Abstract
Objective: The postoperative PCIA effects of dezocine in elderly patients were determined using a large multicenter randomized double-blind prospective study. Methods: A total of 279 patients were randomized into four groups: (1) Control group (C): 2 μg/kg sufentanil plus 10 mg metoclopramide. (2) Dezocine group 1 (D1): 1 μg/kg sufentanil plus 0.1 mg/kg dezocine plus 10 mg metoclopramide. (3) Dezocine group 2 (D2): 1 μg/kg sufentanil plus 0.2 mg/kg dezocine plus 10 mg metoclopramide. (4) Dezocine group 3 (D3): 1 μg/kg sufentanil plus 0.3 mg/kg dezocine plus 10 mg metoclopramide. The index during operation including MAP, HR, SpO2, ETCO2, CVP, and BIS were determined. Analgesia effects including HR, MAP, RR, SpO2, pressing times of PCA demand, pressing times of PCR delivery, total amount of drug, additional sufentanil, VAS at rest and during moving, Ramsay sedation score, and BCS were repeated measured 1 h, 3 h, 6 h, 24 h, and 48 h after surgery. Overall satisfaction index and the side-effects including nausea, urinary retention, skin pruritus and respiratory depression were evaluated 1 h, 3 h, 6 h, 24 h, and 48 h after surgery. Results: Dezocine combining with sufentanil is complement for sufentanil in PCIA at least in its analgesia effects after surgery. Dezocine at a dosage of 0.1 mg/kg or 0.2 mg/kg combining with sufentanil (1 μg/kg) has limited side effects as sufentanil (2 μg/kg) in PCIA. Sufentanil (1 μg/kg) combining Dezocine at a dosage of 0.1 mg/kg or 0.2 mg/kg is better than combining Dezocine at a dosage of 0.3 mg/kg in PCIA at least in Overall satisfaction index. Conclusion: Dezocine combining with sufentanil is a complement drug for sufentanil in PCIA. Considering the side effects and overall satisfaction index, 0.1 mg/kg seems to be an ideal dosage for Dezocine using in the postoperative PCIA in elderly patients.
Keywords: Dezocine, sufentanil, postoperative, analgesia, elderly
Introduction
Ageing is an epidemic [1-3]. The number of ageing population is increasing worldwide [1]. By the year 2050, there are estimated to be at least 108 million Chinese citizens and 31 million American citizens over 80 years old [4-7]. The frequency of the elderly undergo surgery is nearly four times more often than that in other age group [5,7]. In the future, the majority of the patients for the anesthesiologists meet in the surgery might be those older than 65 years and many even older than 80 years [1,8]. Effective postoperative pain control is essential for the care of the surgical patients [9]. Inadequate pain control may lead to increased morbidity or mortality [1].
The incidences of postoperative anesthesia-related events and complications increase with age [9]. Unfortunately, postoperative pain is treated poorly in the elderly. Approximately 50-70% of elderly patients report inadequate postoperative pain relief. Therefore, effectively postoperative analgesia is highly needed for elderly patients as inadequate postoperative pain control is associated with adverse outcomes in elderly patients [8-10]. The ideal postoperative pain management should relieve pain and keeping side effects to a minimum [8-10]. Currently, the mainstay of pain management is still the opioids though opioids can result in life-threatening complication such as respiratory depression [9-12]. Among them, the most commonly used drug in patient controlled intravenous analgesia (PCIA) is sufentanil [9-12].
Dezocine is an analgesic agent and is gaining popularity in China as an alternative for PCIA [8-10]. Several studies have shown that Dezocine combining with sufentanil may be a complement drug for sufentanil in PCIA and is worth for clinical application [8-10]. Thus, a large multicenter randomized double-blind prospective study of the postoperative PCIA effects of dezocine in elderly patients is highly desirable.
Materials and methods
Subjects
This is an independent study which was sponsored by the investigators and the study was approved by the local Ethics committee. Prior to be enrolled into this study, all subjects gave their written informed consent on the basis of verbal and written information. Based on a multicenter randomized double-blind prospective study design, the target enrollment was 279 patients at 9 clinical research centers in Shanghai, China. The patients, aged 65-80 years old, weighing 50-75 kg, and with American Society of Anesthesiologists (ASA) physical status I-II scheduled for elective surgery were enrolled in this study. Exclusive criteria included known allergies, severe respiratory, neurological or cardiovascular diseases, narcotic drug dependence, hepatic and renal dysfunction and recent history of immunosuppressant application. Patients who meet these inclusive criteria during the pre-anestheric evaluation were randomly assigned into four groups using a computer-generated table of random numbers.
Study protocol
All patients received totally intravenous anesthesia and mechanical ventilation. Anesthesia was induced with 0.04 mg/kg midazolam, 0.5-1.0 mg/kg propofol, 3-4 μg/kg sufentanil, and 0.2 mg/kg cisatracurium. Anesthesia was maintained with continuous intravenous infusion of propofol, inhalation anesthesia with sevoflurance, and intermittent intravenous injection of 0.1-0.2 μg/kg/h sufentanil, and 0.1-0.15 mg/kg/h cisatracurium. Monitoring of each patient during the surgery was accomplished by mean arterial pressure (MAP), heart rate (HR), pulse oximetry (SpO2), end-tidal carbondioxide (ETCO2), central venous pressure (CVP), and bispectralindex (BIS). The eligible patients were randomly assigned to four groups as follows: (1) Control group (C): 2 μg/kg sufentanil plus 10 mg metoclopramide. (2) Dezocine group 1 (D1): 1 μg/kg sufentanil plus 0.1 mg/kg dezocine plus 10 mg metoclopramide. (3) Dezocine group 2 (D2): 1 μg/kg sufentanil plus 0.2 mg/kg dezocine plus 10 mg metoclopramide. (4) Dezocine group 3 (D3): 1 μg/kg sufentanil plus 0.3 mg/kg dezocine plus 10 mg metoclopramide.
Patients were given an initial intravenous analgesia of 2 ml drugs according to their groups. The continuous intravenous infusion speed was 2 ml/h. The PCIA pump was set to give aliquots of 1 ml with a lockout interval of 15 min. If the analgesia became incomplete during maintenance (Visual Analogue Scale, VAS, over 4 and Bruggrmann Comfort Scale, BCS, between 0-1), the clinician gave an additional intravenous injection of 25-50 μg sufentanil.
Clinical evaluation
All clinical evaluations were performed by independent anesthesia registrars blinded to group allocation at 1 h, 3 h, 6 h, 24 h, and 48 h after surgery. The index included HR, MAP, respiratory rate (RR), SpO2, pressing times of PCA demand, pressing times of PCR delivery, total amount of drug, additional sufentanil, VAS, Ramsay sedation score, and BCS. VAS was used to assess pain both at rest and during moving by a 10 cm visual analogue scale: 0, no pain; 10, worst imaginable pain. The Ramsay sedation scale (1, anxious, agitated, or restless; 2, cooperative, oriented, and tranquil; 3, response to command; 4, brisk response; 5, a sluggish response; 6, no response) was used to assess sedation. BCS (0, continuous pain; 1, no pain without movement but serious pain when deep breathe or cough; 2, no pain without movement but mild pain when deep breathe or cough; 3, no pain even in deep breath; 4, no pain in cough) were used to evaluate the comfort level.
The side-effects including nausea, urinary retention, skin pruritus and respiratory depression were also evaluated at 1 h, 3 h, 6 h, 24 h, and 48 h after surgery. The degree of nausea was divided as follows: 0, no nausea; 1, mild (once or twice a day); 2, middle (three to five times a day); 3, serve (over 6 times a day). The degree of urinary retention was divided as follows: 0, without urinary retention; 1, dysuria and extension of micturition time; 2, significant dysuria; 3, complete urinary retention. The degree of skin pruritus were divided as mild, middle and serve. Respiratory depression was defined as RR less than 8 bpm and SpO2 less than 90%. In addition, the overall satisfaction index for postoperative analgesic effects was also measured. The overall satisfaction index was divided as follows: 0, complete unsatisfied; 1, kind of unsatisfied; 2, kind of satisfied; 3, satisfied; 4, complete satisfied.
Statistical analysis
Data from all patients who underwent randomization were analyzed using the SPSS 19.0 program. Continuous variables were expressed as mean±SEM. The comparability of the characteristics between the four study groups were assessed using Analysis of Variance (ANOVA) for repeated measurement design data for continuous variables and the chi-square test for categorical variables. If a significant difference was observed, Bonferroni’s post-hoc test was conducted to identify groups with significant differences. P-values that were less than 0.05 were considered to be statistically significant, and all tests were two-tailed.
Results
Index during operation
As shown in Table 1, MAP, HR, SpO2, ETCO2, CVP, and BIS were comparable between Group C, Group D1, Group D2, and Group D3 at the time points as follows: before anesthesia was induced, 5 min after tracheal intubation, 5 min after inducing, 1 h after inducing, 2 h after inducing, 5 min before tracheal extubation, and 5 min after tracheal extubation. These results indicate that Dezocine combining with sufentanil might be a complement drug for sufentanil in PCIA.
Table 1.
Effects of dezocine on index during operation
| Group | Before anesthesia was induced | 5 min after tracheal intubation | 5 min after inducing | 1 h after inducing | 2 h after inducing | 5 min before tracheal extubation | 5 min after tracheal extubation | |
|---|---|---|---|---|---|---|---|---|
| MAP (mmHg) | C | 101±13.4 | 81.4±16.2 | 88.8±14.6 | 85.5±12.2 | 84.4±13.2 | 97.2±13.7 | 95.2±12.5 |
| D1 | 100.9±14.3 | 84.9±14.7 | 87.6±15.6 | 84.5±13.4 | 85.8±11 | 95.4±15.8 | 96.9±12.1 | |
| D2 | 97.6±14.7 | 80.9±12.3 | 84.1±13.1 | 83±12.8 | 83.1±11.8 | 91.6±13.4 | 93.9±12.6 | |
| D3 | 105.4±18.1 | 82.4±15.7 | 88.7±16.2 | 86.3±15.2 | 84.3±12.3 | 96.5±15.8 | 96.8±15.1 | |
| Total | 101.2±15.5 | 82.5±14.6 | 87.2±15 | 84.7±13.5 | 84.4±11.9 | 94.9±14.9 | 95.7±13.1 | |
| HR (bpm) | C | 80.5±13.8 | 73.2±13.7 | 72.8±9.4 | 72.1±9.7 | 72.3±10.6 | 77.8±10.9 | 78.4±10.4 |
| D1 | 80.7±12.6 | 74.3±13.6 | 72±10.6 | 71.4±10.4 | 69.1±10.6 | 78±12.6 | 80.7±10.7 | |
| D2 | 78.3±11.4 | 71.5±10.7 | 69.9±11.6 | 69.8±10.8 | 69.1±9.3 | 77.2±12.7 | 78.5±10.8 | |
| D3 | 77.5±13.2 | 71.6±12.6 | 70.9±12.3 | 70±11 | 70.7±11.7 | 77.7±15.2 | 79.3±12.8 | |
| Total | 79.2±12.7 | 72.6±12.6 | 71.2±11.1 | 70.7±10.5 | 70.1±10.6 | 77.6±13 | 79.3±11.2 | |
| SpO2 | C | 97.4±1.9 | 99.7±0.5 | 99.7±0.4 | 99.7±0.5 | 97.3±15.6 | 99.6±0.9 | 99.2±1.4 |
| D1 | 97.9±1.9 | 99.7±0.5 | 99.4±2.7 | 99.5±2.4 | 99.4±3.1 | 99.5±1.8 | 98.9±2.3 | |
| D2 | 98±1.7 | 99.7±0.5 | 99.8±0.4 | 99.7±0.6 | 99.9±1 | 99.8±0.9 | 99.2±1.5 | |
| D3 | 97.5±2 | 99.6±0.6 | 99.7±0.5 | 99.7±0.5 | 99.7±0.5 | 99.6±0.6 | 99±1.4 | |
| Total | 97.7±1.9 | 99.7±0.5 | 99.7±1.5 | 99.7±1.3 | 99.2±6.9 | 99.6±1.2 | 99.1±1.7 | |
| ETCO2 | C | 35.3±5 | 33±3.5 | 32.9±3.1 | 32.8±3.5 | 33.2±3.2 | 35.3±4 | 36.7±5.8 |
| D1 | 38.2±10.5 | 35.4±8.1 | 34.9±8.2 | 35.3±8.4 | 35.6±8.8 | 37.1±8.6 | 40.5±10.2 | |
| D2 | 36.2±3.8 | 34.1±3.2 | 33.4±3 | 33.8±3.3 | 33.6±3.2 | 35.7±3.8 | 37±3.6 | |
| D3 | 37.1±3.7 | 33.8±3.4 | 33.4±3.4 | 33±3.3 | 33.1±3.2 | 35.3±4 | 37.6±4 | |
| Total | 37±6.9 | 34.2±5.2 | 33.7±5.1 | 33.8±5.3 | 34±5.5 | 35.9±5.6 | 38.1±6.7 | |
| CVP (cmH2O) | C | 6.6±2.7 | 7.4±2.6 | 8.7±3 | 9.1±3.5 | 9.2±3.5 | 9.4±2.9 | 9.6±3.3 |
| D1 | 7.4±4.9 | 8.2±4.4 | 8.7±4.4 | 9.5±4.4 | 10±4.6 | 10.3±4.8 | 9.9±4.6 | |
| D2 | 7.4±3.1 | 8.1±2.5 | 8.8±2.7 | 9.3±2.7 | 9.4±2.5 | 10±2.7 | 9.7±2.8 | |
| D3 | 7±3.2 | 7.9±2.9 | 8.5±3 | 8.7±3.3 | 8.8±3 | 9±2.5 | 9.1±2.9 | |
| Total | 7.2±3.6 | 7.9±3.3 | 8.7±3.4 | 9.1±3.5 | 9.4±3.5 | 9.7±3.4 | 9.6±3.5 | |
| ETSevo | C | - | 0.7±0.4 | 1.1±0.6 | 1.3±0.6 | 1.2±0.6 | 0.2±0.2 | - |
| D1 | - | 0.7±0.3 | 1±0.5 | 1.1±0.5 | 1.1±0.5 | 0.1±0.2 | - | |
| D2 | - | 0.6±0.4 | 0.9±0.4 | 1±0.5 | 1±0.4 | 0.2±0.3 | - | |
| D3 | - | 0.6±0.4 | 2.7±13.3 | 1.1±0.6 | 1.1±0.6 | 0.2±0.3 | - | |
| Total | - | 0.7±0.4 | 1.5±6.9 | 1.1±0.6 | 1.1±0.5 | 0.2±0.3 | - | |
| BIS | C | 92±5.9 | 47.9±6.7 | 47.5±6.7 | 47±7 | 48.9±6 | 75.7±14 | 89.2±6.7 |
| D1 | 93.3±7.4 | 49.6±8.8 | 49.4±7.1 | 49.7±9.4 | 50.9±8.5 | 79.4±13.7 | 90.5±7 | |
| D2 | 88.7±19 | 50.5±9.3 | 49.4±10 | 50±8.9 | 50.3±11.1 | 77.1±17.6 | 86.1±16.3 | |
| D3 | 91.5±12.3 | 47.7±8.3 | 49.2±8.3 | 49.5±8.4 | 50±9.5 | 75.3±17.4 | 88.2±13.2 | |
| Total | 91.3±12.9 | 49.1±8.5 | 49±8.3 | 49.3±8.6 | 50.1±9.2 | 77±15.9 | 88.4±12.1 |
Analgesia effects after surgery
Analgesia effects including HR, MAP, RR, SpO2, pressing times of PCA demand, pressing times of PCR delivery, total amount of drug, additional sufentanil, VAS at rest and during moving, Ramsay sedation score, and BCS were repeated measured 1 h, 3 h, 6 h, 24 h, and 48 h after surgery. No significant difference was observed between Group C, Group D1, Group D2, and Group D3 (Figure 1 and Table 2). These results indicate that Dezocine combining with sufentanil is complement for sufentanil in PCIA at least in its analgesia effects after surgery.
Figure 1.
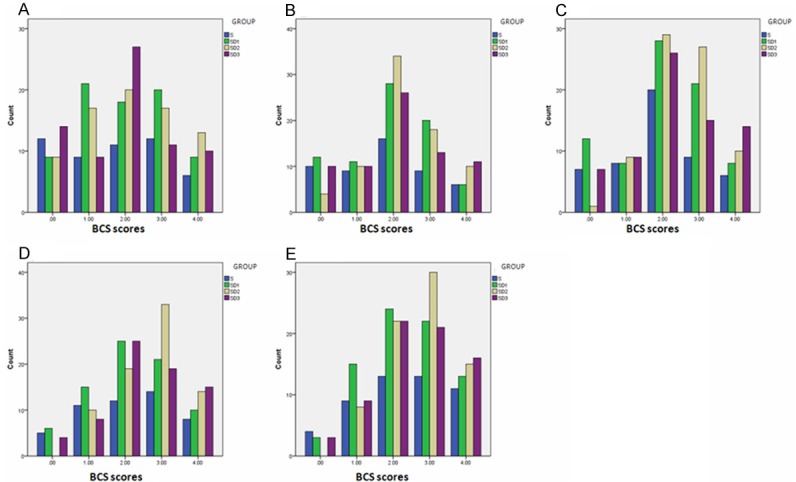
Effects of Dezocine on Bruggrmann Comfort Scale (BCS) at 1 h (A), 3 h (B), 6 h (C), 24 h (D), and 48 h (E) after Surgery.
Table 2.
Effects of dezocine on analgesia after surgery
| Group | 1 h after surgery | 3 h after surgery | 6 h after surgery | 24 h after surgery | 48 h after surgery | |
|---|---|---|---|---|---|---|
| HR (bpm) | C | 77.94±12.33 | 80.6±12.82 | 79.32±14.07 | 79.42±10.85 | 78.1±8.8 |
| D1 | 79.49±11.43 | 79.88±10.28 | 80.71±10.57 | 80.21±9.8 | 79.81±10.07 | |
| D2 | 77.36±10.88 | 78.01±10.5 | 78.4±11.07 | 78.29±10.64 | 76.88±11.55 | |
| D3 | 80.01±12.74 | 79.42±10.7 | 78.96±10.16 | 78.38±10.15 | 78.07±10.16 | |
| Total | 78.75±11.79 | 79.37±10.92 | 79.36±11.29 | 79.05±10.3 | 78.23±10.32 | |
| MAP (mmHg) | S | 95.78±15.3 | 92.18±10.96 | 90.7±10.68 | 91.22±9.37 | 91.08±8.74 |
| D1 | 93.17±10.39 | 92.73±11.2 | 91.99±12.4 | 92.42±13.34 | 91.27±12.13 | |
| D2 | 90.66±12.37 | 89.38±11.4 | 89.17±11.15 | 89.77±11.61 | 88.71±11.39 | |
| D3 | 95.25±13.31 | 93.33±12.54 | 92.32±11.5 | 91.94±12.23 | 91.33±12.21 | |
| Total | 93.49±12.79 | 91.85±11.62 | 91.05±11.53 | 91.34±11.91 | 90.55±11.4 | |
| RR (bpm) | C | 16.58±1.74 | 16.96±2.49 | 16.84±2.05 | 17.02±2.12 | 17.24±2.41 |
| D1 | 16.88±2.33 | 16.77±1.85 | 17.1±1.83 | 17.26±2.51 | 18.01±8.54 | |
| D2 | 16.56±2.04 | 17.26±2.05 | 16.99±1.75 | 17.22±1.91 | 17.08±1.59 | |
| D3 | 16.42±2.51 | 16.58±2.34 | 16.63±2.27 | 16.74±2.21 | 16.82±2.36 | |
| Total | 16.62±2.2 | 16.89±2.17 | 16.9±1.97 | 17.07±2.2 | 17.3±4.86 | |
| SpO2 (%) | C | 99.24±0.82 | 99.14±0.99 | 99±1.16 | 98.92±1.29 | 98.86±1.21 |
| D1 | 98.71±1.5 | 98.71±1.42 | 98.71±1.19 | 98.48±1.3 | 98.31±1.67 | |
| D2 | 99.03±1.06 | 98.94±1.08 | 98.82±0.98 | 98.62±1.57 | 98.67±1.15 | |
| D3 | 99.08±1.14 | 99.03±1.16 | 98.79±1.3 | 98.65±1.41 | 98.61±1.35 | |
| Total | 98.99±1.19 | 98.93±1.2 | 98.82±1.16 | 98.64±1.41 | 98.59±1.39 | |
| Pressing times of PCA demand | S | 0.48±0.79 | 0.98±1.82 | 1.64±3.15 | 3.18±5.37 | 4.12±8.67 |
| D1 | 0.58±0.85 | 1.49±1.96 | 2.38±3.08 | 4.99±7.46 | 6.96±10.62 | |
| D2 | 0.81±1.15 | 1.49±2.87 | 2.1±3.56 | 3.42±5.81 | 4.01±6.79 | |
| D3 | 0.64±1.19 | 0.9±1.48 | 1.19±2.3 | 3.82±17.61 | 4.36±19.16 | |
| Total | 0.64±1.02 | 1.24±2.14 | 1.86±3.08 | 3.92±10.41 | 4.95±12.33 | |
| Pressing times of PCA delivery | C | 0.4±0.57 | 0.76±1.32 | 1.2±1.86 | 2.06±2.54 | 2.4±3.19 |
| D1 | 0.5±0.73 | 1.19±1.35 | 1.89±2.26 | 3.81±4.9 | 5.16±6.75 | |
| D2 | 0.73±0.9 | 1.18±1.59 | 1.78±2.57 | 3.42±6.93 | 5.9±17.67 | |
| D3 | 0.52±0.82 | 0.7±1.11 | 0.91±1.73 | 1.45±2.75 | 1.66±3.52 | |
| Total | 0.55±0.78 | 0.98±1.37 | 1.48±2.19 | 2.77±4.89 | 3.95±10.28 | |
| Total amount of drug | C | 3.21±1.25 | 7.91±1.81 | 14.78±2.89 | 49.68±7.4 | 97.65±3.8 |
| D1 | 3.21±1.25 | 8.17±2.44 | 15.24±3.34 | 52.25±7.05 | 96.68±8.07 | |
| D2 | 3.27±1.73 | 7.79±2.38 | 14.77±3.5 | 51±5.36 | 96.93±4.98 | |
| D3 | 3.2±1.19 | 7.57±1.92 | 14.09±2.99 | 49.75±4.6 | 96.63±4.99 | |
| Total | 3.22±1.37 | 7.86±2.18 | 14.72±3.22 | 50.75±6.18 | 96.92±5.8 | |
| Additional sufentanil | C | 8.26±24.2 | 2.13±8.84 | 3.9±17.3 | 2.63±12.4 | 3.13±16.2 |
| D1 | 5.29±17.57 | 2.01±7.98 | 2.31±9.47 | 1.25±6.77 | 1.99±9.02 | |
| D2 | 5.58±17.88 | 2.31±9.75 | 3.19±14.86 | 1.49±7.38 | 0.89±3.71 | |
| D3 | 7.89±18.39 | 0.89±6.68 | 0.55±3.43 | 0.55±3.43 | 0.09±0.67 | |
| Total | 6.58±19.18 | 1.83±8.32 | 2.4±11.95 | 1.39±7.66 | 1.44±8.76 | |
| VAS (at rest) | C | 1.36±1.38 | 1.44±1.36 | 1.48±1.23 | 1.3±1.16 | 1.16±1.09 |
| D1 | 1.56±1.35 | 1.75±1.38 | 1.84±1.47 | 1.55±1.21 | 1.33±1.11 | |
| D2 | 1.35±1.35 | 1.26±1.02 | 1.23±1.1 | 1.22±1.02 | 1.03±0.97 | |
| D3 | 1.46±1.48 | 1.56±1.81 | 1.26±1.39 | 1.1±1.22 | 1.06±1.03 | |
| Total | 1.44±1.39 | 1.51±1.42 | 1.46±1.33 | 1.29±1.16 | 1.14±1.05 | |
| VAS (during moving) | C | 1.92±1.58 | 2.08±1.5 | 2.18±1.37 | 1.8±1.31 | 1.76±1.36 |
| D1 | 2.25±1.53 | 2.47±1.68 | 2.55±1.62 | 2.34±1.41 | 2.03±1.25 | |
| D2 | 1.93±1.74 | 1.84±1.33 | 2.03±1.42 | 1.87±1.4 | 1.55±1.2 | |
| D3 | 2.07±1.9 | 2.1±1.82 | 1.87±1.68 | 1.83±1.66 | 1.58±1.39 | |
| Total | 2.06±1.69 | 2.13±1.6 | 2.16±1.55 | 1.98±1.47 | 1.73±1.31 | |
| Ramsay | C | 2.3±0.74 | 2.16±0.37 | 2.06±0.24 | 1.98±0.25 | 2±0.2 |
| D1 | 2.32±0.57 | 2.22±0.45 | 2.16±0.43 | 2.08±0.31 | 2.04±0.3 | |
| D2 | 2.16±0.65 | 2.21±0.69 | 2.17±0.57 | 2.14±0.56 | 2.15±0.56 | |
| D3 | 2.36±0.63 | 2.24±0.59 | 2.1±0.48 | 2.08±0.44 | 2.06±0.37 | |
| Total | 2.28±0.64 | 2.21±0.55 | 2.13±0.46 | 2.08±0.42 | 2.07±0.4 |
Side effects
The side-effects including nausea, urinary retention, skin pruritus and respiratory depression were evaluated 1 h, 3 h, 6 h, 24 h, and 48 h after surgery. No significant difference was observed between Group C, Group D1, Group D2, and Group D3 regarding nausea, skin pruritus and respiratory depression that were evaluated 1 h, 3 h, 6 h, 24 h, and 48 h after surgery (Figures 2, 3 and 4). At 24 h after surgery, the degree of urinary retention in Group D3 was higher than in Group D2 (p=0.046) or Group D1 (p=0.046) (Figure 5). These results indicate that Dezocine at a dosage of 0.1 mg/kg or 0.2 mg/kg combining with sufentanil (1 μg/kg) is complement for sufentanil (2 μg/kg) in PCIA at least in its analgesia effects after surgery.
Figure 2.
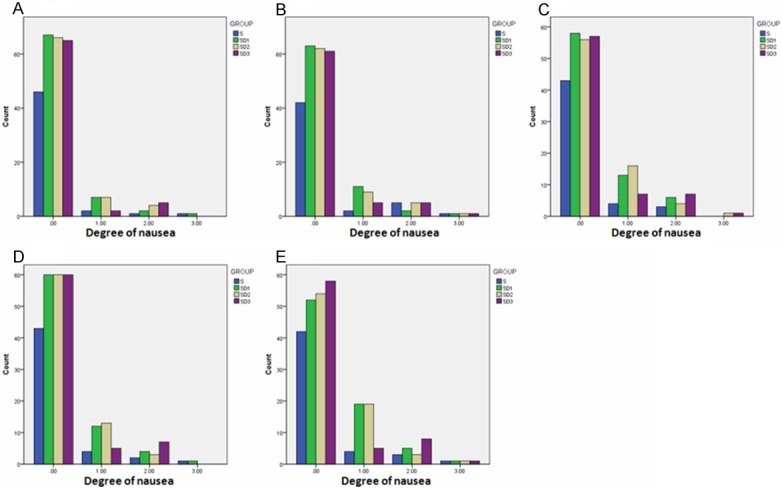
Effects of Dezocine on Nausea at 1 h (A), 3 h (B), 6 h (C), 24 h (D), and 48 h (E) after Surgery.
Figure 3.
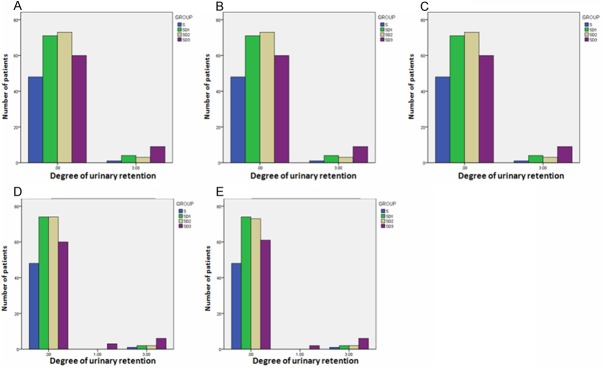
Effects of Dezocine on Urinary Retention at 1 h (A), 3 h (B), 6 h (C), 24 h (D), and 48 h (E) After Surgery.
Figure 4.
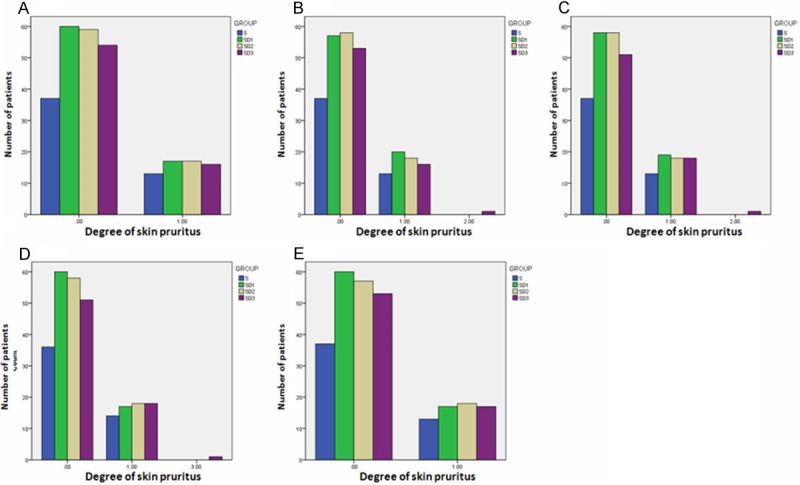
Effects of Dezocine on Skin Pruritus at 1 h (A), 3 h (B), 6 h (C), 24 h (D), and 48 h (E) After Surgery.
Figure 5.
Effects of Dezocine on Respiratory Depression at 1 h (A), 3 h (B), 6 h (C), 24 h (D), and 48 h (E) After Surgery.
Overall satisfaction index
The overall satisfaction index was evaluated 1 h, 3 h, 6 h, 24 h, and 48 h after surgery. At 3 h after surgery, the overall satisfactionin Group D1 was higher than that in Group C (p=0.021) (Figure 6B), indicating that Dezocine at a dosage of 0.1 mg/kg combining with sufentanil (1 μg/kg) is better than sufentanil (2 μg/kg) in PCIA at least in this index. The overall satisfaction index of Group D1 was better than that in Group D3 at 1 h (P=0.015), 3 h (P=0.004), 6 h (P=0.004), and 24 h (P=0.002) after surgery. Moreover, the overall satisfaction index of Group D2 was also better than that in Group D3 at 1 h (P=0.019), 6 h (P=0.016), and 24 h (P=0.034) after surgery. These results indicate that sufentanil (1 μg/kg) combining Dezocine at a dosage of 0.1 mg/kg or 0.2 mg/kg is better than combining Dezocine at a dosage of 0.3 mg/kg in PCIA at least in this index. Overall, considering the overall satisfaction, 0.1 mg/kg seems to be an ideal dosage for Dezocine using in the postoperative PCIA in elderly patients.
Figure 6.
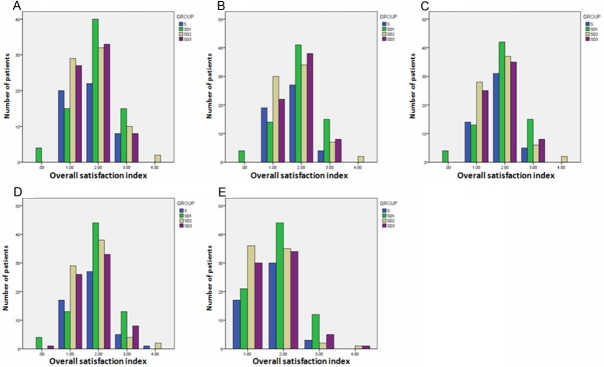
Effects of Dezocine on Overall Satisfaction Index at 1 h (A), 3 h (B), 6 h (C), 24 h (D), and 48 h (E) After Surgery.
Discussion
The major findings of the present study are as follows: 1) Dezocine combining with sufentanil is complement for sufentanil in PCIA at least in its analgesia effects after surgery; 2) Dezocine at a dosage of 0.1 mg/kg or 0.2 mg/kg combining with sufentanil (1 μg/kg) has limited side effects as sufentanil (2 μg/kg) in PCIA; 3) sufentanil (1 μg/kg) combining Dezocine at a dosage of 0.1 mg/kg or 0.2 mg/kg is better than combining Dezocine at a dosage of 0.3 mg/kg in PCIA at least in Overall satisfaction index. Overall, this study presents direct evidence that Dezocine combining with sufentanil is a complement drug for sufentanil in PCIA.
Dezocine is traditionally thought as a full agonist of κ-receptor and partial agonist of μ-receptor without classic μ-receptor dependence [8-10]. Although Dezocine is developed in 1970s by the American Home Products Corporation, it obtains popularity in China as an alternative medication for the management for perioperative pain recently [8,13-16]. In previous studies for the analgesic properties of Dezocine, the potency of 10 mg of dezocine was reported to be equal 10 mg of morphine and 50 mg of meperidine, indicating that it might be an effective analgesic drug for the management of postoperative pain [12,14,17-19]. Moreover, dozocine is not a scheduled drug classified by the World health Organization and no addictive events have been reported since its first use in the clinic [15]. Here using this large multicenter randomized double-blind prospective study of the postoperative PCIA effects of dezocine in elderly patients, we show that Dezocine combining with sufentanil might be a complement drug for sufentanil in PCIA and 0.1 mg/kg of dezocine might be an ideal dosage for its use in the postoperative PCIA in elderly patients.
Interestingly, a recent study has reported that dezocine was actually a μ-receptor agonist, a κ-receptor antagonist, and a norepinephrine and serotonin reuptake inhibitor probably through serotonin transporter and norepinephrine transporter [15]. That study suggests that besides using for pain control, dezocine might also can be used for the treatment of depression and even addition treatment [15]. These unique features need to be further studied especially as depression is present in the elderly patients [15,20,21].
In conclusion, based on the results of this large multicenter randomized double-blind prospective study of the postoperative PCIA effects of dezocine in elderly patients, Dezocine combining with sufentanil is a complement drug for sufentanil in PCIA. Considering the side effects and overall satisfaction index, 0.1 mg/kg seems to be an ideal dosage for Dezocine using in the postoperative PCIA in elderly patient.
Disclosure of conflict of interest
None declared.
References
- 1.Moses H 3rd, Matheson DH, Dorsey ER, George BP, Sadoff D, Yoshimura S. The anatomyof health care in the United States. Jama. 2013;310:1947–1963. doi: 10.1001/jama.2013.281425. [DOI] [PubMed] [Google Scholar]
- 2.Brooks-Wilson AR. Genetics of healthy aging and longevity. Hum Genet. 2013;132:1323–1338. doi: 10.1007/s00439-013-1342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michalakis K, Goulis DG, Vazaiou A, Mintziori G, Polymeris A, Abrahamian-Michalakis A. Obesityin the ageing man. Metabolism. 2013;62:1341–1349. doi: 10.1016/j.metabol.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Degenhardt L, Whiteford HA, Ferrari AJ, Baxter AJ, Charlson FJ, Hall WD, Freedman G, Burstein R, Johns N, Engell RE, Flaxman A, Murray CJ, Vos T. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1564–1574. doi: 10.1016/S0140-6736(13)61530-5. [DOI] [PubMed] [Google Scholar]
- 5.Gupta M, Agarwal M. Understanding medicationerrors in the elderly. N Z Med J. 2013;126:62–70. [PubMed] [Google Scholar]
- 6.Wang YP, Andrade LH. Epidemiology of alcoholand drug use in the elderly. Curr Opin Psychiatry. 2013;26:343–348. doi: 10.1097/YCO.0b013e328360eafd. [DOI] [PubMed] [Google Scholar]
- 7.Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T. Global burden of disease attributable to mental and substance use disorders: findingsfrom the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 8.Sun ZT, Yang CY, Cui Z, Zhang J, Han XP. Effect of intravenous dezocine on fentanyl-induced cough during general anesthesia induction: a double-blinded, prospective, randomized, controlledtrial. J Anesth. 2011;25:860–863. doi: 10.1007/s00540-011-1237-x. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Y, Jing G, Yuan W. Preoperative administrationof intramuscular dezocine reduces postoperativepain for laparoscopic cholecystectomy. J Biomed Res. 2011;25:356–361. doi: 10.1016/S1674-8301(11)60047-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker EA, Picker MJ, Granger A, Dykstra LA. Effects of opioids in morphine-treated pigeons trained to discriminate among morphine, the low-efficacy agonist nalbuphine, and saline. J Pharmacol Exp Ther. 2004;310:150–158. doi: 10.1124/jpet.103.058503. [DOI] [PubMed] [Google Scholar]
- 11.Ramirez-Ruiz M, Smith I, White PF. Use of analgesicsduring propofol sedation: a comparison of ketorolac, dezocine, and fentanyl. J Clin Anesth. 1995;7:481–485. doi: 10.1016/0952-8180(95)00058-p. [DOI] [PubMed] [Google Scholar]
- 12.Strain EC, Preston KL, Liebson IA, Bigelow GE. Opioid antagonist effects of dezocine in opioid-dependent humans. Clin Pharmacol Ther. 1996;60:206–217. doi: 10.1016/S0009-9236(96)90137-X. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien JJ, Benfield P. Dezocine. A preliminary review of its pharmacodynamic and pharmacokineticproperties, and therapeutic efficacy. Drugs. 1989;38:226–248. doi: 10.2165/00003495-198938020-00005. [DOI] [PubMed] [Google Scholar]
- 14.Cohen RI, Edwards WT, Kezer EA, Ferrari DA, Liland AE, Smith ER. Serial intravenous doses of dezocine, morphine, and nalbuphine in the management of postoperative pain for outpatients. Anesth Analg. 1993;77:533–539. doi: 10.1213/00000539-199309000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Liu R, Huang XP, Yeliseev A, Xi J, Roth BL. Novel Molecular Targets of Dezocine and Their ClinicalImplications. Anesthesiology. 2013;20:20. doi: 10.1097/ALN.0000000000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu Y, Ye Z, Wong GT, Dong C, Yu J. Prevention of injection pain due to propofol by dezocine: A comparison with lidocaine. Indian J Pharmacol. 2013;45:619–621. doi: 10.4103/0253-7613.121376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen JC, Smith ER, Cahill M, Cohen R, Fishman JB. The opioid receptor binding of dezocine, morphine, fentanyl, butorphanol and nalbuphine. Life Sci. 1993;52:389–396. doi: 10.1016/0024-3205(93)90152-s. [DOI] [PubMed] [Google Scholar]
- 18.Wilson JM, Cohen RI, Kezer EA, Schange SJ, Smith ER. Single- and multiple-dose pharmacokineticsof dezocine in patients with acute or chronic pain. J Clin Pharmacol. 1995;35:398–403. doi: 10.1002/j.1552-4604.1995.tb04080.x. [DOI] [PubMed] [Google Scholar]
- 19.Picker MJ. Discriminative stimulus effects of the mixed-opioid agonist/antagonist dezocine: cross-substitution by mu and delta opioid agonists. J Pharmacol Exp Ther. 1997;283:1009–1017. [PubMed] [Google Scholar]
- 20.Craven MA, Bland R. Depression in primary care: current and future challenges. Can J Psychiatry. 2013;58:442–448. doi: 10.1177/070674371305800802. [DOI] [PubMed] [Google Scholar]
- 21.Renaud J, Bedard E. Depression in the elderlywith visual impairment and its associationwith quality of life. Clin Interv Aging. 2013;8:931–943. doi: 10.2147/CIA.S27717. [DOI] [PMC free article] [PubMed] [Google Scholar]


