Abstract
Objective: Our objective was to examine whether prior tetanic stimulation of cranial nerves enhances the amplitudes of transcranial motor-evoked potentials (MEPs). Methods: Thirty patients undergoing elective craniotomy under propofol-fentanyl anesthesia with partial neuromuscular blockade were enrolled. Both control and posttetanic MEPs (c-MEPs and p-MEPs) monitoring were performed with a train of five pulses delivered to C3 or C4. c-MEPs were recorded from target muscles and p-MEPs were obtained 1 s after tetanic stimulation to the ulnar nerves and facial nerves. The amplitudes of paired MEPs were compared with Wilcoxon’s signed rank test. Results: When tetanic stimulation was separately applied to the facial nerves, amplitudes of p-MEPs from abductor pollicis brevis, orbicularis oculi or oris were similar with those of c-MEPs. When tetanic stimulations were separately applied to the ulnar nerves, the amplitudes of p-MEPs from the abductor pollicis brevis but not orbicularis oculi or oris were significantly enlarged compared with c-MEP. Conclusions: We found that only prior tetanic stimulation of ulnar nerve but not facial nerve could enlarge the amplitudes of trancranial hand MEPs. Augmentation of MEP amplitude via prior tetanic stimulation of peripheral nerve seems to originate from the subcortical level but not motor cortex.
Keywords: Motor-evoked potentials, tetanic stimulation, ulnar nerve, facial nerve, abductor pollicis brevis, orbicularis oculi, orbicularis oris
Introduction
Intraoperative monitoring of motor-evoked potentials (MEPs) via transcranial stimulation of the motor cortex has become a commonly used technique, which allows preserving functional integrity of descending motor pathways during brain and spinal cord surgery. However, this technique is not reliable in all cases, as the elicited myogenic responses may be very sensitive to suppression by anesthetics and neuromuscular blocker [1-4] although multipulse stimulation is used in this setting [5]. Intraoperative deep anesthesia and/or partial neuromuscular blockade are especially necessary when body movement is a critical consideration. For this reason, the techniques which can improve recording of MEP signals are important and welcomed during general anesthesia. Recently Kakimoto et al [6] developed a technique to augment the amplitudes of MEPs via tetanic stimulation of the ipsilateral peripheral nerve before conducting transcranial electrical stimulation, suggesting its potential usefulness in intraoperative MEP monitoring [7]. Hayashi et al [8] further indicated that the post-tetanic effects on MEP amplitudes can occur at the bilateral limbs under partial neuromuscular blockade or sevoflurane anesthesia [9]. A possible mechanism for this effect is that the subject of speculation relates to an increase in motoneuronal excitability at the level of the spinal cord or brain [10]. However, until now whether the amplitudes of transcranial MEPs can be augmented via tetanic stimulation of cranial nerves has not yet been examined.
Therefore the present study aims to investigate whether tetanic stimulation of the facial nerve or ulnar nerve could augment MEP amplitudes recorded from the abductor pollicis brevis or orbicularis oculi and orbicularis oris, as to verify this effect can be realized by application of tetanic stimulation to the both peripheral and cranial nerve under general anesthesia.
Material and methods
Written informed consent was obtained from each patient after hospital ethical institute approval. Thirty patients undergoing elective resection of intracranial tumors of the hemisphere under propofol-fentanyl anesthesia with partial neuromuscular blockade were enrolled in the study, among which 18 were males and the other 12 females; with the age of 34 ± 5 years and body weight of 55 ± 7 kg. Patients with preoperative motor dysfunction, sensory deficits and seizures were excluded from the study. There was no premedication before anesthesia. Standardized anesthesia was induced with target control infusion (TCI) of propofol (plasma concentration, 4.0 μg/ml), and fentanyl (3 μg/kg). Cisatracurium 0.08-0.1 mg/kg was used to facilitate endotracheal intubation and mechanical ventilation. Anesthesia was maintained with propofol (TCI target plasma concentration: 3.0-5.0 μg/mL) and intermit fentanyl administration for analgesia (total doses of 8-10 μg/kg). Invasive arterial blood pressure, electrocardiogram, pulse oximetry, end-tidal carbon dioxide concentration and oropharynx temperature were monitored continuously to maintain normal physiological parameters in all patients (S/5 Anesthesia Monitor, GE Healthcare, Helsinki, Finland). The level of partial neuromuscular blockade was controlled at a TOF value between 2 and 3, which was achieved via continuous infusion of cisatracurium. The infusion rate of cisatracurium was adjusted according to results monitored from the adductor pollicis muscle with TOF-WATCH®SX (Organon Ltd. Dublin, Ireland) every 5 minutes.
Measurements of MEPs
After stabilizing the level of partial neuromuscular blockade under general anesthesia, the monitoring of MEPs was initiated, as shown in Figure 1. Multipulse transcranial electric stimulation (train-of-five pulses) was performed using EpochXP Neurological Workstation (Axon systems Inc. Hauppauge, NY, United States). The scalp corkscrew stimulating electrodes positioned at C3 or C4 (cathodes), and Cz as reference electrode (anodes) according to International 10-20 System, were used to deliver supramaximal stimuli. The stimulus intensity was set at 500 V with an interstimulus interval of 2 ms (500 Hz) and time constant of 100 ms [11]. Evoked myographic responses were amplified with a 0.2-1.0 kHz bandpass filter. The MEPs were recorded from subdermal paired needles placed in the target muscles.
Figure 1.

The schematic graphic of tetanic stimulation prior to transcranial electric stimulation and motor evoked potentials (MEPs) recording. A: Tetanic stimulation of bilateral ulnar nerves before transcranial electric stimulation and MEPs were unilaterally recorded in target muscles (abductor pollicis brevis, orbicularis oculi or oris). B: Tetanic stimulation of bilateral rami temporales nervi facialis or rami buccales nervi facialis before transcranial electric stimulation and MEPs were unilaterally recorded in target muscles (abductor pollicis brevis, orbicularis oculi or oris).
The protocols of control MEPs (c-MEPs) and post-tetanic stimulation MEPs (p-MEPs) measurements were modified from Hayashi et al [7]. Briefly, c-MEPs were recorded unilaterally as compound muscle action potentials (cMAPs) at the abductor pollicis brevis muscle (c-hand MEPs), orbicularis oculi and oris muscles (c-facial MEPs) without prior tetanic stimulation. For the measurement of p-MEPs, tetanic stimulation (50 Hz, 50 mA of stimulus intensity and 5 s duration) with a peripheral nerve stimulator (LYJS-1 type, Longyi medical device Ltd. Zhangjiagang, China) was separately applied to the bilateral branches of facial nerves (rami temporales nervi facialis or rami buccales nervi facialis) or ulnar nerve 1 s before transcranial electric stimulation and unilateral MEP recordings. For tetanic stimulation of the ulnar nerve or branch of facial nerve, two pre-gelled electrodes are applied over the path of ulnar nerve or facial nerve, for instance, the volar side of the wrist or outer canthus of the eye. The setup of transcranial electric stimulation for p-MEPs is the same as that of c-MEP measurements. The MEP recording sites were located contralateral to the side on which neuromuscular monitoring was performed. Fifteen seconds after c-MEPs were recorded, p-MEPs were recorded as cMAPs at the abductor pollicis brevis (p-hand MEPs), orbicularis oculi and orbicularis oris (p-facial MEPs). Every pair of c-MEP and p-MEP was repeated twice and the amplitudes of MEPs were averaged. The studies indicated that there were no residual effects (augmentation and fatigue) 2 min after p-MEP [12]. So the interval of p-MEP recordings was set at more than 2 min. The ground electrode was placed on the left or right arm proximal to the elbow. Peak-to-peak amplitude was determined from the average of two individual responses. When the average MEP amplitude was < 30 uV, the MEP response was defined as “no response”.
Statistical analysis
Sample sizes were determined based on the data in previous and preliminary studies. It was assumed that it was clinically important that the amplitude of the MEPs was augmented by 75% after the application of tetanic stimulation to the motor branches of the facial nerve and ulnar nerve [8]. Based on the formula for normal theory and assuming a Type I error of 0.05 and a power of 0.8, 30 patients were required for each comparison. Comparisons of amplitudes of the c-MEPs and p-MEPs at each recording site were performed using Wilcoxon’s signed rank test. P < 0.05 was considered different significantly.
Results
There were no complaints of seizures or skin burns after their operations. Under partial neuromuscular blockade during general anesthesia, c-MEP amplitudes were obtained reliably in 26 out of 30 patients (87%) from all recording sites (c-hand MEP and c-facial MEP). When tetanic stimulation was separately applied to the bilateral rami temporales nervi facialis or rami buccales nervi facialis, the success rate of p-facial MEP monitoring recorded from orbicularis oculi or oris musculus and p-hand MEP monitoring recorded from abductor pollicis brevis were also 87% (26 of 30 patients). However, when tetanic stimulation was separately applied to the bilateral ulnar nerve, as for the abductor pollicis brevis, the success rate of p-hand MEP-monitoring reached 100% (30 of 30 patients); while the success rate for p-facial MEP-monitoring of the orbicularis oculi or oris musculus still was 87% (26 of 30 patients, Table 1). No patient exhibited body movement during tetanic stimulation and transcranial electric stimulation.
Table 1.
Success rates of c-MEP and p-MEP recordings when tetanic stimulation was separately applied to the bilateral ulnar nerves
| Recording sites | Control % (n) | i-TS % (n) | c-TS % (n) |
|---|---|---|---|
| orbicularis oculi | 87 (26/30) | 87 (26/30) | 87 (26/30) |
| orbicularis oris | 87 (26/30) | 87 (26/30) | 87 (26/30) |
| abductor pollicis brevis | 87 (26/30) | 100 (30/30) | 100 (30/30) |
Note: Values are proportion (number). i-TS represents C3 or C4 stimulation 1 second after tetanic stimulation of the ipsilateral ulnar nerve; and similarly, c-TS represents transcranial stimulation after tetanic stimulation of the contralateral ulnar nerve.
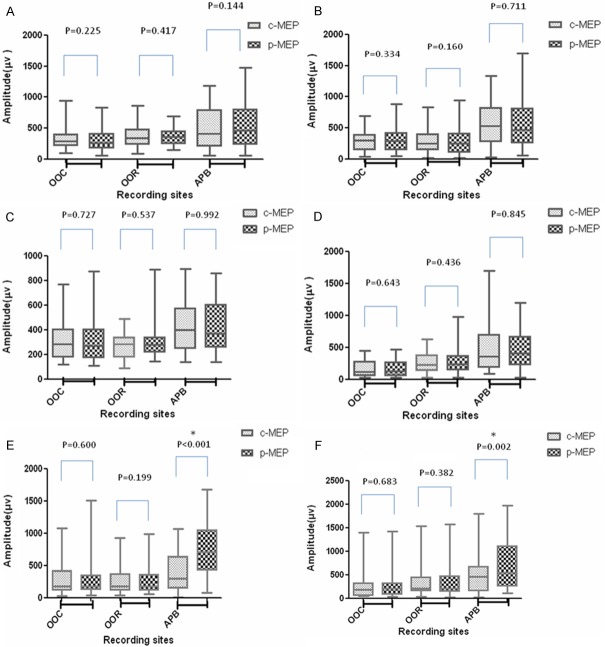
Comparisons of the amplitudes of c-MEPs and p-MEPs are shown in a box plot (Figure 2). The representative recordings of c-MEPs and p-MEPs for the same patient are shown in Figure 3. When tetanic stimulation was separately applied to the bilateral rami temporales nervi facialis or rami buccales nervi facialis, the amplitudes of p-facial MEPs from the orbicularis oculi and oris musculus and p-hand MEPs from the abductor pollicis brevis did not increase compared with those of c-facial MEPs and c-hand MEPs. However, when tetanic stimulation was separately applied to the bilateral ulnar nerve, the amplitudes of p-hand MEPs significantly increased compared with those of c-hand MEPs. The amplitudes of p-facial MEPs remained unchanged compared with those of c-facial MEPs.
Figure 2.
Amplitudes of control motor-evoked potentials (c-MEPs) and post-tetanic motor-evoked potentials (p-MEPs) from orbicularis oculi (OOC), orbicularis oris (OOR) and abductor pollicis brevis (APB). The Wilcoxon’s signed rank test is used to compare the amplitudes of paired MEPs. A: Tetanic stimulation of the ipsilateral rami temporales nervi facialis; B: Tetanic stimulation of the ipsilateral rami buccales nervi facialis; C: Tetanic stimulation of the contralateral rami temporales nervi facialis; D: Tetanic stimulation of the contralateral rami buccales nervi facialis; E: Tetanic stimulation of the ipsilateral ulnar nerve; F: Tetanic stimulation of the contralateral ulnar nerve.
Figure 3.
The representative waveforms of paired motor-evoked potentials (c-MEPs and p-MEPs) recorded from target muscles. A: The waveforms of paired MEPs recorded from orbicularis oculi with prior tetanic stimulation of the ipsilateral (1-2 channels) and contralateral (3-4 channels) rami temporales nervi facialis in the same patient; B: The waveforms of paired MEPs recorded from abductor pollicis brevis with prior tetanic stimulation of the ipsilateral (1-2 channels) and contralateral (3-4 channels) rami temporales nervi facialis in the same patient; C: The waveforms of paired MEPs recorded from orbicularis oculi with prior tetanic stimulation of the ipsilateral (1-2 channels) and contralateral (3-4 channels) ulnar nerve in the same patient; D: The waveforms of paired MEPs recorded from abductor pollicis brevis with prior tetanic stimulation of the ipsilateral (1-2 channels) and contralateral (3-4 channels) ulnar nerve in the same patient.
Discussion
The results of our study revealed, contrary to our hypothesis, that the application of tetanic stimulation to the bilateral branches of facial nerves or ulnar nerves before transcranial stimulation does not significantly change the amplitudes of p-facial MEPs compared with those of c-facial MEPs in patients under propofol–fentanyl anesthesia with partial neuromuscular blockade. Similarly, compared with c-hand MEPs, prior tetanic stimulation of bilateral facial nerve does not significantly increase amplitudes of p-hand MEPs. However, the amplitudes of p-MEPs recorded from abductor pollicis brevis are significantly larger than those of c-MEPs following tetanic stimulation of bilateral ulnar nerves.
Which is consistent with the results of previous studies [6], in present study we bilaterally tetanically stimulated ulnar nerve or one branch of facial nerve, but only recorded unilateral p-hand MEPs and p-facial MEPs from the muscles which side is tetanically stimulated. This may raise the question of whether augmenting the amplitudes of MEPs is associated with tetanic stimulation’s self-induced neuromuscular junction potentiation, but not remote MEP enhancement. Therefore when MEPs were recorded from one side, we also delivered tetanic stimulation to contralateral ulnar nerve or branch of facial nerve to exclude this possibility. Another technical concern is electric intensity to transcranially stimulate the central cortex. Although the stimulating thresholds (in electric current mA) of hand MEPs and facial MEPs seem basically equal [13,14] and reduced electric intensity can decrease the problems of the current spread effect, a supramaximal stimulation method (500 V voltages) but not a threshold-level stimulation method was selected for the purpose to obtain maximal MEPs and avoid fluctuation of threshold level during procedure [13].
Recent findings indicate that tetanic stimulation of the peripheral nerve before transcranial stimulation can be a feasible technique used to augment MEP amplitudes during general anesthesia [6-9]. Others reported that double-train transcranial electrical stimulation technique could result in magnified MEPs in adult and pediatric patients [15]. These strategies can be useful for those cases in which MEP responses to transcranial stimulation are small or absent due to suppression by anesthetics and neuromuscular blockade [4] which have the purpose of preventing patient movement during neurosurgical procedures. The studies demonstrate that tetanic stimulations of peripheral nerves such as ulnar or posterior tibial nerve prior to conventional transcranial electric stimulation not only augment ipsilateral but also contralateral amplitudes of myogenic MEPs in both upper and lower extremities [8]. In this study we used temporal facilitation (consisting of a train-of-five pulse, 500 V; time constant 100 ms; interstimulus interval 2 ms) in conjunction with spatial facilitation (by tetanic stimulating the peripheral or cranial nerves 1 s before transcranial electric stimulus) [16]. It seems evident that facilitatory stimuli results in improved MEP signals after tetanic stimulation of peripheral nerves but not cranial nerves, although we don’t test other cranial nerves such as glossopharyngeal nerve or vagus nerve.
Although synaptic transmission has been regarded as the primary mechanism of this effect, the precise site at which myogenic MEPs are augmented by prior tetanic stimulation of peripheral nerve is unknown. The changes of amplitudes of MEPs under tetanic stimulation of peripheral nerve prior to transcranial electric stimulation can be motor cortical or spinal in origin. Peripheral stimulation has been reported to modulate corticomotoneuronal excitability [17-19]. Kaelin-Lang et al [10] demonstrated that ulnar nerve stimulation at the wrist for 2 h enhanced MEP amplitudes to transcranial magnetic stimulation from abductor digiti minimi muscles in humans, and this effect was blocked by lorazepam, a γ-aminobutyric acid type A agonist. This suggests that somatosensory stimulation elicited an increase in corticomotoneuronal excitability, probably at the level of the cortex. Andersson et al [17] demonstrated that a train of stimuli to the foot sole within the receptive field of the withdrawal reflex of the tibialis anterior muscle before transcranial stimulation augmented MEP responses. This indicates that the cortically elicited responses were spatially facilitated, probably at the level of the spine. To distinguish which level of central nervous system this effect occurs on, we set out to tetanically stimulate nerves in different sites before conventional MEPs and facial MEPs recordings, observing whether this phenomenon is common. The design of our study is based on the assumption that an increase in cortical motoneuron excitability via tetanic stimulation of peripheral nerves or cranial nerves before transcranial stimulation can result in amplitude augmentation of MEPs recorded from target muscles. Instead, our findings indicated this augmentation effect on amplitudes of MEP only worked in tetanic stimulation of ulnar nerve regardless of which side was stimulated. While tetanic stimulation of rami temporales nervi facialis or rami buccales nervi facialis, two motor branches of VII cranial nerve (facial nerve), does not increase the amplitudes of MEPs from either orbicularis oculi and oris, the muscles innervated by cranial nerve, nor abductor pollicis brevis, the muscle innervated by peripheral nerve. Interestingly, tetanic stimulation of peripheral nerves (bilateral ulnar nerves) also does not increase the amplitudes of MEPs recorded from the muscles innervated by the cranial nerve. These results suggest that tetanic stimulation of nerves in different motor pathways prior to transcranial electric stimulation has a different role on amplitudes of p-MEPs, implying the level of the central nervous system that may contribute to the effect of this post-tetanic stimulation on the amplitudes of MEPs.
According to the anatomy of descending motor pathways, fibers of corticobulbar tract, which separate from the corticospinal tract in the course of the latter’s descent through the pons and medulla oblongata, innervate the motor nuclei of the trigeminal, facial, and hypoglossal nerves (perhaps also the nucleus ambiguus). Therefore myogenic MEPs can be obtained from muscles innervated by peripheral or cranial motor nerve fibers dependent on the functional integrity of the corticospinal or corticobulbar tract. Similar to conventional MEPs, facial MEPs, obtained from electrical stimulation given through electrodes placed at the area corresponding to the cortex controlling the facial muscles adjacent to the cortex controlling the limb muscles, are recently introduced for monitoring the integrity of facial nerve pathways in surgery for acoustic neuromas and microvascular decompression [11,20]. Facial MEPs can be recorded as cMAP in target muscles including orbicularis oculi and oris muscles although its measurement is more difficult than measuring MEP of the extremities. In this study we separately monitored hand MEPs and facial MEPs following peripheral or cranial nerve tetanic stimulation, successfully obtaining most of the p-MEPs waveforms under general anesthesia. Zentner et al. [21] suggested that the descending impulse elicited by electrical stimulation of the motor cortex during anesthesia with inhaled anesthetics was inhibited mainly at the level of the spinal interneuronal or motoneuronal systems. This inhibition might just be counteracted by tetanic stimulation of peripheral nerves. On the other hand, peripheral nerve (mixed population of nerve fibers) stimulation produces mixed effects, including afferents and efferents. The studies showed that somatosensory afferent input has an inhibitory effect on motor cortex excitability [22]. This seems to indicate that cortical origin is unlikely. Therefore we consider that augmentation of MEP amplitude most likely occurred at the level of the spinal cord or subcortex but not in the motor cortex when tetanic stimulation was applied to peripheral nerve. However, we still do not know the exact mechanism of remote augmentation on MEPs with tetanic stimulation of the peripheral nerves, on the spinal cord level. It is possible that the afferent signals from tetanic stimulation of the peripheral nerves to spinal cord are processed and integrated, and properties (excitatory postsynaptic potentials) of spatial and temporal summation are potentiated and facilitated with transcranial electric stimulation. Therefore amplitudes of MEPs presented as cMAPs are enlarged.
There are several limitations in this study. Firstly, MEPs monitored may not be on the same levels of neuromuscular blockade. For example, hand MEPs were monitored at TOF = 2 while facial MEPs at TOF = 3. However, every pair of c-MEP and p-MEP is thought to be monitored at the same level of neuromuscular blockade, because the interval of c-MEP and p-MEP monitoring is very short (2 minutes). Secondly, the response sensitivity of facial muscles to the neuromuscular blockers may be different from that of hand muscles [23]. Thereby someone will argue that we may have overlooked the differential influence of partial neuromuscular blockade on amplitudes of facial and hand MEPs. However, by this study we just want to verify the concept that tetanic stimulation of motor nerves can enlarge the ensued transcranial MEP recordings, but not its feasibility in each of MEP monitoring with partial blockade [24]. Thirdly, we used tetanic stimulation at a stimulus intensity of 50 mA with duration of 5 s and a posttetanic interval of 1 s to obtain maximal augmentations of conventional MEPs, 15 seconds between each pair of c-MEP and p-MEP, based on the results in the previous study [6,11,25]. However, whether these settings were also optimal for augmentation of facial MEPs from the target muscles is unclear. Finally, the stimulus intensity of facial MEPs via transcranial stimulation ranged between 200 V and 600 V in previous studies [11,25], and 500 V was selected in this study considering the application of muscle relaxant. It would be more clinically significant if the same findings were yielded with lower stimulus intensity, and this leaves for further research.
In summary, we investigated whether tetanic stimulation of the facial nerve and ulnar nerve before transcranial stimulation could augment myogenic MEPs from orbicularis oculi, orbicularis oris and abductor pollicis brevis in patients under propofol-fentanyl anesthesia with partial neuromuscular blockade. Our results showed that this augmentation of MEP amplitude is effective only when peripheral nerves but not cranial nerves (eg. facial nerve) are tetanically stimulated prior to transcranial stimulation. Furthermore, this effect seems to involve in the subcortical level (maybe spinal cord), but not the motor cortex.
Acknowledgements
This work was supported by Initiation Fund of Huashan Hospital.
Disclosure of conflict of interest
None.
References
- 1.Kawaguchi M, Sakamoto T, Inoue S, Kakimoto M, Furuya H, Morimoto T, Sakaki T. Low dose propofol as a supplement to ketamine-based anesthesia during intraoperative monitoring of motor-evoked potentials. Spine (Phila Pa 1976) 2000;25:974–979. doi: 10.1097/00007632-200004150-00013. [DOI] [PubMed] [Google Scholar]
- 2.Lotto ML, Banoub M, Schubert A. Effects of anesthetic agents and physiologic changes on intraoperative motor evoked potentials. J Neurosurg Anesthesiol. 2004;16:32–42. doi: 10.1097/00008506-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto Y, Kawaguchi M, Kakimoto M, Inoue S, Furuya H. The effects of dexmedetomidine on myogenic motor evoked potentials in rabbits. Anesth Analg. 2007;104:1488–1492. doi: 10.1213/01.ane.0000261518.62873.91. table of contents. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto Y, Kawaguchi M, Kakimoto M, Takahashi M, Inoue S, Goto T, Furuya H. The effects of xenon on myogenic motor evoked potentials in rabbits: a comparison with propofol and isoflurane. Anesth Analg. 2006;102:1715–1721. doi: 10.1213/01.ane.0000208992.83093.5c. [DOI] [PubMed] [Google Scholar]
- 5.Kawaguchi M, Sakamoto T, Ohnishi H, Shimizu K, Karasawa J, Furuya H. Intraoperative myogenic motor evoked potentials induced by direct electrical stimulation of the exposed motor cortex under isoflurane and sevoflurane. Anesth Analg. 1996;82:593–599. doi: 10.1097/00000539-199603000-00029. [DOI] [PubMed] [Google Scholar]
- 6.Kakimoto M, Kawaguchi M, Yamamoto Y, Inoue S, Horiuchi T, Nakase H, Sakaki T, Furuya H. Tetanic stimulation of the peripheral nerve before transcranial electrical stimulation can enlarge amplitudes of myogenic motor evoked potentials during general anesthesia with neuromuscular blockade. Anesthesiology. 2005;102:733–738. doi: 10.1097/00000542-200504000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi H, Kawaguchi M, Yamamoto Y, Inoue S, Koizumi M, Ueda Y, Takakura Y, Furuya H. Evaluation of reliability of post-tetanic motor-evoked potential monitoring during spinal surgery under general anesthesia. Spine (Phila Pa 1976) 2008;33:E994–E1000. doi: 10.1097/BRS.0b013e318188adfc. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi H, Kawaguchi M, Yamamoto Y, Inoue S, Koizumi M, Ueda Y, Takakura Y, Furuya H. The application of tetanic stimulation of the unilateral tibial nerve before transcranial stimulation can augment the amplitudes of myogenic motor-evoked potentials from the muscles in the bilateral upper and lower limbs. Anesth Analg. 2008;107:215–220. doi: 10.1213/ane.0b013e318177082e. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi H, Kawaguchi M, Abe R, Yamamoto Y, Inoue S, Koizumi M, Takakura Y, Furuya H. Evaluation of the applicability of sevoflurane during post-tetanic myogenic motor evoked potential monitoring in patients undergoing spinal surgery. J Anesth. 2009;23:175–181. doi: 10.1007/s00540-008-0733-0. [DOI] [PubMed] [Google Scholar]
- 10.Kaelin-Lang A, Luft AR, Sawaki L, Burstein AH, Sohn YH, Cohen LG. Modulation of human corticomotor excitability by somatosensory input. J Physiol. 2002;540:623–633. doi: 10.1113/jphysiol.2001.012801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acioly MA, Liebsch M, Carvalho CH, Gharabaghi A, Tatagiba M. Transcranial electrocortical stimulation to monitor the facial nerve motor function during cerebellopontine angle surgery. Neurosurgery. 2010;66:354–361. doi: 10.1227/01.neu.0000369654.41677.b7. discussion 362. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto Y, Kawaguchi M, Hayashi H, Horiuchi T, Inoue S, Nakase H, Sakaki T, Furuya H. The effects of the neuromuscular blockade levels on amplitudes of posttetanic motor-evoked potentials and movement in response to transcranial stimulation in patients receiving propofol and fentanyl anesthesia. Anesth Analg. 2008;106:930–934. doi: 10.1213/ane.0b013e3181617508. table of contents. [DOI] [PubMed] [Google Scholar]
- 13.Goto T, Muraoka H, Kodama K, Hara Y, Yako T, Hongo K. Intraoperative Monitoring of Motor Evoked Potential for the Facial Nerve Using a Cranial Peg-Screw Electrode and a “Threshold-level” Stimulation Method. Skull Base. 2010;20:429–434. doi: 10.1055/s-0030-1261270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe K, Watanabe T, Takahashi A, Saito N, Hirato M, Sasaki T. Transcranial electrical stimulation through screw electrodes for intraoperative monitoring of motor evoked potentials. Technical note. J Neurosurg. 2004;100:155–160. doi: 10.3171/jns.2004.100.1.0155. [DOI] [PubMed] [Google Scholar]
- 15.Journee HL, Polak HE, de Kleuver M, Langeloo DD, Postma AA. Improved neuromonitoring during spinal surgery using double-train transcranial electrical stimulation. Med Biol Eng Comput. 2004;42:110–113. doi: 10.1007/BF02351019. [DOI] [PubMed] [Google Scholar]
- 16.Frei FJ, Ryhult SE, Duitmann E, Hasler CC, Luetschg J, Erb TO. Intraoperative monitoring of motor-evoked potentials in children undergoing spinal surgery. Spine (Phila Pa 1976) 2007;32:911–917. doi: 10.1097/01.brs.0000259836.84151.75. [DOI] [PubMed] [Google Scholar]
- 17.Andersson G, Ohlin A. Spatial facilitation of motor evoked responses in monitoring during spinal surgery. Clin Neurophysiol. 1999;110:720–724. doi: 10.1016/s1388-2457(98)00049-2. [DOI] [PubMed] [Google Scholar]
- 18.Hamdy S, Rothwell JC, Aziz Q, Singh KD, Thompson DG. Long-term reorganization of human motor cortex driven by short-term sensory stimulation. Nat Neurosci. 1998;1:64–68. doi: 10.1038/264. [DOI] [PubMed] [Google Scholar]
- 19.Luft AR, Kaelin-Lang A, Hauser TK, Buitrago MM, Thakor NV, Hanley DF, Cohen LG. Modulation of rodent cortical motor excitability by somatosensory input. Exp Brain Res. 2002;142:562–569. doi: 10.1007/s00221-001-0952-1. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda M, Oishi M, Hiraishi T, Fujii Y. Facial nerve motor-evoked potential monitoring during microvascular decompression for hemifacial spasm. J Neurol Neurosurg Psychiatry. 2010;81:519–523. doi: 10.1136/jnnp.2009.181495. [DOI] [PubMed] [Google Scholar]
- 21.Zentner J, Albrecht T, Heuser D. Influence of halothane, enflurane, and isoflurane on motor evoked potentials. Neurosurgery. 1992;31:298–305. doi: 10.1227/00006123-199208000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Tokimura H, Ridding MC, Tokimura Y, Amassian VE, Rothwell JC. Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroencephalogr Clin Neurophysiol. 1996;101:263–272. doi: 10.1016/0924-980x(96)95664-7. [DOI] [PubMed] [Google Scholar]
- 23.Lee HJ, Kim KS, Jeong JS, Cheong MA, Shim JC. Comparison of the adductor pollicis, orbicularis oculi, and corrugator supercilii as indicators of adequacy of muscle relaxation for tracheal intubation. Br J Anaesth. 2009;102:869–874. doi: 10.1093/bja/aep064. [DOI] [PubMed] [Google Scholar]
- 24.Sloan TB. Muscle relaxant use during intraoperative neurophysiologic monitoring. J Clin Monit Comput. 2013;27:35–46. doi: 10.1007/s10877-012-9399-0. [DOI] [PubMed] [Google Scholar]
- 25.Fukuda M, Oishi M, Takao T, Saito A, Fujii Y. Facial nerve motor-evoked potential monitoring during skull base surgery predicts facial nerve outcome. J Neurol Neurosurg Psychiatry. 2008;79:1066–1070. doi: 10.1136/jnnp.2007.130500. [DOI] [PubMed] [Google Scholar]