Abstract
Methylenetetrahydrofolate reductase (MTHFR) is a crucial enzyme in homocysteine/methionine metabolism. It catalysis the formation of 5-methyltetrahydrofolate (5-methyl-THF), which is the methyl donor for synthesis of methionine from homocysteine (Hcy). Decreases in folate consumption due to MTHFR polymorphism may affect production rate of keratinocytes of which had faster reproduction rates with a continuous DNA turnover and this may affect the clinical picture of psoriasis. This study aimed to investigate correlation of C677T polymorphisms in the MTHFR gene with severity of psoriasis and to evaluate the status of plasma Hcy, folate and vitamin B12 levels in patient with chronic plaque psoriasis. The study included 60 patients with chronic plaque psoriasis. The C677T polymorphisms were genotyped using PCR (Qiagen). Psoriasis Area and Severity Index (PASI) score below 7 was defined as mild, between 7 and 12 as moderate, and above 12 as severe disease. There was a significant difference between the severity of disease classification (p<0.05) with respect to the C677T polymorphism in the MTHFR gene. Severe involvement (PASI score >12) was observed in 38.46% of wild type (CC), but only 12.50% of homozygote (TT) and 7.69% of heterozygote (CT) patients. Significant differences between gene polymorphism and Hcy levels were noted in TT and CT genotypes respectively (p=0.025 and p=0.040). Plasma Hcy, folate and vitamin B12 levels were not correlated with the PASI score. Our data indicate a possible correlation of MTHFR polymorphism with severity of psoriasis.
Keywords: Psoriasis vulgaris, MTHFR, polymorphism, homocysteine, folate
Introduction
Psoriasis is a chronic, immune-mediated inflammatory skin disease that affects 2% to 3% of the general population [1] and is characterized by epidermal hyperproliferation, abnormal keratinocyte differentiation, increased cutaneous T lymphocyte activity. Although the pathogenesis of the disease has not been clearly understood so far, genetic tendency, infections, physical trauma, stress, various medicines and, as yet undefined, environmental factors seems to act together or individually to precipitate the disease [2].
Deoxyribonucleic acid (DNA) methylation plays an important role in the regulation of gene expression and cellular differentiation [3-5]. Therefore, methylation state of the genes can be speculated to modify phenotypes of psoriasis as well as the responsiveness to therapy. Actually, it is emphasized lately that DNA methylation disorders might play a role in etiopathogenesis of psoriasis [6,7]. Moreover, the lower frequency of p16 gene methylation and SHP-1 (tyrosine phosphatase) gene demethylation have been observed in psoriatic skin lesions [6,7].
Methylenetetrahydrofolate reductase (MTHFR) is a crucial enzyme in Hcy/methionine metabolism. It catalysis the formation of 5-methyltetrahydrofolate (5-methyl-THF), which is the methyl donor for synthesis of methionine from Hcy [8]. This cycle is important for maintaining the methyl donors for DNA methylation, and hence gene regulation and cellular differentiation. Polymorphism of the MTHFR gene involves the substitution of the nucleotide C with T at position 677. This gene polymorphism results in a valine to alanine exchange at amino acid position 222 of the MTHFR enzyme, leading to a reduced enzyme activity, therefore interferes with the Hcy levels.
Folate and vitamin B12 levels are major determinants of Hcy levels and MTHFR gene had strict interactions with those molecules in metabolic cycle. Therefore, the deficiencies of the Vitamin B12 and folate will lead to hyperhomocysteinanemia [9-11]. Additionally, polymorphism of the MTHFR gene has previously been shown to affect plasma Hcy levels [12,13]. In recent studies, plasma Hcy concentrations were found to be significantly higher in psoriatic patients than among control subjects [14-17]. Actually, there are conflicting results in association of MTHFR gene polymorphism with psoriasis so far; while significant association of the polymorphism of the MTHFR gene and psoriasis vulgaris was reported by Wang et al. in the Chinese population [18], and it was refuted by the studies conducted in Austria (Weger et al., 2008) [16], Czech Republic (Vasku et al., 2009) [19] and the Malaysia (Liew et al., 2012) [20].
Decreases in utilization of folate due to MTHFR polymorphism may affect production rate of keratinocytes of which had faster reproduction rates with a continuous DNA turnover. Consequently this may affect the severity of psoriasis which is characterized by keratinocyte proliferation. Therefore, we herein aimed to investigate the correlation of MTHFR gene polymorphism with psoriasis severity by using Psoriasis Area and Severity Index (PASI) scores and evaluated the status of Hcy, vitamin B12 and folate levels in chronic plaque psoriasis.
Material and methods
Subjects
From the patients admitted to dermatology outpatient clinic, 60 patients with chronic plaque psoriasis (49 male, 11 female) aged between 19 and 77 were included in the study. Diagnosis of chronic plaque psoriasis was based on clinical and/or histopathological findings. The psoriasis activity was evaluated by Psoriasis Area and Severity Index (PASI). A PASI-score below 7 was defined as mild, between 7 and 12 as moderate, and above 12 as severe disease [21].
All patients were signed an informed consent. Patients with ischemic heart disease, history of myocardial infarction, history of thromboembolic and cerebrovascular events were excluded from the study. None of the patients included in the study was treated with systemic drug therapy or photo chemotherapy. The study was approved by Local Ethics Committee.
Laboratory analysis
Blood samples were taken after an overnight fasting, and total Hcy, vitamin B12 and folate analysis were made by using chemiluminescent immunoassay kits (Abbott Laboratories, IL, USA). The reference ranges for Hcy, vitamin B12 and folate were accepted as 5-12 μmol/L, 200-600 pg/mL and 4-17 ng/mL, respectively. C677T polymorphisms in MTHFR gene were detected by using real-time PCR kit after extraction of DNA (QIAGEN Hamburg GmbH Hamburg, Germany) from blood samples. After dividing patients according to their mutation states (wild type: CC, heterozygote: CT and homozygote: TT), the distributions of laboratory parameters were analyzed.
Statistical analysis
Parametric or non-parametric statistical test were performed with regard to the normal distribution or not. The statistical relationships were calculated by using Pearson’s or Spearman correlation coefficients. For statistical significance, p<0.05 was accepted. All statistical calculations were made by using SPSS 15.0 version (SPSS Inc., Chicago, IL).
Results
Demographic features
The study included 60 patients with chronic plaque psoriasis (49 male and 11 female). Age of the patients ranged between 19 and 77 (median age of 29 years). The demographic characteristics are shown in Table 1. PASI score of the patients ranged from 1.2 to 19.5 and the mean PASI score was 7.2±4.6. According to disease activity, 35 of the patients were evaluated as mild (58.33%), 12 as moderate (20%) and 13 as severe (21.66%). Disease duration was ranged between 1-42 years (mean: 10.7±8.1 years).
Table 1.
Demographic data of patients with psoriasis
| Males, n (%) | 49 (81.66) |
| Females, n (%) | 11 (18.33) |
| Mean age, (years ± SD) | 33.7±14.1 |
| BMI | 24.3±4.4 |
| PASI ort. ± SD | 7.2±4.6 |
| Vit B12 (pg/ml) | 344.9±251.9 |
| Hcy (μmol/l) | 15.8±9.2 |
| Folate (pg/ml) | 3.7±1.7 |
MTHFR gene polymorphism
MTHFR C677T gene polymorphism frequencies, Hcy levels and PASI scores are shown in Table 2. Twenty-six patients (43.33%) were CC genotype, 26 (43.33%) were CT genotype and 8 (13.33%) were TT genotype.
Table 2.
MTHFR C677T gene polymorphism frequencies, Hcy, Vit B12, folate levels and PASI scores
| MTHFR 677 genotypes | n (%) | PASI | Hcy (μmol/l) | Vit B12 (pg/ml) | Folate (pg/ml) |
|---|---|---|---|---|---|
| CC | 26 (43.33) | 8.3±5.3 | 12.6±3.1 | 340.6±233.9 | 3.5±1.3 |
| TT | 8 (13.33) | 6.8±4.5 | 24.3±20.7 | 382.1±275.3 | 3.1±0.6 |
| CT | 26 (43.33) | 6.2±3.8 | 16.1± 6.4 | 337.9±270.6 | 4.3±2.6 |
Mean PASI scores were 8.3±5.3 in patient with CC genotype, 6.8±4.5 in TT genotype, and 6.2±3.8 in CT genotype. There was no significant difference between the PASI scores of the patients according to the MTHFR polymorphism (p=0.262). While 10 patients (38.46%) in CC genotype group had severe involvement, there was only 1 patient (12.50%) with severe involvement in TT genotype group, and 2 patients (7.6%) in CT genotype group (Table 3). There was a statistically significant difference between the severity of disease classification (p<0.05) with respect to the C677T polymorphism. The relationship between duration of illness or average age of onset of the disease and PASI scores was not statistically significant (p>0.05).
Table 3.
MTHFR C677T polymorphism and disease severity
| MTHFR 677 | PASI | Total | ||
|---|---|---|---|---|
|
|
||||
| <7 (mild) n (%) | 7-12 (moderate) n (%) | >12 (severe) n (%) | ||
| CC | 11 (42.30) | 5 (19.23) | 10 (38.46) | 26 |
| TT | 5 (62.50) | 2 (25) | 1 (12.50) | 8 |
| CT | 19 (73.07) | 5 (19.23) | 2 (7.69) | 26 |
| Total | 35 (58.33) | 12 (20) | 13 (21.66) | 60 |
The relationships between Hcy, vitamin B12, folate and disease severity
Plasma Hcy, vitamin B12 and folate levels are shown in Table 1. Hcy levels were highest in patients with TT genotype compared to those with CC and CT genotypes. Hcy levels of patients with TT and CT polymorphisms were significantly higher than those with CC genotype (p=0.025, p=0.040, respectively). Plasma Hcy, folate and vitamin B12 levels were not correlated with the PASI score. Figure 1 shows the relationship between Hcy levels and PASI score.
Figure 1.
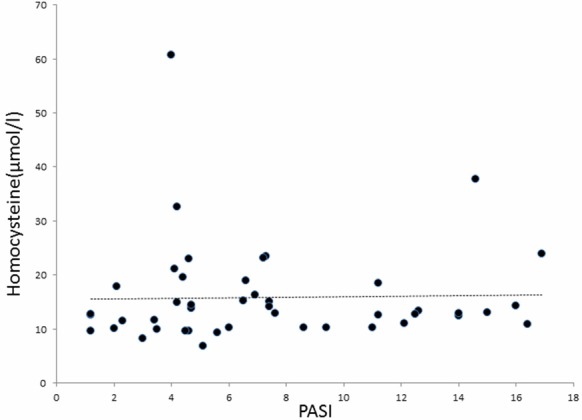
Relationship between Hcy and PASI score.
Discussion
The present study showed a possible correlation of MTHFR polymorphism with severity of psoriasis and to our knowledge this is the first in the literature. We found that PASI score of patients with MTHFR polymorphism of TT and CT genotype was lower than CC genotype patients in our study. While severe involvement (PASI score >12) was observed in 38.46% of normal phenotype, it was found only 12.50% in homozygote (TT) and 7.69% in heterozygote patients. Most of the patients with MTHFR polymorphism had mild and moderate severity of psoriasis. Decrease in folate usage due to MTHFR polymorphism may affect the reproduction rates of the keratinocytes which have faster turnover and the cells which continues DNA synthesis and consequently may lead milder forms of psoriasis. Furthermore, DNA methylation may also decrease especially psoriasis severity by modifying or changing transcription of tumor suppression genes in occurrence of the disease. Ruchusatsawat K et al. reported that the promoter demethylation may play an important role in skin pathogenesis by enhancing SHP-1 isoform II transcription in psoriatic skin lesions [7].
Investigations about MTHFR gene polymorphism association with psoriasis vulgaris in different countries showed conflicting results [16,18-20]. These contradictory findings were postulated to the probable differences in ethnicities. The frequency of the MTHFR 677T allele varies substantially in different regions of the world and among ethnic groups. MTHFR C677T polymorphism prevalence is ranges from 0.20 to 0.55 among different populations [22-25]. It is hard to claim the association of psoriasis of which 2-3% incidence, with such a gene mutation with a higher incidence in population.
Folate and vitamin B12 levels are major determinants of Hcy levels [26,27] and MTHFR gene had strict interactions with those molecules in metabolic cycle. Notably, low plasma folate concentrations have previously been reported among psoriatic patients [15,28,29]. Malabsorption and increased utilization of folate for DNA synthesis in skin cells have been proposed to be the cause [28,29]. In contrast to these findings, no correlation between plasma folate concentrations and PASI scores was observed by Weger et al. [16]. In our study, patients also had normal plasma folate levels and there was no association between folate levels and PASI score. As for vitamin B12, two previous studies yielded conflicting results [14-16]. While Vanizor et al. [14] reported significantly lower plasma vitamin B12 levels among psoriatic patients, Malerba et al. [15] and Weger et al. [16] were unable to confirm this finding. In the present study, plasma vitamin B12 levels were in the normal range in our patients as well.
The MTHFR gene polymorphism was reported to be associated with hyperhomocysteinanemia which has deleterious effects on the cardiovascular system [9,29]. Therefore, psoriasis vulgaris patients with MTHFR gene polymorphism may be at greater risk of cardiovascular diseases and thromboembolic events. Recent studies showing significantly increased mean plasma Hcy concentrations in psoriasis patients compared with control subjects [14-17]. Plasma Hcy levels were also high in our study group (>12 μmol/L). Furthermore, we demonstrated a significant relationship between MTHFR gene polymorphism and Hcy levels. In comparison to Hcy levels with gene polymorphism, the patients with TT genotype had significantly higher levels of Hcy than the patients with CC and CT genotype; statistically significant differences between gene polymorphism and Hcy levels were noted in TT and CT genotype consequently (p=0.025, p=0.040 respectively).
Subsequently, we thought that the higher Hcy levels may be related to the MTHFR gene polymorphism rather than folate and vitamin B12 deficiency in our study. Interestingly, Malerba et al. [15] recently reported a significant correlation between plasma Hcy concentrations and PASI scores; in contrast to these findings, no correlation between plasma Hcy concentrations and PASI scores was observed in our patients. Large further prospective studies are clearly warranted to investigate a potential role of Hcy, folate and vitamin B12 in psoriasis.
In conclusion, we found a relationship between MTHFR polymorphism and severity of psoriasis by PASI score for the first time in the literature. The genetic variation of the psoriasis patients may have an impact on clinical appearance of the disease and/or the treatment results in the clinical ground. Our study is a cross-sectional study, so our findings must be further studied with large prospective studies to evaluate the long-term severity of the disease and MTHFR enzyme activities of psoriatic patients with MTHFR polymorphism with more accurate results. Since plasma Hcy levels with regard to the MTHFR mutation were high in our study group (>12 μmol/L). Psoriasis vulgaris patients with MTHFR gene polymorphism may also be at greater risk of cardiovascular diseases and thromboembolic events.
Disclosure of conflict of interest
None.
References
- 1.Balta I, Balta S, Demirkol S, Celik T, Ekiz O, Cakar M, Sarlak H, Ozoguz P, Iyisoy A. Aortic Arterial Stiffness is a Moderate Predictor of Cardiovascular Disease in Patients With Psoriasis Vulgaris. Angiology. 2014;65:74–8. doi: 10.1177/0003319713485805. [DOI] [PubMed] [Google Scholar]
- 2.Braun-Falco O, Plewig G, Wolff HH, Burgdorf WHC. Dermatology. 2nd edition. Berlin: Springer-Verlag; 2000. pp. 585–607. [Google Scholar]
- 3.Feinberg AP. Methylation meets genomics. Nat Genet. 2001;27:9–10. doi: 10.1038/83825. [DOI] [PubMed] [Google Scholar]
- 4.Bird AP, Wolffe AP. Methylation-induced repression – belts, braces, and chromatin. Cell. 1999;99:451–454. doi: 10.1016/s0092-8674(00)81532-9. [DOI] [PubMed] [Google Scholar]
- 5.Razin A, Riggs AD. DNA methylation and gene function. Science. 1980;210:604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- 6.Zhang K, Zhang R, Li X, Yin G, Niu X, Hou R. The mRNA expression and promoter methylation status of the p16 gene in colony-forming cells with high proliferative potential in patients with psoriasis. Clin Exp Dermatol. 2007;32:702–708. doi: 10.1111/j.1365-2230.2007.02458.x. [DOI] [PubMed] [Google Scholar]
- 7.Ruchusatsawat K, Wongpiyabovorn J, Shuangshoti S, Hirankarn N, Mutirangura A. SHP-1 promoter 2 methylation in normal epithelial tissues and demethylation in psoriasis. J Mol Med (Berl) 2006;84:175–82. doi: 10.1007/s00109-005-0020-6. [DOI] [PubMed] [Google Scholar]
- 8.Selhub J, Miller JW. The pathogenesis of homocysteinemia: interruption of the coordinate regulation by S-adenosylmethionine of the remethylation and transsulfuration of homocysteine. Am J Clin Nutr. 1992;55:131–138. doi: 10.1093/ajcn/55.1.131. [DOI] [PubMed] [Google Scholar]
- 9.Santos M, Silva F, Gomes K, Fernandes AP, Freitas FR, Faria MC, Mota AP, Carvalho MG. Mutations in methylenetetrahydrofolate reductase and in cysthationine beta synthase: is there a link to homocysteine levels in peripheral arterial disease? Mol Biol Rep. 2010;38:3361–6. doi: 10.1007/s11033-010-0443-1. [DOI] [PubMed] [Google Scholar]
- 10.Kang SS, Zhou J, Wong PW, Kowalisyn J, Strokosch G. Intermediate homocysteinemia: a thermolabile variant of methylenetetrahydrofolate reductase. Am J Hum Genet. 1988;43:414–21. [PMC free article] [PubMed] [Google Scholar]
- 11.Pereira AC, Schettert IT, Filho AA, Guerra-Shinohara EM, Krieger JE. Methylenetetrahydrofolate reductase (MTHFR) C677T gene variant modulates the homocysteine folate correlation in a mild folate-deficient population. Clin Chim Acta. 2004;340:99–105. doi: 10.1016/j.cccn.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Engbersen AM, Franken DG, Boers GH, Stevens EM, Trijbels FJ, Blom HJ. Thermolabile 5,10-methylenetetrahydrofolate reductase as a cause of mild hyperhomocysteinemia. Am J Hum Genet. 1995;56:142–150. [PMC free article] [PubMed] [Google Scholar]
- 13.Harmon DL, Woodside JV, Yarnell JW, McMaster D, Young IS, McCrum EE, Gey KF, Whitehead AS, Evans AE. The common thermolabile variant of the methylene tetrahydrofolate reductase is a major determinant of mild hyperhomocysteinemia. QJM. 1996;89:571–577. doi: 10.1093/qjmed/89.8.571. [DOI] [PubMed] [Google Scholar]
- 14.Vanizor Kural B, Orem A, Cimsit G, Uydu HA, Yandi YE, Alver A. Plasma homocysteine and its relationships with atherothrombotic markers in psoriatic patients. Clin Chim Acta. 2003;332:23–30. doi: 10.1016/s0009-8981(03)00082-2. [DOI] [PubMed] [Google Scholar]
- 15.Malerba M, Gisondi P, Radaeli A, Sala R, Calzavara Pinton PG, Girolomoni G. Plasma homocysteine and folate levels in patients with chronic plaque psoriasis. Br J Dermatol. 2006;155:1165–1169. doi: 10.1111/j.1365-2133.2006.07503.x. [DOI] [PubMed] [Google Scholar]
- 16.Weger W, Hofer A, Stanger O, Wolf P, El-Shabrawi Y, Renner W, Kerl H, Salmhofer W. The methylenetetrahydrofolate reductase 677C>T gene polymorphism is not associated with chronic plaque psoriasis. Exp Dermatol. 2008;17:748–51. doi: 10.1111/j.1600-0625.2008.00713.x. [DOI] [PubMed] [Google Scholar]
- 17.Karabudak O, Ulusoy RE, Erikci AA, Solmazgul E, Dogan B, Harmanyeri Y. Inflammation and hypercoagulable state in adult psoriatic men. Acta Derm Venereol. 2008;88:337–40. doi: 10.2340/00015555-0456. [DOI] [PubMed] [Google Scholar]
- 18.Baiqiu W, Songbin F, Guiyin Z, Pu L. Study of the relationship between psoriasis and the polymorphic site C677T of methylenetetrahydrofolate reductase. Chin Med Sci J. 2000;15:119–120. [PubMed] [Google Scholar]
- 19.Vasku V, Bienertova-Vasku J, Necas M, Vasku A. MTHFR (methylenetetrahydrofolate reductase) C677T polymorphism and psoriasis. Clin Exp Med. 2009;9:327–31. doi: 10.1007/s10238-009-0054-0. [DOI] [PubMed] [Google Scholar]
- 20.Liew SC, Das-Gupta E, Wong SF, Lee N, Lee N, Safdar N, Jamil A. Association of methylentetraydrofolate reductase (MTHFR) 677C>T gene polymorphism and homocysteine levels in psoriasis vulgaris patients from Malaysia: a case-control study. Nutr J. 2012;11:1–7. doi: 10.1186/1475-2891-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitt J, Wozel G. The psoriasis area and severity index is the adequate criterion to define severity in chronic plaque-type psoriasis. Dermatology. 2005;210:194–9. doi: 10.1159/000083509. [DOI] [PubMed] [Google Scholar]
- 22.Van der Put NM, Eskes TK, Blom HJ. Is the common 677CT mutation in the methylenetetrahydrofolate reductase gene a risk factor for neural tube defects? A meta-analysis. QJM. 1997;90:111–5. doi: 10.1093/qjmed/90.2.111. [DOI] [PubMed] [Google Scholar]
- 23.Pepe G, Venegas OC, Giusti B, Brunelli T, Marcucci R, Attanasio M, Rickards O, De Stefano GF, Prisco D, Gensini GF, Abbate R. Heterogeneity in world distribution of thermolabile C677T mutation in 5,10-methylenetetrahydrofolate reductase. Am J Hum Genet. 1998;63:917–20. doi: 10.1086/302015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider JA, Rees DC, Liu YT, Clegg JB. Worldwide distribution of a common methylenetetrahydrofolate reductase mutation. Am J Hum Genet. 1998;62:1258–60. doi: 10.1086/301836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansoor A, Mazhar K, Ali L, Muazzam AG, Siddiqi S, Usman S. Prevalence of the C677T single-nucleotide polymorphism in the methylenetetrahydrofolate reductase gene among Pakistani ethnic groups. Genet Test Mol Biomarkers. 2009;13:521–6. doi: 10.1089/gtmb.2009.0012. [DOI] [PubMed] [Google Scholar]
- 26.Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- 27.Brattström L. Vitamins as homocysteine-lowering agents. J Nutr. 1996;126:1276S–1280S. doi: 10.1093/jn/126.suppl_4.1276S. [DOI] [PubMed] [Google Scholar]
- 28.Fry L, Macdonald A, Almeyda J, Griffin CJ, Hoffbrand AV. The mechanism of folate deficiency in psoriasis. Br J Dermatol. 1971;84:539–544. doi: 10.1111/j.1365-2133.1971.tb02543.x. [DOI] [PubMed] [Google Scholar]
- 29.Hild DH. Folate losses from the skin of exfoliative dermatitis. Arch Intern Med. 1969;123:51–57. [PubMed] [Google Scholar]
- 30.Norimah A, Safiah M, Jamal K, Haslinda S, Zuhaida H, Rohida S, Fatimah S, Norazlin S, Poh BK, Kandiah M, Zalilah MS, Wan Manan WM, Fatimah S, Azmi MY. Food Consumption Patterns: Findings from the Malaysian Adult Nutrition Survey (MANS) Malays J Nutr. 2008;14:25–39. [PubMed] [Google Scholar]


