Abstract
Objective: It is controversial that whether the GABA receptors contribute to the hypnotic action of volatile anesthetics. This study was to detect the effect of GABA receptors on the hypnotic action of volatile anesthetics by evaluation of the effect of intravenous flumazenil on sevoflurane minimum alveolar anesthetic concentration–awake (MAC-Awake) and emergence mental status. Methods: This study included two steps. Firstly, 49 healthy patients, aged 20-40 years scheduled for elective surgeries, were randomly assigned to two groups, a flumazenil group (n=24) and a saline group (n=25). The flumazenil group received 0.006 mg/Kg IV, and the control group received the same volume of saline 20 min before induction. The flumazenil group and the control group were compared with regard to MAC-Awake (anesthetic concentration achieving 50% probability of eye opening in response to a verbal command). We used the mask inhalation to measure the MAC-Awake by up-and-down method. The second steps, 60 patients undergoing lower abdomen surgeries were randomly divided into two groups, a experimental group (n=30) and a saline group (n=30). All patients were anesthetized with sevoflurane/sulfentanil. The experimental group received flumazenil at 0.006 mg/Kg IV, and the control group received the same volume of saline at the end of surgery. We recorded the time to awake and extubation. After extubation, the patients’ recovery status was scored with the Mini-Mental state examination (MMSE) system in post anesthesia care unit (PACU). Results: The MAC-Awake was 0.65% in the control group and 0.82% in the flumazenil group (p=0.34). After extubation, the recovery time and time to extubation showed no difference between the flumazenil group and the saline group (p>0.05). But the 10 min and 15 min MMSE scores after extubation were better in the flumazenil group than those in the saline group (p<0.05). There was no difference for MMSE scores after 30 min between two groups. Conclusion: We found that an IV flumazenil (0.006 mg/Kg) has no effect on sevoflurane MAC-Awake in humans. A single intravenous injection of flumazenil (0.006 mg/Kg) can partially reverse the hypnotic effect of sevoflurane/sulfentanil but do not contribute to reduction in the time to recovery and extubation.
Keywords: Flumazenil, sevoflurane, sulfentanil, MAC-awake
Introduction
Flumazenil is an imidazobenzodiazepine that promptly reverses the sedative/hypnotic effects of benzodiazepines via competitive inhibition on gamma-aminobutyric acid (GABA) receptors [1]. It was approved for the reversal of sedation from benzodiazepines used during therapeutic procedures [2]. It has a dose-independent antagonistic effect on all actions of overdosed benzodiazepine, including amnesia, sedation and respiratory depression. Anesth-esiologists typically use flumazenil in patients to reverse the effect of midazolam, which is a kind of benzodiazepine. The question is whether the effect of flumazenil extends to volatile anesthetics. On this question, previous studies indicate either no significant effect of flumazenil on volatile anesthetics or a significant effect [3-10]. Such an inconsistency in findings represent controversy on the issue of whether the GABA receptors contribute to the hypnotic action of volatile anesthetics [11,12].
We conducted a randomized trial to investigate the controversial issue of the effect of flumazenil on hypnotic action of volatile anesthetics. Patients in the trial were had lower abdominal surgery for tumor excision. The specific objective of the trial was to compare those who had been administered flumazenil with those who had not, in order to determine what effect, if any, flumazenil has on a volatile anesthetic, particularly sevoflurane/sulfentanil.
Minimum alveolar anesthetic concentration-awake (MAC-Awake) is the measure of volatile agent’s potency with respect to loss of consciousness [13], which is the alveolar anesthetic concentration achieving 50% probability of eye opening in response to a verbal command. In this study, MAC-awake of sevoflurane in patients administrated flumazenil comparing with patients without flumazenil. Meanwhile, we scored the mental status at different points in PACU after sevoflurane/sulfentanil anesthesia between patients with and without flumazenil.
Methods
Study composition
The study was composed of two parts. In the first part, MAC-awake values of sevoflurane were obtained by up-and-down method for patients who had been administered flumazenil and those who had not. Having determined the MAC-awake values, the purpose of the second part was, to detect the recovery time, extubation time and the recovery status at different points from sevoflurane/sulfentanil anesthesia after flumazenil was or was not administered.
Research site and ethical review
The Study was conducted at West China Hospital of Sichuan University, which is a large-scale, (4,300 impatient beds) comprehensive hospital in Chengdu, Sichuan Province, China. The study protocol was approved by the institutional Review Board of West China Hospital, Sichuan University and registered in Chinese Clinical Trial Registry (ChiCTR-TRC-11001509). Informed written consents for the study were obtained from all patients included in both part 1 and part 2 of the study.
Patient population
Patients ages 18 to 40 were included in part 1 of the study and ages 18 to 60 in part 2 of the study. All included patients were graded I or II according to the American Society of Anesth-esiologists physical status measure. The reason why ages differed for the two parts of the study was that the age will affect the MAC-awake value of volatile anesthetics. The patients in part 1 were scheduled for the surgeries about removal of implants in orthopedic ward. The patients in part 2 were scheduled for selective rectum or colon surgeries.
Exclusion criteria for both parts of the study were history or presence of neurologic diseases, hearing disorders, a pre-existing pain problem, and malignant hyperthermia history. All baseline laboratory results were normal.
Anesthesia protocol, part 1 of study
In the first part, we measured MAC-Awake before surgery. The unpremedicated patients, aged 20-40 years, were randomly assigned to flumazenil group and saline group. All patients took three maximum (vital capacity) breaths, using modified Ruffle and Snider’s triple-breath technique [14], and were induced by 8% sevoflurane in oxygen 6 L/min using the mask till loss of consciousness and then maintained with the target concentration for 20 min. We observed the response to the command of eye opening onverbal, which was finished by the same record voice previously of “please open your eyes”. The recording was applied 500 click stimuli of 0.1 ms at 80 dB and frequency of 5 Hz using earphones. The initial concentration of sevoflurane was 1.2% and varied by steps with ± 0.2% using the Dixon’s up-and-down method. No other stimuli were used during the study period. If the previous patient failed to follow three commands, the end-tidal concentration of sevoflurane of next patient was reduced by 0.2%. If patients could open their eyes after commend, they were judged awake and the concentration of next patient was increased by 0.2%. As previous studies, MAC-awake was defined as the concentration of 50% patients were response to the command of eye opening onverbal, as well as the concentration midway between the value permitting the first response to command and the value just preventing it [15,16]. When the cross over midpoint was up to 6, the study will be discontinued and MAC-wake would be analyzed by calculating the midpoint concentration of all independent pairs of patients involving a cross-over (i.e., response or no response).
Protocol, part 2 of study
In the second part, the patients, ASA I-II and aged from 18-60 years, scheduled for selective rectum or colon surgeries were enrolled into the study. After obtained the information consent form, the patients were randomly assigned into two groups using a SPSS-generated random number assignment. The study was double-blind and placebo controlled, including flumazenil group and control group. Oxygen saturation, electrocardiogram (ECG), noninvasive blood pressure (NBP), end-tidal carbon dioxide values (ETCO2), Bispectral index (BIS) and ETSevo were monitored continuously. Anesthesia was induced with 8% sevoflurane in 100% oxygen via a facemask. After loss of consciousness and injected sulfentanil 0.3 μg/Kg and rocuronium bromide 0.6 mg/kg, the patients were intubated. Maintenance of anesthesia was with 2-4% sevoflurane in nitrogen/oxygen at 0.5 fraction of inspired oxygen (FiO2) to keep the BIS with range of 40-60. ETCO2 was maintained 35-45 mmHg and BIS value was kept 40-60. Sulfentainil were added 0.3 μg/Kg before skin incision and 0.1 μg/Kg at 30 min before the end of snuggery. After the end of surgery, all patients were randomly assigned to receive 5 mL saline (control group, n=30), or flumazenil 6 μg/Kg diluted in 5 mL of saline (flumazeni group, n=30) injected. All syringes with study drugs or placebo were prepared by the same investigator. All patients were injected 40 mg Parecoxib Sodium and 8 mg Ondansetron for analgesia and preventing postoperative nausea and vomiting. Sevoflurane and nitrous oxide were discontinued simultaneously at the end of surgery. Ventilation was continued at the same parameter setting and a total gas flow of 4 L/min of oxygen, without any attempt to stimulate the patient. After return of sufficient spontaneous ventilation (VT>8 mL/kg and respiratory rate>12 breaths/min) and the gag reflex, the endotracheal tube was removed.
After anesthesia, patients were transferred to the postanesthesia care unit (PACU) after monitored 5 min and evaluated every 2 minutes. Time of response to verbal commands, spontaneous eye opening, date of birth, place of stay, were recorded. Patients were scored at 5 min, 10 min, 15 min, and 30 min after extubation by the MMSE system [17] and visual analogue scales for pain. The MMSE was used to gauge the severity of dementia by assessing cognitive functions. It consists of tests on orientation, short-term memory, registration, language use, comprehension, and basic motor skills. Patients are considered to be in a mild mental decline when score≥20; in a moderate stage when score between 10 and 19; and in a severe stage when scoring<10.
The patients were transferred to the ward from the PACU when they satisfied with a modified Aldrete score≥9 [18]. The stay in PACU and complications, for example nausea, vomiting, delirium, hypoxemia also were recorded.
The MAC-awake estimates using the up and down method for the two groups and the difference between the MAC-awake estimates for the two groups were obtained by using the SAS 9.1 (North Carolina, USA). Statistical analysis was performed for other parameters using SPSS software (version 18.0, SPSS Inc., Chicago, IL). The parameters related to the recovery from general anesthesia were expressed as means ± standard deviation (SD). A normality test was performed with respect to each of the parameters to compare the time to reach the index representing recovery from anesthesia between the two groups. After verifying that the parameters satisfied normality, a two-sample t-test was performed. A P value of less than 0.05 was considered significant.
Results
There were no significant differences between two groups in the demographic data in both parts of studies (Table 1).
Table 1.
Demographic data of two parts of studies
| Control group | Flumazenil group | |||
|---|---|---|---|---|
|
|
|
|||
| Part of studies | Part 1 | Part 2 | Part 1 | Part 2 |
| Number of patients | 24 | 30 | 25 | 30 |
| Gender (Female/Male) | 14/10 | 12/18 | 12/13 | 11/19 |
| Age (y) | 26 ± 3.6 | 43 ± 7.5 | 25 ± 4 | 44 ± 10.7 |
| Index of body (kg/m2) | 22.7 ± 6.4 | 21.02 ± 2.12 | 22.1 ± 2.08 | 22.00 ± 2.22 |
| Basic Hb (g/L) | 139.6 ± 19.8 | 129.0 ± 16.5 | 131.1 ± 15.5 | 126.8 ± 13.1 |
| Basic ALT (IU/L) | 22.2 ± 16.5 | 26.1 ± 10.4 | 21.5 ± 8.5 | 22.1 ± 4.2 |
| Basic creatine (mmol/L) | 71.8 ± 19.3 | 59.7 ± 9.3 | 62.4 ± 16.9 | 60.7 ± 18.1 |
| Basic BIS | 95.7 ± 3.6 | 95.1 ± 3.9 | 95.4 ± 3.0 | 95.0 ± 3.3 |
| BIS at loss of consciousness | 82.1 ± 22.4 | 87.9 ± 12.7 | 87.8 ± 11.3 | 82.0 ± 22.0 |
| ETSevo at loss of consciousness (%) | 5.1 ± 1.6 | 4.3 ± 1.6 | 4.6 ± 1.4 | 3.9 ± 0.8 |
| Time to loss of consciousness during induction (s) | 78.0 ± 33.6 | 80.1 ± 22.5 | 73.8 ± 25.8 | 76.4 ± 28.2 |
MAC-awake test
In this part, 49 patients were finished the tests and 25 patients were in control group, 24 in flumazenil group. The MAC-Awake was 0.65% in the control group and 0.82% in the flumazenil group. The differences in MAC-Awake between the two groups were not statistically (p=0.34) (Figures 1 and 2).
Figure 1.
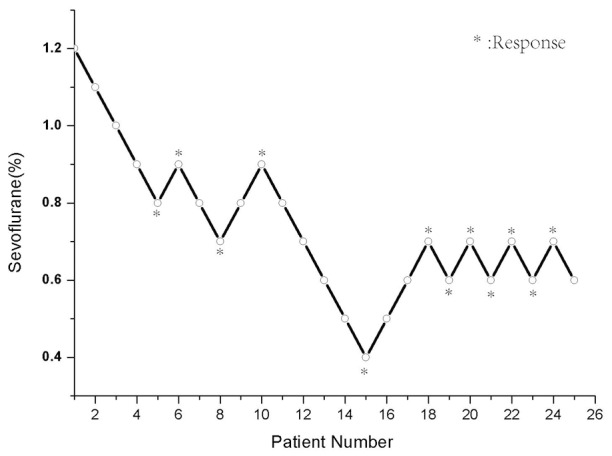
Verbal command of 25 patients in the control group was attempted, and the concentration of end-tidal sevoflurane in oxygen. Each patient’s data are showed with a circle. The minimum alveolar anesthetic concentration for awake of sevoflurane on verbal command was possible in 50% of patients was 0.66%.
Figure 2.
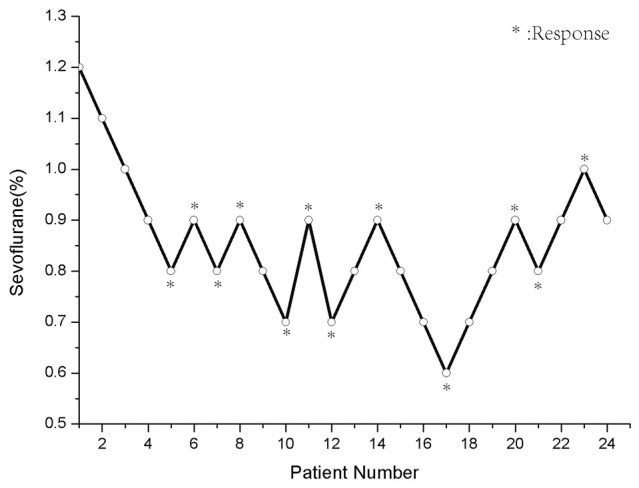
24 patients in the flumazenil group (0.006 mg/Kg) in whom verbal command was attempted, and the concentration of end-tidal sevoflurane in oxygen. Each patient’s data are showed with a circle. The minimum alveolar anesthetic concentration for awake of sevoflurane on verbal command was possible in 50% of patients was 0.82%.
Recovery status test
60 patients were involved in the second part and there were no differences between the groups in time to opening eyes, extubation, birth to date, and stay in PACU. Table 2 is the summary of Recovery data of study 2.
Table 2.
Recovery data of part 2
| Control group | Flumazenil group | |
|---|---|---|
| Time to recovery of spontaneous breathing (min) | 8.0 ± 5.6 | 7.0 ± 5.2 |
| BIS at recovery of spontaneous breathing | 70.1 ± 14.3 | 64.8 ± 17.7 |
| ETSevo at recovery of spontaneous breathing (%) | 0.8 ± 0.6 | 0.8 ± 0.5 |
| Time to extubation (min) | 7.9 ± 7.1 | 6.4 ± 6.7 |
| BIS at extubation | 80.6 ± 7.5 | 79.8 ± 11.1 |
| ETSevo at extubation (%) | 0.5 ± 0.1 | 0.4 ± 0.3 |
| Time to opening eyes on verbal command (min) | 14.6 ± 5.6 | 11.4 ± 3.4 |
| BIS at opening eyes on verbal command | 83.8 ± 5.9 | 79.4 ± 10.7 |
| Birth to date (min) | 23.0 ± 4.7 | 19.4 ± 10.4 |
For the recovery status, the MMSE scores at 5 min and 10 min in PACU after extubation in flumazenil group were better than in saline group (p<0.05). There was no difference for MMSE score at 15 min and 30 min in PACU between two groups. The 0 min value after in PACU was similar because of most of patients were not awake. (Figure 3: MMSE score at different points in PACU).
Figure 3.
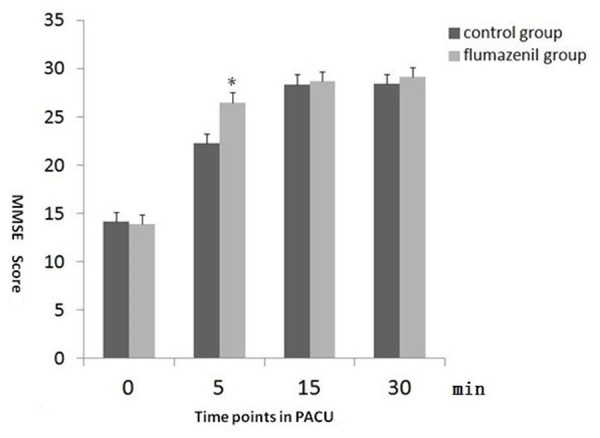
MMSE scores at 0 min after extubation was low in both groups because of the patients were not wake. But the 10 min and 15 min after extubation in flumazenil group were higher than the control group. The MMSE score were no difference above 30 min after extubation between two groups and all the patients were fully awake. ﹡: Flumazenil group vs. control group, p<0.05.
Discussion
Flumazenil is the specific benzodiazepine antagonist, which can inhibit GABA receptors in the central nervous system. This effect of flumazenil is specifically caused by competitive inhibition of the GABAA receptors, the targets for benzodiazepines [19]. So it is used to reverse the overdose of benzodiazepines, such as diazepam and in operation room for overly sedating with midazolam and failing to recovery from anesthesia [20]. However, for reverse of volatile agents, there were different opinions from previous studies. Because of the unclear mechanism of volatile agents for hypnotic effect, GABAA receptors’ action for volatile agents also still unknown. In this study, first step we tested the MAC-awake of sevoflurane between patients with or without flumazenil. Second steps, sevoflurane/sulfentanil anesthesia were performed in unpremeditated patients without benzodiazepines and the results showed that injection of flumazenil (0.006 mg/Kg) at the end of operation significantly could not reduce the time taken to recovery from the anesthesia but increased the MMSE score when compared to the control group.
Kochs concluded in 1989 that flumazenil does not have such an effect: flumazenil was injected to the groups that with or without midazolam, only in the midazolam-treated group, the auditory and somatosensory evoked cortical reaction was increased [21]. Other studies also showed that the reversal action of flumazenil for other general anesthetics. Weinbroum found that flumazenil can improved in the cognitive, motor abilities and the subjective feelings of the patients after halothane, enflurane and isoflurane anesthesia [5]. Roald found that cerebral metabolic rate of oxygen (CMRO2) of dogs anesthetized with isoflurane was increased after injected with flumazenil, but not in the non-anesthetized dogs [3]. These studies concluded that flumazenil may have a partial antagonizing effect to volatile agents. Dahaba reported that flumazenil can enhance the recovery from propofol/remifentanil anesthesia and significantly inc-rease the BIS value [22].
Karakosta recently found that 0.5 mg of flumazenil can improve the parameters of recovery from sevoflurane/ remifentanil anaesthesia after administrated to unpremedicated patients 30 minutes before the end of operation [10]. Kim also reported that a single dose of 0.3 mg of flumazenil accelerated the emergence from anesthesia and increased the BIS value after sevoflurane/fentanyl anesthesia without benzodiazepines [9]. A recent study suggested that sevoflurane has separate binding sites and converging pathways on the GABAA receptor, although unknown the accurately act way [11].
However, there are some studies with opposite conclusion that flumazenil did not reverse the hypnotic effect of volatile anesthetics. Schwieger reported that flumazenil do not affect the MAC of enflurane, isoflurane, or a fentanyl-enflurane combination [8]. Moreover, Schwartz found that flumazenil may play the role of an agonist but not an antagonist in reducing the MAC in dogs anesthetized with isoflurane [6]. Murayama also found that flumazenil does not antagonize halothane anesthesia in rats [23]. Hosaka reported that a single intraoral injection of flumazenil (0.2 mg) do not immediately reverse oversedation with triazolam [24]. These studies suggest that volatile anesthetics may do not interact with the benzodiazepine receptor.
In our studies, we found that flumazenil can increases the value of MAC-awake of sevoflurane for healthy adult patients but the differences were not statistically significant (p<0.05). 0.006 mg/Kg of flumazenil did not decrease the time to awake and intubation, but improve the MMSE score, after sevoflurane/sulfentanil anesthesia. As we known, the sedating effect maybe provoked after administrated single sulfentanil. We don’t know the relationship between sedation of sulfentanil and GABA receptors, which may cover partially the antagonist effect of flumazenil for sevoflurane. MMSE score can reflect the recognition status after anesthesia. The increasing of the MMSE score showed that flumazenil may partially reverse the hypnotic effect of sevoflurane. This result concluded that flumazenil may affect partially the hypnotic action of sevoflurane, which may result from the hypothesis of flumazenil antagonizing intrinsic benzodiazepines and also might help to explain that GABAA receptors may contribute to hypnosis of sevoflurane. Although flumazenil is not useful to accelerate the emergence but help to improve the recognition level after sevoflurane/sulfentanil anesthesia, which could be helpful to some aged patients or neurosurgery patients.
Conclusion
This study showed that an IV flumazenil (0.006 mg/Kg) has no effect on sevoflurane MAC-awake. A single intravenous injection of flumazenil (0.006 mg/Kg) to unpremedicated patients anesthetized with sevoflurane/sulfentanil can improve the recognition level but do not contribute the time to recovery and extubation. Flumazenil may partially reverse the hypnotic effect of sevoflurane.
Disclosure of conflict of interest
None.
References
- 1.Amrein R, Hetzel W, Hartmann D, Lorscheid T. Clinical pharmacology of flumazenil. Eur J Anaesthesiol Suppl. 1988;2:65–80. [PubMed] [Google Scholar]
- 2.Bell GD, Spickett GP, Reeve PA, Morden A, Logan RF. Intravenous midazolam for upper gastrointestinal endoscopy: a study of 800 consecutive cases relating dose to age and sex of patient. Br J Clin Pharmacol. 1987;23:241–243. doi: 10.1111/j.1365-2125.1987.tb03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roald OK, Forsman M, Steen PA. Partial reversal of the cerebral effects of isoflurane in the dog by the benzodiazepine antagonist flumazenil. Acta Anaesthesiol Scand. 1988;32:209–212. doi: 10.1111/j.1399-6576.1988.tb02716.x. [DOI] [PubMed] [Google Scholar]
- 4.Goto T, Nakata Y, Ishiguro Y, Niimi Y, Suwa K, Morita S. Minimum alveolar concentration-awake of Xenon alone and in combination with isoflurane or sevoflurane. Anesthesiology. 2000;93:1188–1193. doi: 10.1097/00000542-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Weinbroum AA, Geller E. Flumazenil improves cognitive and neuromotor emergence and attenuates shivering after halothane-, enflurane- and isoflurane-based anesthesia. Can J Anaesth. 2001;48:963–972. doi: 10.1007/BF03016585. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz AE, Maneksha FR, Kanchuger MS, Sidhu US, Poppers PJ. Flumazenil decreases the minimum alveolar concentration isoflurane in dogs. Anesthesiology. 1989;70:764–766. doi: 10.1097/00000542-198905000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Araki H, Fujiwara Y, Shimada Y. Effect of flumazenil on recovery from sevoflurane anesthesia in children premedicated with oral midazolam before undergoing herniorrhaphy with or without caudal analgesia. J Anesth. 2005;19:204–207. doi: 10.1007/s00540-005-0314-4. [DOI] [PubMed] [Google Scholar]
- 8.Schwieger IM, Szlam F, Hug CC Jr. Absence of agonistic or antagonistic effect of flumazenil (Ro 15-1788) in dogs anesthetized with enflurane, isoflurane, or fentanyl-enflurane. Anesthesiology. 1989;70:477–480. doi: 10.1097/00000542-198903000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Kim YJ, Lee H, Kim CH, Lee GY, Baik HJ, Han JI. Effect of flumazenil on recovery from anesthesia and the bispectral index after sevoflurane/ fentanyl general anesthesia in unpremedicated patients. Korean J Anesthesiol. 2012;62:19–23. doi: 10.4097/kjae.2012.62.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karakosta A, Andreotti B, Chapsa C, Pouliou A, Anastasiou E. Flumazenil expedites recovery from sevoflurane/remifentanil anaesthesia when administered to healthy unpremedicated patients. Eur J Anaesthesiol. 2010;27:955–959. doi: 10.1097/EJA.0b013e3283398ef9. [DOI] [PubMed] [Google Scholar]
- 11.Sebel LE, Richardson JE, Singh SP, Bell SV, Jenkins A. Additive effects of sevoflurane and propofol on gamma-aminobutyric acid receptor function. Anesthesiology. 2006;104:1176–1183. doi: 10.1097/00000542-200606000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Nishikawa K, Harrison NL. The actions of sevoflurane and desflurane on the gamma-aminobutyric acid receptor type A: effects of TM2 mutations in the alpha and beta subunits. Anesthesiology. 2003;99:678–684. doi: 10.1097/00000542-200309000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Davidson AJ, Wong A, Knottenbelt G, Sheppard S, Donath S, Frawley G. MAC-awake of sevoflurane in children. Paediatr Anaesth. 2008;18:702–707. doi: 10.1111/j.1460-9592.2008.02664.x. [DOI] [PubMed] [Google Scholar]
- 14.Ruffle JM, Snider MT. Comparison of rapid and conventional inhalation inductions of halothane oxygen anesthesia in healthy men and women. Anesthesiology. 1987;67:584–587. doi: 10.1097/00000542-198710000-00027. [DOI] [PubMed] [Google Scholar]
- 15.Katoh T, Suguro Y, Ikeda T, Kazama T, Ikeda K. Influence of age on awakening concentrations of sevoflurane and isoflurane. Anesth Analg. 1993;76:348–352. [PubMed] [Google Scholar]
- 16.Stoelting RK, Longnecker DE, Eger EI 2nd. Minimum alveolar concentrations in man on awakening from methoxyflurane, halothane, ether and fluroxene anesthesia: MAC awake. Anesthesiology. 1970;33:5–9. doi: 10.1097/00000542-197007000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Funk W, Moldaschl J, Fujita Y, Taeger K, Hobbhahn J. [Sevoflurane or halothane in inhalational anesthesia induction in childhood. Anesthesia quality and fluoride level] . Anaesthesist. 1996;45:22–30. doi: 10.1007/s001010050236. [DOI] [PubMed] [Google Scholar]
- 19.Philip BK, Simpson TH, Hauch MA, Mallampati SR. Flumazenil reverses sedation after midazolam-induced general anesthesia in ambulatory surgery patients. Anesth Analg. 1990;71:371–376. doi: 10.1213/00000539-199010000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Salmi E, Kaisti KK, Metsähonkala L, Oikonen V, Aalto S, Någren K, Hinkka S, Hietala J, Korpi ER, Scheinin H. Sevoflurane and propofol increase 11C-flumazenil binding to gamma-aminobutyric acidA receptors in humans. Anesth Analg. 2004;99:1420–1426. doi: 10.1213/01.ANE.0000135409.81842.31. table of contents. [DOI] [PubMed] [Google Scholar]
- 21.Kochs E, Wust P, Blanc I, Schulte am Esch J. [Acoustic and somatosensory evoked cortical potentials in sedation with midazolam and drug antagonism with flumazenil] . Anasth Intensivther Notfallmed. 1989;24:49–56. [PubMed] [Google Scholar]
- 22.Dahaba AA, Bornemann H, Rehak PH, Wang G, Wu XM, Metzler H. Effect of flumazenil on bispectral index monitoring in unpremedicated patients. Anesthesiology. 2009;110:1036–1040. doi: 10.1097/ALN.0b013e31819db2c4. [DOI] [PubMed] [Google Scholar]
- 23.Murayama T, Shingu K, Ogawa T, Tomoda K, Shindo K, Tamai S, Mori K. Flumazenil does not antagonize halothane, thiamylal or propofol anaesthesia in rats. Br J Anaesth. 1992;69:61–64. doi: 10.1093/bja/69.1.61. [DOI] [PubMed] [Google Scholar]
- 24.Hosaka K, Jackson D, Pickrell JE, Heima M, Milgrom P. Flumazenil reversal of sublingual triazolam: a randomized controlled clinical trial. J Am Dent Assoc. 2009;140:559–566. doi: 10.14219/jada.archive.2009.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]


