Abstract
Increased expression of apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3G (APOBEC3G) in human primary colorectal tumors and hepatic metastasis has been detected. However, the clinical relevance of APOBEC3G in colon carcinoma hepatic metastasis remains uncertain. The aim of this study was to assess the prognostic value of APOBEC3G in colon carcinoma patients with hepatic metastasis after hepatic resection. APOBEC3G expression was evaluated by immunohistochemistry in paraffin-embedded primary colon carcinoma and paired hepatic metastasis tissues from 136 patients with liver metastasis from colon carcinoma that underwent hepatic resection. The relation between APOBEC3G expression and clinicopathologic factors and long-term prognosis in these 136 patients was retrospectively examined. The prognostic significance of negative or positive APOBEC3G expression in colon carcinoma hepatic metastasis was assessed using Kaplan-Meier survival analysis and log-rank tests. Positive expression of APOBEC3G was correlated with liver metastasis of colon cancer. Univariate analysis indicated significantly worse overall survival (OS) for patients with a positive APOBEC3G expression in colon carcinoma hepatic metastasis than for patients with a negative APOBEC3G expression. Multivariate analysis showed positive-APOBEC3G in colon carcinoma hepatic metastasis to be an independent prognostic factor for OS after hepatic resection (P = 0.000). Positive expression of APOBEC3G was statistically significantly associated with poor prognosis of colon carcinoma patients with hepatic metastasis. APOBEC3G could be a novel predictor for poor prognosis of colon carcinoma patients with hepatic metastasis after hepatic resection.
Keywords: Colon carcinoma, hepatic metastasis, APOBEC3G, prognosis
Introduction
Colon cancer is one of the most common leading causes of cancer-related deaths worldwide [1]. The primary cause of death in patients with colon cancer is hepatic metastasis, and 5-year overall survival is only 25% to 40% [1]. Hepatic metastasis is the most common form of distant spread of primary colon cancer. It is estimated that approximately 50% of patients with colon cancer develop hepatic metastases synchronously or metachronously, and in advanced disease the mortality of colon cancer is principally attributable to the development of hepatic metastases [1].
Despite the improvements of systemic chemotherapy in terms of both tumor response rates and overall survival (OS) benefit, hepatic resection is the most effective and potentially curative therapy for patients with hepatic metastasis from colon cancer [2]. However, many colon cancer patients with hepatic metastasis undergoing surgery ultimately die of their disease, which means that surgery is not sufficient in a many patients [3,4]. The development of effective chemotherapy regimens, such as 5-fluorouracil (5-FU) with leucovorin (LV) plus oxaliplatin or irinotecan, and the addition of molecular targeted agents, such as anti-vascular endothelial growth factor (VEGF)-based and anti-epidermal growth factor receptor (EGFR)-based antibodies to the existing cytotoxic chemotherapy has improved colon cancer patients with hepatic metastasis response rates [5-8]. However, the OS benefits remain modest despite exceedingly high financial costs. It is important to uncover the biological mechanisms underlying hepatic metastasis of colon cancer and accelerate the development of new treatment strategies. Early treatment targeting colon cancer hepatic metastatic might be important for improving patient survival. Therefore, there is an urgent need to identify molecules that are correlated with colon cancer hepatic metastasis and facilitate the metastasis of colon cancer to the liver, which would be potential therapeutic targets for treating colon cancer patients with hepatic metastases.
The apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3G (APOBEC3G) is a cytidine deaminase that induces the guanine-to-adenine (G-to-A) hypermutation of the viral DNA by editing cytosines to uracils in the minus-strand DNA copies of the retrovirus RNA genome [9,10]. APOBEC3G has been reported to play an important role in host defense mechanisms [11]. Overexpression of APOBEC3G was detected in renal carcinoma tissues and cell lines [12], colorectal carcinoma and hepatic metastasis [13]. However, the clinical relevance of APOBEC3G in colon cancer hepatic metastasis remains uncertain.
In this study, we evaluated the relationship between APOBEC3G expression and clinicopathologic features, and assessed the utility of APOBEC3G as a new prognostic marker in patients with colon carcinoma hepatic metastasis after hepatic resection.
Materials and methods
Patients and tumor tissue samples
Paraffin-embedded primary colon carcinoma and paired hepatic metastasis tissue samples were obtained from 136 patients undergoing surgical resection of primary colon carcinoma and liver metastasis at the Department of Gastrointestinal Surgery, Taizhou Hospital, Wenzhou Medical University from January 1999 to December 2006. None of 136 patients had received chemotherapy, radiotherapy or molecularly targeted therapy before resection. After resection, patients were followed up every three months. Sections were reviewed by two experienced pathologists to verify the histologic assessment. Prior informed consent was obtained and the study protocol was approved by the Ethics Committee of the Taizhou Hospital, Wenzhou Medical University. The locations of tumors and distant metastases were determined by colonoscopy, computerized tomography (CT), and magnetic resonance imaging (MRI). Patients were enrolled into this study if synchronous metastasis confined to the liver as assessed by preoperative radiological imaging assessment and confirmed by surgery at the time of initial diagnosis. All patients were classified as stage IV (TXNXM1) according to UICC staging of resected specimens.
Immunohistochemistry
Selected tumor specimen were fixed in 10% neutral-buffered formalin and embedded in paraffin. Five micromolar sections were cut, dewaxed, rehydrated, and subjected to antigen retrieval. After blocking endogenous peroxidase activity, the sections were incubated with the primary antibody against APOBEC3G (Proteintech Group, Inc. Chicago, IL) (1:100) (overnight at 4 at 4°C). Immunohistochemistry was performed using the streptavidin-biotin peroxidase complex method (Lab Vision, Fremont, CA). The slides were examined and pictures were taken using an Olympus BX60 (Olympus, Japan). Sections known to stain positively were incubated in each batch and negative controls were also prepared by replacing the primary antibody with preimmune sera.
Expression analysis of APOBEC3G in tumor tissue was performed by comparing staining intensity and the percentage of immunoreactive cells. Staining intensity was arbitrarily scored on a scale of four grades: 0 (no staining of cancer cells), 1 (weak staining), 2 (moderate staining), and 3 (strong staining), and the percentage of positive cells was scored as follows: 0 (0%), 1 (1% to 25%), 2 (26% to 50%), and 3 ( > 50%). APOBEC3G staining positivity was determined using the following formula: overall score = positive percentage score × intensity score. A score of 0 was defined as “0”, > 0 to ≤ 2 as “1”, > 2 to ≤ 6 as “2”, and > 6 to ≤ 9 as “3”. In the end, tumor samples rated as level 0 or 1 were defined as negative for expression, whereas samples rated as level 2 or 3 were defined as positive.
Follow-up
Patient follow-up consisted of physical examination, assessment of serum carcinoembryonic antigen (CEA) levels, and thoracoabdominal computed tomographic scan every 3 months for the first 5 years, then annually thereafter. The patients were followed up until death or until the date of last follow-up. Follow-up was finished on December 31, 2012. The median follow-up was 47.2 months (range, 5-126 months).
Statistical analysis
Data are expressed as a mean ± SEM. Clinicopathologic parameters were analyzed using the two-tailed chi-square test, and the two-tailed t test was used to evaluate association between APOBEC3G expression and clinicopathologic parameters. OS curves for positive- and negative-APOBEC3G patients were estimated with the Kaplan-Meier method, and the survival functions were compared by the log rank test. Univariate and multivariate analyses were based on the Cox proportional hazards regression model. Factors that significantly influenced overall survival were used in the Cox proportional regression model for multivariate analysis. All P-values were considered statistically significant when the associated probability was less than 0.05.
Results
APOBEC3G protein overexpression in colon carcinoma hepatic metastatic tissue
We first evaluated the expression of APOBEC3G in 136 primary colon cancer samples and paired liver metastatic samples using the method of immunohistochemical staining. We found that among these 136 paired samples, APOBEC3G was positive in 89 primary colon tumors (65.4%) and in 91 paired hepatic metastases (66.9%) (Figure 1). The expression of APOBEC3G in the hepatic metastases was noticeably higher than that in the paired primary colon cancer tissues (Figure 2). These results suggested that APOBEC3G might play a key role in the colorectal cancer liver metastasis.
Figure 1.
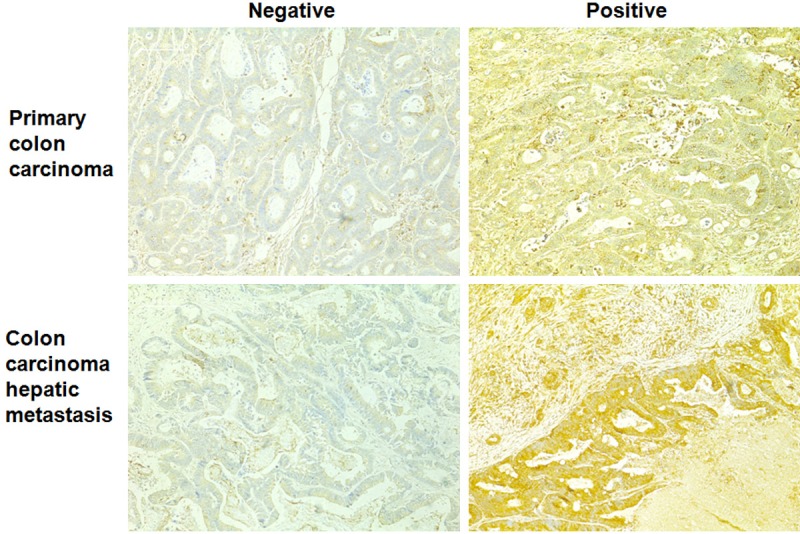
Expression of APOBEC3G in human primary colon cancer and heptic metastasis tissues. Negative and positive staining of APOBEC3G in primary colon cancer tissues and heptic metastatic tissues (original magnification × 100).
Figure 2.
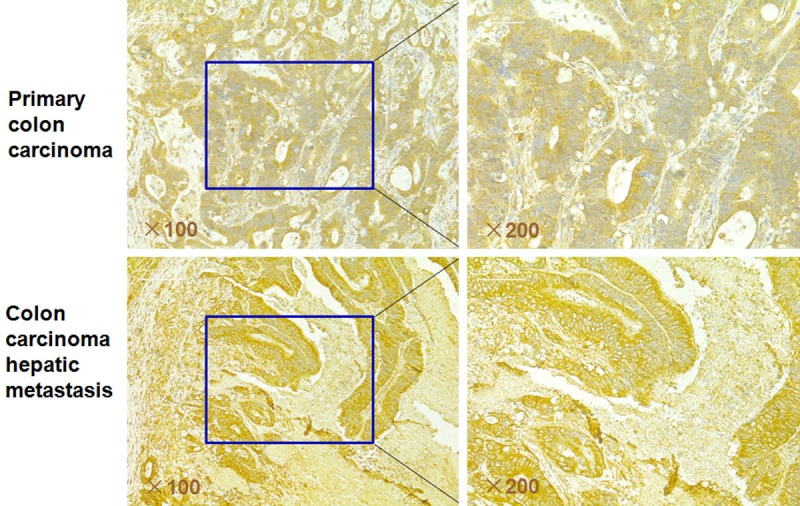
Representative immunohistochemical staining of APOBEC3G in primary colon cancer tissues and paired hepatic metastatic tissues. The expression of APOBEC3G is significantly greater in the colon cancer hepatic metastasis tissues than that of in the primary colon cancer tissues (original magnification, left column, × 100; right column, × 200).
Positive expression of APOBEC3G is associated with clinicopathological parameters
Table 1 showed the distribution of APOBEC3G expression level in 136 primary colon cancer tissues and paired hepatic metastases and the relationship between APOBEC3G expression level and clinicopathologic characteristics, including age, gender, primary colon tumor location, serum CEA level, tumor size, histological grade and differentiation, vascular invasion and lymph node involvement. Univariate and multivariate analysis showed that lymph node involvement, serum CEA level, and positive-APOBEC3G expression were independently predictive factors for synchronous hepatic metastasis (Table 2).
Table 1.
Clinicopathological characteristics in relation to APOBEC3G expression in patients with primary colon carcinoma and hepatic metastasis (n = 136)
| APOBEC3G expression in primary colon cancer | ||||
|
| ||||
| Factor | Positive | Negative | P value | |
|
| ||||
| Patients (n/%) | 89/65.4 | 47/34.6 | ||
| Age (years; mean ± SEM) | 66.3 ± 9.1 | 65.2 ± 8.9 | 0.725 | |
| Gender | Female (n) | 17 | 10 | 0.827 |
| Male (n) | 72 | 37 | ||
| Primary colon tumor location | Ascending colon (n) | 14 | 8 | 0.536 |
| Transverse colon (n) | 4 | 3 | ||
| Descending colon (n) | 71 | 36 | ||
| Primary colon tumor size | < 5 cm (n) | 55 | 30 | 0.681 |
| ≥ 5 cm (n) | 34 | 17 | ||
| Primary colon tumor histology grade | Low grade (n) | 43 | 21 | 0.877 |
| High grade (n) | 46 | 26 | ||
| Primary colon tumor differentiation | Well (n) | 14 | 12 | 0.633 |
| Moderate (n) | 68 | 32 | ||
| Poor (n) | 7 | 3 | ||
| Lymph node involvement | Yes (n) | 68 | 22 | 0.000 |
| No (n) | 21 | 25 | ||
| Vascular invasion | Yes (n) | 34 | 15 | 0.281 |
| No (n) | 55 | 32 | ||
| Serum CEA level | > 6 ng/mL (n) | 68 | 17 | 0.000 |
| ≤ 6 ng/mL (n) | 21 | 30 | ||
| HBV infection | Positive (n) | 8 | 9 | 0.752 |
| Negative (n) | 81 | 38 | ||
| HCV infection | Positive (n) | 2 | 3 | 0.878 |
| Negative (n) | 87 | 44 | ||
|
| ||||
| APOBEC3G expression in colon cancer hepatic metastasis | ||||
|
| ||||
| Factor | Positive | Negative | P value | |
|
| ||||
| Patients (n/%) | 91/66.9 | 45/33.1 | ||
| Age (years; mean ± SEM) | 67.3 ± 9.2 | 65.8 ± 9.3 | 0.885 | |
| Gender | Female (n) | 19 | 8 | 0.732 |
| Male (n) | 72 | 37 | ||
| Hepatic metastatic tumor number | Single (n) | 76 | 40 | 0.832 |
| Multiple (n) | 15 | 5 | ||
| Hepatic metastatic tumor size | < 5 cm (n) | 54 | 27 | 0.789 |
| ≥ 5 cm (n) | 37 | 18 | ||
| Hepatic metastatic histology grade | Low grade (n) | 46 | 17 | 0.875 |
| High grade (n) | 45 | 28 | ||
CEA, carcinoembryonic antigen. HBV, hepatitis C virus infection. HCV, hepatitis C virus infection.
Table 2.
Univariate and multivariate analysis for synchronous hepatic metastasis
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
| ||||
| P value | HR | 95% CI | P value | |
| APOBEC3G expression (Positive vs. negative) | < 0.001 | 2.672 | 1.368-4.322 | 0.023 |
| Serum CEA level (> 6 ng/mL vs. ≤ 6 ng/mL) | < 0.001 | 4.51 | 1.37-28.37 | < 0.001 |
| Lymph node involvement (Yes vs. no) | < 0.001 | 3.23 | 1.53-8.36 | < 0.001 |
| Vascular invasion (Yes vs. no) | 0.057 | 7.38 | 1.22-53.8 | 0.083 |
HR, hazards ratio. CI, confidence interval. CEA, carcinoembryonic antigen. HBV, hepatitis C virus infection. HCV, hepatitis C virus infection.
Positive-APOBEC3G is associated with poor survival in colon cancer patients with synchronous hepatic metastasis
The OS curves for colon cancer patients with hepatic metastasis subdivided on the basis of APOBEC3G expression are shown in Figure 3. Positive-APOBEC3G expression was associated with poor prognosis in patients with synchronous hepatic metastasis (log-rank test, P = 0.000).
Figure 3.
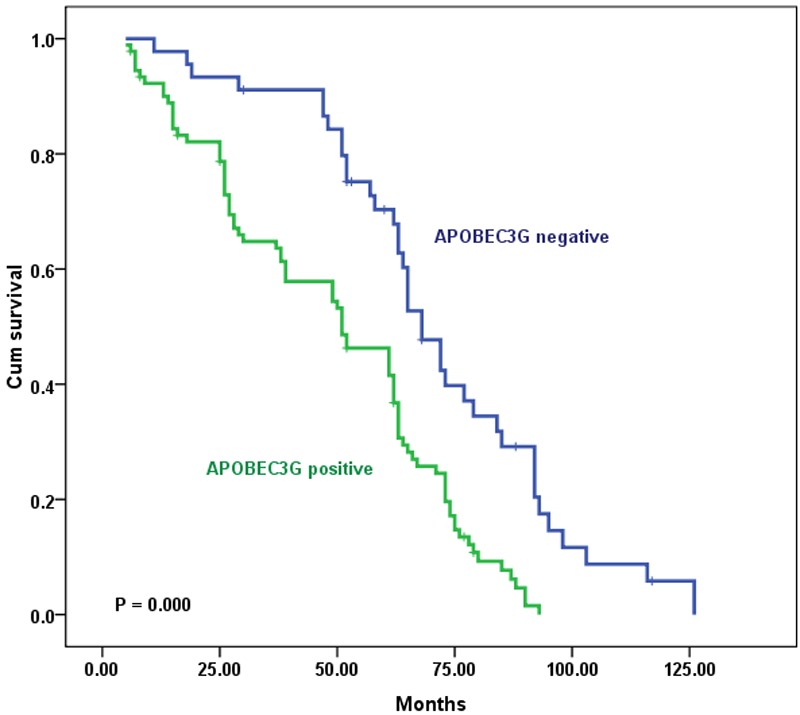
Kaplan-Meier survival curves of patients with metastatic colon carcinoma undergoing liver resection with curative intent, grouped by APOBEC3G expression in metastatic tumor tissues. The survival rate for patients with hepatic metastasis from colon cancer in the APOBEC3G-negative expression group (n = 45) was significantly higher than that for patients in the APOBEC3G-positive expression group (n = 91, log-rank, P = 0.000).
Table 3 lists the relationship between the clinicopathologic variables and overall survival after hepatic resection. Univariate analysis showed that APOBEC3G-positive patients had a significantly poorer prognosis than APOBEC3G-negative colon cancer patients with synchronous hepatic metastasis (P < 0.001; Table 3). Multivariate analysis showed that lymph node involvement, serum CEA level, and positive-APOBEC3G expression were independent and significant predictors in overall survival (Table 4).
Table 3.
Univariate analysis of overall survival after hepatic resection
| Factor | Overall survival | ||
|---|---|---|---|
|
| |||
| Patients (n) | P value | ||
| APOBEC3G expression in colon carcinoma hepatic metastasis | Positive | 91 | < 0.001 |
| Negative | 45 | ||
| Serum CEA level | > 6 ng/mL | 83 | < 0.001 |
| ≤ 6 ng/mL | 53 | ||
| Lymph node involvement | Yes | 90 | < 0.001 |
| No | 46 | ||
CEA, carcinoembryonic antigen.
Table 4.
Multivariate analysis of overall survival after hepatic resection
| Factor | HR (95% CI) | P value |
|---|---|---|
| APOBEC3G expression in colon carcinoma hepatic metastasis (Positive) | 2.582 (1.831-3.633) | < 0.001 |
| Serum CEA level (> 6 ng/mL) | 4.527 (2.002-16.670) | 0.0022 |
| Lymph node involvement (Yes) | 4.377 (1.936-11.822) | 0.0035 |
HR, hazards ratio. CI, confidence interval. CEA, carcinoembryonic antigen.
Discussion
Colon cancer remains the most common malignant disease worldwide [1]. After lymph nodes, the liver is the most common site for colorectal cancer metastasis, and liver metastasis is a common cause of cancer-related mortality [14-17]. It is estimated that approximately 50% of patients develop hepatic metastases (15% to 25% synchronous metastases and 20% metachronous metastases) [18]. Despite recent advances in diagnostic and therapeutic modalities, the prognosis of colon cancer patients with hepatic metastasis remains poor. Hepatic metastasis is a crucial issue for the treatment of colon cancer and it would be invaluable to develop predictive markers for screening high risk groups of patients for hepatic metastasis and prognostic markers for recurrence following hepatic resection. Although numerous studies have reported prognostic factors for recurrence and survival following hepatectomy and predictive factors for hepatic metastasis, the current knowledge remains incomplete. It is imperative that we uncover the underlying mechanisms and genetic alterations that predispose to the metastatic phenotype in colon carcinoma. Such an understanding has the potential to improve early detection and prevention in addition to helping with developing novel targeted therapies for late stage disease. However, detailed molecular mechanisms that mediate colon carcinoma metastasis to liver have not been systemically characterized. Therefore, identification of the specific tumor metastasis-associated genes or proteins responsible for colon cancer metastasis to the liver would be beneficial to a large proportion of the colon cancer patient population.
Overexpression of APOBEC3G was detected in colorectal carcinoma and hepatic metastasis [13]. However, the clinical relevance of APOBEC3G in colon cancer hepatic metastasis remains unclear. The aim of this study was to evaluate the prognostic value of APOBEC3G in patients with colon carcinoma hepatic metastasis after hepatic resection. We evaluated the APOBEC3G expression in primary colon carcinoma and paired hepatic metastasis tissues from 136 colon carcinoma patients with liver metastasis who underwent hepatic resection, which had clinical follow-up records. The result also demonstrated that the positive expression of APOBEC3G was correlated with liver metastasis and was coupled with shorter OS. These data further imply that APOBEC3G has distinct roles in colon cancer liver metastasis and is worthy of further investigation.
Conclusion
In summary, this study showed the expression of APOBEC3G in the hepatic metastases of colon carcinoma and revealing that APOBEC3G overexpression is significantly associated with hepatic metastasis in patients with colon carcinoma. Our results also showed that positive expression of APOBEC3G was statistically significantly associated with poor prognosis of colon carcinoma patients with hepatic metastasis. Our results indicated APOBEC3G can be a novel predictor for poor prognosis of colon carcinoma patients with hepatic metastasis after hepatic resection and APOBEC3G could be a potential target for diagnosis and treatment for colon carcinoma hepatic metastasis.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 81374014), Zhejiang Provincial Medical and Healthy Science and Technology Projects (Grant No. 2013KYA228), Science Research Fund of Taizhou (Grants No. A121KY08 and A131KY13-3), and Enze Medical Research Fund (Grants No. 12EZA1, 13EZA2 and 13EZB6).
Disclosure of conflict of interest
The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Primrose JN. Surgery for colorectal liver metastases. Br J Cancer. 2010;102:1313–1318. doi: 10.1038/sj.bjc.6605659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kemeny N. The management of resectable and unresectable liver metastases from colorectal cancer. Curr Opin Oncol. 2010;22:364–373. doi: 10.1097/CCO.0b013e32833a6c8a. [DOI] [PubMed] [Google Scholar]
- 4.Hebbar M, Pruvot FR, Romano O, Triboulet JP, de Gramont A. Integration of neoadjuvant and adjuvant chemotherapy in patients with resectable liver metastases from colorectal cancer. Cancer Treat Rev. 2009;35:668–675. doi: 10.1016/j.ctrv.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Masi G, Loupakis F, Pollina L, Vasile E, Cupini S, Ricci S, Brunetti IM, Ferraldeschi R, Naso G, Filipponi F, Pietrabissa A, Goletti O, Baldi G, Fornaro L, Andreuccetti M, Falcone A. Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann Surg. 2009;249:420–425. doi: 10.1097/SLA.0b013e31819a0486. [DOI] [PubMed] [Google Scholar]
- 6.Adam R, Aloia T, Lévi F, Wicherts DA, de Haas RJ, Paule B, Bralet MP, Bouchahda M, Machover D, Ducreux M, Castagne V, Azoulay D, Castaing D. Hepatic resection after rescue cetuximab treatment for colorectal liver metastases previously refractory to conventional systemic therapy. J. Clin. Oncol. 2007;25:4593–4602. doi: 10.1200/JCO.2007.10.8126. [DOI] [PubMed] [Google Scholar]
- 7.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Collette L, Praet M, Bethe U, Van Cutsem E, Scheithauer W, Gruenberger T EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD) Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kopetz S, Abbruzzese JL. Hidden biases in an observational study of bevacizumab beyond progression. J. Clin. Oncol. 2009;27:1732–1733. doi: 10.1200/JCO.2009.21.2084. [DOI] [PubMed] [Google Scholar]
- 9.Harris RS, Bishop KN, Sheehy AM, Craig HM, Petersen-Mahrt SK, Watt IN, Neuberger MS, Malim MH. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113:803–809. doi: 10.1016/s0092-8674(03)00423-9. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Yang B, Pomerantz RJ, Zhang C, Arunachalam SC, Gao L. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature. 2003;424:94–98. doi: 10.1038/nature01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- 12.Komohara Y, Suekane S, Noguchi M, Matsuoka K, Yamada A, Itoh K. Expression of APOBEC3G in kidney cells. Tissue Antigens. 2007;69:95–98. doi: 10.1111/j.1399-0039.2006.00725.x. [DOI] [PubMed] [Google Scholar]
- 13.Ding Q, Chang CJ, Xie X, Xia W, Yang JY, Wang SC, Wang Y, Xia J, Chen L, Cai C, Li H, Yen CJ, Kuo HP, Lee DF, Lang J, Huo L, Cheng X, Chen YJ, Li CW, Jeng LB, Hsu JL, Li LY, Tan A, Curley SA, Ellis LM, Dubois RN, Hung MC. APOBEC3G promotes liver metastasis in an orthotopic mouse model of colorectal cancer and predicts human hepatic metastasis. J Clin Invest. 2011;121:4526–4536. doi: 10.1172/JCI45008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiang AC, Massagué J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814–2823. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curley SA, Izzo F, Abdalla E, Vauthey JN. Surgical treatment of colorectal cancer metastasis. Cancer Metastasis Rev. 2004;23:165–182. doi: 10.1023/a:1025875332255. [DOI] [PubMed] [Google Scholar]
- 16.Manfredi S, Bouvier AM, Lepage C, Hatem C, Dancourt V, Faivre J. Incidence and patterns of recurrence after resection for cure of colonic cancer in a well defined population. Br J Surg. 2006;93:1115–1122. doi: 10.1002/bjs.5349. [DOI] [PubMed] [Google Scholar]
- 17.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254–259. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMillan DC, McArdle CS. Epidemiology of colorectal liver metastases. Surg Oncol. 2007;16:3–5. doi: 10.1016/j.suronc.2007.04.008. [DOI] [PubMed] [Google Scholar]


