Abstract
Objective: To observe the effect of Cbfa1 on biological characteristics of marrow mesenchymal stem cells under hypoxia. Methods: The second passage of the MSCs were transfected with Cbfa1 and then cultured in 20% O2 and 3% O2 condition individually. The biological features of the cultured MSCs were assessed by the Real-time PCR. Results: After transfected with Cbfa1, the morphology of MSCs was no significant difference between two oxygen concentrations; The RT-PCR examination revealed that the expression of Cbfa1, BMP, OPN and VEGF in MSCs was higher than those before Cbfa1 transfection (P<0.05), especial the expression of Cbfa1 (P<0.05). Conclusion: After transfected with Cbfa1, cell morphology or growth cycle of MSCs was not significantly affected, but its osteogenic differentiation potential enhanced, particularly its osteogenic differentiation potential under hypoxia enhanced.
Keywords: Mesenchymal stem cell, hypoxia, Core binding factor alpha l
Introduction
Avascular necrosis of femoral head (ANFH) is a common disease in the Department of Orthopedics and has high disability. Currently, surgical and non-surgical interventions have limited effectiveness for ANFH. In recent years, numerous basic studies have shown that mesenchymal stem cells (MSCs) may provide seed cells for the repair of injured femoral head and favorable environment for the osteogenesis [1-3]. In addition, some clinicians have applied MSCs in clinical treatment of ANFH achieving favorable effectiveness [2,4,5].
Our in depth studies showed MSCs have limited efficacy in the treatment of ANFH. This might be explained as that severe ischemia/hypoxia is present in the injured femoral head after ANFH, and focal oxygen concentration is at a very low level (about <1%), which significantly compromises the survival osteogenic differentiation of MSCs [6-8]. Currently, the influence of hypoxia on the biological activity of MSCs is still controversial. Some investigators propose that hypoxia is beneficial for the maintenance of characteristics of stem cells and may inhibit the differentiation of these stem cells into osteoblasts [6,8-11], and oxygen at the concentration of <0.02% may even cause the loss of differentiation of MSCs into osteoblasts [12]. However, other investigators postulate that hypoxia may inhibit the aging of MSCs, promote the proliferation of these cells and enhance their differentiation into osteoblasts [13-15]. This discrepancy is attributed to multiple factors including difference in oxygen concentrations (0.02%~5%) [12,16,17], different time of cell culture (0 h~28 d) [8,12,18], the generation of cells (primary, secondary or multiple) [8], difference in cell sources (mouse, rat, human, etc) [8], and incomplete hypoxia during the cell culture (such as during medium refreshing and passaging) [18]. However, most studies confirm that moderate gene modification may enhance the tolerance of MSCs against ischemia/hypoxia. For example, modification of hemeoxygenase-1 (HO-1), Akt1 or HIF-1α may significantly increase the survival rate and prolong the survival time of MSCs under ischemia/hypoxia conditions [11].
Core binding factor alpha l (Cbfa1) is one of members in Cbfa family and a determinant in the differentiation of MSCs into osteoblasts. Cbfa1 is the first and unique osteogenesis-specific transcription factor and can regulate the osteogenesis specific genes (such as OCN, BSP, OPN and type I collagen). Studies have shown that Cbfa1 is closely related to the growth and development of the bone [19-22]. In vitro studies demonstrate that Cbfa1 gene modification may promote the secretion of Cbfa1 by MSCs, elevate the ALP activity and increase the expressions of OCN, BSP and OPN, which facilitate the differentiation into osteoblasts and increase the calcium deposition in the extracellular matrix [23,24]. In vivo studies reveal that Cbfa1 gene modification may promote the repair of injured bones and has favorable prospect of clinical application [23-25]. However, few studies have been conducted to investigate the survival of MSCs with Cbfa1 gene modification. In the present study, the survival, proliferation and osteogenic differentiation of MSCs with Cbfa1 gene modification were investigated under the hypoxia condition, which may provide evidence for future treatment of ANFH with MSCs after Cbfa1 gene modification.
Materials and methods
Main reagents and instrument
L-DMEM, fetal bovine serum (FBS), Trizol (Gibco, USA), AMV reverse transcription kit (Promega, USA), SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) (TAKARA, Japan), tetracycline hydrochloride (Sigma, USA), alkaline phosphatase kit (Beijing Zhongshan Biotech, China), Methylthiazolyldiphenyl-tetrazolium bromide (MTT, Beijing Tongbaoda Technique Center, China), primers (Shanghai Sangon Biotech Co., Ltd, China), low temperature centrifuge (Sigma, USA), electrophoresis tank (Beijing Liuyi Instrument Factory, China), CO2 incubator, three-gas incubator (FORMA, USA), 2100 centrifuge (KUBOTA, Japan), inverted phase contrast microscope (LEICA, Germany), YJ-875 Clean Bench (Beijing Changping purification equipment factory, China) and culture plates and flasks (NUNC, Sweden) were used in the present study.
Construction of Ad-Cbfa1 expressing plasmids
The target gene Cbfa1 with 1015 bp-2559 bp in length was synthesized in Beijing Aoke Biotech Co., Ltd and cloned into Teasy vectors. Cbfa1 gene carrying XhoI and BglII restriction sites was amplified by PCR and cloned into pShuttle-CMV vectors. The resulting vectors were pShuttle-CMV-Cbfa1 which was confirmed by digestion with restriction enzyme and sequencing. pShuttle-CMV-Cbfa1 was digested with PmeI for linearization followed by dephosphorylation. Then, extraction and purification were done with phenol-chloroform. The linearized plasmids (1 μg) were used to infect competent BJ5183-Adeasy cells (100 μl). LB-kanamycin agar plates were employed to screen positive colonies, which were confirmed by digestion with PacI. After digestion with PacI, Ad5-pSh-CMV-Cbfa1 was extracted with phenol-chloroform and its purity and concentration were determined. Purity was calculated as follow: OD260 nm/OD280 nm = 1.8652, and concentration was 425 ng/μl. Then, Ad5-pSh-CMV-Cbfa1 (0.8 μg and 1.2 μg) was used to transfect HEK293 cells in the presence of liposomes. The transfected cells were then observed: presence of phagocytizing plaques on day 7 and complete destruction on day 10.
Separation, culture, identification and observation of cells
The separation, culture and identification of MSCs were done as previously described [26]. In brief, bone marrow was collected from the upper terminal of right tibia of adult rabbits aged 6 months. MSCs were separated by gradient centrifugation with Percoll, and then seeded into 6-well plate at a density of 3 × 104/well in low glucose - DMEM containing 10% FBS, 100 U/ml penicillin and 100 U/ml streptomycin followed by incubation at 37°C in an environment with 5% CO2 and 100% humidity. The medium was refreshed 3 days later and then once every two days. When the cell confluence reached 85%, cells were passaged. MSCs of the second passage were seeded into 6-well plate and maintained an incubator with 3% O2. Cells grown in normal condition (37°C, 5% CO2, 95% air, 100% humidity) served as controls. The morphology of MSCs was observed under an inverted microscope 16 days later. Rabbits (6 months old, body weight 2.5-3.2 kg) were provided by the Animal Facility of the Chinese Academy of Military Medical Sciences (Lenience No. SCXK Beijing 2007-0003), China. This study was approved by the Ethics Committee of our university.
Ad-Cbfa1 infected MSCs
One day before infection, cells of the second generation were passaged and counted. The cell count was higher than 106, and then further maintained. Cells were infected with Ad-Cbfa1 at an MOI of 80% in an environment with 5% CO2 and 100% humidity at 37°C One day later, cells in 5 randomly selected fields were counted in each well, and the transfection efficiency was determined as follow: transfection efficiency = cells expressing GFP/total cells × 100%. Cells were further cultured, and detection was done when the cell confluence reached 80%.
Detection of cell proliferation by MTT assay
The growth of MSCs was detected under normoxia and hypoxia conditions before and after Cbfa1 transfection. Curve was delineated with time as horizontal axis and optical density (OD) as vertical axis. In brief, single layer cells were digested with 0.25% trypsin and cell suspension was prepared with L-DMEM containing 10% FBS. These cells were then seeded into 96-well plate at a density of 1.5 × 104 per well (200 μl per well). Cells were incubated under the hypoxia or normoxia condition for 1, 2, 4, 6, 8, 10 and 12 d. Then, cells in 3 wells were collected and incubated with 5 mg/ml MTT at 37°C for 4 h. The supernatant was removed and cells were treated with 150 μl of DMSO for 10 min under continuous shaking. The optical density (OD) was detected at 490 nm in a microplate reader.
Detection of ALP activity
The ALP activity was measured according to manufacturer’s instructions. The supernatant of transfected and untransfected MSCs was collected for the detection of ALP activity. Briefly, the working solution containing 16 mmol/L nitrophenylphosphate, 350 mmol/L 2-amino-2- methyl-1-propanol buffer (30°C, pH 0.9) and 2 mmol/L MgCl2 was prepared before detection. Then, 1.00 ml of working solution was mixed with 0.02 ml of supernatant followed by incubation at 30°C for 60 s. OD was measured at 405 nm, which was done once every 60 s for a total of 4 times. The mean change in OD in 1 min was calculated as (ΔA), and the ALP activity was calculated according to the following formula: ALP (U/L) = ΔA × 0.665.
Labeling of MSCs with tetracycline
MSCs of the second passage were seeded into 4 6-well plates at a density of 3 × 104/well. Cells in two plates were maintained under the hypoxia or normoxia condition, and cells in one of plates under the hypoxia or normoxia condition were transfected with Ad-Cbfa1. Once cells formed nodules in these plates, tetracycline at 100 μg/ml was added followed by incubation for 30 min. The medium was refreshed followed by further incubation for 30 min. Cells were washed with Hank’s solution and then fixed in absolute ethanol for 15 min. These cells were observed under a fluorescence microscope.
Detection of mRNA expressions of Cbfa1, OPN, BMP and VEGF
Primer synthesis was done according to previously described by Halvorsen et al [7]. The gene sequences of rabbit Cbfa1, OPN, BMP and VEGF were identified in NCBI, according to which the corresponding primers were designed with Premier 5. The primers were synthesized in Shanghai Sangon Biotech Co., Ltd. The primers were as follows: Cbfa1: 5’-cccagccacctttacctaca-3’ (forward), 5’-tatggagtgctgctggtctg- 3’ (reverse); BMP: 5’-cttggaggagaagcaaggtg-3’ (forward), 5’-gctgtttgtgtttcgcttga-3’ (reverse); VEGF: 5’-cgagaccttggtggacatct-3’ (forward), 5’-tatggagtgctgctggtctg-3’ (reverse); OPN 5’- aggatgaggacgatgaccac-3’ (forward), 5’-cacggccgtcgtatatttct-3’ (reverse); β-actin: 5’-TCACCCACACTGTGCCCCATCTACGA-3’ (forward), 5’-CAGCGGAACCGCT CATTGCCAATGG-3’ (reverse). After incubation under the hypoxia or normoxia condition for 72 h, the mRNA expressions of Cbfa1, OPN, BMP and VEGF were determined.
In brief, MSCs were seeded into 6-well plates at a density of 2 × 105 cells/well. On the second day, MSCs were infected with Ad-cbfa1 at a MOI of 150, and cells without infection served as a control. Two days later, cells were collected and total RNA was extracted and stored at -80°C for use. Then, 2.0 μg of RNA was used for reverse transcription with cDNA first strand synthesis kit (Promega). The products were stored at -20°C. mRNA expression was detected by Real-time PCR. The reaction mixture (30 μl) included 15 μl of PCR reaction solution, 1 μl of forward primer, 1 μl of reverse primer, 1 μl of cNDA template, and 12 μl of sterilized water. The reaction conditions were as follows: 41 cycles of 50°C for 2 min, 95°C for 2 min, 95°C for 3 s and 60°C for 30 s. The mRNA expression of target genes was determined and normalized to that of β-actin.
Statistical analysis
All the data were expressed as mean ± standard deviation (SD), and analysis of variance was employed for comparisons. A value of P<0.05 was considered statistically significant. Statistical analysis was done with SAS 6.12.
Results
Morphology of MSCs
At 6~12 d after culture, MSCs presented with rapid growth, were spindle-shaped and had thick and big processes. A few cells were triangular or polygonal. The nucleus located in the center and 1-2 nuclei were present in one cell. Colonies were presented within about 12 d. Two weeks after culture, cells merged into a single-layer, and the spindle-shaped processes were elongated. These cells arranged in a direction and in a whirlpool, network or radiation manner. Hypoxia and Cbfa1 gene transfection had no influence on the morphology of MSCs and the time of MSCs merging into a single-layer (Figure 1).
Figure 1.
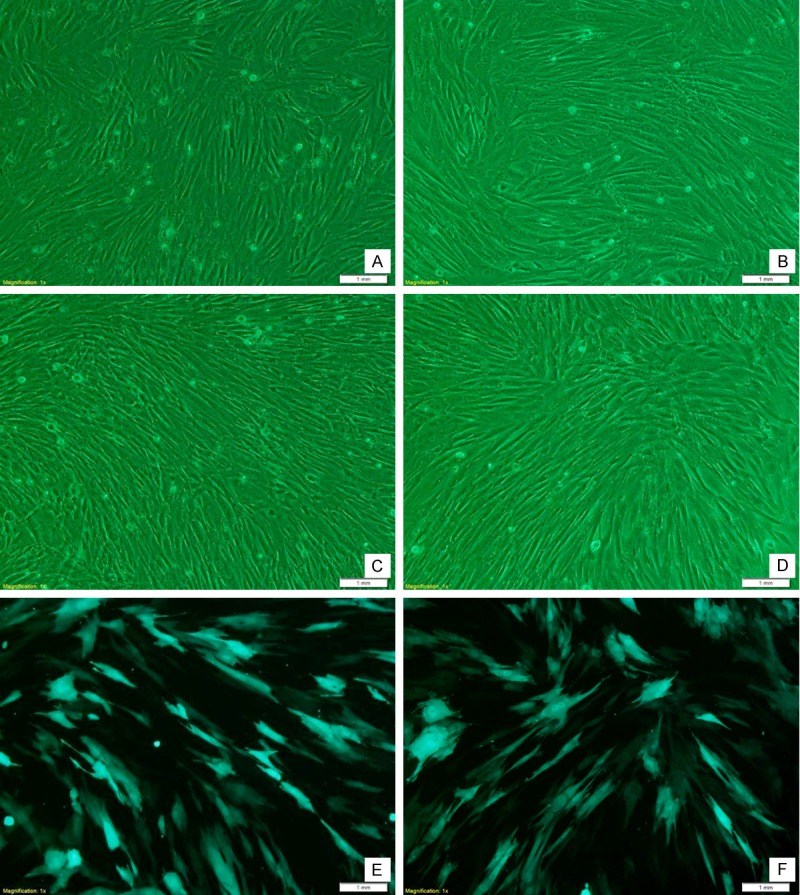
MSCs under an inverted microscope. A, C, E: 9 days after culture under a normoxia condition; B, D, F: 9 days after culture under a hypoxia condition; C-F: 9 days after Cbfa1 gene transfection; E, F: MSCs under a fluorescence microscope. MSCs after incubation under hypoxia condition were similar to those under normoxia condition. Cbfa1 gene transfection had no influence on the morphology of MSCs.
Cell growth curve
During the culture under a hypoxia condition within 12 days, the absorbance increased. At 4 days after culture, the absorbance was significantly higher than that after culture under a normoxia condition. Under a normoxia condition, the proliferation rare of MSCs began to reduce after culture for 10 days. However, under the hypoxia condition, the proliferation rate was still increasing after culture for 14 days. In addition, the absorbance of Cbfa1 transfected cells was slightly higher than that of non-tranfected cells (Figure 2).
Figure 2.
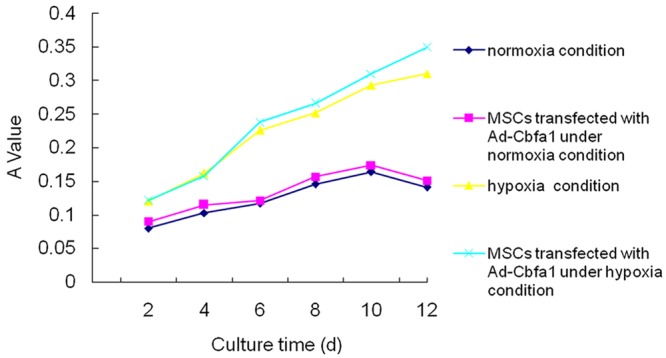
Cell growth curve. As shown in figure, hypoxia significantly changed the proliferation of MSCs, prolonged the doubling time and increased their proliferation rate. However, Cbfa1 gene transfection had no influence on the doubling time and proliferation rate of MSCs.
ALP activity
The ALP activity is related to the amount of deposited minerals, and thus ALP activity may indirectly reflect the osteogenic ability of MSCs. As shown in Figure 3, the ALP activity increased gradually within 14 days of cell culture. After 14-day culture, the ALP activity began to reduce. The ALP activity of MSCs under the normoxia condition was significantly higher than that under the hypoxia condition. In addition, the ALP activity of Cbfa1 transfected MSCs was markedly increased when compared with non-transfected MSCs.
Figure 3.
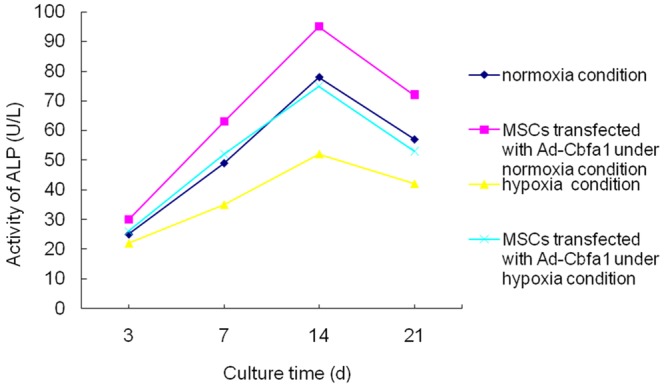
ALP activity in different groups. As shown in figure, the ALP activity was increasing within 14 days of culture. After 14-day culture, the ALP activity began to increase. The ALP activity of MSCs under the normoxia condition was significantly higher than that under the hypoxia condition. In addition, the ALP activity of Cbfa1 transfected MSCs was markedly increased when compared with non-transfected MSCs.
Labeling with fluorescent tetracycline
Tetracycline hydrochloride is a specific marker of new bones because it can selectively bind to calcium and deposit in bone like tissues. Under a fluorescence microscope, the tetracycline hydrochloride labeled bone-like tissues present with yellow and green fluorescence. After culture of MSCs, the extracellular matrix may form nodules in which a lot of calcium-containing compounds lie. Thus, these compounds may also present with yellow and green fluorescence after labeling with tetracycline hydrochloride under a fluorescence microscope. The amount of extracellular matrix can be determined according to the area of fluorescence, which indirectly reflects the osteogenic ability of MSCs. As shown in Figure 4, the fluorescence area of MSCs after culture under a normoxia condition was significantly larger than that after culture under a hypoxia condition. In addition, the fluorescence area of Cbfa1 transfected cells was markedly larger than that of non-transfected cells. This suggests that hypoxia may inhibit the differentiation of MSCs into osteoblasts, and Cbfa1 transfection promotes the differentiation of MSCs into osteoblasts.
Figure 4.
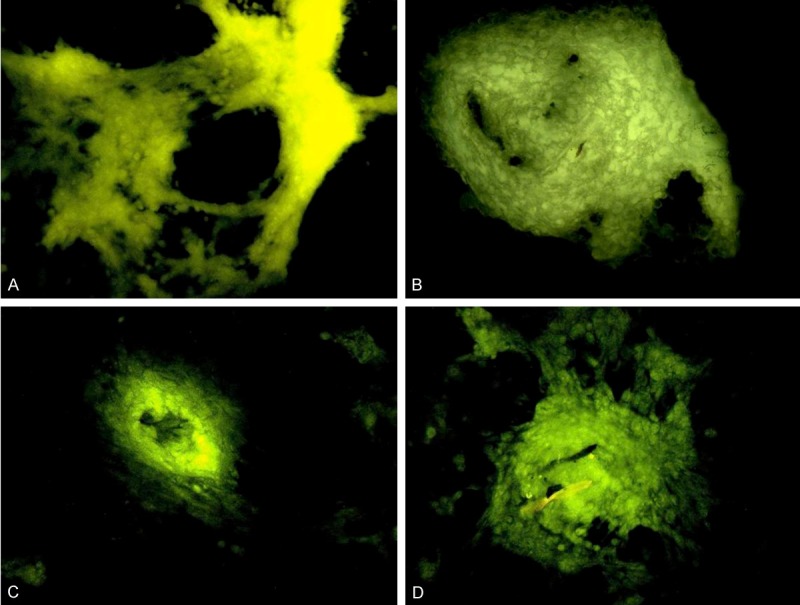
Labeling with tetracycline hydrochloride fluorescence. A, B: MSCs under the normoxia condition; C, D: MSCs under the hypoxia condition; A, C: Non-transfected cells; B, D: Cbfa1 transfected cells. The fluorescence area of MSCs under normoxia condition was significantly larger than that under hypoxia condition. The fluorescence area of Cbfa1 transfected MSCs was markedly increased when compared with non-transfected cells.
Osteogenic differentiation of MSCs
As shown in Figure 5, under the normoxia condition, the mRNA expressions of Cbfa1, OPN, BMP and VEGF were significantly higher than those under the hypoxia condition (P<0.05). As shown in Figure 6, the Cbfa1 mRNA expression in Cbfa1 transfected MSCs was markedly increased and about 9 folds of that before transfection. The mRNA expressions of BMP and OPN were slightly higher than those before transfection, but the VEGF mRNA expression was slightly reduced when compared with that before trasnfection. In addition, after Cbfa1 gene trasnfection, the MSCs had increased mRNA expressions of Cbfa1, OPN, BMP and VEGF under the hypoxia condition, and the maximal increase was observed in Cbfa1 mRNA expression (P<0.05).
Figure 5.
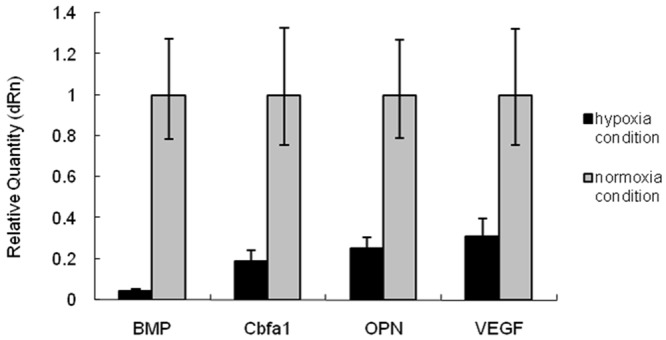
mRNA expressions of BMP, Cbfa1, OPN and VEGF. Under the normoxia condition, the mRNA expressions of Cbfa1, OPN, BMP and VEGF were significantly higher than those under the hypoxia condition (P<0.05).
Figure 6.
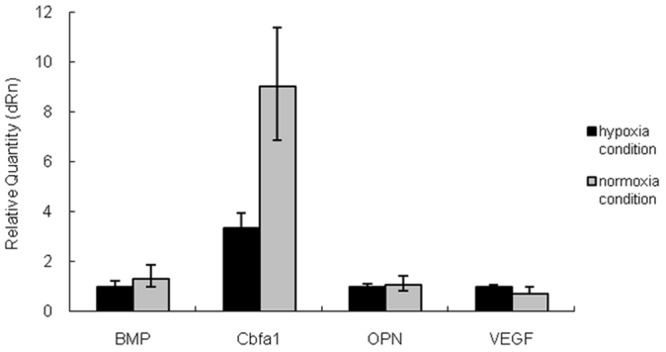
mRNA expressions of BMP, Cbfa1, OPN and VEGF in Cbfa1 transfected cells. After Cbfa1 transfection, under the normoxia condition, the MSCs had significantly increased Cbfa1 mRNA expression, which was about 9 folds of that before transfection. The mRNA expressions of BMP and OPN were slightly increased when compared with those before transfection, but the VEGF mRNA expression reduced slightly. After Cbfa1 transfection, the mRNA expressions of Cbfa1, OPN, BMP and VEGF increased under the hypoxia condition, and the maximal increase was observed in Cbfa1 mRNA expression (P<0.05).
Discussion
MSCs have potent self-renewal and multi-lineage differentiation potentials and have been used as common seed cells in the treatment of some refractory diseases such as ischemic heart diseases, cerebral diseases, limb necrosis, bone fracture and bone necrosis. In some diseases, local hypoxia or even anoxia is present. Studies have shown that the oxygen concentration at focal lesions is as low as 1% [27], and in vitro culture of cells is done at the oxygen concentration of 20%. In healthy subjects, the oxygen concentration is 4%-7% in the bone marrow where the majority of MSCs locate [13]. Thus, to maintain the biological activity of MSCs collected after in vitro culture is crucial for the treatment of diseases with MSCs [11]. There is evidence showing that hypoxia is beneficial for the cell proliferation during the in vitro culture. Results from studies of Ranera [28], D’Ippolito [6], Grayson [7] and Bosch [8] indicated that the phenotype of MSCs remained unchanged under the hypoxia condition, and they still presented with undifferentiated phenotype, but their ability to form colonies increased significantly, the expressions of markers of stem cells elevated markedly, and the proliferation of MSCs was dramatically facilitated. On the contrary, the osteogenic differentiation of MSCs is inhibited under the hypoxia condition, and the expressions of osteocalcin, bone sialoprotein (BSP), Osterix, Cbfa1 and ALP (markers of osteoblasts) reduced significantly [6,9,13,29]. Our results showed, under the hypoxia (3%) and normoxia conditions, the morphology and growth curve remained unchanged, but the expressions of Cbfa1 and BMP (two determinants of osteogenesis) were markedly inhibited under the hypoxia condition. Although expressions of OPN and VEGF (two proteins related to osteogenesis) were also reduced, the extent of reduction was lower than that in the expressions of Cbfa1 and BMP. This suggests that the hypoxia induced inhibition of differentiation of MSCs into osteoblasts is more closely associated with the reduction of Cbfa1 and BMP expressions.
To maintain the osteogenic capability of MSCs under the hypoxia condition, MSCs were transfected with Cbfa1 to elevate the osteogenic ability of MSCs under the hypoxia condition. Cbfa1 is the first and unique osteogenesis-specific factor. In 1998, Komori et al [30] confirmed that Cbfa1 was a determinant transcriptional factor in the osteogenic differentiation of MSCs. Cbfa1 has 3 isoforms, and Cbfa1/Osf2 is expressed in only bone tissues and osteoblasts but not in other tissues. In the present study, Cbfa1 was transfected into MSCs, which were then maintained under the hypoxia or normoxia condition. The morphology, time of cell confluence and proliferation rate were determined. Results showed Cbfa1 transfection had no influence on the morphology and proliferation rate of these cells under the hypoxia and normoxia condition. At the same time, Cbfa1 transfection significantly increased the mRNA expressions of Cbfa1, OPN, BMP and VEGF in MSCs, especially the Cbfa1 expression. This suggests that Cbfa1 transfection may favorably increase the osteogenic differentiation of MSCs under the hypoxia condition.
Our results showed hypoxia may inhibit the osteogenic differentiation of MSCs, and Cbfa1 transfection can favorably increase the osteogenic ability of MSCs under the hypoxia condition. Our findings provide evidence for the treatment of avascular bone injury (such as ischemic bone necrosis and severe bone defects).
Acknowledgements
The study was supported by National Natural Science Foundation of China (No: 30700854, 8107146); Natural Science Foundation of Hubei Province (No: 2009CDB414).
Disclosure of conflict of interest
None.
References
- 1.Jager M, Hernigou P, Zilkens C, Herten M, Li X, Fischer J, Krauspe R. Cell therapy in bone healing disorders. Orthop Rev (Pavia) 2010;2:e20. doi: 10.4081/or.2010.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan ZQ, Chen YS, Li WJ, Yang Y, Huo JZ, Chen ZR, Shi JH, Ge JB. Treatment of osteonecrosis of the femoral head by percutaneous decompression and autologous bone marrow mononuclear cell infusion. Chin J Traumatol. 2006;9:3–7. [PubMed] [Google Scholar]
- 3.Sen RK. Management of avascular necrosis of femoral head at pre-collapse stage. Indian J Orthop. 2009;43:6–16. doi: 10.4103/0019-5413.45318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muller I, Vaegler M, Holzwarth C, Tzaribatchev N, Pfister SM, Schutt B, Reize P, Greil J, Handgretinger R, Rudert M. Secretion of angiogenic proteins by human multipotent mesenchymal stromal cells and their clinical potential in the treatment of avascular osteonecrosis. Leukemia. 2008;22:2054–2061. doi: 10.1038/leu.2008.217. [DOI] [PubMed] [Google Scholar]
- 5.Tzaribachev N, Vaegler M, Schaefer J, Reize P, Rudert M, Handgretinger R, Muller I. Mesenchymal stromal cells: a novel treatment option for steroid-induced avascular osteonecrosis. Isr Med Assoc J. 2008;10:232–234. [PubMed] [Google Scholar]
- 6.D’Ippolito G, Diabira S, Howard GA, Roos BA, Schiller PC. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone. 2006;39:513–522. doi: 10.1016/j.bone.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 7.He J, Genetos DC, Yellowley CE, Leach JK. Oxygen tension differentially influences osteogenic differentiation of human adipose stem cells in 2D and 3D cultures. J Cell Biochem. 2010;110:87–96. doi: 10.1002/jcb.22514. [DOI] [PubMed] [Google Scholar]
- 8.Potier E, Ferreira E, Andriamanalijaona R, Pujol JP, Oudina K, Logeart-Avramoglou D, Petite H. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone. 2007;40:1078–1087. doi: 10.1016/j.bone.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 9.Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am J Physiol Cell Physiol. 2006;290:C1139–1146. doi: 10.1152/ajpcell.00415.2005. [DOI] [PubMed] [Google Scholar]
- 10.Holzwarth C, Vaegler M, Gieseke F, Pfister SM, Handgretinger R, Kerst G, Muller I. Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol. 2010;11:11. doi: 10.1186/1471-2121-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geng YJ. Molecular mechanisms for cardiovascular stem cell apoptosis and growth in the hearts with atherosclerotic coronary disease and ischemic heart failure. Ann N Y Acad Sci. 2003;1010:687–697. doi: 10.1196/annals.1299.126. [DOI] [PubMed] [Google Scholar]
- 12.Salim A, Nacamuli RP, Morgan EF, Giaccia AJ, Longaker MT. Transient changes in oxygen tension inhibit osteogenic differentiation and Runx2 expression in osteoblasts. J Biol Chem. 2004;279:40007–40016. doi: 10.1074/jbc.M403715200. [DOI] [PubMed] [Google Scholar]
- 13.Lennon DP, Edmison JM, Caplan AI. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: effects on in vitro and in vivo osteochondrogenesis. J Cell Physiol. 2001;187:345–355. doi: 10.1002/jcp.1081. [DOI] [PubMed] [Google Scholar]
- 14.Tsai CC, Yew TL, Yang DC, Huang WH, Hung SC. Benefits of hypoxic culture on bone marrow multipotent stromal cells. Am J Blood Res. 2012;2:148–159. [PMC free article] [PubMed] [Google Scholar]
- 15.Das R, Jahr H, van Osch GJ, Farrell E. The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev. 2010;16:159–168. doi: 10.1089/ten.TEB.2009.0296. [DOI] [PubMed] [Google Scholar]
- 16.Huang YC, Zhu HM, Cai JQ, Huang YZ, Xu J, Zhou Y, Chen XH, Li XQ, Yang ZM, Deng L. Hypoxia inhibits the spontaneous calcification of bone marrow-derived mesenchymal stem cells. J Cell Biochem. 2012;113:1407–1415. doi: 10.1002/jcb.24014. [DOI] [PubMed] [Google Scholar]
- 17.Sheehy EJ, Buckley CT, Kelly DJ. Oxygen tension regulates the osteogenic, chondrogenic and endochondral phenotype of bone marrow derived mesenchymal stem cells. Biochem Biophys Res Commun. 2012;417:305–310. doi: 10.1016/j.bbrc.2011.11.105. [DOI] [PubMed] [Google Scholar]
- 18.Pattappa G, Thorpe SD, Jegard NC, Heywood HK, de Bruijn JD, Lee DA. Continuous and uninterrupted oxygen tension influences the colony formation and oxidative metabolism of human mesenchymal stem cells. Tissue Eng Part C Methods. 2013;19:68–79. doi: 10.1089/ten.TEC.2011.0734. [DOI] [PubMed] [Google Scholar]
- 19.Frendo JL, Xiao G, Fuchs S, Franceschi RT, Karsenty G, Ducy P. Functional hierarchy between two OSE2 elements in the control of osteocalcin gene expression in vivo. J Biol Chem. 1998;273:30509–30516. doi: 10.1074/jbc.273.46.30509. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida CA, Furuichi T, Fujita T, Fukuyama R, Kanatani N, Kobayashi S, Satake M, Takada K, Komori T. Core-binding factor beta interacts with Runx2 and is required for skeletal development. Nat Genet. 2002;32:633–638. doi: 10.1038/ng1015. [DOI] [PubMed] [Google Scholar]
- 21.Selvamurugan N, Brown RJ, Partridge NC. Regulation of collagenase-3 gene expression in osteoblastic and non-osteoblastic cell lines. J Cell Biochem. 2000;79:182–190. doi: 10.1002/1097-4644(20001101)79:2<182::aid-jcb20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Komori T. A fundamental transcription factor for bone and cartilage. Biochem Biophys Res Commun. 2000;276:813–816. doi: 10.1006/bbrc.2000.3460. [DOI] [PubMed] [Google Scholar]
- 23.Zheng H, Guo Z, Ma Q, Jia H, Dang G. Cbfa1/osf2 transduced bone marrow stromal cells facilitate bone formation in vitro and in vivo. Calcif Tissue Int. 2004;74:194–203. doi: 10.1007/s00223-003-0004-x. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Z, Zhao M, Xiao G, Franceschi RT. Gene transfer of the Runx2 transcription factor enhances osteogenic activity of bone marrow stromal cells in vitro and in vivo. Mol Ther. 2005;12:247–253. doi: 10.1016/j.ymthe.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Z, Wang Z, Ge C, Krebsbach P, Franceschi RT. Healing cranial defects with AdRunx2-transduced marrow stromal cells. J Dent Res. 2007;86:1207–1211. doi: 10.1177/154405910708601213. [DOI] [PubMed] [Google Scholar]
- 26.Li ZH, Liao W, Cui XL, Zhao Q, Liu M, Chen YH, Liu TS, Liu NL, Wang F, Yi Y, Shao NS. Intravenous transplantation of allogeneic bone marrow mesenchymal stem cells and its directional migration to the necrotic femoral head. Int J Med Sci. 2011;8:74–83. doi: 10.7150/ijms.8.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordillo GM, Sen CK. Revisiting the essential role of oxygen in wound healing. Am J Surg. 2003;186:259–263. doi: 10.1016/s0002-9610(03)00211-3. [DOI] [PubMed] [Google Scholar]
- 28.Ranera B, Remacha AR, Alvarez-Arguedas S, Romero A, Vazquez FJ, Zaragoza P, Martin-Burriel I, Rodellar C. Effect of hypoxia on equine mesenchymal stem cells derived from bone marrow and adipose tissue. BMC Vet Res. 2012;8:142. doi: 10.1186/1746-6148-8-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JH, Kemp DM. Human adipose-derived stem cells display myogenic potential and perturbed function in hypoxic conditions. Biochem Biophys Res Commun. 2006;341:882–888. doi: 10.1016/j.bbrc.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 30.Komori T, Kishimoto T. Cbfa1 in bone development. Curr Opin Genet Dev. 1998;8:494–499. doi: 10.1016/s0959-437x(98)80123-8. [DOI] [PubMed] [Google Scholar]


