Abstract
Objective: To assess numerical sex chromosomal abnormalities of the sperms before and after radiotherapy in seminoma patients and to evaluate their reproduction risks. Methods: Three color Fluorescence in situ hybridization (FISH) was performed on sperms harvested from one seminoma patient before and after radiotherapy and before surgery. The numerical sex chromosomal abnormalities were compared. Results: The ratio of 18-X and 18-Y sperm cells among the counted 40944 ones was close to 1:1 at three time points. The incidence of chromosome aneuploidy and diploid rate (18, X, Y) significantly increased after radiotherapy when compared with that before surgery and before radiotherapy. However, no significance was observed in the aneuploid and diploid rate between pre-operation group and pre-radiotherapy (post-operation) group except for the 18-YY karyotype (0.095% vs 0.026%, p<0.05). Conclusion: Our study shows increased incidence of numerical sex chromosomal abnormalities and high risk for reproductive and genetic diseases in patients treated with radiotherapy. Three colored FISH test is recommended to evaluate the rate of numerical chromosomal abnormalities; PGD and prenatal diagnosis are advised to improve the likelihood of a successful pregnancy.
Keywords: Seminoma, spermatozoa, numerical sex chromosomal abnormalities, radiotherapy
Introduction
Though the morbidity of seminoma is relatively low in adult males, it’s one of the most common cancers of the reproductive system that has demonstrated an increasingly high incidence in recent years [1]. The five-year survival of seminoma patients can be significantly improved via surgeries combined with post-operative radiotherapy and chemotherapy [2]. Nevertheless, since the majority of seminoma patients are of their reproductive age, the fertility, especially the change in the sperm chromosomes, hasn’t yet been comprehensively and systemically studied. This study was conducted to investigate the numerical abnormalities of 18, X and Y chromosome (aneuploid and diploid) in one patient with testicular seminoma before surgery, before and after radiotherapy, aiming to assess the genetic reproductive risk.
Methods and materials
Materials
A physical examination of a married 32-year-old patient with a swollen left testicle for four years indicated the lack of boundaries between testicle and epididymis and a hard lump (4 cm × 3 cm × 4 cm) in his scrotum with uneven surface, moderate mobility and slight tenderness. The enlargement of the spermatic cord, as well as the abnormalities of the right scrotum and its contents was absent. The left testicular resection was conducted after examinations, and the pathological examination showed seminoma confined to the testicular tissues. Due to the normal alpha fetal protein (AFP) concentration, the patient was diagnosed as having stage IA seminoma according to the latest NCCN criteria in 2012. At four weeks after surgery, radiotherapy was carried out in an area ranging from the abdominal aortic lymphatic drainage area to the ipsilateral iliac vessels. The total radiation dosage was 26 Gy with an average of 2 Gy per day. The patient had no particular discomfort during radiotherapy and systemic chemotherapy was not performed.
This study was carried out after obtaining the patient’s consent. The semen samples were divided into three groups: 1). Pre-operation group: Semen samples were obtained through masturbation four days before operation; 2). Post-operation group: Semen samples were obtained through masturbation three days before radiotherapy; 3). Post-radiotherapy group: Semen samples were obtained through masturbation two months after radiotherapy. The patient was asked to be abstinent for three to five days before sample collection. Detection was conducted according to the WHO laboratory manual for the Examination and processing of human semen (5th edition) [3]. Semen parameters in three groups are shown in Table 1.
Table 1.
Semen parameters of a seminoma patient at three time points: before operation, before radiotherapy (after operation) and after radiotherapy
| Before operation | Before radiotherapy | After radiotherapy | |
|---|---|---|---|
| Semen volume (ml) | 3.0 | 3.0 | 2.5 |
| Sperm concentration (× 106) | 24 | 22 | 13 |
| pH | 7.5 | 7.5 | 7.5 |
| Liquefaction time (min) | 30 | 30 | 45 |
| Progressive motility (%) | 32 | 30 | 12 |
| Non-progressive motility (%) | 27 | 25 | 20 |
| Immotility (%) | 41 | 45 | 68 |
Specimen processing
In brief, after thawing at 37°C, 100 μl of semen was collected and washed twice with PBS by centrifugation at 4000 r/min for 2 min. The cells were resuspended in DTT-Tris (pH=8.0) containing 10 mmol/L DTT and 100 mmol/L Tris to process the sperm head for approximately 40 min while mixing the suspension continuously.
In situ fluorescence hybridization
Sperm sections were denatured for 4 min in 70% formamide/2 × SSC solution (74°C) and then sequentially treated with 70%, 90% and 100% ethanol for 1 min, respectively. Probe mixture (Chr 18, X, Y, abbott, US) was added for hybridization after denaturation at 90°C for 10 min. Sections were hybridized in a humidified chamber overnight at 3°C and then washed in a solution containing 0.4 × SSC and 0.3% Nonidet P-40 at 73°C for 2 min and then in a solution containing 2 × SSC and 0.1% NP-40 at room temperature for 1 min. Following being dehydrated in graded ethanol series, sections were air-dried. Then, counterstaining was performed with 10 μl of 4’,6-diamidino-2-phenylindol (DAPI) (including Antifade), followed by mounting.
Observations and signal assay
The Olympus BX51 fluorescence microscope with a three-color filter (FITC/Rhodamine/DAPI) and the FISH-View (Applied Spectral Image, Israel) software was used for the detection and analysis of fluorescence signals.
Signal count and determination of abnormality Specimens with a hybridization of more than 95% were considered valid. More than 10,000 sperm cells were analyzed in both the experimental and the control groups. The signal counting was conducted in accordance with the signal determination and count criteria [4]. The sperm cells with integrated nuclear morphology and without overlapping with other nuclei were counted, and comparability was required in the determination of signal strength. When two signals were present in one sperm nucleus, it was required to distinguish whether two sperms overlapped; they were classified as one signal when two signals were very close or intersecting each other. The normal sperms showed green (X chromosome) or red (Y chromosome) fluorescence with an extra aqua color (chromosome 18), while sperms with numerical chromosomal abnormalities presented several forms below. Null signal sperm were the nucleus without any color signal (red, green and aqua).
Statistical analysis
Statistical analysis was conducted with SPSS version 16.0. The rate of bunerical chromosomal abnormalities was compared with chi square test among three groups. A value of P<0.05 was considered statistically significant.
Results
Parameters of semen samples
The parameters of semen samples before operation, and pre- and post-radiotherapy are shown in Table 1. in briefly, there was little variant of the semen condensation between the pre-operation and post-operation group, while obvious decrease was found of semen condensation after radiotherapy treatment while compared with the pre-operation and post-operation groups.
Sperm chromosomes detected by FISH
The nuclei of sperms before operation and pre- and post-radiotherapy after operation were blue, with clear boundaries after DAPI staining. The normal spermatic nucleus showed aqua signal (chromosome 18) with a red or green signal representing the Y/X chromosome centromeric region (red: X chromosome, green: Y chromosome). Thus, a sperm with normal karyotype showed two colors (23, X or 23, Y). In terms of numerical chromosomal abnormalities, several conditions were observed: chromosome 18 aneuploidy was characterized by 2 aqua signals with another red or green signal (18, 18 + X/Y). Likewise, the types of sex chromosome aneuploidy were illustrated as two sex chromosomal signals (2 red, 2 green or 1 red, 1 green signals) with an additional aqua signal (chromosome 18), representing the 18, XX, 18XY and 18YY aneuploidy. Chromosomal diploidy showed two aqua signals with two sex chromosome signals (1818, XX, 1818, XY and 1818, YY). Numerical chromosomal abnormalities in details are illustrated in Figure 1A-J.
Figure 1.
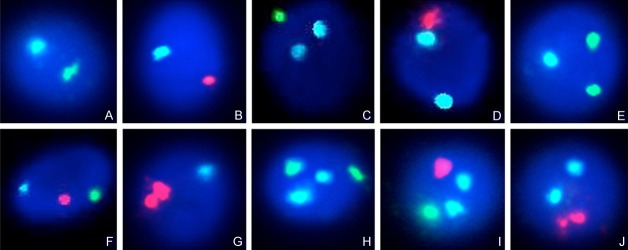
Three-color fluorescence in situ hybridization analysis on sperm before and after radiotherapy of a seminona patient. Aqua color- chromosome 18, red color-Y chromosome, green color-X chromosome. Normal sperm karyotype: 18, X (A) and 18, Y (B), chromosome aneuploidy: 1818, X (C), 1818, Y (D), 18, XX (E), 18, XY (F), 18, YY (G). Chromosome diploidy: 1818, XX (H), 1818, XY (I), 1818, YY (J).
Hybridization efficiency
A number of 40944 sperms were counted in this study, including 12678 after radiotherapy, 13099 before radiotherapy and 15167 after radiotherapy. The hybridization efficiency (HE) was calculated as follow: HE = (sperms-sperms without signals)/sperms.
Statistical analysis
The ratio of sperms carrying X chromosome and Y chromosome among three groups was near to 1:1 (18, X and 18, Y).
There were no significant differences in both the chromosomal aneuploidy rate and diploid rate between pre-operation group and pre-radiotherapy group except in the 18, XX karyotype (0.095% vs. 0.026%, P<0.05). The incidence of numerical chromosomal abnormalities (aneuploidy and diploidy) in post-radiotherapy group, however, was significantly higher than that in pre-radiotherapy group (aneuploidy: 18, 18, X: 0.171% vs. 0.069, 18, 18Y: 0.191% vs. 0.099%, 18, XX: 0.422% vs. 0.163%, 18, XY: 0.343% vs. 0.168%, 18, YY: 0.429% vs. 0.206%; P<0.05. Diploidy: 1818, XX: 0.138% vs. 0.061%, 1818, XY: 0.145 vs. 0.038%, 1818, YY: 0.171% vs. 0.053%; P<0.05). Similar findings were obtained when compared post-radiotherapy group with pre-operation group in terms of the numerical chromosomal abnormalities (Table 2).
Table 2.
Three-colored fluorescent in situ hybridization of sperm samples collected from one seminoma patient at different time points: before operation, before radiotherapy (after operation) and after radiotherapy. [n (100%)]
| Total sperms (n) | Normal karyotype sperms | Null signal sperms | Aneuploidy | Diploid | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Chromosome 18 | Sex chromosome | 1818, XX | 1818, XY | 1818, YY | ||||||||
|
|
|
|||||||||||
| 18, X | 18, Y | 1818, X | 1818, Y | 18, XX | 18, XY | 18, YY | ||||||
| Before operation | 12678 | 6313 | 6201 | 101 | 10 (0.079) | 9 (0.071) | 16 (0.126) | 14 (0.110) | 12 (0.095) | 6 (0.047) | 5 (0.039) | 6 (0.047) |
| Before radiotherapy (after operation) | 13099 | 6519 | 6372 | 122 | 9 (0.069) ns | 13 (0.099) ns | 21 (0.163) ns | 22 (0.168) ns | 27 (0.206)* | 8 (0.061) ns | 5 (0.038) ns | 7 (0.053) ns |
| After radiotherapy | 15167 | 7451 | 7282 | 147 | 26 (0.171)†,‡ | 29 (0.191)†,‡ | 64 (0.422)†,‡ | 52 (0.343)†,‡ | 65 (0.429)†,‡ | 21 (0.138)†,‡ | 22 (0.145)†,‡ | 26 (0.171)†,‡ |
P<0.05 vs. pre-operation group.
ns: no statistical significance while compared with pre-operation group.
P<0.05 vs. pre-operation group.
P<0.05 vs. after operation group.
Discussion
Seminoma is highly sensitive to radiotherapy. Surgeries combined with postoperative radiotherapy contribute to a good prognosis of patients suffering from Stage I seminoma, and the five-year survival rate of such patients is higher than 90% [5]. As the numerical chromosomal abnormality is a major cause of pregnancy failure and genetic diseases, its detection, therefore, is important for reproductive male patients with seminoma.
In this study, semen samples were collected before operation, and pre- and post-radiotherapy after operation from one reproductive male patient with Stage IA seminoma. Detection of spermatic numerical chromosomal abnormality was carried out via FISH. In addition, the influence of radiotherapy on the meiosis of spermatic chromosomes was also investigated. Previous studies reported that 18, X and Y chromosomes were most prone to numeric abnormalities [6]. Thus, three-colored fluorescent probe was used to detect the chromosome 18 and sex chromosomes (XY) in this study so as to explore the spermatic numerical chromosomal abnormality (including chromosomal aneuploidy and diploidy) pre- and post-radiotherapy after operation. In this study, the post-operation semen samples were harvested 70 days after radiotherapy, because the average cycle for spermatogenesis was about 70 days, which followed the physiological process. Results demonstrated that there was no significant difference between pre-operation group and pre-radiotherapy (after operation) group except in, the 18, YY type. Moreover, no marked difference was observed in the ratio of numerical chromosomal abnormality. This suggests that operation alone has a minimal impact on the spermatic numerical sex chromosomal abnormalities. However, significant differences were found in the chromosomal aneuploidy and diploidy among post-radiotherapy group, pre-operation group and pre-radiotherapy group, suggesting that radiotherapy is a main factor causing the spermatic numerical sex chromosomal abnormality.
The main cause of numerical sex chromosomal abnormality is that the homologous chromosomes do not separate in the Phase I and Phase II meiosis [7,8]. Since factors (such as environmental change) were capable of leading to the separation failure and destruction of spermatic chromosomes [9], strict restrictions were set on the subjects and conditions aiming to define the radiotherapy as a single variable and to make the results more convincing.
The radiation field in the radiotherapy of seminoma includes the peri-aortic and ipsilateral iliac lymph drainage area. Although the contralateral testis was not irradiated directly, the radiation exposure was inevitable. The numerical chromosomal abnormality rate after radiotherapy was higher than that in control group, suggesting that radiotherapy has significant and destructive impact on the spermatogenesis and separation of homologous chromosomes during the meiosis period.
It has been reported that routine orchiectomy and radiotherapy may cause the reduction in fertility of seminoma patients [10]. With the development of assisted reproduction, a large number of seminoma patients, with the fertility requirements, select in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) as an auxiliary strategy after the conventional surgery and postoperative radiotherapy.
However, there is no genetic screening on the chromosomal abnormalities in both IVF and ICSI. Thus, for seminoma patients suffering from radiotherapy, it is crucial to conduct Pre-implantation Genetic Diagnosis and Prenatal Diagnosis as an effective method to prevent the pregnancy termination and reduce the risk for genetic diseases to a great extent.
In present study, the semen samples were collected from one seminoma patient before surgery and before and after radiotherapy which spans about 70 days (an average cycle for spermatogenesis). However, it is no doubt that further studies are required to reveal the long-term effects of radiotherapy on sperm numerical chromosomal abnormalities.
Acknowledgements
This study was sponsored by the Shanghai Committee of Science and Technology Fund (No: 09411960100). The study was designed by Professor Jinfu Zhang and was performed by Wei Le and Shengsong Huang.
Disclosure of conflict of interest
None.
References
- 1.Diamantopoulos N, Kortsaris A. Testicular germ cell tumors. J BUON. 2010;15:421–434. [PubMed] [Google Scholar]
- 2.Horwich A, Shipley J, Huddart R. Testicular germ-cell cancer. Lancet. 2006;367:754–765. doi: 10.1016/S0140-6736(06)68305-0. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO laboratory manual for the Examination and processing of human semen. Geneva: World Health Organization; 2010. [Google Scholar]
- 4.Liehr T. Fluorescence in situ hybridization (FISH): application guide. Springer; 2009. [Google Scholar]
- 5.Majewski W, Majewski S, Maciejewski A, Kolosza Z, Tarnawski R. Adverse effects after radiotherapy for early stage (I,IIa,IIb) seminoma. Radiother Oncol. 2005;76:257–263. doi: 10.1016/j.radonc.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Shi Q, Martin RH. Aneuploidy in human sperm: a review of the frequency and distribution of aneuploidy, effects of donor age and lifestyle factors. Cytogenet Cell Genet. 2000;90:219–226. doi: 10.1159/000056773. [DOI] [PubMed] [Google Scholar]
- 7.Martin RH. Mechanisms of nondisjunction in human spermatogenesis. Cytogenet Genome Res. 2005;111:245–249. doi: 10.1159/000086895. [DOI] [PubMed] [Google Scholar]
- 8.Asada H, Sueoka K, Hashiba T, Kuroshima M, Kobayashi N, Yoshimura Y. The effects of age and abnormal sperm count on the nondisjunction of spermatozoa. J Assist Reprod Genet. 2000;17:51–59. doi: 10.1023/A:1009454114973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril. 2010;93:1027–1036. doi: 10.1016/j.fertnstert.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 10.Matos E, Skrbinc B, Zakotnik B. Fertility in patients treated for testicular cancer. J Cancer Surviv. 2010;4:274–278. doi: 10.1007/s11764-010-0135-9. [DOI] [PubMed] [Google Scholar]


