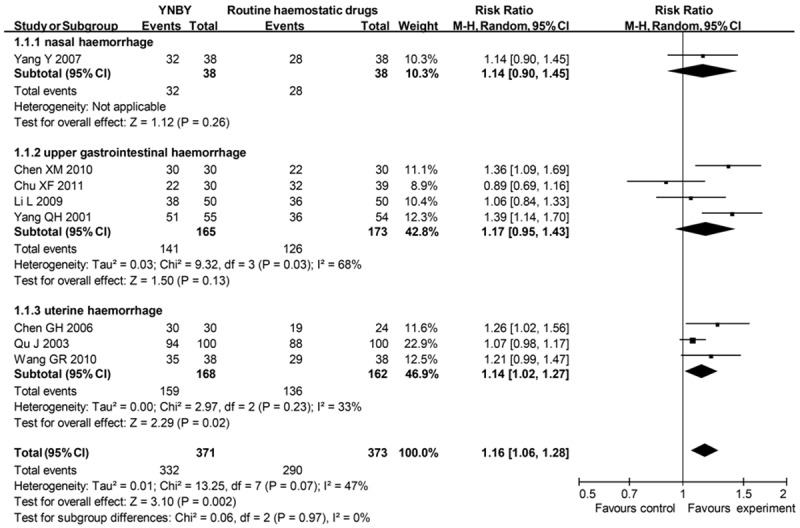
Effects of YNBY versus routine haemostatic drugs in treatment of haemorrhage YNBY, Yunnan Baiyao; 95% CI, 95% confidence interval. Note that Risk Ratio < 1 means numerically lower response rate than control group and RR > 1 numerically higher response rate than control group. 95% CI doesn’t include the number 1 means statistical difference between the 2 groups.
Table 1.
Sensitivity analysis of efficacy of Yunnan Baiyao
| Group | Number of studies at low risk of bias | Number of subjects | RR | 95% CI |
|---|---|---|---|---|
| YNBY versus routine haemostatic drugs | 6 | 468 | 1.18 | [1.04, 1.35] |
| YNBY versus routine antiulcer drugs | 3 | 281 | 1.16 | [0.99, 1.36] |
| YNBY plus routine haemostatic drugs versus the same haemostatic drugs | 18 | 1533 | 1.28 | [1.20, 1.37] |
| YNBY plus routine antiulcer drugs versus the same antiulcer drugs | 5 | 506 | 1.18 | [0.99, 1.41] |
YNBY, Yunnan Baiyao; 95% CI, 95% confidence interval.
Of these 8 studies, one assessed epistaxis. The routine haemostatic drug used was carbazochrome. In this study, 32 (84.2%) of 38 patients assigned to experimental groups responded to therapy compared with 28 (73.7%) of 38 patients allocated to control groups (RR = 1.14; 95% CI 0.90 to 1.45).
4 of the 8 studies assessed upper gastrointestinal haemorrhage. The routine haemostatic drugs used were famotidine, ranitidine, omeprazole, or dicynone. 141 (85.5%) of 165 patients assigned to experimental groups responded to therapy compared with 126 (72.8%) of 173 patients in control groups (RR = 1.17; 95% CI 0.95 to 1.43), with significant heterogeneity between studies (I² = 68%).
3 of the 8 studies assessed uterine haemorrhage caused by abortion or surgery. The routine haemostatic drugs used were Vitamin C, Vitamin K, or gel foam. 159 (94.6%) of 168 patients assigned to experimental groups responded to therapy compared with 136 (84.0%) of 162 patients allocated to control groups (RR = 1.14; 95% CI 1.02 to 1.27) with no significant heterogeneity (I² = 33%).
YNBY versus routine antiulcer drugs
7 studies assessed the effects of YNBY versus routine antiulcer drugs on ulcer. There were 317 (81.7%) of 388 patients receiving YNBY who responded to therapy, compared with 170 (65.6%) of 259 allocated to control groups (RR = 1.26; 95% CI 1.03 to 1.53) (Figure 3), with significant heterogeneity between studies (I² = 81%). When subgroup analysis were conducted according to ulcer locations, the heterogeneity vanished. There was no significant funnel plot asymmetry (Begg’s test, p = 0.230) suggesting no evidence of publication bias or other small study effects. After excluding 3 studies at high risk of bias, the RR was not stable (RR = 1.16; 95% CI 0.99 to 1.36) (Table 1).
Figure 3.
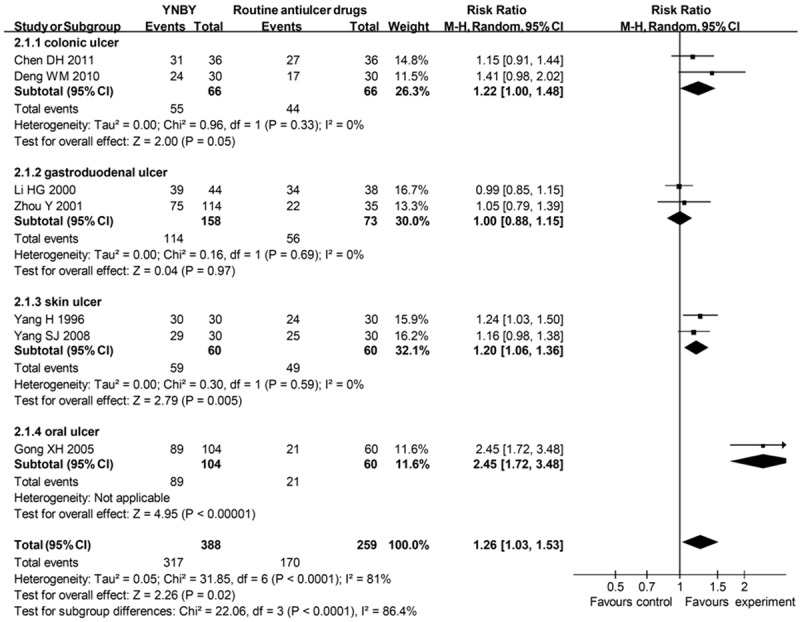
Effects of YNBY versus routine antiulcer drugs in treatment of ulcer YNBY, Yunnan Baiyao; 95% CI, 95% confidence interval. Note that Risk Ratio < 1 means numerically lower response rate than control group and RR > 1 numerically higher response rate than control group. 95% CI doesn’t include the number 1 means statistical difference between the 2 groups.
Among these 7 studies, 2 assessed ulcerative colitis. The drugs the control groups used were sulfasalazine, hydrocortisone, and gentamicin. In these studies, 55 (83.3%) of 66 patients assigned to experimental groups responded to therapy compared with 44 (66.7%) of 66 patients allocated to control groups (RR = 1.22; 95% CI 1.00 to 1.48) with no significant heterogeneity between studies (I² = 0%).
2 studies assessed peptic ulcer. The drugs used in control groups were sucralfate tablets or bismuth potassium citrate tablets. 114 (72.2%) of 158 patients assigned to experimental groups responded to therapy compared with 56 (76.7%) of 73 patients allocated to control groups (RR = 1.00; 95% CI 0.88 to 1.15), with no significant heterogeneity between studies (I² = 0%).
2 studies assessed skin ulcer caused by bedsore. The control drug was Vaseline. Overall, 59 (98.3%) of 60 patients assigned to experimental groups responded to therapy compared with 49 (81.7%) of 60 patients allocated to control groups (RR = 1.20; 95% CI 1.06 to 1.36), with no significant heterogeneity between studies (I² = 0%).
One study assessed oral ulcer. 89 (85.6%) of 104 patients assigned to experimental group responded to therapy compared with 170 (65.6%) of 259 patients allocated to control group (RR = 2.45; 95% CI 1.72 to 3.48). The control group used hydrogen peroxide and iodine glycerol.
YNBY combined with routine haemostatic drugs versus the same haemostatic drugs
Dichotomous data were reported by 33 RCTs, containing 3,053 patients. 1572 (94.3%) of 1667 patients assigned to groups of YNBY plus routine haemostatic drugs responded to the therapy, compared with 1055 (76.1%) of 1386 in control groups (RR = 1.23; 95% CI 1.17 to 1.29) (Figure 4), with a borderline heterogeneity (I² = 57%). There was a significant funnel plot asymmetry (Begg’s test, p = 0), suggesting publication bias or small study effects. After excluding 15 studies with low quality, the RR was relatively stable (RR = 1.28; 95% CI 1.20 to 1.37) (Table 1).
Figure 4.
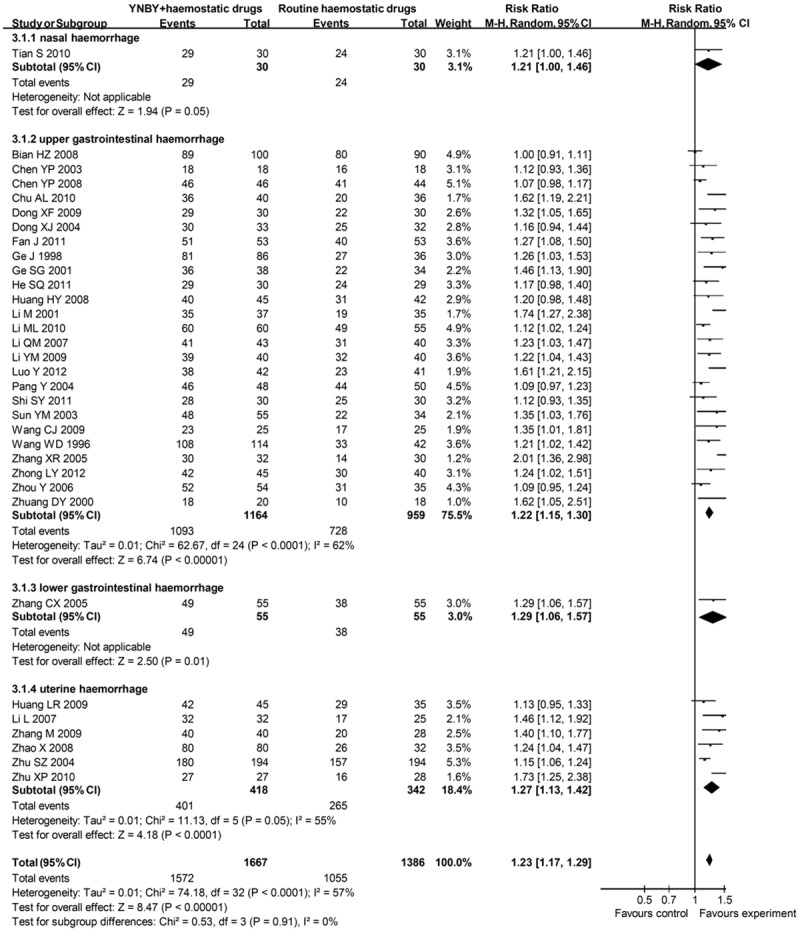
Effects of YNBY combined with routine haemostatic drugs versus routine haemostatic drugs in treatment of haemorrhage YNBY, Yunnan Baiyao; 95% CI, 95% confidence interval. Note that Risk Ratio < 1 means numerically lower response rate than control group and RR > 1 numerically higher response rate than control group. 95% CI doesn’t include the number 1 means statistical difference between the 2 groups.
One study assessed epistaxis. The routine haemostatic drugs were epinephrine and antibiotics. 29 (96.7%) of 30 patients assigned to experimental group responded to therapy compared with 24 (80.0%) of 30 patients allocated to control group (RR = 1.21; 95% CI 1.00 to 1.46).
25 studies assessed upper gastrointestinal haemorrhage. The routine haemostatic drugs contained ethamsylate, cimetidine, norepinephrine, Vitamin B, omeprazole, somatostatin, ranitidine or pantoprazole. Overall, 1093 (93.9%) of 1164 patients assigned to experimental groups responded to therapy compared with 728 (75.9%) of 959 patients allocated to control groups (RR = 1.22; 95% CI 1.15 to 1.30), with a significant heterogeneity between studies (I² = 62%). Begg’s test (p = 0) showed a great possibility of publication bias.
One study assessed lower gastrointestinal haemorrhage. The routine haemostatic drug was norepinephrine. There were 49 (89.1%) of 55 patients assigned to experimental group responding to therapy, compared with 38 (69.1%) of 55 in the control group (RR = 1.29; 95% CI 1.06 to 1.57).
6 studies assessed uterine haemorrhage. The routine haemostatic drugs used in both groups were norethindrone or leonurus japonicus houtt. 401 (95.9%) of 418 patients assigned to experimental groups responded to therapy compared with 265 (77.5%) of 342 patients in control groups (RR = 1.27; 95% CI 1.13 to 1.42), with a borderline heterogeneity between studies (I² = 55%).
YNBY combined with routine antiulcer drugs versus the same antiulcer drugs
7 studies reported dichotomous data, containing 706 patients. 337 (92.3%) of 365 patients assigned to groups of YNBY plus routine antiulcer drugs responded to the therapy, compared with 263 (77.1%) of 341 allocated to the control groups (RR = 1.18; 95% CI 1.05 to 1.33) (Figure 5), with a significant heterogeneity (I² = 78%). There was no significant funnel plot asymmetry (Begg’s test, p = 0.133), suggesting no evidence of publication bias or other small study effects. After excluding 2 studies with low quality, the RR was not stable (RR = 1.18; 95% CI 0.99 to 1.41) (Table 1).
Figure 5.
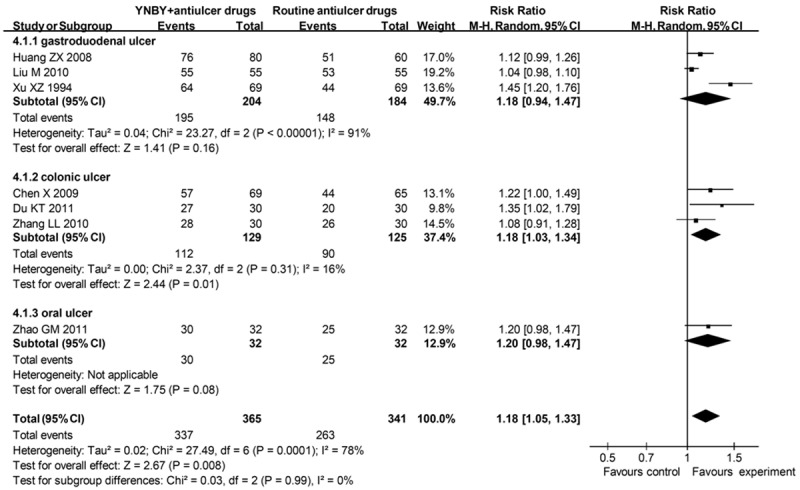
Effects of YNBY combined with routine antiulcer drugs versus routine antiulcer drugs in treatment of ulcer YNBY, Yunnan Baiyao; 95% CI, 95% confidence interval. Note that Risk Ratio < 1 means numerically lower response rate than control group and RR > 1 numerically higher response rate than control group. 95% CI doesn’t include the number 1 means statistical difference between the 2 groups.
3 studies assessed peptic ulcer. The routine antiulcer drugs were omeprazole and ranitidine. 195 (95.6%) of 204 patients assigned to experimental groups responded to therapy compared with 148 (80.4%) of 184 patients allocated to control groups (RR = 1.18; 95% CI 0.94 to 1.47), with a significant heterogeneity between studies (I² = 91%).
3 studies assessed ulcerative colitis. The routine drugs were sulfasalazine, mesalazine, dexamethasone and metronidazole. Overall, 112 (86.8%) of 129 patients assigned to experimental groups responded to therapy compared with 90 (72.0%) of 125 patients allocated to control groups (RR = 1.18; 95% CI 1.03 to 1.34), with no significant heterogeneity between studies (I² = 16%).
Discussion
Principal findings
We performed this meta-analysis of fifty-five RCTs to evaluate the effects of YNBY on haemorrhage and ulcer. Most of the studies were not clearly reported in accordance with the CONSORT statement. Synthesized results from 8 studies evaluating the effects of YNBY alone on local haemostasis found a positive effect. The 8 studies had investigated a variety of illnesses including nasal, upper gastrointestinal and uterine haemorrhage. Subgroup analysis indicated that YNBY alone was only effective on uterine haemorrhage. The results for uterine haemorrhage should be interpreted with caution as only 3 studies with a total of 330 patients were included. Subgroup analysis comparing the effects of YNBY plus routine haemostatic drugs to routine haemostatic drugs alone suggested a complementary role of YNBY on gastrointestinal and uterine haemorrhage but the heterogeneity and possible publication bias should be noted.
Meta analysis also showed an efficacy of YNBY with or without routine antiulcer drugs on ulcer. Subgroup analysis found that YNBY alone or plus routine antiulcer drugs was effective in treating ulcerative colitis. These results were supported from animal studies showing that YNBY was effective in treating inflammatory bowel disease including Crohn’s disease and ulcerative colitis by anti-inflammation and promoting intestinal epithelial wound-healing and repair [19]. However, as unstable results were shown by sensitivity analysis, the effects of YNBY on ulcer should not be taken as certain.
Strengths and limitations
This systematic review had several strengths. 1) To our knowledge, this was the first meta-analysis focusing on the efficacy of YNBY. Subgroup analysis was also performed and found a curing effect of YNBY on specific diseases such as gastrointestinal haemorrhage, uterine haemorrhage, ulcerative colitis and skin ulcer suggesting strong potential of YNBY as an alternative herbal therapy. Sensitivity analysis was also conducted according to the risk of bias of these trials. 2) Our study has found poor reports of clinical trials on YNBY suggesting an urgent need for Traditional Chinese Medicine doctors to learn and obey the rules of the CONSORT statement. 3) Although the Traditional Chinese Medicine theory hypothesized a shared pathophysiological mechanisms of ulcer and haemorrhage [23,26], modern medicine has found a greater discrepancy than similarity between ulcer and haemorrhage and also among different illnesses pertaining to either ulcer or haemorrhage. Our inconsistent results from group and subgroup analysis also supported that more studies were needed to investigate the exact pharmacology of YNBY and YNBY should be cautiously used clinically to treat different kinds of ulcer or haemorrhage.
Limitations of our meta-analysis, as with any systematic review and meta-analysis, arose from the quality and reporting of the trials included. Only 32 of the 55 eligible RCTs were at low risk of bias. We arbitrarily chose “13” as the cutting point for studies at high or low bias during the sensitivity analysis. The number “13” was based on the lower 95% confidence interval (CI) of the mean score of the included trials. The 95% CI was 13.40-15.18 with a mean of 14.29. However, as the score based on the CONSORT statement was not always in accordance with the quality of trials. The clinical trial may be conducted under the principles of RCT trials but failed to report them. Previous study also found that the foreign language studies had lower mean score than the studies published in English [32]. Thus sensitivity analysis results using this cutting point should not be considered definite.
The methodology quality in the studies was generally poor. For most of the trials, the method of randomization was not reported clearly, and few trials reported blinding of assessors of outcomes. Blinding and allocation concealment were not reported in these RCTs, which meant potential risk of bias [33,34]. As all of the 55 studies were conducted in less-developed China and most of them were published in Chinese, there was a great possibility of selection or language bias. As reported before [35,36], the bias might be due to the incomplete search of related articles although we have searched most of the database resources or the selected publication of positive results in a journal indexed in a literature database. These potential biases added uncertainty to the synthesized results and following interpretation. Further well designed; randomized; double-blinded multi-centre studies were needed to make a more definite conclusion.
Conclusions
In conclusion, evidence from this systematic review and meta-analysis supports that YNBY alone has efficacy on uterine haemorrhage, ulcerative colitis and skin ulcer. YNBY combined with routine antiulcer drugs is effective in treating ulcer (especially colonic ulcer). The great heterogeneity and publication bias make the conclusion that YNBY plus routine haemostatic drugs is effective on haemorrhage should be taken with caution. More well designed and reported RCTs are needed.
Acknowledgements
This study was supported by funds from National Nature Science Foundation of China (81000525), Shanghai Chen-Guang Program (10CG40), Shanghai Health bureau (XYQ2011022 and 2009Y062), Shanghai Municipal Education Commission E-Institute Program (E03008) and Shanghai Three Years’ Program for the Development of TCM (No.zysnxd-cczdyj023).
Disclosure of conflict of interest
None.
References
- 1.Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, Dewan Y, Gando S, Guyatt G, Hunt BJ, Morales C, Perel P, Prieto-Merino D, Woolley T. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011;377:1096–1101. e1–2. doi: 10.1016/S0140-6736(11)60278-X. [DOI] [PubMed] [Google Scholar]
- 2.Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichaishvili T, Gupta S, Herrera J, Hunt B, Iribhogbe P, Izurieta M, Khamis H, Komolafe E, Marrero MA, Mejia-Mantilla J, Miranda J, Morales C, Olaomi O, Olldashi F, Perel P, Peto R, Ramana PV, Ravi RR, Yutthakasemsunt S. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 3.White CM, Fan C, Chow M. An evaluation of the hemostatic effect of externally applied notoginseng and notoginseng total saponins. J Clin Pharmacol. 2000;40:1150–1153. [PubMed] [Google Scholar]
- 4.Mannucci PM. Hemostatic drugs. N Engl J Med. 1998;339:245–253. doi: 10.1056/NEJM199807233390407. [DOI] [PubMed] [Google Scholar]
- 5.Choudhary M, Kumar V, Singh S. Gastric antisecretory and cytoprotective effects of hydroalcoholic extracts of Plumeria alba Linn. leaves in rats. J Integr Med. 2014;12:42–51. doi: 10.1016/S2095-4964(14)60002-9. [DOI] [PubMed] [Google Scholar]
- 6.Marti-Carvajal AJ, Sola I, Marti-Carvajal PI. Antifibrinolytic amino acids for upper gastrointestinal bleeding in patients with acute or chronic liver disease. Cochrane Database Syst Rev. 2012;9:CD006007. doi: 10.1002/14651858.CD006007.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Schulman S. Pharmacologic tools to reduce bleeding in surgery. Hematology Am Soc Hematol Educ Program. 2012;2012:517–521. doi: 10.1182/asheducation-2012.1.517. [DOI] [PubMed] [Google Scholar]
- 8.Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Hunt BJ, Komadina R, Nardi G, Neugebauer E, Ozier Y, Riddez L, Schultz A, Stahel PF, Vincent JL, Spahn DR. Management of bleeding following major trauma: an updated European guideline. Crit Care. 2010;14:R52. doi: 10.1186/cc8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurusamy KS, Pissanou T, Pikhart H, Vaughan J, Burroughs AK, Davidson BR. Methods to decrease blood loss and transfusion requirements for liver transplantation. Cochrane Database Syst Rev. 2011:CD009052. doi: 10.1002/14651858.CD009052.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doren M. Hormonal replacement regimens and bleeding. Maturitas. 2000;34(Suppl 1):S17–23. doi: 10.1016/s0378-5122(99)00069-9. [DOI] [PubMed] [Google Scholar]
- 11.Han SM, Lee KG, Pak SC. Effects of cosmetics containing purified honeybee (Apis mellifera L. ) venom on acne vulgaris. J Integr Med. 2013;11:320–326. doi: 10.3736/jintegrmed2013043. [DOI] [PubMed] [Google Scholar]
- 12.Holle GE. Pathophysiology and modern treatment of ulcer disease. Int J Mol Med. 2010;25:483–491. doi: 10.3892/ijmm_00000368. [DOI] [PubMed] [Google Scholar]
- 13.Ghassemi KA, Kovacs TO, Jensen DM. Gastric acid inhibition in the treatment of peptic ulcer hemorrhage. Curr Gastroenterol Rep. 2009;11:462–469. doi: 10.1007/s11894-009-0071-x. [DOI] [PubMed] [Google Scholar]
- 14.Jull A, Walker N, Parag V, Molan P, Rodgers A. Randomized clinical trial of honey-impregnated dressings for venous leg ulcers. Br J Surg. 2008;95:175–182. doi: 10.1002/bjs.6059. [DOI] [PubMed] [Google Scholar]
- 15.Leontiadis GI, Sharma VK, Howden CW. Proton pump inhibitor treatment for acute peptic ulcer bleeding. Cochrane Database Syst Rev. 2006:CD002094. doi: 10.1002/14651858.CD002094.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Molan PC. The potential of honey to promote oral wellness. Gen Dent. 2001;49:584–589. [PubMed] [Google Scholar]
- 17.Ladas EJ, Karlik JB, Rooney D, Taromina K, Ndao DH, Granowetter L, Kelly KM. Topical Yunnan Baiyao administration as an adjunctive therapy for bleeding complications in adolescents with advanced cancer. Support Care Cancer. 2012;20:3379–3383. doi: 10.1007/s00520-012-1598-1. [DOI] [PubMed] [Google Scholar]
- 18.He H, Ren X, Wang X, Shi X, Ding Z, Gao P, Xu G. Therapeutic effect of Yunnan Baiyao on rheumatoid arthritis was partially due to regulating arachidonic acid metabolism in osteoblasts. J Pharm Biomed Anal. 2012;59:130–137. doi: 10.1016/j.jpba.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Li R, Alex P, Ye M, Zhang T, Liu L, Li X. An old herbal medicine with a potentially new therapeutic application in inflammatory bowel disease. Int J Clin Exp Med. 2011;4:309–319. [PMC free article] [PubMed] [Google Scholar]
- 20.Tang ZL, Wang X, Yi B, Li ZL, Liang C, Wang XX. Effects of the preoperative administration of Yunnan Baiyao capsules on intraoperative blood loss in bimaxillary orthognathic surgery: a prospective, randomized, double-blind, placebo-controlled study. Int J Oral Maxillofac Surg. 2009;38:261–266. doi: 10.1016/j.ijom.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Salgado B, Paramo R, Sumano H. Successful treatment of canine open cervix-pyometra with Yun-Nan-Pai-Yao, a Chinese herbal preparation. Vet Res Commun. 2007;31:405–412. doi: 10.1007/s11259-006-3438-6. [DOI] [PubMed] [Google Scholar]
- 22.Fan C, Song J, White CM. A comparision of the hemostatic effects of notoginseng and yun nan bai yao to placebo control. J Herb Pharmacother. 2005;5:1–5. [PubMed] [Google Scholar]
- 23.Xiu RJ. Microcirculation and traditional Chinese medicine. JAMA. 1988;260:1755–1757. doi: 10.1001/jama.260.12.1755. [DOI] [PubMed] [Google Scholar]
- 24.Zhu TZ. [Progress in the pharmacological and clinical study of Yunnan baiyao] . Zhong Xi Yi Jie He Za Zhi. 1987;7:377–379, 384. [PubMed] [Google Scholar]
- 25.Yang T. [Anti-inflammatory effect of the saponins in Yunnan bai-yao] . Zhong Yao Tong Bao. 1986;11:47–50. [PubMed] [Google Scholar]
- 26.Li FL, Wang YF, Li X, Li F, Xu R, Chen J, Geng L, Li B. Characteristics and clinical managements of chronic skin ulcers based on traditional chinese medicine. Evid Based Complement Alternat Med. 2012;2012:930192. doi: 10.1155/2012/930192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 30.Biljana M, Jelena M, Branislav J, Milorad R. Bias in meta-analysis and funnel plot asymmetry. Stud Health Technol Inform. 1999;68:323–328. [PubMed] [Google Scholar]
- 31.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 32.Thoma A, Chew RT, Sprague S, Veltri K. Application of the CONSORT statement to randomized controlled trials comparing endoscopic and open carpal tunnel release. Can J Plast Surg. 2006;14:205–210. doi: 10.1177/229255030601400401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He J, Du L, Liu G, Fu J, He X, Yu J, Shang L. Quality assessment of reporting of randomization, allocation concealment, and blinding in traditional Chinese medicine RCTs: a review of 3159 RCTs identified from 260 systematic reviews. Trials. 2011;12:122. doi: 10.1186/1745-6215-12-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, Gluud C, Martin RM, Wood AJ, Sterne JA. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008;336:601–605. doi: 10.1136/bmj.39465.451748.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williamson PR, Gamble C, Altman DG, Hutton JL. Outcome selection bias in meta-analysis. Stat Methods Med Res. 2005;14:515–524. doi: 10.1191/0962280205sm415oa. [DOI] [PubMed] [Google Scholar]
- 36.Juni P, Holenstein F, Sterne J, Bartlett C, Egger M. Direction and impact of language bias in meta-analyses of controlled trials: empirical study. Int J Epidemiol. 2002;31:115–123. doi: 10.1093/ije/31.1.115. [DOI] [PubMed] [Google Scholar]


