Abstract
The present work tested the hypotheses that: 1) short-term dietary deficiency of magnesium (Mg; 21 days) in rats (MgD) would result in a downregulation of telomerase in cardiac and aortic smooth muscle cells, 2) low levels of Mg2+ added to drinking water (DW) would either prevent or greatly reduce the downregulation of telomerase in MgD, 3) MgD in rats would cause an upregulation of neutral-sphingomyelinase (N-SMAse) and p53, 4) short-term MgD would result in oxidation of DNA in diverse cardiac muscle and aortic smooth muscle cells as exemplified by measurement of 8-hydroxydeoxyguanosine (8-OH-dG), and 5) cross-talk between telomerase, N-SMase, p53, and 8-OH-dG would be evident in left ventricular (LV), right ventricular (RV), atrial and aortic smooth muscle obtained from rats subjected to short-term MgD. The data indicated that short-term MgD (10% normal dietary intake) resulted in downregulation of telomerase in LV, RV, atrial and aortic muscle cells; even very low levels of water-bourne Mg2+ (e.g., 15-40 mg/lday) either prevented or ameliorated the downregulation of telomerase. Our experiments also showed that MgD resulted in a 7-10 fold increased formation of 8-OH-dG in the cardiac and aortic muscle cells. The experiments also confirmed that short-term dietary deficiency of Mg resulted in greatly increased upregulation of N-SMAse and p53 in the cardiac and aortic muscle tissues. These new experiments point to a sizeable cross-talk among telomerase, N-SMAse, and p53 in rat cardiac and peripheral vascular muscle exposed to a short-term MgD. These studies would be compatible with the idea that even short-term MgD could cause alterations of the genome in diverse cell types leading to mutations of cardiac, vascular, and endothelial cells seen in aging and atherogenesis. Since we have shown, previously, that activation of N-SMAse in MgD leads to synthesis and release of ceramide in cardiovascular tissues and cells, we believe this pathway, most likely, helps to result in downregulation of telomerase, upregulation of transcription factors (e.g., p53; NF-kB), cytokine release, mutations, transformations, and dysfunctional growth seen in the cardiac and vascular cells observed in the normal aging process, atherogenesis, hypertension, and cardiac failure. Lastly, we suggest ways in which this hypothesis can be tested.
Keywords: Cardiac muscle, vascular muscle, sphingolipids, p53, 8-hydroxydeoxyguanosine, ceramide, epigenetics, telomeres
Introduction
Over the past 100 years, evidence has accumulated, from multiple sources, that a number of diverse control mechanisms, including physiological, nutritional, and biochemical factors are responsible for normal functions of the cardiovascular systems and in the aging process. These homeostatic factors maintain the patency of blood vessels, cardiac output, and the fluidity of the blood. Pathophysiological changes in peripheral blood vessels and the chambers of the heart can result in alterations of vascular wall geometry, disturbances in oxygenation, and nutritional status of the blood vessels and myocardium as well as the tissues they perfuse. Although multiple theories and hypotheses have been generated to account for the hypertrophy of resistance blood vessels and alterations in the etiology of the aging process, atherogenesis, and essential hypertension, there is no agreement as to the precise mechanisms [1-3].
Disturbances in diet are known to promote lipid deposition and accelerate the growth and transformation of smooth muscle cells in the vascular walls and to promote cardiac dysfunction [3]. Autopsies of children, who have died as a consequence of accidents, have demonstrated early signs of atherogenesis (i.e., fatty streaks) on aortic and carotid arterial blood vessel walls even in young children as early as five to six years of age [4]. Several epidemiological studies in North America and Europe have shown that people consuming Western-type diets are low in magnesium (Mg) content (i.e., <30-50% of the RDA for Mg) [5-7]); most such diets in the U.S.A. show that 60-80% of Americans are consuming only 185-235 mg/day of Mg [5-8]. Low Mg content in drinking water, found in areas of soft water and Mg-poor soil, is associated with high incidences of ischemic heart disease, coronary vasospasm, hypertension, and sudden cardiac death [5,6,9-15]. Both animal and human studies have shown an inverse relationship between dietary intake of Mg and atherosclerosis [5,6,16-21]. The myocardial level of Mg has consistently been observed to be lower in subjects dying from ischemic heart disease and sudden cardiac death in soft water areas than those in hard water areas [5,6,10-12,14,15,22-24]. Mg plays an essential role in more than 350 enzymatic reactions in the body and is required for all energy-generating reactions and oxidative phosphorylation. Mg is a natural calcium (Ca2+) channel blocker on myocardial and vascular smooth muscle (VSM) [5,6,25,26], which was first demonstrated by The Alturas, and is a natural statin in that it lowers blood cholesterol, LDL and triglycerides as well as acting like a vasodilator agent [4-6,27-30]. Hypermagnesemic diets have been shown to ameliorate hypertension and atherogenesis [5,6,9,13,16,17,24,27-32]. Using sensitive, specific Mg2+ -selective electrodes it has been shown that patients with hypertension, ischemic heart disease, cardiac failure, strokes, diabetes, gestational diabetes, renal-induced vascular changes, and atherosclerosis exhibit a significant depletion of serum/plasma ionized, but not total, Mg [5,6,32-44]. Dietary deficiency of Mg in rats and rabbits has been shown to cause vascular remodeling concomitant with hypertension and atherosclerosis (i.e., arteriolar wall hypertrophy and alterations in the matrices) of unknown origin [5,6,9,13,16,17,28-31,36-38]. In addition, Mg deficiency has been shown to result in an acceleration of the cellular aging process via unknown mechanisms [45].
Several years ago, our laboratories showed in primary cerebral and peripheral VSM cells, in culture, that a variation in free Mg ions (Mg2+) causes sustained changes in membrane phospholipids and several second messengers, as well as the activation of apoptotic pathways, membrane oxidation and truncation of membrane fatty acids [46-48]. Decreases in extracellular Mg2+ ([Mg2+]0) produced a fall in membrane sphingomyelin (SM), whereas increases in [Mg2+]0 resulted in increases in membrane SM and phosphatidylcholine [46]; intracellular ceramide formation and activation of neutral-sphingomyelinase (N-SMAse) was inversely proportional to Mg2+ [47]. Ceramides, released as a consequence of sphingomyelinases (SMAses) acting on SM, are now thought to play important roles in fundamental processes such as angiogenesis, cell proliferation, immuno inflammatory responses, atherogenesis, programmed cell death, and the aging process [49-56]. Recent findings suggest that MgD may induce upregulation of N-SMAse in intact cardiac tissues and VSM cells exposed to deficiency in dietary Mg [47,48,57].
Telomeres are critical in regulating genome integrity and hence chromosomal integrity [58,59]. Telomeric DNA characteristics are highly conserved molecules among all eukaryotes [58,59]. The telomeres are found at the ends of the chromosomes, consisting of short nucleotide repeats and specialized proteins, which are regulated by telomerase [58,59]. Chromosomes devoid of telomeres or low telomerase activity usually undergo apoptosis [60-62]. Thus, cells with normal telomerase activities are thought to survive periods of oxidative stress (e.g., production of reactive oxygen and nitrogen species) and curtail apoptosis and the aging process [58-62]. Since Mg deficient states have recently been shown, both in-vivo and in-vitro, to promote apoptosis in cardiovascular tissues and cells [48,63], oxidative stress [46-48,64-69], DNA damage-fragmentation [48], alter carbohydrate and lipid metabolism and calcium content [64], cardiac bioenergetics [64,72], and result in accumulation of the tumor suppressor protein p53 [70] coupled to cardiac and VSM cell production of ceramide [47,57,70,71], we hypothesized that dietary deficiency of Mg probably results in inhibition of telomerase activity in both cardiac and VSM cells which are correlated to increased N-SMAse contents and p53 levels as well as to oxidative DNA damage.
Materials and methods
Animals, diets, sera, organ tissue collections, and measurement of serum total and ionized Mg
Mature male and female Wistar rats (200±65 g) were used for all experiments. All experiments were approved by The Animal Use and Care Committee of the State University of New York Downstate Medical Center. Equal numbers of paired male and female animals were used for all experiments. Control (600 ppm Mg) and magnesium deficient (MgD; 60 ppm Mg) pellet diets were obtained from DYETS (Bethlehem, PA; AIN-93Gdiets). All animals were given their respective diets for 21 days as previously described [48,57,67,70,71]. MgD animals were allowed to drink triply distilled water (Mg2+<10-6 M) containing one of four different levels of Mg aspartate-HCl (0, 15, 40, or 100 mg/l, Verla Pharm, Tutzing, Germany), where appropriate. All control animals received a normal Mg-containing diet (i.e., 600 ppm Mg) as well as triply distilled water to drink. On the 22nd day, sera and tissues (the left and right ventricles, atria, abdominal aorta between the superior mesenteric arteries and renal arteries) cleaned of all connective tissues were collected quickly after anesthesia (45 mg/kg im pentobarbital sodium) [48,57,67,71]. Tissues were stored rapidly under liquid nitrogen (-85°C) until use. Whole blood was collected under anaerobic conditions in red-stoppered tubes (no anticoagulant present), allowed to clot under anaerobic conditions, and then centrifuged under anaerobic conditions in capped Vacutainer tubes [48,57,67,71]. The sera were then collected into additional red-stoppered Vacutainer tubes under anaerobic conditions for processing shortly thereafter, similar to previously described methods [33]. Serum samples were analyzed within 2 hr after collection, as previously described [48,57,67,71]. Total Mg levels were measured by standard techniques in our laboratory (Kodak DT-60 Analyzer, Ektachem Colorimetric Instruments, Rochester, NY). The method favorably compares with atomic absorption techniques for total Mg [33]. A Mg2+-selective electrode with a novel neutral carrier-based membrane (NOVA 8 Analyzer, NOVA Biomedical Instruments, Waltham, MA) was used to measure the free divalent cation in the sera [33]. The ion-selective electrode was used in accordance with established procedures developed in our laboratory, having an accuracy and precision of 3% [33].
Biochemical tissue measurements of N-SMAse and p53
For the assay of tissue N-SMAse, tissues (0.1 gm) were placed in a buffer (pH=7.4). The buffer consisted of: 100 mM Tris-HCl, 50 mM MgCl2, 5 mM DTT, and protease inhibitor. Activity of N-SMAse was measured using a Cayman Chemical Co. colormetric ELISA sphingomyelinase kit (Ann Arbor, MI). The samples were read in a microplate reader. Standard curves were plotted using authentic N-SMAse and the experimental N-SMAse sample values were then read from the standard curves [57].
For the p53 assay, we used a p53 ELISA Assay Kit from Assay Designs (Ann Arbor, MI) as described previously [70]. Briefly, the kit uses a monoclonal antibody to p53, which is immobilized on a microplate reader so as to bind p53 to either samples or standards. Tissues were homogenized in 1-5 volumes of RIPA buffer (20 mM Tris-HCl, 0.5 mM EDTA, 1% Nonidet P-40, 0.5% sodium deoxylate, 0.05% SDS, 1.0 mM PMSF, 1 ug/ml aprotinin, and 2 ug/ml leupeptin) on ice. Homogenates were then centrifuged, supernatants collected and diluted in the assay buffer (1:5) [70]. Lyophilized authentic p53 standards were utilized along with a polyclonal antibody to p53 labeled with horseradish peroxidase added to the samples and standards [70]. The polyclonal antibody binds to the p53 protein captured on the microliter plates. Standards and samples (100 ul) were then placed in contact with 100 ul of the antibody working solution (1:15) into the appropriate wells and washed as described previously [70]. TMB substrate solution (3,3’, 5,5’-tetramethylbenzidine and H2O; 200 ul) was then added to each well and the samples incubated at room temperature on a plate shaker for 30 min at ~500 rpm, stop solution added and the samples optical density read at 450 nm. The sample values were then derived from the standard curve for p53 (in pg/ml).
Measurement of telomerase activity in tissues and sera
Telomerase activities were quantitatively measured using an ELISA kit utilizing a monoclonal antibody for the target antigen, i.e., telomerase, and the target antigen HRP conjugate (Biocompare, South San Francisco, CA). The assay uses a colormetric assay, forming a blue colored complex and a stop solution which turns yellow. The intensity of the color is inversely proportional to the target concentration since the target antigen from samples and the target antigen HRP conjugate to compete for the antibody binding site. Tissue and sera samples are minced, where appropriate, in PBS, and homogenized, samples centrifuged for ~15 min at 1500 xg (~5000 rpm), supernatant removed and assayed after washing three-times in PBS. A standard curve is plotted relating the intensity of the color (O.D. read with microplate reader at 450 nm) to the concentration of standards. The target concentration in each sample is interpolated from the standard curve.
Measurement of 8-hydroxydeoxguanosine-8-OHdG in tissues
The ELISA assay (Abcam Biochemicals, Cambridge, MA) is based upon the competition between 8-hydroxy-2-deoxyguanosine (8-OH-dG) and an 8-OH-dG-acetylcholinesterase (AchE) conjugate (8-OH-dG Tracer) for a limited amount of monoclonal antibody. The amount of 8-OH-dG is inversely proportional to the concentration of 8-OH-dG in the wells. This antibody -8-OH-dG complex binds to a polyclonal antimouse IgG that has been previously attached to the wells. The product of the enzymatic reaction has a distinct yellow color and absorbs at 412 nm. The intensity of this yellow color, determined by a plate reader, is proportional to the amount of 8-OH-dG tracer bound to the wells, which is inversely proportional to the amount of free 8-OH-dG present in the wells during the incubation. Briefly, the frozen (-80°C) tissue homogenates are placed in a homogenization buffer (0.1 M phosphate buffer, pH 7.4, containing 1.0 mM EDTA/gm tissue, centrifuged at 1,000 xg for 10 min), then the supernatant is purified using a commercially- available DNA extraction kit. The DNA is then digested using nuclease P1 following the manufacturer’s instructions (see Abcam ELISA kit), the pH adjusted to 7.5-8.5 using 1.0 M Tris, followed by the addition of 1 unit of alkaline phosphatase/100 ug of DNA, and then incubated at 37°C for 30 min, and finally boiled for 10 min and placed on ice until ready for the assay.
Statistical analyses
Where appropriate, means and Means ± SE were calculated. Differences between means were assessed for statistical significance by Student’s t-test and ANOVA followed by a Newman-Keuls test. In some cases, linear correlation coefficients were calculated by the method of least squares. P values of <0.05 were considered significant.
Results
Influence of diet on water consumption, food intake, and overall physiological conditions
As recently shown, using an identical protocol [48,57,67,70,71], in controls and MgD animals, there were no significant differences in either water consumption or food intake between the diverse subgroups of rats (i.e., controls-600 ppm M) or MgD + 15 mg/l Mg/day, MgD + 40 mg/l Mg/day, or MgD + 100 mg/l Mg/day in drinking water [48,57,67,70,71]. All of the Mg subgroups (10-16 animals/group), irrespective of the amount of Mg in the diets or drinking water, did not demonstrate any loss of gait, fur, or any other outward signs of pathology or behavior over the 21 days, similar to that seen previously [48,57,67,70,71].
Serum total and ionized Mg levels
Feeding the animals a normal (600 ppm Mg) AIN-93G diet (n=10-14 animals/group) resulted in a total serum Mg level of ~1.00±0.005 mM, whereas animals receiving the MgD diet showed a total serum level of 0.42±0.036 mM Mg (P<0.01). The serum level of ionized Mg in the control group had a mean value of 0.58±0.005 mM, whereas in the MgD group, the serum ionized level was reduced to 0.28±0.002 mM, an approximate loss of 54% (P<0.01).
Feeding MgD rats various levels of Mg in their drinking water resulted in concentration-dependent rises in both the total and ionized serum levels of Mg, similar to previous studies [48,57,67,70,71]. Feeding the MgD animals 15 and 40 mg/l Mg/day in the drinking water elevated the total serum Mg levels 75 and 85%, respectively, whereas feeding the MgD 100 mg/l lMg/day in their drinking water elevated the total serum level of Mg to normal levels (i.e., 1.05±0.006. Feeding the MgD animals 100 mg/l Mg/day restored the serum ionized to normal levels (i.e., 0.38±0.004 mM), while the addition of 15 or 40 mg/l Mg/day to the drinking water of the MgD animals elevated the serum ionized Mg level to 65 and 70%, respectively of normal, control levels.
Influence of dietary Mg intake on telomerase levels in cardiac and vascular smooth muscle and sera
Figure 1 shows that feeding rats a MgD diet for 21 days resulted in an approximate 70-88% reduction in telomerase activity, depending upon the cardiovascular tissue, with the LV exhibiting the greatest degree of downregulation. Feeding these MgD animals various levels of Mg, in the drinking water, demonstrated a range of sensitivities in the tissues. For example, although the RV showed that as little as 15 mg Mg/l in the drinking water could significantly elevate the telomerase activity close to normal, much more of a Mg intake was required for the other tissues. However, 40 mg Mg/l added to the drinking water was able to bring the telomerase levels to almost normal in the atrial muscle.
Figure 1.
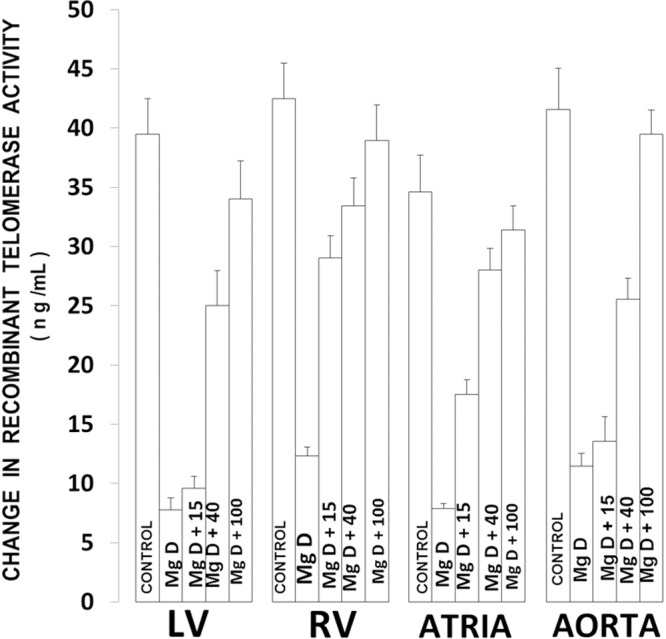
Telomerase levels in cardiovascular tissues in normal and MgD rats with and without Mg2+ added to the drinking water (DW). All values are Means ± SE. N=14-16 animals per group. All mean values are significantly different from controls, except MgD + 100 mg Mg2+/l (P<0.01, ANOVA).
With respect to serum levels, our experiments indicate that animals subjected to MgD diets demonstrate a profound lowering of telomerase activity (Figure 2), whereas addition of Mg to the drinking water resulted in a concentration-dependent rise of telomerase towards control levels. However, unlike the cardiovascular tissues ~100 mg Mg/l was required in the drinking water to achieve close to normal serum levels.
Figure 2.
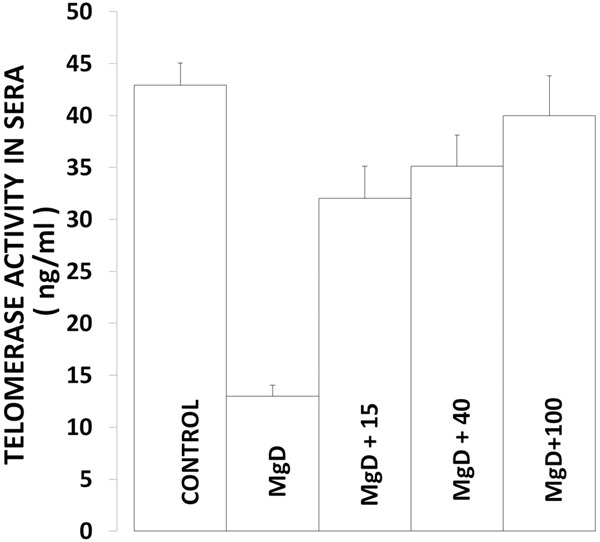
Serum telomerase levels in normal and MgD rats with and without Mg2+ added to the DW. All values are Means ± SE. N=14-16 animals per group. All mean values are significantly different from controls, except MgD + 100 mg Mg2+/l (P<0.01, ANOVA).
Correlation of telomerase activities with serum ionized Mg levels in cardiovascular tissues
Figure 3 indicates high positive correlations of telomerase activities in LV, RV, atria and aortic smooth muscle with the serum ionized Mg level (r values=0.85-0.99; P<0.001) with LV and aortic smooth muscle exhibiting the highest degrees of correlation (r=0.98; P<0.01). It is clear from our data, that the lower the serum ionized Mg, the lower the telomerase activities both for the muscular tissues and the blood-serum.
Figure 3.
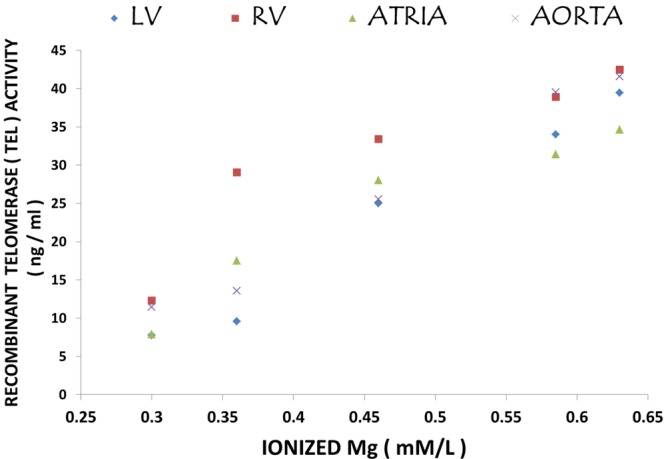
Correlation of cardiovascular tissue levels of telomerase with serum ionized levels of Mg. Regression equations with r-values are as follows: 1) left ventricle (LV) vs. Mg2+: y=99.514x-23.296; r=0.9784; 2) right ventricle (RV) vs. Mg2+: y=76.663x-4.565; r=0.848; 3) atria vs. Mg2+: y=74.566x-10.939; r=0.9165; and 4) aorta vs. Mg2+: y=98.784x-19.813; r=0.9866.
Influence of dietary Mg deficiency on N-SMAse levels in cardiac and vascular smooth muscles
Figure 4 indicates that all four cardiovascular muscle tissues, removed from MgD animals , exhibit 150-450% rises in N-SMAse levels, with the aortic smooth muscle showing the greatest upregulation, similar to data recently published [57].
Figure 4.
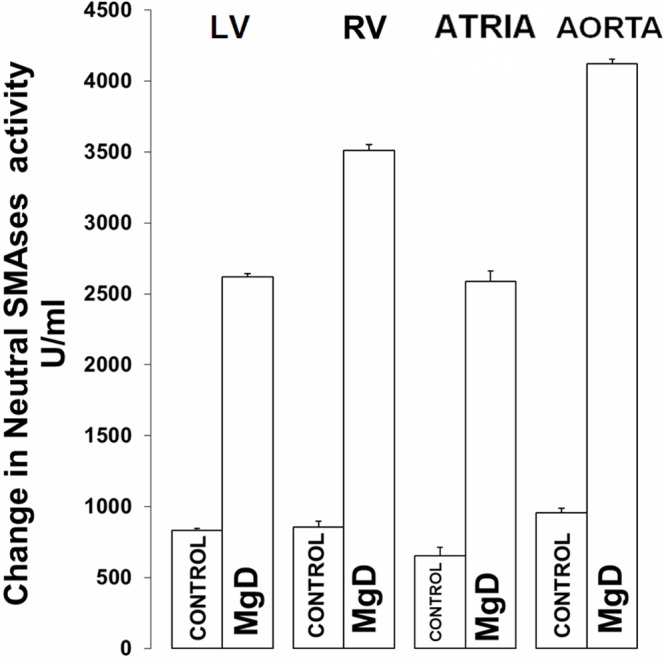
N-SMAse levels in LV, RV, atria and aortic smooth muscle in normal and MgD rats. All values are Means ± SE. N=14-16 animals per group. All MgD mean values are significantly different from control rats (P<0.01, ANOVA).
Inverse correlation of N-SMAse with telomerase in MgD animals
The regression coefficients shown in Figure 5 demonstrate strong inverse correlations of telomerase levels with N-SMAse levels (r=-0.66 to -0.98), with the RV and LV muscular tissues exhibiting the greatest inverse correlations, suggesting cross-talk between telomerase and N-SMAse.
Figure 5.
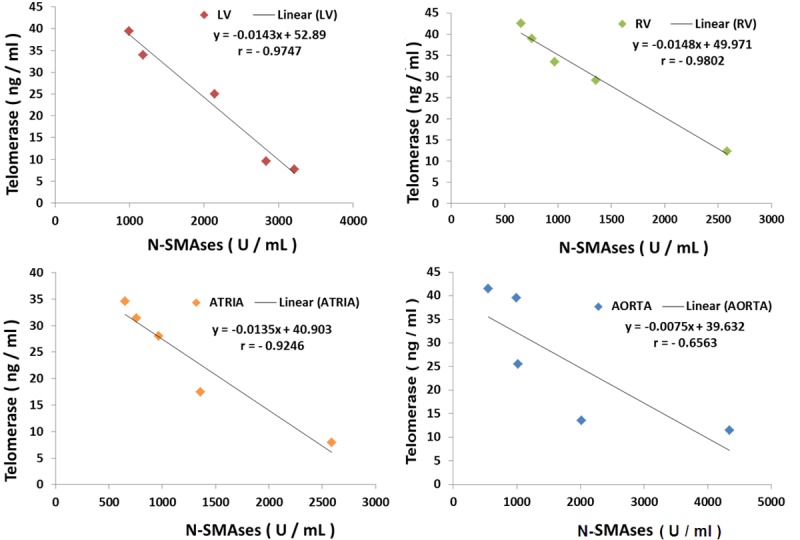
Inverse correlations of telomerase levels with N-SMASes in cardiovascular tissues.
Influence of dietary MgD on p53 levels in cardiac and vascular smooth muscles
The data presented in Figure 6 show that feeding rats a MgD diet for 21 days resulted in >250% rises in ventricular and vascular muscle cells and more than a 300% rise in atrial muscle cells, thus supporting other recent results [70].
Figure 6.
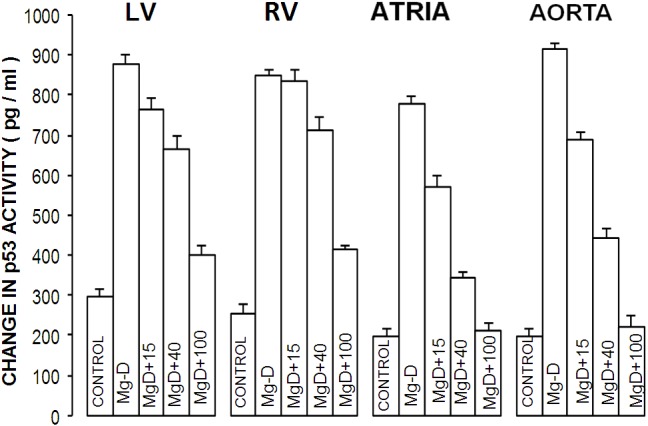
p53 levels in cardiovascular tissues in normal and MgD rats. All values are Means ± SE. All MgD mean values are significantly different from controls (P<0.001, ANOVA).
Inverse correlation of p53 levels with telomerase in MgD animals
Linear regression analyses shown in Figure 7 demonstrate strong inverse correlations of telomerase levels with p53 levels (r=-0.66 to -0.99; P<0.01) with the LV, atrial and aortic smooth muscular tissues exhibiting the greatest inverse correlations, suggesting cross-talk between telomerase and p53.
Figure 7.
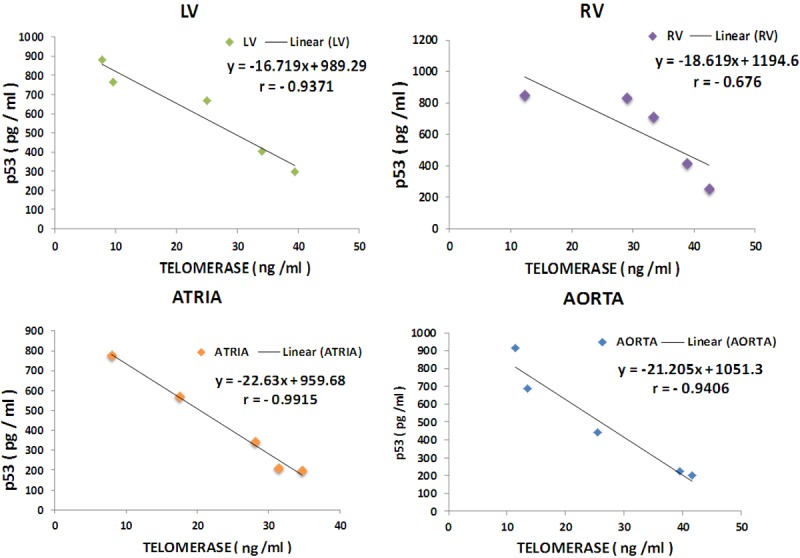
Inverse correlations of telomerase levels with p53 in cardiovascular tissues. Regression equations with r-values are as follows: 1) left ventricle vs. p53: y=-16-719x+989.29; r=0.9371; 2) right ventricle vs. p53: y=-18.619x+1194.6; r=0.676; 3) atria vs. p53: y=-22.63x+959.68; r=0.9915; and 4) aortic smooth muscle vs. p53: y=-1.205x+1051.3; r=0.9406.
Multiple regression analyses and correlations of p53 and N-SMAse levels with telomerase in MgD animals
The data summarized in Figure 8 using multiple regression analysis indicates high degrees of correlation of p53 and N-SMAse levels with the levels of telomerase (P<0.001). These data are suggestive of strong cross-talk between p53, N-SMAses and telomerases.
Figure 8.
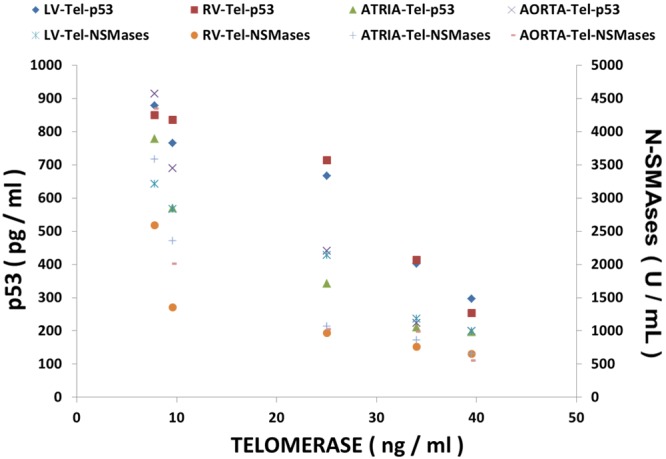
Multiple regression analysis of and N-SMAses and p53 with telomerase in cardiovascular tissues from normal and MgD rats with and without Mg2+ added to the DW.
Influence of MgD on 8-OHdG levels in cardiovascular tissues with and without Mg added to the drinking water: correlations to serum ionized Mg and tissue levels of N-SMASe, p53 and telomerase
Figure 9 indicates that dietary deficiency of Mg for 21 days results in a 7-10-fold increase in the formation of 8-OHdG in the four cardiovascular tissues. Between 40-100 mg/l of Mg in the drinking water is needed to restore the 8-OHdG to control levels. Although not shown, linear regression analysis of the data indicated that the lower the serum ionized Mg level, the greater the rise in oxidative DNA damage with high degrees of regression coefficients (r=-0.8 to -0.96). Likewise, analysis of the linear regression data indicated high positive correlations of the tissue levels of 8-OHdG with the upregulation of N-SMAse and p53 as well as with the downregulation of the tissue levels of telomerase (e.g., r=0.75-0.98; data not shown).
Figure 9.
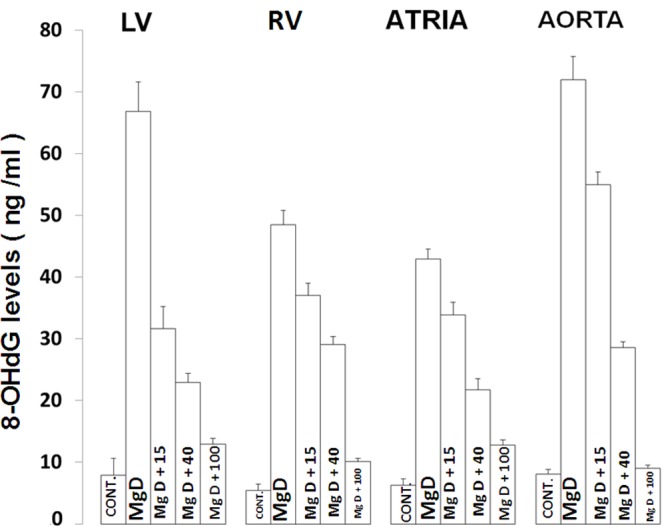
8-OHdG levels in cardiovascular tissues of MgD and control rats with and without Mg2+ added to the drinking water. N=14-16 animals per group. All values are Means ± SE. All mean values are significantly different from controls (P<0.01).
Discussion
The results reported here is the first demonstration that the enzyme telomerase, required for length of telomeres in all eukaryote cell types, is down-regulated in all chambers of the heart as well as in aortic smooth muscle in animals exposed to short-term magnesium deficiency. We also show for the first time that 21 days of Mg deficiency results in increased formation of 8-OH-dG which strongly suggests that the DNA in all the cardiovascular tissues assayed has undergone oxidation. In addition, we also confirm that short-term Mg deficiency upregulates N-SMAse and p53 in cardiovascular cardiac and vascular smooth muscle cells demonstrated recently [57,70]. When taken together with other data, generated by our laboratories [5,6,46-48,57,64,67,70-74], we believe our new data strengthens our hypothesis that even short-term Mg deficiency will result in atherosclerosis, hypertension, and cardiac failure. The new data on 8-OH-dG could strengthen the hypothesis suggested many years ago [75] that Mg deficiency, unless checked early, probably will contribute in a major way to the aging process. The fact that short-term Mg deficiency leads to an upregulation of N-SMAse in all cardiovascular muscular tissues examined [47,57] lends considerable support to the idea, advanced previously, that ceramide production and possibly other sphingolipid products are pivotal pathways in atherogenesis, hypertension, and heart failure [5,6,47,48], all of which are involved in the aging process. Our new results also demonstrate that addition of 40-100 mg/Mg added to the drinking water can completely prevent all of the vascular and molecular anomalies produced by Mg deficient diets reported herein.
Aging is now agreed to be critical in the etiology of metabolic decline in most subjects as they close-in on their 65th birthday. Many subjects at 65 years of age show signs of metabolic decline, atherosclerosis, high blood pressure, cardiovascular diseases, and often type 2 diabetes, which eventually contribute to congestive heart failure by their 75th-85th year. It is, thus, of considerable interest, here, to remind the reader that all of these attributes have been associated, both experimentally and clinically, with the presence of Mg-deficient states where they have been looked for and measured properly [5,6,28,32,33,38,39,41-43]. The aging process is also associated with an increase in the levels of proinflammatory cytokines in tissues and cells. Interestingly, recent findings in Mg-deficient animals, tissues, and different cell types have shown elevated levels of many of the proinflammatory cytokines such as IL-1, IL-6, TNF-alpha, among others [57,71,76,77]. TNF-alpha is known to be associated negatively on telomerase activity in some cell types [59-61]. Recent experiments in short-term Mg -deficient animals and vascular smooth muscle cells have clearly demonstrated cross-talk between cytokines [and chemokines], ceramide generation, proto-oncogenes, p53, and activation of nuclear factor-kB pathways [51,70,71,76,77]. The present study lends further support for the hypothesis that even short-term Mg deficiency probably leads to the decline and activation of multiple molecular pathways which would contribute (or be in large measure responsible for cardiovascular dysfunction) in the normal aging process, particularly as only 20-30% or the North American population is ingesting the approximate RDA of Mg intake for adequate body functions.
The present results indicate strong correlations and cross-talk of telomerase downregulation in cardiovascular tissues and cells with serum ionized Mg levels, N-SMAse, p53 and 8-OHdG. The increased formation of 8-OHdG in all cardiovascular tissues and cells studied, herein, with declining serum ionized Mg levels which indicates increasing oxidation of DNA, supports ideas advanced years ago, that Mg deficiency could lead to multiple mutations in the genomes of multiple cell types [78-80]. Previous studies indicate that smoking [81-83], high BMI [84,85], and consumption of unprocessed meats [86] have been reported to correlate with short telomere length. A number of studies have recently reported distinct relationships between dietary biomarkers and telomere length. Interestingly, all of these latter factors have been shown to be associated with declining serum/blood levels of Mg, particularly ionized serum/plasma/blood levels of Mg [4-6,24,28,32,38,41-43,87-109]. A number of studies have recently reported distinct relationships between certain dietary markers and telomere length [59]. At least one study has reported a correlation of multivitamin intake with telomere length [110]. The present study, when viewed in light of findings on isolated endothelial cells, in culture, showing erosion of telomere length [45], would suggest that mutations and transformation of vascular and endothelial cells caused by Mg deficiency and oxidation of DNA (seen in atherogenesis, hypertension, and congestive heart failure) may be pivotal in the aging process, thus leading to multiple cardiovascular changes including high blood pressure, cardiac dysfunctions, and eventual cardiac failure. Twenty-five years ago, our group demonstrated, for the first time, the importance of the endothelial lining of blood vessels in the responses of arterial muscle to lack of Mg [111]. Although the critical role of endothelial cells in the vascular responses to Mg deficiency was made by us long ago, including their importance in control of the microcirculation [6,37,44] and in lipid deposition on the arterial walls in experimental Mg deficiency [16], these seminal results appear to be overlooked by more recent, younger investigators [112]. Chronic stress has been long-associated with Mg deficiency [4] and recently with decreases in telomere length and decreased telomerase activity [59]. Oxidative stress (e.g., increased cell levels of 8-OhdG) has recently been shown to promote telomeric erosion [113]. In this regard, we have shown recently that short-term Mg deficiency, using the same animal model, herein, was demonstrated to cause marked reductions in the cellular levels of the free radical scavenger glutathione in cardiovascular tissues and cells [67] coupled to fragmentation of DNA and activation of several nitric oxide synthases [48,67], which would support the hypothesis that Mg deficiency can cause mutations in many types of cells. Oxidative stress caused by Mg deficiency has been demonstrated to promote the induction of reactive oxygen species (e.g., the hydroxyl radical) and reactive nitrogen species such as peroxynitrite, both of which we have shown to be formed in cardiovascular tissues and cells exposed to Mg deficiency (unpublished findings). It should be pointed out that normal amounts of telomerase in all cell types are required to promote efficient cell cycle kinetics and normal cell growth [58-61]. Mg deficiency betes, which eventually contribute to congestive heart failure by their 75th-85th year. It is, thus, of considerable interest, here, to remind the reader that all of these attributes have been associated, both experimentally and clinically, with the presence of Mg-deficient states where they have been looked for and measured properly [5,6,28,32,33,38,39,41-43]. The aging process is also associated with an increase in the levels of proinflammatory cytokines in tissues and cells. Interestingly, recent findings in Mg-deficient animals, tissues, and different cell types have shown elevated levels of many of the proinflammatory cytokines such as IL-1, IL-6, TNF-alpha, among others [57,71,76,77]. TNF-alpha is known to be associated negatively on telomerase activity in some cell types [59-61]. Recent experiments in short-term Mg -deficient animals and vascular smooth muscle cells have clearly demonstrated cross-talk between cytokines [and chemokines], ceramide generation, proto-oncogenes, p53, and activation of nuclear factor-kB pathways [51,70,71,76,77]. The present study lends further support for the hypothesis that even short-term Mg deficiency probably leads to the decline and activation of multiple molecular pathways which would contribute (or be in large measure responsible for cardiovascular dysfunction) in the normal aging process, particularly as only 20-30% or the North American population is ingesting the approximate RDA of Mg intake for adequate body functions. The present results indicate strong correlations and cross-talk of telomerase downregulation in cardiovascular tissues and cells with serum ionized Mg levels, N-SMAse, p53 and 8-OHdG. The increased formation of 8-OHdG in all cardiovascular tissues and cells studied, herein, with declining serum ionized Mg levels which indicates increasing oxidation of DNA, supports ideas advanced years ago, that Mg deficiency could lead to multiple mutations in the genomes of multiple cell types [78-80]. Previous studies indicate that smoking [81-83], high BMI [84,85], and consumption of unprocessed meats [86] have been reported to correlate with short telomere length. A number of studies have recently reported distinct relationships between dietary biomarkers and telomere length. Interestingly, all of these latter factors have been shown to be associated with declining serum/blood levels of Mg, particularly ionized serum/plasma/blood levels of Mg [4-6,24,28,32,38,41-43,87-109]. A number of studies have recently reported distinct relationships between certain dietary markers and telomere length [59]. At least one study has reported a correlation of multivitamin intake with telomere length [110]. The present study, when viewed in light of findings on isolated endothelial cells, in culture, showing erosion of telomere length [45], would suggest that mutations and transformation of vascular and endothelial cells caused by Mg deficiency and oxidation of DNA (seen in atherogenesis, hypertension, and congestive heart failure) may be pivotal in the aging process, thus leading to multiple cardiovascular changes including high blood pressure, cardiac dysfunctions, and eventual cardiac failure. Twenty-five years ago, our group demonstrated, for the first time, the importance of the endothelial lining of blood vessels in the responses of arterial muscle to lack of Mg [111]. Although the critical role of endothelial cells in the vascular responses to Mg deficiency was made by us long ago, including their importance in control of the microcirculation [6,37,44] and in lipid deposition on the arterial walls in experimental Mg deficiency [16], these seminal results appear to be overlooked by more recent, younger investigators [112]. Chronic stress has been long-associated with Mg deficiency [4] and recently with decreases in telomere length and decreased telomerase activity [59]. Oxidative stress (e.g., increased cell levels of 8-OhdG) has recently been shown to promote telomeric erosion [113]. In this regard, we have shown recently that short-term Mg deficiency, using the same animal model, herein, was demonstrated to cause marked reductions in the cellular levels of the free radical scavenger glutathione in cardiovascular tissues and cells [67] coupled to fragmentation of DNA and activation of several nitric oxide synthases [48,67], which would support the hypothesis that Mg deficiency can cause mutations in many types of cells. Oxidative stress caused by Mg deficiency has been demonstrated to promote the induction of reactive oxygen species (e.g., the hydroxyl radical) and reactive nitrogen species such as peroxynitrite, both of which we have shown to be formed in cardiovascular tissues and cells exposed to Mg deficiency (unpublished findings). It should be pointed out that normal amounts of telomerase in all cell types are required to promote efficient cell cycle kinetics and normal cell growth [58-61]. Mg deficiency is well-known to promote disturbances in cell cycle kinetics [79,114] via reactive oxygen and reactive nitrogen species [77] most-likely acting to help downregulate telomerase.
Based on the data shown, herein, and published elsewhere [47,48,57,71], we believe that the upregulation of N-SMAse, which is induced in all types of rat cardiovascular tissues and cells, in short-term Mg deficiency (results herein and elsewhere [47,48,57,71]), results in the formation of ceramide, which probably acts to downregulate telomerase observed in the present study. Previously, other workers utilizing a variety of cell types in normal and tumorous cells have demonstrated that ceramide can inhibit telomerase activities [115,116]. Most of these studies, however, were done with cell lines, not primary cells. Two of us have shown in primary cultured rat aortic VSM cells and canine cerebral VSM cells that the lower the concentration of Mg2+, in the medium, the higher the level of ceramide production in the primary cultured VSM cells [47,48,57,71,73]. SM synthesis, which is catalyzed by SM synthase [117], requires the transfer of a choline phosphate moiety from phosphatidyl choline (PC) to ceramide, generating 1,2-diacylglycerol. N-SMAse regenerates ceramide and choline phosphate. Recently, we clearly showed that Mg deficiency does, indeed, result in production of ceramide when using radiolabeling techniques in the cardiovascular tissues and cells [47,48,57,71,73]. Other experiments performed by our labs seem to indicate that products of ceramide metabolism such as sphingosine, sphingosine-1-phosphate, or glycerylsphingolipids are not produced in high concentration on hydrolysis of SM in either primary cultured peripheral VSM cells or primary cultured cerebral VSM cells [71]. In the present study, we confirm that the tumor suppressor p53 is upregulated in cardiovascular tissues obtained from animals subjected to 21 days of Mg deficiency [70]. P53 (often found in diverse normal cell types) functions , primarily, as a transcription factor [118] and has been shown by several of us to be important in Mg-deficient induced apoptosis in rat cardiovascular tissues and cells [70], and is known to be important in ceramide-induced cell death (i.e., apoptosis and necrosis) in the model of Mg deficiency used herein [70], as well as in other models of disease states [118,119], it would seem that ceramide and p53 act in concert to inhibit telomerase.
The new data presented, in the study herein, showing a large increase in 8-OHdG in all the cardiovascular tissues and cells assayed, when viewed in light of other data [5,6,46-48,57,64-67,70-72,74] which demonstrate increased DNA fragmentation, reduced cellular levels of glutathione and increased generation of nitric oxide synthases, make a strong case for low Mg-induced oxidative cellular stress, which could lead to proinflammatory states (e.g., increased cellular levels of cytokines and chemokines [71,76]), atherogenesis, hypertension and eventual cardiac failure. This scenario could, in part, be a consequence of some mutations in the genome caused by the Mg-deficient state activating a cascade of transcription factors, e.g., NF-kB, protein kinases, and proto-oncogenes. Interestingly, all of the latter transcription factors have been shown by our group to be activated in cardiovascular tissues and cells obtained from rats subjected to 21 days of Mg deficiency [48,57,67,70,71,73]. It is important, here, to recall that using this same model of Mg deficiency, we demonstrated upregulation of several cytokines and chemokines, such as IL-1beta, IL-6, TNF-alpha, and interferon-gamma, among others in the cardiovascular tissues and cells [71,76]. N-SMAse has recently been shown, in several cell types to increase in rat strial and hippocampal cells along with concomitant elevations in several of the latter proinflammatory biomarkers as the animals aged [120]. It is of considerable interest to point out that, using the 21 day model of short-term Mg deficiency, we found that the N-SMAse inhibitor, scyphostatin, attenuated greatly the elevation in N-SMASe cellular levels as well as the diverse cytokine levels in the VSM cells concomitant with reduction in upregulation of NF-kB [57,76]. When taken, together, with our new findings, we believe that Mg-deficiency- induced downregulation of telomerase activities are in all likelihood a consequence of upregulation of N-SMASe, p53 upregulation, stress-induced oxidation, and cytokine formation (and release) coupled to an upregulation of NF-kB. We believe this hypothesis can be tested in Mg-deficient animals treated with scyphostatin or another more specific inhibitor of N-SMAse.
Approximately 20 years ago, two of us advanced the hypothesis that Mg2+ ions function as extracellular signals in cardiovascular pathobiology [121]. The new studies presented, herein, when taken together with other in-vivo and in-vitro studies [5,6,9,13,22,25-28,34,38,41,46-48,57,64-67,70-74,76,87,108,111,121-134], support our hypothesis that Mg2+ ions play important regulatory roles in the evolution of cardiovascular pathobiology and are extracellular signaling ions. Mg2+ plays pivotal roles in regulation of cardiac hemodynamics, vascular tone, vascular reactivity, endothelial functions, carbohydrate, nucleotide and lipid metabolism, prevention of free radical formation and stabilization of the genome. As reviewed above and elsewhere [5,6,25-29,64,74,121-126,129,132], Mg2+ exerts key roles in control of Ca2+ uptake, subcellular content, and subcellular distribution in smooth muscle cells, endothelial cells, and cardiac muscle cells. The advent, and clinical utilization, of new Mg2+ ion-selective electrodes has ushered-in a new era of technology for the diagnosis and potential treatment of hypertension, ischemic heart disease, atherogenesis, peripheral vascular diseases, diabetic-related vascular diseases, preeclamptic-eclamptic disorders in women, sickle cell anemic-related vascular disease, trauma-shock injuries, cardiac failure, strokes, and organ transplantation [5,6,28,32-43,87-108]. Since most of these disorders (or clinical situations) become worsened with age or take place in the natural-aging process, and are now known (from our studies) to be associated with varying degrees of Mg deficiency, the role of Mg in carbohydrate, lipid, nucleotide, RNA, DNA, and protein metabolism, and the effects of Mg deficiency, particularly on sphingolipid metabolism, appear to play pivotal roles on telomerase activities and telomeres and, most likely, the genome.
A few years ago, we suggested that Mg deficiency, by itself, probably acts as a genotoxic agent [70]. As is now known, one of ceramides major pathophysiological actions is its ability to induce cell differentiation and transformation [135,136]. Abnormal cell differentiation, transformation, and growth are pivotal events in the development of atherogenesis, hypertension, and cardiac failure. Hyperplasia and cardiovascular hypertrophy are common events in aging, atherosclerosis, hypertension, and cardiac failure. However, the precise mechanisms regulating alterations in tissue mass are not completely understood [3]. The tumor suppressor protein p53, ceramide, and telomerases are now known to play key roles in cell transformation, growth, apoptotic events, and the aging process [58-62,118,119,137]. Both ceramide and p53 can induce cell cycle arrest (and senescence), induce programmed cell death, and are associated with DNA damage (genotoxic events) [118,119,137-139]. As reviewed above, and elsewhere [5,6,9,13,16,17,24,27-29,30,37,45,48,57,67,70-76,78,121,134], MgD can produce all three of these pathophysiological events in multiple cell types, including cardiac and vascular smooth muscle cells. Recently, and herein, we have demonstrated that magnesium deficiency (via activation of p53) is a “driver” of ceramide synthesis through the activation of N-SMAses and ceramide synthase in diverse cardiac and vascular smooth muscle cells [47,48,57,70]. Interestingly, atherosclerotic plaques in vascular walls of hypertensive subjects demonstrate considerable DNA damage, activation of DNA repair pathways, increased expression of p53, oxidation, and apoptosis [3,137,139], as well as increased levels of ceramide [70]. Experimentally, Mg deficiency results in accelerated atherogenesis in rabbit arterial walls [16] which has been associated with increased levels of p53 in the thickened atherosclerotic plaques [70], and decreased levels of telomerase (unpublished findings). We, thus, hypothesize that Mg deficiency plays key roles in the generation of atherosclerotic plaques and hypertension via the upregulation of N-SMAses, p53, ceramide synthase, and ceramide production as well as the downregulation of telomerases, particularly as the majority of individuals who consume Western-type diets have 45-70% short falls in daily dietary intake of Mg. These events would all be accelerated in the aging population, particularly in old-age and nursing homes where the daily intake of Mg often shows short falls in Mg often approaching 80-90% [3].
In view of the present findings and those previously published [5,6,46-48,57,64,65,67,70,71,73,74,76,121], we would be remiss if some discussion regarding the potential role of epigenetics to Mg deficiency’s long-term effects on the aging process was not pointed out here. Overall, the process of epigenetics provides every cell with its special identity. All organisms begin as a single cell, which divides through a process of stem cells creating a mass, via a series of carefully-designed changes in gene expression, which is required to form the tissues and cells of the fetal organism. The process of epigenetics orchestrates which genes have to be turned-on in each type of cell, and then maintains the particular type of gene expression or, in other words, the particular cell’s molecular identity via how DNA encodes the gene [140,141]. Anything that produces modifications in the chromatin structure can affect a particular gene expression via transcription [140,141]. Thus, if Mg-deficient states are, indeed, genotoxic as we have suggested [70], then the chromatin structure of one or more cell types (e.g., cardiac, endothelial or vascular) could be modified and affect one or more genes and cell phenotype. DNA methylation, histone modification, and microRNA alterations are known epigenetic mechanisms [140-142]. We believe in view of the current report, and other works recently published by our labs (reviewed above), prolonged Mg deficiency should be categorized as another epigenetic mechanism. Hopefully, this hypothesis will be tested shortly and prove to be critical in the senescent process and its amelioration of numerous events associated with aging.
Last, but not least, our new results bolster the idea that water intake (e.g., from tap waters, well waters, bottled waters, beverages using tap/well/spring waters, or desalinated waters) in humans should contain at least 25-40 mg Mg2+/l [57,67,70,71,73,76]. The latter inclusion in our diets should go a long-way towards the prevention of cardiovascular diseases and ameliorate the aging process of bodily tissues and cells in humans worldwide.
Acknowledgements
The authors appreciate the gratis supply of magnesium aspartate-HCl which was provided by Dr. Angela Weigert of Verla Pharm (Tutzing, Germany). The authors also appreciate the invaluable technical assistance of J-P Liu and Malik Zulqarnain. This work was supported, in part, by NIH grants to B.M. Altura and X-C. Jiang).
Disclosure of conflict of interest
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- 1.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 2.Mulvany MJ. Small artery remodeling and significance in the development of hypertension. News Physiol Sci. 2002;17:105–109. doi: 10.1152/nips.01366.2001. [DOI] [PubMed] [Google Scholar]
- 3.Kumar V, Abbas AK, Fasuto N, Aster JC, editors. Robbins and Cotran Pathologic Basis of Disease. 8th edition. 2010. pp. 487–506. [Google Scholar]
- 4.Seelig MS. Magnesium Deficiency in the Pathogenesis of Disease. New York: Plenum; 1980. [Google Scholar]
- 5.Altura BM, Altura BT. Magnesium: forgotten mineral in cardiovascular biology and angiogenesis. In: Nishizawa N, Morii H, Durlach J, editors. New Perspectives in Magnesium Research. 2007. pp. 239–260. [Google Scholar]
- 6.Altura BM, Altura BT. Magnesium and cardiovascular biology: an important link between cardiovascular risk factors and atherogenesis. Cell Mol Biol Res. 1995;41:347–359. [PubMed] [Google Scholar]
- 7.Ford ES, Mokdad AH. Dietary magnesium intake in a national sample of US adults. J Nutr. 2003;121:2879–2882. doi: 10.1093/jn/133.9.2879. [DOI] [PubMed] [Google Scholar]
- 8.Mosfegh A, Goldman J, Abuja J, Rhodes D, LaComb R. What We Eat in America. NHANES 2005-2006: Usual Intakes from Food and Water Compared to 1997 Dietary Reference Intakes for Vitamin D, Calcium, Phosphorus, and Magnesium. Washington, DC. U.S. Department of Agricultural Research Service. 2009 [Google Scholar]
- 9.Altura BM, Altura BT, Gebrewold A, Ising H, Günther T. Magnesium deficiency and hypertension: correlation between magnesium deficient diets and microcirculatory changes in situ. Scirnce. 1984;223:1315–1317. doi: 10.1126/science.6701524. [DOI] [PubMed] [Google Scholar]
- 10.Marier JR, Neri LC. Quantifying the role of magnesium in the interrelationship between human mortality/morbidity and water hardness. Magnesium. 1985;4:53–59. [PubMed] [Google Scholar]
- 11.Leary WP. Content of magnesium in drinking water and deaths from ishemic heart disease in white South Africans. Magnesium. 1986;5:150–153. [PubMed] [Google Scholar]
- 12.Chipperfield B, Chipperfield JR. Relation of myocardial metal concentration to water hardness and death from ischemic heart disease. Lancet. 1967;2:709–712. doi: 10.1016/s0140-6736(79)90641-x. [DOI] [PubMed] [Google Scholar]
- 13.Altura BM, Altura BT, Gebrewold A, Gunther T, Ising H. Noise-induced hypertension and magnesium: relationship to microcirculation and magnesium. J Appl Physiol. 1992;172:194–202. doi: 10.1152/jappl.1992.72.1.194. [DOI] [PubMed] [Google Scholar]
- 14.Marx A, Neutra RR. Magnesium in drinking water and deaths from ischemic heart disease. Epidemiol Rev. 1997;19:258–272. doi: 10.1093/oxfordjournals.epirev.a017957. [DOI] [PubMed] [Google Scholar]
- 15.Rubenowitz E, Molin L, Axellson G, Rylander R. Magnesium in drinking water in relation to morbidity and mortality from acute myocardial infarction. Epidemiology. 2003;11:416–421. doi: 10.1097/00001648-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Altura BT, Brust M, Bloom S, Barbour RL, Stempak JG, Altura BM. Magnesium deficiency modulates blood lipid levels and atherogenesis. Proc Natl Acad Sci U S A. 1990;87:1840–1844. doi: 10.1073/pnas.87.5.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouchi Y, Tabata RE, Stegiopoulos K, Sato K, Hattori A, Orimo H. Effect of dietary magnesium on development of atherosclerosis in cholesterol-fed rabbits. Arteriosclerosis. 1990;10:732–737. doi: 10.1161/01.atv.10.5.732. [DOI] [PubMed] [Google Scholar]
- 18.Ma J, Folsom AR, Melnick SL, Eckfeddt JH, Sharett AR, Nabulsi AA. Association of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: the ARIC study. J Clin Epidemiol. 1995;48:927–940. doi: 10.1016/0895-4356(94)00200-a. [DOI] [PubMed] [Google Scholar]
- 19.Song Y, Manson J, Cook N, Albert C, Buring J, Liu S. Dietary magnesium intake and risk of cardiovascular disease among women. Am J Cardiol. 2005;96:1135–1141. doi: 10.1016/j.amjcard.2005.06.045. [DOI] [PubMed] [Google Scholar]
- 20.King JL, Miller RJ, Blue JP Jr, O’Brien WD Jr, Erdman JW Jr. Inadequate dietary magnesium intake increases atherossclerotic plaque development in rabbits. Nutr Res. 2009;29:343–349. doi: 10.1016/j.nutres.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amighi J, Sabeti S, Schlager O, Mlekusch W, Exner M, Labouschek W. Low serum magnesium predicts neurological events in patients with advanced atherosclerosis. Stroke. 2004;35:22–27. doi: 10.1161/01.STR.0000105928.95124.1F. [DOI] [PubMed] [Google Scholar]
- 22.Turlapaty PDMV, Altura BM. Magnesium deficiency produces spasms of coronary arteries: relationship to etiology of sudden death ischemic heart disease. Science. 1980;208:198–200. doi: 10.1126/science.7361117. [DOI] [PubMed] [Google Scholar]
- 23.Crawford T, Crawford MD. Prevalance and pathological changes of ischemic heart disease in a hard-water area. Lancet. 1967;1:229–232. doi: 10.1016/s0140-6736(67)91297-4. [DOI] [PubMed] [Google Scholar]
- 24.Altura BM. Ischemic heart disease and magnesium. Magnesium. 1988;7:57–67. [PubMed] [Google Scholar]
- 25.Turlapaty PDMV, Altura BM. Extracellular magnesium ions control calcium exchange and content of vascular smooth muscle. Eur J Pharmacol. 1978;52:421–423. doi: 10.1016/0014-2999(78)90303-5. [DOI] [PubMed] [Google Scholar]
- 26.Altura BM, Altura BT. Magnesium modulates calcium entry and contractility in vascular smooth muscle. In: Onishi T, Endo M, editors. The Mechanism of Gated Calcium Transport Across Biological Membranes. 1981. pp. 137–145. [Google Scholar]
- 27.Altura BM. Magnesium and regulation of contractility of vascular smooth muscle. Adv in Microcirculation. 1982;11:77–113. [Google Scholar]
- 28.Altura BM, Altura BT. Magnesium in cardiovascular biology. Scientific Am Sci Med. 1995;2:28–37. [Google Scholar]
- 29.Touyz RM. Role of magnesium in the pathogenesis of hypertension. Mol Aspects Med. 2003;24:107–136. doi: 10.1016/s0098-2997(02)00094-8. [DOI] [PubMed] [Google Scholar]
- 30.Gimenez MS, Oliveros LB, Gomez NN. Nutritional deficiencies and phospholipid metabolism. Int J Mol Sci. 2011;12:2408–2433. doi: 10.3390/ijms12042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luthringer C, Rayssiguier Y, Gueux E, Berthelot A. Effect of moderate magnesium deficiency on serum lipids, blood pressure and cardiovascular reactivity in normotensive rats. Br J Nutr. 1988;59:243–250. doi: 10.1079/bjn19880031. [DOI] [PubMed] [Google Scholar]
- 32.Saris NE, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A. Magnesium, an update on physiological, clinical and analytical aspects. Clin Chim Acta. 2000;294:1–26. doi: 10.1016/s0009-8981(99)00258-2. [DOI] [PubMed] [Google Scholar]
- 33.Altura BT, Altura BM. Measurement of ionized magnesium in whole blood, plasma and serum with a new ion-selective electrode in healthy and diseased human subjects. Magnes Trace Elem. 1991;10:90–98. [PubMed] [Google Scholar]
- 34.Altura BT, Memon ZI, Zhang A, Cracco RQ, Altura BM. Low levels of serum ionized magnesium are found in patients early after stroke which result in rapid elevation in cytosolic free calcium and spasm in cerebral vascular smooth muscle cells. Neurosci Lett. 1997;230:37–40. doi: 10.1016/s0304-3940(97)00471-0. [DOI] [PubMed] [Google Scholar]
- 35.Handwerker SM, Altura BT, Royo B, Altura BM. Ionized magnesium and calcium levels in umbilical cord serum of pregnant women with transient hypertension during labor. Am J Hypertens. 1993;6:542–545. doi: 10.1093/ajh/6.6.542. [DOI] [PubMed] [Google Scholar]
- 36.Altura BM, Altura BT. Role of magnesium and calcium in alcohol-induced hypertension and strokes as probed by in-vivo television microscopy, digital image microscopy, optical spectroscopy, 31P-NMR spectroscopy and a unique magnesium ion-sensitive electrode. Alcohol Clin Exp Res. 1994;18:1057–1068. doi: 10.1111/j.1530-0277.1994.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 37.Altura BM, Altura BT. Role of magnesium in the pathogenesis of hypertension updated: Relationship to its actions on cardiac, vascular smooth muscle, and endothelial cells. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis, and Management. 2nd edition. New York: Raven Press; 1995. pp. 1213–1242. [Google Scholar]
- 38.Altura BM, Altura BT. Role of magnesium in pathophysiological processes and the clinical utility of magnesium ion selective electrodes. Scand J Clin Lab Invest. 1996;56:211–234. doi: 10.3109/00365519609088642. [DOI] [PubMed] [Google Scholar]
- 39.Markell MS, Altura BT, Barbour RL, Altura BM. Ionized and total magnesium levels in cyclosporin-treated renal transplant recipients: relationship with cholesterol and cyclosporin levels. Clin Sci. 1993;85:315–318. doi: 10.1042/cs0850315. [DOI] [PubMed] [Google Scholar]
- 40.Markell MS, Altura BT, Sarn Y, Delano BG, Hudo O, Friedman EA, Altura BM. Deficiency of serum ionized magnesium in patients receiving hemodialysis or peritoneal dialysis. ASAIO J. 1993;39:M801–M804. [PubMed] [Google Scholar]
- 41.Altura BM, Lewenstam A. Unique magnesium ion-selective electrodes. Scand J Clin Lab Invest. 1994;54:1–100. [Google Scholar]
- 42.Resnick LM, Altura BT, Gupta RK, Alderman MH, Altura BM. Intracellular and extracellular magnesium depletion in type 2 diabetes (non-insulin-dependent) diabetes mellitus. Diabetologia. 1993;36:767–770. doi: 10.1007/BF00401149. [DOI] [PubMed] [Google Scholar]
- 43.Bardicef M, Bardicef O, Sorokin Y, Altura BM, Altura BT, Cotton DB, Resnick LM. Extracellular and intracellular magnesium depletion in pregnancy and gestational diabetes. Am J Obstet Gynecol. 1996;172:1009–1013. doi: 10.1016/0002-9378(95)90035-7. [DOI] [PubMed] [Google Scholar]
- 44.Yang ZW, Gebrewold A, Nowakowski M, Altura BT, Altura BM. Mg2+-induced endothelium-dependent relaxation of blood vessels and blood pressure lowering: role of NO. Am J Physiol Regul Integr Comp Physiol. 2000;278:R628–R639. doi: 10.1152/ajpregu.2000.278.3.R628. [DOI] [PubMed] [Google Scholar]
- 45.Kilililea DW, Ames BN. Magnesium deficiency accelerates cellular senescence in cultured human fibroblasts. Proc Natl Acad Sci U S A. 2008;105:5768–5773. doi: 10.1073/pnas.0712401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrill GA, Gupta RK, Kostellow AB, Ma GY, Zhang A, Altura BT, Altura BM. Mg2+ modulates membrane lipids in vascular smooth muscle: a link to atherogenesis. FEBS Lett. 1997;408:191–197. doi: 10.1016/s0014-5793(97)00420-1. [DOI] [PubMed] [Google Scholar]
- 47.Morrill GA, Gupta RK, Kostellow AB, Ma GY, Zhang A, Altura BT, Altura BM. Mg2+ modulates membrane sphingolipids and lipid second messengers in vascular smooth muscle cells. FEBS Lett. 1998;440:167–171. doi: 10.1016/s0014-5793(98)01446-x. [DOI] [PubMed] [Google Scholar]
- 48.Altura BM, Shah NC, Jiang XC, Li Z, Perez-Albela JL, Sica AC, Altura BT. Short-term magnesium deficiency results in decreased levels of serum sphingomyelin, lipid peroxidation, and apoptosis in cardiovascular tissues. Am J Physiol Heart Circ Physiol. 2009;297:H86–H92. doi: 10.1152/ajpheart.01154.2008. [DOI] [PubMed] [Google Scholar]
- 49.Andrieu-Abadie N, Gouaze V, Salvayre R, Levade T. Ceramide in apoptosis signaling: relationship with oxidative stress. Free Radic Biol Med. 2001;31:717–728. doi: 10.1016/s0891-5849(01)00655-4. [DOI] [PubMed] [Google Scholar]
- 50.Birbes H, Bawab SE, Hannun YA, Obeid LM. Selective hydrolysis of a mitochondrial pool of sphingomyelin induces apoptosis. FASEB J. 2001;14:2669–2679. doi: 10.1096/fj.01-0539com. [DOI] [PubMed] [Google Scholar]
- 51.Hannun YA, Obeid LM. The ceramide-centric universe of lipid -mediated regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277:25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- 52.Okazaki T, Bielawska AE, Bell RM, Hannun YA. Role of ceramide as a lipid mediator of 1, alpha, 25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J Biol Chem. 1990;265:15823–15831. [PubMed] [Google Scholar]
- 53.Pandey S, Murphy RF, Agrawal DK. Recent advances in the immunobiology of ceramide. Exp Mol Pathol. 2007;82:298–309. doi: 10.1016/j.yexmp.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wyman M, Schneider R. Lipid signaling in disease. Nat Rev Mol Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 55.Hage-Sleiman R, Esmerian MO, Kobeissy H, Dbaibo G. p53 and ceramide as collaborators in the stress response. Int J Mol Sci. 2013;14:4982–5012. doi: 10.3390/ijms14034982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aguilera-Romero A, Gehin C, Reizman H. Sphingolipid homeostatsis in the web of metabolic routes. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbalip.2013.10.014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 57.Altura BM, Shah NC, Shah GJ, Li W, Zhang A, Zheng T, Li Z, Jiang XC, Perez-Albela JL, Altura BT. Magnesium deficiency upregulates sphingomyelinases in cardiovascular tissues and cells: cross-talk among proto-oncogenes, Mg2+, NF-kB and ceramide and their potential relationships to resistant hypertension, atherogenesis and cardiac failure. Int J Clin Exp Med. 2013;6:861–879. [PMC free article] [PubMed] [Google Scholar]
- 58.Fleisig HB, Wong JMY. Telomerase promotes efficient cell cycle kinetics and confers growth advantage to telomerase-negative transformed cells. Oncogene. 2012;31:954–965. doi: 10.1038/onc.2011.292. [DOI] [PubMed] [Google Scholar]
- 59.Lin J, Epel E, Blackburn E. Telomeres and life style factors: Roles in cellular aging. Mutat Res. 2012;730:85–89. doi: 10.1016/j.mrfmmm.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Wong JM, Collins K. Telomere maintenance and disease. Lancet. 2003;362:983–988. doi: 10.1016/S0140-6736(03)14369-3. [DOI] [PubMed] [Google Scholar]
- 61.Dong CK, Mastomi K, Han WC. Telomerase: regulation, function and transformation. Crit rev Oncol Hematol. 2005;54:85–93. doi: 10.1016/j.critrevonc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 62.Matsutomi K, Yu EY, Khurts S, Ben-Porath I, Currier JL, Metz GB. Telomerase maintains telomere structure in normal human cells. Cell. 2003;114:241–253. doi: 10.1016/s0092-8674(03)00550-6. [DOI] [PubMed] [Google Scholar]
- 63.Tejero-Taido MI, Chmielinska JJ, Weglicki WB. Chronic dietary Mg2+ deficiency induces cardiac apoptosis in the rat heart. Magnes Res. 2007;20:208–212. [PubMed] [Google Scholar]
- 64.Altura BM, Gebrewold A, Altura BT, Brautbar N. Magnesium depletion impairs cardiac carbohydrate and lipid metabolism and cardiac bioenergetics and raises myocardial calcium content in vivo: relationship to etiology of cardiac diseases. Biochem Molec Biol Int. 1996;40:1183–1190. doi: 10.1080/15216549600201823. [DOI] [PubMed] [Google Scholar]
- 65.Altura BM, Gebrewold A, Zhang A, Altura BT. Low extracellular magnesium ions induces lipid peroxidation and activation of nuclear factor kB in canine cerebral vascular smooth muscle: possible relation to traumatic brain injury and strokes. Neurosci Lett. 2003;341:189–192. doi: 10.1016/s0304-3940(03)00134-4. [DOI] [PubMed] [Google Scholar]
- 66.Wu F, Altura BT, Gao J, Barbour RL, Altura BM. Ferrylmyoglobin formation induced by acute magnesium deficiency in perfused rat heart causes cardiac failure. Biochim Biophys Acta. 1994;1225:158–164. doi: 10.1016/0925-4439(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 67.Shah NC, Liu JP, Iqbal J, Hussain M, Jiang XC, Li Z, Li Y, Zheng T, Li W, Sica AC, Perez-Albela JL, Altura BT, Altura BT. Mg deficiency results in modulation of serum lipids, glutathione, and NO synthase isozyme activation in cardiovascular tissues: relevance to de novo synthesis of ceramide, serum Mg and atherogenesis. Int J Clin Exp Med. 2011;4:103–118. [PMC free article] [PubMed] [Google Scholar]
- 68.Dickens BF, Weglicki WB, Li YS, Mak IT. Magnesium deficiency in vitro enhances free radical-induced intracellular oxidation and cytotoxicity in endothelial cells. FEBS Lett. 1992;311:187–191. doi: 10.1016/0014-5793(92)81098-7. [DOI] [PubMed] [Google Scholar]
- 69.Weglicki WB, Mak IT, Kramer JH, Dickens BF, Cassidy MM. Role of free radicals and substance P in magnesium deficiency. Cardiovasc Res. 1996;31:677–682. [PubMed] [Google Scholar]
- 70.Altura BM, Shah NC, Li Z, Jiang XC, Zhang A, Li W, Zheng T, Perez-Albela JL, Altura BT. Short-term magnesium deficiency upregulates sphingomyelin synthase and p53 in cardiovascular tissues and cells: relevance to de novo synthesis of ceramide. Am J Physiol Heart Circ Physiol. 2010;299:H2046–H2055. doi: 10.1152/ajpheart.00671.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Altura BM, Shah NC, Shah GJ, Zhang A, Li W, Zheng T, Perez-Albela JL, Altura BT. Short-term Mg-deficiency upregulates protein kinase C isoforms in cardiovascular tissues and cells; relation to NF-kB, cytokines, ceramide salvage sphingolipid pathway and PKC-zeta: hypothesis and review. Int J Clin Exp Med. 2014;6:1–21. [PMC free article] [PubMed] [Google Scholar]
- 72.Altura BM, Barbour RL, Dowd TL, Wu F, Altura BT, Gupta RK. Low extracellular magnesium induces intracellular free Mg deficits, depletion of high-energy phosphates and cardiac failure in intact working rat hearts: a 31P-NMR study. Biochim Biophys Acta. 1993;1182:329–332. doi: 10.1016/0925-4439(93)90077-e. [DOI] [PubMed] [Google Scholar]
- 73.Altura BM, Shah NC, Li Z, Jiang XC, Perez-Albela JL, Altura BT. Magnesium deficiency upregulates serine palmitoyltransferase (SPT 1 and SPT 2) in cardiovascular tissues: relationship to serum ionized Mg and cytochrome c. Am J Physiol Heart Circ Physiol. 2010;299:H932–938. doi: 10.1152/ajpheart.01076.2009. [DOI] [PubMed] [Google Scholar]
- 74.Altura BM, Kostellow AB, Zhang A, Li W, Morrill GA, Gupta RK, Altura BT. Expression of the nuclear factor-kB and proto-oncogenes c-fos and c-jun are induced by low extracellular Mg2+ in aortic and cerebral vascular smooth muscle cells: possible links to hypertension, atherogenesis and stroke. Am J Hypertens. 2003;16:701–707. doi: 10.1016/s0895-7061(03)00987-7. [DOI] [PubMed] [Google Scholar]
- 75.Durlach J. Magnesium and aeging. II. Clinical data: aettiological mechanisms and pathophysiological consequences of magnesium deficiency in the elderly. Magnes Res. 1993;6:379–394. [PubMed] [Google Scholar]
- 76.Altura BM, Shah NC, Shah GJ, Zhang A, Li W, Zheng T, Perez-Albela JL, Altura BT. Short-term magnesium deficiency upregulates ceramide synthase in cardiovascular tissues and cells: cross-talk among cytokines, Mg2+, NF-kB and de novo ceramide. Am J Physiol Heart Circ Physiol. 2012;302:H319–H332. doi: 10.1152/ajpheart.00453.2011. [DOI] [PubMed] [Google Scholar]
- 77.Weglicki WB. Hypomagnesemia and inflammation: Clinical and basic aspects. Annu Rev Nutr. 2012;32:55–71. doi: 10.1146/annurev-nutr-071811-150656. [DOI] [PubMed] [Google Scholar]
- 78.Rubin H. Differences in growth requirement and retentiveness for magnesium in non-transformed and transformed mouse 3T3 cells. Magnes. 1982;1:41–48. [Google Scholar]
- 79.Walker G. Magnesium and cell cycle control: an update. Magnesium. 1986;5:9–33. [PubMed] [Google Scholar]
- 80.Vernon WB. The role of magnesium in nucleic-acid and protein metabolism. Magnesium. 1988;7:234–248. [PubMed] [Google Scholar]
- 81.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 82.Babizhayev MA, Yegorov YE. Smoking and health: association between telomere length and factors impacting on human disease, quality of life and life span in a large population-based cohort under the effect of smoking duration. Fundam Clin Pharmacol. 2011 Aug;25:425–42. doi: 10.1111/j.1472-8206.2010.00866.x. [DOI] [PubMed] [Google Scholar]
- 83.Nawrot TS, Staessen JA, Holvoet P, Struijker-Boudier HA, Schiffers P, Van Bortel LM, Fagard RH, Gardner JP, Kimura M, Aviv A. Telomere length and its associations with oxidized-LDL, carotid artery distensibility and smoking. Front Biosci (Elite Ed) 2010;2:1164–1168. doi: 10.2741/e176. [DOI] [PubMed] [Google Scholar]
- 84.O’Callaghan NJ, Clifton PM, Noakes M, Fenech Ml. Weight loss in obese men is associated with increased telomere length and decreased abasic sites in rectal mucosa. Rejuvenation Res. 2009;12:169–176. doi: 10.1089/rej.2008.0819. [DOI] [PubMed] [Google Scholar]
- 85.Nettleton JA, Steffen LM, Palmas W, Burke GL, Jacobs DR Jr. Inverse association between adiposity and telomere length: the fels longitudinal study. Am J Human Biol. 2011;23:100–106. doi: 10.1002/ajhb.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nettleton JA, Steffen LM, Palmas W, Burke GL, Jacobs DR Jr. Associations between microalbuminuria and animal foods, plant foods, and dietary patterns in the Multiethnic Study of Atherosclerosis. Am J Clin Nutr. 2008;87:1825–1826. doi: 10.1093/ajcn/87.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Altura BT, Shirey T, Young CC, Hiti J, Dell’Orfano K, Handwerker S, Altura BM. A method for the rapid determination of ionized Mg2+ in whole blood, serum,and plasma. Methods Find Exp Clin Pharmacol. 1992 May;14:297–304. [PubMed] [Google Scholar]
- 88.Altura BT, Altura BM. A method for distingushing ionized, complex and protein-bound Mg in normal and diseased subjects. Scand J Clin Lab Invest. 1994;54:83–88. doi: 10.3109/00365519409095214. [DOI] [PubMed] [Google Scholar]
- 89.Altura BT, Wilimzig C, Trnovec T, Nyulassy S, Altura BM. Comparative effects of a Mg-enriched diet and different orally administered magnesium oxide preparations on ionized Mg, Mg metabolism and electrolytes in serum of human volunteers. J Am Coll Nutr. 1994;13:447–454. doi: 10.1080/07315724.1994.10718433. [DOI] [PubMed] [Google Scholar]
- 90.Memon Z, Altura BT, Benjamin JL, Cracco RQ, Altura BM. Predictive value of serum ionized but not total magnesium levels in head injuries. Scand J Clin Lab Invest. 1995;55:671–677. doi: 10.3109/00365519509075397. [DOI] [PubMed] [Google Scholar]
- 91.Handwerker S, Altura BT, Jones KY, Altura BM. Maternal-fetal transfer of ionized serum magnesium during stress of labor and delivery. J Am Coll Nutr. 1995;14:376–381. doi: 10.1080/07315724.1995.10718524. [DOI] [PubMed] [Google Scholar]
- 92.Fogh-Andersen N, Altura BM, Altura BT, Sigaard-Andersen O. Composition of interstitial fluid. Clin Chem. 1995;41:1522–1525. [PubMed] [Google Scholar]
- 93.Scott VL, DeWolf AM, Kang Y, Altura BT, Virji MA, Cook DR, Altura BM. Ionized hypomagnesemia in patients undergoing orthotopic liver transplantation: a complication of citrate intoxication. Liver Transpl Surg. 1996;2:343–347. doi: 10.1002/lt.500020503. [DOI] [PubMed] [Google Scholar]
- 94.Fogh-Andersen N, Altura BM, Altura BT, Sigaard-Andersen O. Changes in plasma ionized calcium and magnesium in blood donors after donation of 450 ml blood. Scand J Clin Lab Invest Suppl. 1996;224:245–250. doi: 10.3109/00365519609088644. [DOI] [PubMed] [Google Scholar]
- 95.Resnick LM, Bardicef D, Altura BT, Alderman MH, Altura BM. Serum ioized magnesium: relation to blood pressure and racial factors. Am J Hypertens. 1997;10:1420–1424. doi: 10.1016/s0895-7061(97)00364-6. [DOI] [PubMed] [Google Scholar]
- 96.Seelig MS, Altura BM. How best to determine magnesium status:a new laboratory test worth trying. Nutrition. 1997;13:376–377. [PubMed] [Google Scholar]
- 97.Seelig MS, Altura BM. How best to determine magnesium requirement: need to consider cardiotherapeutic drugs that affect its retention. J Am Coll Nutr. 1997;16:4–6. doi: 10.1080/07315724.1997.10718643. [DOI] [PubMed] [Google Scholar]
- 98.Muneyyrici-Delale O, Nacharaju VL, Altura BM, Altura BT. Sex steroid hormones modulate serum ionized magnesium throughout the menstrual cycle in women. Fertil Steril. 1998;69:958–962. doi: 10.1016/s0015-0282(98)00053-3. [DOI] [PubMed] [Google Scholar]
- 99.Muneyyrici-Dalale O, Nacharaju VL, Dalloul M, Altura BM, Altura BT. Serum ionized magnesium and calcium in women after menopause: inverse relationship of estrogen with ionized magnesium. Fertil Steril. 1999;71:869–873. doi: 10.1016/s0015-0282(99)00065-5. [DOI] [PubMed] [Google Scholar]
- 100.Djurhuus S, Henriksen JE, Klitgaard NAH, Blaaberg O, Thye-Ron P, Altura BM, Altura BT, Beck-Nielsen H. Effect of moderate improvement in metabolic control on magnesium and lipid concentrations in patients with type I diabetes. Diabetes Care. 1999;22:546–554. doi: 10.2337/diacare.22.4.546. [DOI] [PubMed] [Google Scholar]
- 101.Muneyyrici-Dalale O, Nacharaju VL, Altura BM, Altura BT. Serum ionized magnesium and calcium and sex hormones in healthy young men: importance of serum progesterone level. Fertil Steril. 1999;72:817–822. doi: 10.1016/s0015-0282(99)00386-6. [DOI] [PubMed] [Google Scholar]
- 102.Muneyyrici-Dalale O, Nacharaju VL, Dalloul M, Jalou S, Rahman M, Altura BM, Altura BM, Altura BT. Divalent cations in PCOS: implications for cardiovascular disease. Gynecol Endocrinol. 2001;15:198–201. [PubMed] [Google Scholar]
- 103.Djurhuus S, Klitgaard NAH, Pedersen KK, Blaaberg O, Altura BM, Altura BT, Henriksen JE. Magnesium reduces insulin-stimulated glucose uptake and serum lipid concentrations in type I diabetes. Metabolism. 2002;50:1409–1417. doi: 10.1053/meta.2001.28072. [DOI] [PubMed] [Google Scholar]
- 104.Djurhuus S, Vaag A, Altura BM, Altura BT, Klitgaard NAH. Skeletal muscle magnesium content in identical twins discordant for type 2 diabetes. Diabetes Metab. 2002;29:201–206. [PubMed] [Google Scholar]
- 105.Altura RA, Wang WC, Wynn L, Altura BM, Altura BT. Hydroxyurea therapy associated with declining serum levels of magnesium in children with sickle cell anemia. J Pediatr. 2002;140:565–569. doi: 10.1067/mpd.2002.122644. [DOI] [PubMed] [Google Scholar]
- 106.Zehtabchi S, Sinert R, Rinnert S, Chang B, Henis R, Altura R, Altura BT, Altura BM. Serum ionized magnesium levels and ionized calcium-to-magnesium ratios in adult patients with sickle cell anemia. Am J Hematol. 2004;77:215–222. doi: 10.1002/ajh.20187. [DOI] [PubMed] [Google Scholar]
- 107.Sinert R, Zehtabchi S, Desai S, Peacock P, Altura BT, Altura BM. Seum ionized magnesium and calcium levels in adult patients with seizures. Scand J Clin Lab Invest. 2007;67:317–326. doi: 10.1080/00365510601051441. [DOI] [PubMed] [Google Scholar]
- 108.Apostol A, Apostol R, Ali E, Choi A, Ehsuni N, Lei L, Altura BT, Altura BM. Cerebral spinal fluid and serum ionized magnesium and calcium levels in preeclamptic women during administration of magnesium sulfate. Fertil Steril. 2009;94:276–282. doi: 10.1016/j.fertnstert.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 109.Li W, Zheng T, Altura BT, Altura BM. Magnesium modulates contractile responses of rat aorta to thiocyanate: A possible relationship to smoking-induced atherosclerosis. Toxicol Appl Pharmacol. 1999;157:77–84. doi: 10.1006/taap.1999.8666. [DOI] [PubMed] [Google Scholar]
- 110.Xu Q, Parks CG, DeRoo LA, Cawthon RM, Sandler DP, Chen Hl. Multivitamin use and telomere length in women. Am J Clin Nutr. 2009;89:1857–1865. doi: 10.3945/ajcn.2008.26986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Altura BT, Altura BM. Endothelium-dependent relaxation in coronary arteries requires magnesium ions. Br J Pharmacol. 1987;91:449–451. doi: 10.1111/j.1476-5381.1987.tb11235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maier JAM. Endothelial cells and magnesium: implications in atherosclerosis. Clin Sci. 2012;122:397–407. doi: 10.1042/CS20110506. [DOI] [PubMed] [Google Scholar]
- 113.Grahame T, Schlesinger RB. Oxidative stress-induced telomeric erosion as a mechanism underlying airborne particulate matter-related cardiovascular disease. Part Fibre Toxicol. 2012;9:21. doi: 10.1186/1743-8977-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wolf FI, Cittadini A. Magnesium in cell proliferation and differentiation. Front Biosci. 1999;4:d607–617. doi: 10.2741/wolf. [DOI] [PubMed] [Google Scholar]
- 115.Ogretmen B, Kraveka JM, Schady D, Ustal J, Hannun YA. Molecular mechanisms of ceramide- induced telomerase inhibition in the A549 human lung adenocarcinoma cell line. J Biol Chem. 2002;276:32506–32514. doi: 10.1074/jbc.M101350200. [DOI] [PubMed] [Google Scholar]
- 116.Ogretmen B, Pettus BJ, Rossi MJ, Wood R, Ustal J, Szulc Z, Bielawska A, Obeid LM, Hannun YA. Biochemical mechanisms of the generation of endogenous long chain ceramide in response to exogenous short chain ceramide in A549 human lung adenocarcinoma cell line. J Biol Chem. 2002;277:12960–12969. doi: 10.1074/jbc.M110699200. [DOI] [PubMed] [Google Scholar]
- 117.Voelker DR, Kennedy EP. Cellular and enzymatic synthesis of sphingomyelin. Biochem. 1982;21:2753–2759. doi: 10.1021/bi00540a027. [DOI] [PubMed] [Google Scholar]
- 118.Meek DK. Tumor suppression by p53: a role for DNA damage response? Nat Rev Cancer. 2009;9:714–723. doi: 10.1038/nrc2716. [DOI] [PubMed] [Google Scholar]
- 119.Menendez D, Inga A, Resnick MA. The expanding universe of p53 targets. Nat Rev Cancer. 2009;9:724–737. doi: 10.1038/nrc2730. [DOI] [PubMed] [Google Scholar]
- 120.Crivello NA, Rosenberg IH, Dallal GE, Bielinski D, Joseph JA. Age-related changes in neutral sphingomyelin-specific phospholipase C activity in striatum, hippocampus, and frontal cortex: Implication for sensitivity to stress and inflammation. Neurochem Int. 2005;47:573–579. doi: 10.1016/j.neuint.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 121.Altura BM, Altura BT. Magnesium as an extracellular signal in cardiovascular pathobiology. Jap J Soc Magnes Res. 1996;15:17–32. [Google Scholar]
- 122.Altura BM, Altura BT. Magnesium and contraction of arterial smooth muscle. Microvasc Res. 1974;7:145–155. doi: 10.1016/0026-2862(74)90001-6. [DOI] [PubMed] [Google Scholar]
- 123.Altura BT, Altura BM. Withdrawal of magnesium causes vasospasm while elevated magnesium produces relaxation of tone in cerebral arteries. Neurosci Lett. 1980;20:323–327. doi: 10.1016/0304-3940(80)90168-8. [DOI] [PubMed] [Google Scholar]
- 124.Altura BM, Altura BT. Magnesium ions and contraction of vascular smooth muscles: relationship to some vascular diseases. Federation Proc. 1981;40:2672–2679. [PubMed] [Google Scholar]
- 125.Zhang A, Cheng TPO, Altura BM. Magnesium regulates intracellular free ionized calcium concentration and cell geometry in vascular smooth muscle cells. Biochim Biophys Acta. 1992;1134:25–29. doi: 10.1016/0167-4889(92)90024-6. [DOI] [PubMed] [Google Scholar]
- 126.Zhang A, Cheng TPO, Altura BT, Altura BM. Mg2+ and caffeine-induced intracellular Ca2+ release in human vascular endothelial cells. Br J Pharmacol. 1993;109:291–292. doi: 10.1111/j.1476-5381.1993.tb13568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu F, Zou LY, Altura BT, Barbour RL, Altura BM. Low extracellular magnesium results in cardiac failure in isolated perfused hearts. Magnes Trace Elem. 1992;10:364–373. [PubMed] [Google Scholar]
- 128.Friedman HS, Nguyen TN, Mokroaui AM, Barbour RL, Murakawa T, Altura BM. Effects of magnesium chloride on cardiovascular dynamics in the neurally intact dog. J Parmacol Exp Ther. 1987;243:126–130. [PubMed] [Google Scholar]
- 129.Delpiano M, Altura BM. Modulatory effect of extracellular Mg2+ ions on K+ and Ca2+ currents of capillary endothelial cells. FEBS Lett. 1996;394:335–339. doi: 10.1016/0014-5793(96)00980-5. [DOI] [PubMed] [Google Scholar]
- 130.Zhang A, Cheng TP-O, Wu XY, Altura BT, Altura BM. Extracellular Mg2+ regulates intracellular Mg2+ and its subcellular compartmentation in fission yeast, Schizosaccharomyces pombe . Cell Molec Life Sci. 1997;53:69–72. doi: 10.1007/PL00000581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang ZW, Wang J, Altura BT, Altura BM. Extracellular magnesium deficiency induces contraction ofarterial muscle: role of PI3-kinases and MAPK signaling pathways. Pfliug Arch European J Physiol. 2000;439:240–247. doi: 10.1007/s004249900179. [DOI] [PubMed] [Google Scholar]
- 132.Yang ZW, Wang J, Zheng T, Altura BT, Altura BM. Low extracellular Mg2+ induces contraction and [Ca2+] i rises in cerebral arteries: roles of Ca2+, PKC and PI-3 kinases. Am J Physiol Heart Circ Physiol. 2000;279:H2898–H2907. doi: 10.1152/ajpheart.2000.279.6.H2898. [DOI] [PubMed] [Google Scholar]
- 133.Yang ZW, Wang J, Zheng T, Altura BT, Altura BM. Low extracellular Mg induces contraction of cerebral arteries: roles of tyrosine and mitogen- activated protein kinases. Am J Physiol Heart Circ Physiol. 2000;279:H185–H194. doi: 10.1152/ajpheart.2000.279.1.H185. [DOI] [PubMed] [Google Scholar]
- 134.Zheng T, Li W, Altura BT, Shah NC, Altura BM. Sphingolipids regulate [Mg2+] o uptake and [Mg2+] i content in vacular smooth muscle cells: potential mechanisms and importance to membrane transport of Mg2+ Am J Physiol Heart Circ Physiol. 2011;300:H486–H492. doi: 10.1152/ajpheart.00976.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hannun YA, Obeid LM. The ceramide-centric universe of lipid-mediated regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277:25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- 136.Villani M, Suathra M, Im VH, Choi Y, Signorelli P, Del Poeta M, Luberto C. Sphingomyelin sunthases regulate production of diacylglycerol at the Golgi. Biochem J. 2008;414:31–41. doi: 10.1042/BJ20071240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mercer J, Mahmoundi M, Bennett M. DNA damage, p53, apoptosis and vascular disease. Mutat Res. 2007;621:75–86. doi: 10.1016/j.mrfmmm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 138.Dbalbo GS, Pushkareva MY, Rachid RA, Alter N, Smyth MJ, Obeid LM, Hannun YA. A p53-dependent ceramide response to genotoxic stress. J Clin Invest. 1998;102:329–339. doi: 10.1172/JCI1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vousden KH, Ryan KM. P53 and metabolism. Nat Rev Cancer. 2009;9:691–700. doi: 10.1038/nrc2715. [DOI] [PubMed] [Google Scholar]
- 140.Katada S, Imhof A, Sasson-Corsi P. Connecting threads: Epigenetics and metabolism. Cell. 2012;148:24–28. doi: 10.1016/j.cell.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 141.Lu C, Thompson CB. Metabolic regulation of epigenetics. Cell Metabolism. 2012;16:9–17. doi: 10.1016/j.cmet.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bartel DP. MicroRNAS: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


