Abstract
PURPOSE
This study sought to elucidate the effects of a low- and high-load jump resistance exercise (RE) training protocol on cortical bone of the tibia and femur mid-diaphyses.
METHODS
Sprague-Dawley rats (male, 6-mos-old) were randomly assigned to high-load RE (HRE; n = 16), low-load RE (LRE; n = 15) or cage control (CC; n = 11) groups. Animals in the HRE and LRE groups performed 15 sessions of jump RE for 5 weeks. Load in the HRE group was progressively increased from 80g added to a weighted vest (50 repetitions) to 410g (16 repetitions). The LRE rats completed the same protocol as the HRE group (same number of repetitions) with only a 30g vest applied.
RESULTS
Low- and high-load jump RE resulted in 6–11% higher cortical bone mineral content (BMC) and cortical bone area compared to controls as determined by in vivo pQCT measurements. In the femur, however, only LRE demonstrated improvements in cortical volumetric bone mineral density (vBMD; +11%) and cross-sectional moment of inertia (CSMI; +20%) versus CC group. Three-point bending to failure revealed a marked increase in tibial max force (25–29%), stiffness (19–22%), and energy to max force (35–55%), and a reduction in elastic modulus (−11–14%) in both LRE and HRE compared to controls. Dynamic histomorphometry assessed at the tibia mid-diaphysis determined that both LRE and HRE resulted in 20–30% higher periosteal mineralizing surface versus CC group. Mineral apposition rate (MAR) and bone formation rate (BFR) were significantly greater in LRE animals (27%, 39%) than in the HRE group.
CONCLUSION
These data demonstrate that jump training with minimal loading is equally, and sometimes more, effective at augmenting cortical bone integrity compared to overload training in skeletally mature rats.
Keywords: bone formation, biomechanics, bone mass, diaphysis, tibia
INTRODUCTION
Bone is a dynamic tissue that responds to the external stimuli imparted upon it. In accordance with Wolff’s Law, the size, shape, and strength of bone depend upon the mechanical environment it must withstand. When gravitational load is removed (e.g., spinal cord injury and spaceflight), bone turnover begins to favor resorption, leading to subsequent losses in bone mass and strength (7, 19). Conversely, physical exercise can induce skeletal anabolism. That is, increased mechanical load via exercise aids in the accrual of bone in youth and the preservation or prevention of bone accretion in adulthood. Various types of exercise in humans, including jogging (5,1 4), resistance training (17, 41), and high-impact jumping (1, 11) elicit an osteogenic response from exercise-induced mechanical loading. In fact, high-impact, dynamic training, such as jumping (10), produces a larger anabolic response when compared to low-impact, high-repetition exercise, such as walking (18). Similar results have been shown in animal research
Free-fall impact landing in rats, a variation of jump resistance exercise (RE), enhanced mineral apposition rate (MAR) (16), volumetric bone mineral density (vBMD) (37), and bone strength (16, 36) versus non-exercising controls. High-impact jump training in rats increased bone formation rate (BFR) (24,28) vBMD (15), mRNA levels of osteocalcin (38), and mechanical properties (35) compared to ambulatory controls. Voluntary jump RE in animals has been performed with (32, 38) and without (15, 24, 26, 35) added resistance (i.e., weighted vests), yet testing for a graded response in bone formation response to increasing intensity of RE in weight-bearing animals has not been performed. Furthermore, measures of cortical bone remodeling, mass, and mechanical properties following voluntary jump training in skeletally mature rats are sparse (2, 38).
The RE protocol used in this study has previously been shown to increase proximal tibia cancellous vBMD, BFR, and relative bone volume (BV/TV, %), and elastic modulus (32). Contrary to other external loading paradigms commonly used to test bone outcomes, this RE training increases muscle mass and protein synthesis, and is performed voluntarily in conscious animals (8, 9, 12). The physiological response elicited by the lower-limb squat action performed by the animals in this study closely resembles that of weightlifting in humans (4, 29).
To our knowledge, no study has assessed the effects of low- and high-load jump RE on weightbearing cortical bone. Additionally, few RE studies have investigated the effects of voluntary rodent RE on cortical bone formation, geometry, and mechanical properties in skeletally mature animals. The purpose of our investigation was to evaluate the effects of low-and high-load voluntary rodent jump RE on changes in mechanically sensitive cortical bone mass, structure, and mechanical properties at the tibia and femur mid-diaphyses. Furthermore, we sought to define the effects of these two exercise loads on cortical bone formation. We hypothesized that high-load jump RE would result in a significantly greater osteogenic response leading to enhanced cortical bone formation and geometry, and greater cortical bone material properties as compared to low-load jump RE.
MATERIALS AND METHODS
Animals
Forty-two male Sprague-Dawley rats (6 months old, ~400 g) were obtained from Harlan (Houston, TX) and individually housed in a climate-controlled room (23 ± 2°C) with a 12 hour light (0600–1800) and dark cycle (1800–0600) in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal care facility. Rats were fed an ad libitum diet (Purina Test Diet, 5001) that was comprised of 24% protein, 12% fat, 54% carbohydrate, 7% ash, 5% fiber, and vitamins; and randomized by body mass to high-load resistance exercise (HRE, n = 16), low-load resistance exercise control (LRE, n = 15) or cage control (CC, n = 11) groups. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Texas A&M University and adhere to ACSM animal care standards. Calcein injections (25 mg/kg body mass) were administered subcutaneously 9 and 2 days prior to sacrifice to label mineralizing bone for histomorphometric analyses. At necropsy, right tibia and femur were removed, cleaned of soft tissue, and stored at -80 °C in PBS soaked gauze for ex vivo pQCT scans and/or mechanical testing, whereas left tibia were removed of soft tissue, cleaned, and stored in 70% ethanol at 4 °C for histology.
Operant Conditioning
The HRE and LRE animals were operantly conditioned to depress an illuminated bar located 12 in high on a Plexiglas exercise cage with dimensions 9 in x 9 in x 16 in (2, 39). Each training session, the exercised rat was first placed in the Plexiglas cage atop an electrical grid. Negative reinforcement via a brief electrical foot shock (1 mA, 60Hz) from an electrical grid was used to train the rats to perform the desired movements. After four sessions of operant conditioning (on alternating days for 8 days), a Velcro vest (30 g) was strapped over the scapulae and the rats were required to touch the illuminating bar 50 times for two additional sessions. The CC animals did not perform any conditioning or exercise activities and remained single-housed over the course of the study.
Voluntary Jump Resistance Exercise Paradigm
The HRE and LRE rats performed 15 training sessions (three sessions per week separated by at least 48 hours of rest) over a 5-week period as previously reported (9, 12, 25, 38). In brief, the HRE group completed a progressive resistance training program with a starting weight of 80 g for 50 repetitions on session one and increasing to 410 g for 16 repetitions on session 15. Training volume for each session was computed by multiplying the total number of repetitions times the added weight (in excess of body mass). The intent of this progressive resistance training paradigm was to overload the lower body skeletal muscle during each successive training session and provide anabolic stimulus to musculoskeletal tissue. As a result, total exercise volume decreased (increased added resistance to the weighted pack and decreased number of repetitions/jump) by 5% per week for a total of 25% over the 5 week training period (32). The LRE rats completed the same protocol as the HRE group, performing the same number of repetitions and receiving the same number of electrical foot shocks, wearing only the 30g Velcro vest (~8% body mass). Few, if any, shocks were necessary to elicit a positive response during the training sessions, and a minimum of 5–10 seconds of inserted rest was given between each repetition.
Peripheral Quantitative Computed Tomography (pQCT)
Tibia Mid-diaphysis (in vivo)
On days 1 and 35 of the study, in vivo tomographic scans were performed on anesthetized animals at the mid-diaphysis of the left tibia with a Stratec XCT Research-M device (Norland Corp., Fort Atkinson, WI), using a voxel size of 100 μm and a scanning beam thickness of 500 μm. Daily calibration of this machine was performed with a hydroxyapatite standard cone phantom. Two transverse images of the left tibia mid-diaphysis (50% of the total tibia length), 1 mm apart, were averaged to obtain a mean value for each of the cortical bone outcomes. A standardized analysis for diaphyseal bone (separation 1, threshold of 0.605 g/cm3) was applied to each section.
Femur Mid-diaphysis (ex vivo)
Thawed right femora were placed in a 1 mol/L PBS-filled vial to maintain proper hydration during the course of the scan, after which time they were returned to the −80 °C freezer. Two serial slices (1 mm apart) were taken at the femur mid-diaphysis and averaged to yield a mean value. A standard analysis for diaphyseal bone was again applied to each section, as described above, with a voxel resolution of 70 μm x 70 μm x 500 μm.
Values of cortical volumetric bone mineral density (vBMD), cortical bone mineral content (BMC) and cortical bone area were averaged across slices at each bone site. Additionally, mid-diaphyseal cross-sectional moment of inertia (CSMI) was obtained with respect to the bending axis during three-point bending for later estimation of material properties of tibia and femur bone. Machine precision (based on manufacturer’s data) is ±9 mg/cm3 for cortical vBMD. In vivo coefficients of variation for cortical vBMD (0.7%), cortical BMC (1.2%), cortical bone area (1.5%), and CSMI (3.0%) were determined from repeat scans completed on a subset of animals (n = 6).
Biomechanical Testing
Whole right tibiae and femora were tested by three-point bending to assess cortical bone structural and material properties at the mid-diaphysis pQCT sampling site (50% of total bone length). Femur and tibia mid-diaphyseal cortical bone mechanical properties were estimated using three-point bending to failure on an Instron 1125 machine as previously described (28). The anterioposterior (AP) and mediolateral (ML) surface diameters were measured with digital calipers (Mitutoyo Corp., Japan) at each mid-diaphysis. The day of testing, bones were thawed at room temperature and placed either anterior (femur) or lateral (tibia) side down on metal pin supports having a span of 18 mm (tibia) and 15 mm (femur). Quasi-static loading was applied at a rate of 2.54 mm/min at mid-diaphysis to the posterior surface of the femora and medial surface of the tibia until fracture. All specimens were sprayed with PBS immediately preceding testing to maintain hydration. Displacements were monitored by a linear variable differential transformer (LVDT) interfaced with a personal computer (Gardener Systems software) and loads were recorded using a 1000 N load cell calibrated to 100 N maximum load. Raw data were collected at 10 Hz as load vs. displacement curves and analyzed with Table-Curve 2.0 (Jandel Scientific; San Rafael, CA). Structural variables were obtained directly from load/displacement curves. The maximum load attained was defined as maximum force (MF), and the slope of the linear elastic portion of the curve defined as stiffness (S). Material properties of tibia and femur were calculated as previously mentioned (33). Briefly, material properties were estimated by normalizing structural properties to bone geometry at the site of testing using cross-sectional moment of inertia (CSMI; from pQCT), bone diameter (D) measured by calipers, and the support span distance (L) of either 18 mm (tibia) or 15 mm (femur). Formulas for elastic modulus (EM) and ultimate stress (US) were as follows:
Histomorphometry Analysis
Undemineralized distal left tibia were subjected to serial dehydration and embedded in methylmethacrylate (Sigma-Aldrich M5, 590–9). Serial cross-sections (150–200 μm) of mid-shaft cortical bone were cut starting 2.5 mm proximal to the tibial-fibular junction with an Isomet diamond wafer low-speed saw (Buehler, Lake Bluff, IL). Sections were hand ground to reduce thickness (<80 μm) before mounting on glass slides. The histomorphometric analyses were performed using the OsteoMeasure Analysis System, Version 1.3 (OsteoMetrics, Atlanta, GA). Measures of labeled surfaces and interlabel widths were obtained at 200x magnification, and standardized calculations and terminology were used (6). Periosteal and endocortical mineral apposition rates (MAR, μm/d) were calculated by dividing the average interlabel width by the time between labels (7 days), and mineralizing surface (MS) for both periosteal and endocortical bone surfaces was calculated using the formula . Bone formation rate (BFR) was calculated as (MAR x %MS/BS).
Statistical Analyses
All data are expressed as mean ± standard error, and their statistical relationships were evaluated using the statistical package SPSS (v.15). All in vivo and ex vivo variables were analyzed using a one-way ANOVA. When a significant main effect was found, Tukey’s post-hoc analyses were performed for pairwise comparisons. For all data, statistical significance was accepted at p ≤ 0.05.
RESULTS
Femur and tibia cortical bone benefits from jump resistance exercise, regardless of applied load
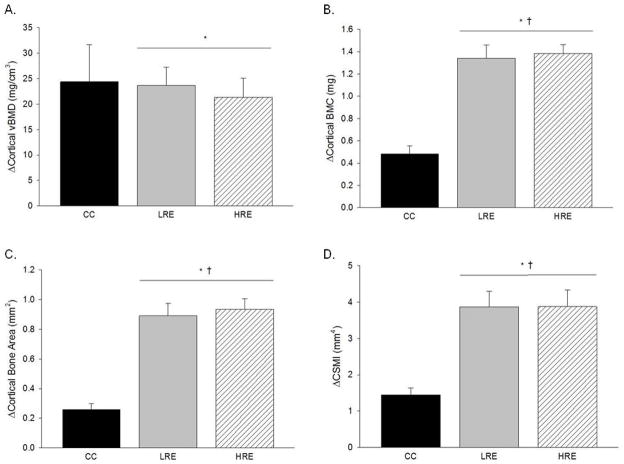
In vivo pQCT scans, taken on days 1 and 35, assessed longitudinal changes in mid-diaphysis tibia cortical bone. Both LRE and HRE training protocols significantly increased tibial cortical vBMD (2%), BMC (17%), bone area (14%), and CSMI (26%) during the 5 weeks of training (Fig 1). No significant pre-to-post changes were found in CC animals. Both LRE and HRE training protocols resulted in significantly greater femur mid-diaphysis cortical BMC and bone area, 6% to 11%, as compared to CC (Table 1). However, only LRE (and not HRE) demonstrated beneficial effects for femur cortical vBMD and CSMI (11% and 20% higher, respectively, vs. CC). In addition, there was a 2.6 to 3.4-fold increase in these parameters when the change (pre- vs. post) in BMC, bone area, and CSMI values in the LRE and HRE groups were normalized to that of the CC group.
Figure 1.
Effects of low- and high-load resistance exercise (LRE, HRE) on changes in densitometric and geometric properties of the tibial mid-diaphysis as taken by in vivo pQCT scans. Changes are for the duration of the study (day 35 values minus day 0 values). A: Cortical volumetric bone mineral density (vBMD). B: Cortical bone mineral content (BMC). C: Cortical bone area. D: Polar cross-sectional moment of inertia (CSMI). Each bar represents the group mean ± standard error of the mean. † vs. CC (p ≤ 0.05); * vs. day 0 (p ≤ 0.05).
Table 1.
Effects of low- and high-load resistance exercise (LRE, HRE) on densitometric and geometric properties of the mid-diaphysis femur as taken by ex vivo pQCT scans at study end (day 35).
| CC | LRE | HRE | |
|---|---|---|---|
| Cortical vBMD (mg/cm3) | 1445.20 ± 4.80 | 1458.97 ± 3.95† | 1457.15 ± 5.42 |
| Cortical BMC (mg) | 11.61 ± 0.20 | 12.93 ± 0.21† | 12.40 ± 0.24† |
| Cortical Bone Area (mm2) | 8.03 ± 0.14 | 8.86 ± 0.13† | 8.50 ± 0.15† |
| CSMI (mm4) | 21.30 ± 0.80 | 25.51 ± 0.87† | 23.58 ± 0.84 |
Values are group mean ± standard error of the mean.
vs. CC (p<0.05).
Mechanical properties of cortical bone are enhanced with both LRE and HRE
The effects of low- and high-load jump RE on tibia cortical bone mechanical properties were determined by three-point bending to failure. Both LRE and HRE resulted in significantly greater tibia max force (25–29%), stiffness (19–22%), and energy to max force (35–55%) compared to CC group (Table 2). Estimated elastic modulus values were lower for both LRE and HRE (by 11% and 14%, respectively).
Table 2.
Effects of low- and high-load resistance exercise (LRE, HRE) on mechanical properties of tibia and femur mid-diaphysis cortical bone at study end (day 35).
| CC | LRE | HRE | |
|---|---|---|---|
| Tibia | |||
| Max Force (N) | 114.57 ± 3.46 | 147.95 ± 2.60† | 144.06 ± 3.81† |
| Stiffness (N/mm) | 371.90 ± 12.20 | 454.97 ± 9.87† | 442.37 ± 12.32† |
| Elastic Modulus (GPa) | 3.41 ± 0.11 | 2.92 ± 0.06† | 3.05 ± 0.09† |
| Ultimate Stress (MPa) | 64.18 ± 2.79 | 62.32 ± 1.43 | 63.35 ± 1.58 |
| Energy to Max Force (mJ) | 32.27 ± 2.16 | 49.90 ± 3.02† | 43.46 ± 1.90† |
| Femur | |||
| Max Force (N) | 184.02 ± 15.70 | 260.74 ± 8.42†‡ | 234.23 ± 10.89† |
| Stiffness (N/mm) | 675.92 ± 42.06 | 844.42 ± 26.59†‡ | 752.06 ± 27.34† |
| Elastic Modulus (GPa) | 4.53 ± 0.39 | 4.71 ± 0.19 | 4.54 ± 0.18 |
| Ultimate Stress (MPa) | 114.59 ± 11.31 | 141.68 ± 4.89† | 137.37 ± 5.72 |
| Energy to Max Force (mJ) | 48.96 ± 11.01 | 104.77 ± 7.91† | 91.45 ± 10.63† |
Values are group mean ± standard error of the mean.
vs. CC (p<0.05);
vs. HRE (p<0.05).
Similar to the tibia, biomechanical properties of femur from LRE and HRE groups demonstrated greater max force (27–42%), stiffness (14–25%), and energy to max force (87% to 2-fold) vs. CC (Table 2). In addition, ultimate stress was 23% higher after LRE as compared to controls. However, the greatest effects on femur mechanical properties were evidenced in the LRE group. Compared to HRE, LRE resulted in significantly greater femur max force (11%) and stiffness (12%).
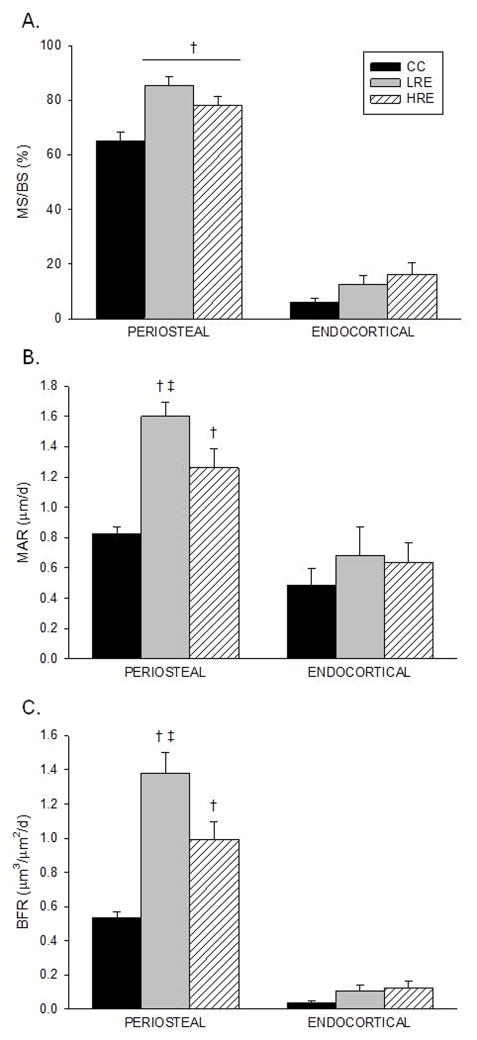
Jump resistance exercise increases periosteal but not endocortical bone formation
Low-and high-load jump RE significantly increased mid-diaphysis tibia cortical bone dynamic measures of osteoblast activity as compared to controls (Fig 2). HRE and LRE resulted in 20–30% higher periosteal %MS/BS, respectively, vs. the CC group (Fig. 2A). Although both LRE and HRE significantly increased cortical MAR and BFR vs. controls, the greatest effects were demonstrated by low-load jump RE (Fig. 2B-C). Compared to the HRE group, LRE training resulted in significantly greater MAR (27%) and BFR (39%). Extensive flurochrome labeling and large interlabel width on the periosteal and endocortical surfaces were evidenced in the HRE and LRE groups (Figure 3B,C).
Figure 2.
Effects of low- and high-load resistance exercise (LRE, HRE) on dynamic histomorphometry analyses measured at the tibia diaphysis (on day 35). A: Mineralizing Surface (%MS/BS). B: Mineral Apposition Rate (MAR). C: Bone Formation Rate (BFR). Values are group mean ± standard error of the mean. † vs. CC; ‡ vs. HRE (p ≤ 0.05).
Figure 3.
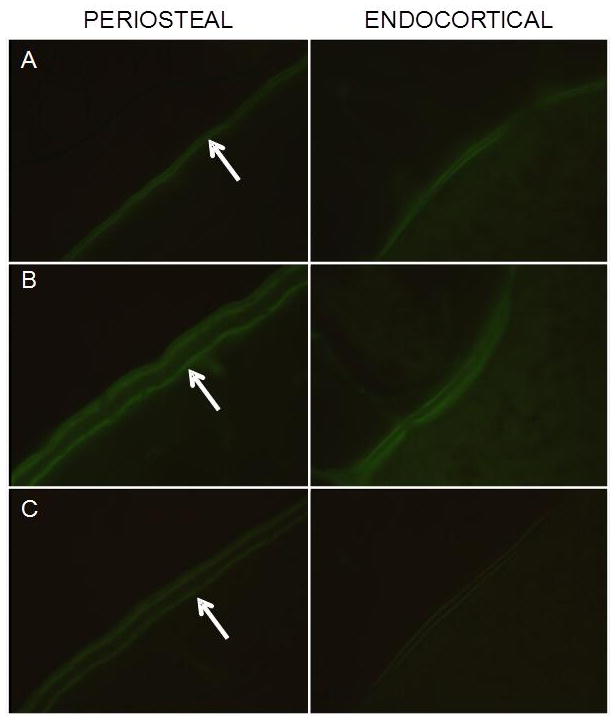
Visual depiction (100x) of calcein labeling on the periosteal and endocortical surfaces of cortical bone at the tibia diaphysis. A: CC B: LRE C: HRE. Note the extensive flurochrome labeling (arrows) and large interlabel width (LRE and HRE).
DISCUSSION
The purpose of this study was to evaluate changes in cortical bone densitometric properties, structure, strength, and turnover in skeletally mature rodents exposed to either low- or high-load voluntary jump resistance exercise (RE). Our hypothesis that increased load during jump RE would result in a significantly greater osteogenic response was not supported by these data. Although cortical bone accretion was significantly higher in both LRE and HRE compared to controls, HRE was not shown to be more osteogenic than LRE. Moreover, periosteal bone formation rate (BFR) was 40% greater in LRE than HRE. This enhanced BFR improved mid-diaphysis tibial mass, area, and moment of inertia, although the differences between LRE and HRE were not significant. Furthermore, gains in cortical mass and structure from jump RE at both loading levels caused an enhancement of tibial and femoral mechanical properties. Contrary to our hypothesis, overload was not required to produce a greater anabolic response in cortical bone properties versus low-load resistance exercise. Minimal loading (~8% body weight) was equally, and sometimes more, effective at augmenting cortical bone properties compared to overload training (100% of body weight).
Other studies have investigated the relationship between loading and osteogenesis in animals. External stimuli, such as axial compression, applied to anesthetized mice caused an increase in tibial bone formation, structure, and mechanical properties (31). Increasing the number of loading cycles per day, however, does not always lead to further increases in bone mass. Externally applied loading (0.5 Hz frequency) in rooster ulna using four cycles each day was equally successful at enhancing bone mass and formation compared to 36 and 1800 cycles/day (27). Furthermore, five jumps per day in growing rats improved bone mass and strength with few differences versus animals jumped between 10 and 40 times per day (34). In fact, bending stress in the 5-jump group was higher than all other groups, including animals jumped 100 times per day. These data are in agreement with the data presented herein, where increasing the load is not necessarily superior for bone modeling.
Both LRE and HRE resulted in increased cortical bone area and mass at the mid-diaphysis femur and tibia, but vBMD was only improved in the femur of the LRE group. Similar site-specific reactions to jump RE have been shown previously by our lab, where cancellous vBMD in the proximal tibia, but not in the femoral neck, was improved in both low- and high-load RE groups (32). Enhanced vBMD, however, is not necessary for improvements in mechanical properties. Femoral max force, stiffness, and energy to max force all improved despite the absence of vBMD gains (HRE group). These mechanical improvements are likely the product of periosteal apposition. Tibial MAR and BFR were improved in both LRE (88%, 155%) and HRE (47%, 82%) groups, occurring along the periosteum. This increased periosteal apposition corresponds to larger cross-sectional area and moment of inertia, both well-established determinants of strength in bending, at the mid-diaphysis tibia. All mechanical property measurements from three-point bending, with the exception of ultimate stress, were augmented with jump RE exercise in the tibia, with no significant differences between RE groups. Previous data investigating femoral neck strength provided evidence of improved bone strength where enhanced endocortical apposition and increased bone mass were the likely determinants (32).
Although LRE and HRE both improved mechanical properties in the tibia and femur, LRE unexpectedly led to larger gains in femoral maximum force and stiffness compared to HRE. Furthermore, the dynamic histomorphometric variables MAR and BFR were higher in LRE animals. A potential explanation for differences in bone formation is the window of time in which fluorchrome labels were administered and the ability of animals in the different groups to land squarely on their feet during that time frame. During the final training sessions, which corresponded to the first and second calcein injections, animals in the HRE group were jumping with 360–410 g affixed to their backs. This appended weight was approximately 100% of the animals’ body weight, making it difficult to land solely on their feet. Although only observational, animals in the HRE group, and most pronounced towards the latter training sessions, often landed on their flanks rather than on their limbs. Since it is well understood that dynamic strain is highly osteogenic, one may conjecture that the HRE animals’ inability to consistently land on their feet led to less cumulative strain, and subsequently less bone formation, than animals landing with only 30 g (~8% of body weight) attached to their backs. This observation suggests that impact forces generated by approximately one body weight of force are optimal for osteogenic adaptation, at least over the time duration of this experiment.
The evidence provided here that high-load impact exercise is not necessary for bone hypertrophy has vast clinical relevance. In youth, bone accretion can be augmented by implementing a thrice weekly school-based jumping program consisting of 10 jumps each session (23). Similar studies in children have proven successful at promoting bone hypertrophy with low-load, high-impact exercise (20, 21, 40). Although youth are not likely at risk for low bone mass, heightening peak bone mass in adolescence is a proven countermeasure for preventing adult-onset osteoporosis (22). Jump training has also been investigated in adults, particularly post-menopausal women. Five years of weighted vest jump training completely prevented age-related bone loss in the femoral neck and trochanter of post-menopausal women (30). It is worth noting that compliance reported by Snow et al. was over 80% during the course of their long-term study. With fractures of the femoral neck and trochanter comprising up to 90% of all hip fractures (13), low-repetition jump exercise in the aging population has shown to be a safe and practical training modality that adult patients adhere to with high compliance. Furthermore, an additional clinical benefit of this study is that it demonstrates that low-load jumping is equally, and sometimes more beneficial to bone health, thus decreasing the likelihood of injury in a jump training exercise prescription.
This study is not without limitations. First, neither strain measurements nor ground reaction forces during the landing phase were assessed in this study, making conclusions about exercise-induced landing deformations and impacts between groups somewhat conjectural. Although other studies have demonstrated that peak strain magnitude during high-impact exercise is the probable determinant of osteogensis (3, 28), we cannot definitively conclude here that differences in strain and/or impact forces upon landing were responsible for variations in skeletal anabolism between LRE and HRE animals. Additionally, CC animals were not subjected to the same stresses as the LRE and HRE groups. More specifically, CC animals were not placed in the jump boxes on training days and thus were not subjected to negative reinforcement shock treatment. Furthermore, a comparison of corticosterone levels between LRE and HRE may have aided in the explanation of group differences. Although limited, this is the first study to our knowledge to show absolute gains in in vivo longitudinal measurements of cortical bone in adult rodents subjected to voluntary jump RE. Additionally, the comparison of two different intensities of RE in fully weightbearing rodents has not previously been investigated.
In summary, our data suggest that low-load jump RE was effective at enhancing bone mass, structure, and material properties in tibial and femoral cortical bone. Contrary to our hypothesis, high-load jumping (up to 100% of body weight) did not produce a more robust osteogenic response when compared to low-load jumping (~8% body weight). In fact, LRE proved more beneficial for tibial bone formation and femoral mechanical strength versus HRE. These data demonstrate that jump training with minimal loading is equally, and sometimes more, effective at augmenting cortical bone integrity compared to overload training in skeletally mature rats.
Acknowledgments
This study was funded by the Sydney and JL Huffines Institute for Sports Medicine and Human Performance (HGG), through the NASA Cooperative Agreement NCC 9-58 with the National Space Biomedical Research Institute (SAB), and by the National Institutes of Health (AG01025; JDF). RDB and JMS were supported by a National Space Biomedical Research Institute Graduate Fellowship NSBRI-RFP-05-02. The authors gratefully acknowledge the technical assistance of CJ Walthal, Scott Bouse, Bryan Harpster and David Oliphant. The results of this study do not constitute endorsement by ACSM.
Footnotes
CONFLICTS OF INTEREST
None
References
- 1.Bassey EJ, Rothwell MC, Littlewood JJ, Pye DW. Pre- and postmenopausal women have different bone mineral density responses to the same high-impact exercise. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1998;13(12):1805–13. doi: 10.1359/jbmr.1998.13.12.1805. [DOI] [PubMed] [Google Scholar]
- 2.Buhl KM, Jacobs CR, Turner RT, Evans GL, Farrell PA, Donahue HJ. Aged bone displays an increased responsiveness to low-intensity resistance exercise. Journal of applied physiology. 2001;90(4):1359–64. doi: 10.1152/jappl.2001.90.4.1359. [DOI] [PubMed] [Google Scholar]
- 3.Carter DR. Mechanical loading histories and cortical bone remodeling. Calcified tissue international. 1984;36 (Suppl 1):S19–24. doi: 10.1007/BF02406129. [DOI] [PubMed] [Google Scholar]
- 4.Casey DP, Joyner MJ. Skeletal muscle blood flow responses to hypoperfusion at rest and during rhythmic exercise in humans. Journal of applied physiology. 2009;107(2):429–37. doi: 10.1152/japplphysiol.00331.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chien MY, Wu YT, Hsu AT, Yang RS, Lai JS. Efficacy of a 24-week aerobic exercise program for osteopenic postmenopausal women. Calcified tissue international. 2000;67(6):443–8. doi: 10.1007/s002230001180. [DOI] [PubMed] [Google Scholar]
- 6.Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013;28(1):2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards WB, Schnitzer TJ, Troy KL. Torsional stiffness and strength of the proximal tibia are better predicted by finite element models than DXA or QCT. Journal of biomechanics. 2013;46:1655–62. doi: 10.1016/j.jbiomech.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrell PA, Fedele MJ, Hernandez J, Fluckey JD, Miller JL, 3rd, Lang CH, et al. Hypertrophy of skeletal muscle in diabetic rats in response to chronic resistance exercise. Journal of applied physiology. 1999;87(3):1075–82. doi: 10.1152/jappl.1999.87.3.1075. [DOI] [PubMed] [Google Scholar]
- 9.Fluckey JD, Kraemer WJ, Farrell PA. Pancreatic islet insulin secretion is increased after resistance exercise in rats. Journal of applied physiology. 1995;79(4):1100–5. doi: 10.1152/jappl.1995.79.4.1100. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs RK, Bauer JJ, Snow CM. Jumping improves hip and lumbar spine bone mass in prepubescent children: a randomized controlled trial. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2001;16(1):148–56. doi: 10.1359/jbmr.2001.16.1.148. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs RK, Snow CM. Gains in hip bone mass from high-impact training are maintained: a randomized controlled trial in children. The Journal of pediatrics. 2002;141(3):357–62. doi: 10.1067/mpd.2002.127275. [DOI] [PubMed] [Google Scholar]
- 12.Gasier HG, Riechman SE, Wiggs MP, Buentello A, Previs SF, Fluckey JD. Cumulative responses of muscle protein synthesis are augmented with chronic resistance exercise training. Acta physiologica. 2011;201(3):381–9. doi: 10.1111/j.1748-1716.2010.02183.x. [DOI] [PubMed] [Google Scholar]
- 13.Greenspan SL, Myers ER, Maitland LA, Kido TH, Krasnow MB, Hayes WC. Trochanteric bone mineral density is associated with type of hip fracture in the elderly. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1994;9(12):1889–94. doi: 10.1002/jbmr.5650091208. [DOI] [PubMed] [Google Scholar]
- 14.Heinonen A, Oja P, Sievanen H, Pasanen M, Vuori I. Effect of two training regimens on bone mineral density in healthy perimenopausal women: a randomized controlled trial. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1998;13(3):483–90. doi: 10.1359/jbmr.1998.13.3.483. [DOI] [PubMed] [Google Scholar]
- 15.Honda A, Sogo N, Nagasawa S, Shimizu T, Umemura Y. High-impact exercise strengthens bone in osteopenic ovariectomized rats with the same outcome as Sham rats. Journal of applied physiology. 2003;95(3):1032–7. doi: 10.1152/japplphysiol.00781.2002. [DOI] [PubMed] [Google Scholar]
- 16.Hsin-Shih Lin T-HH, Mao Shih-Wei, Tai Yuh-Shiou, Chiu Hung-Ta, Cheng Kuang-You B, Yang Rong-Sen. A short-term free-fall landing enhances boen formation and bone material properties. J Mech Med Biol. 2011;11(5):1125–39. [Google Scholar]
- 17.Kerr D, Morton A, Dick I, Prince R. Exercise effects on bone mass in postmenopausal women are site-specific and load-dependent. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1996;11(2):218–25. doi: 10.1002/jbmr.5650110211. [DOI] [PubMed] [Google Scholar]
- 18.Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR American College of Sports M. American College of Sports Medicine Position Stand: physical activity and bone health. Medicine and science in sports and exercise. 2004;36(11):1985–96. doi: 10.1249/01.mss.0000142662.21767.58. [DOI] [PubMed] [Google Scholar]
- 19.LeBlanc AD, Spector ER, Evans HJ, Sibonga JD. Skeletal responses to space flight and the bed rest analog: a review. Journal of musculoskeletal & neuronal interactions. 2007;7(1):33–47. [PubMed] [Google Scholar]
- 20.MacKelvie KJ, Khan KM, Petit MA, Janssen PA, McKay HA. A school-based exercise intervention elicits substantial bone health benefits: a 2-year randomized controlled trial in girls. Pediatrics. 2003;112(6 Pt 1):e447. doi: 10.1542/peds.112.6.e447. [DOI] [PubMed] [Google Scholar]
- 21.MacKelvie KJ, Petit MA, Khan KM, Beck TJ, McKay HA. Bone mass and structure are enhanced following a 2-year randomized controlled trial of exercise in prepubertal boys. Bone. 2004;34(4):755–64. doi: 10.1016/j.bone.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Matkovic V, Jelic T, Wardlaw GM, Ilich JZ, Goel PK, Wright JK, et al. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross-sectional model. The Journal of clinical investigation. 1994;93(2):799–808. doi: 10.1172/JCI117034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKay HA, Petit MA, Schutz RW, Prior JC, Barr SI, Khan KM. Augmented trochanteric bone mineral density after modified physical education classes: a randomized school-based exercise intervention study in prepubescent and early pubescent children. The Journal of pediatrics. 2000;136(2):156–62. doi: 10.1016/s0022-3476(00)70095-3. [DOI] [PubMed] [Google Scholar]
- 24.Nagasawa S, Honda A, Sogo N, Umemura Y. Effects of low-repetition jump exercise on osteogenic response in rats. Journal of bone and mineral metabolism. 2008;26(3):226–30. doi: 10.1007/s00774-007-0812-6. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson MI, Greene NP, Dobson JP, Wiggs MP, Gasier HG, Macias BR, et al. Insulin resistance syndrome blunts the mitochondrial anabolic response following resistance exercise. American journal of physiology Endocrinology and metabolism. 2010;299(3):E466–74. doi: 10.1152/ajpendo.00118.2010. [DOI] [PubMed] [Google Scholar]
- 26.Ooi FK, Singh R, Singh HJ, Umemura Y. Minimum level of jumping exercise required to maintain exercise-induced bone gains in female rats. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2009;20(6):963–72. doi: 10.1007/s00198-008-0760-6. [DOI] [PubMed] [Google Scholar]
- 27.Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. The Journal of bone and joint surgery. 1984;66(3):397–402. American volume. [PubMed] [Google Scholar]
- 28.Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcified tissue international. 1985;37(4):411–7. doi: 10.1007/BF02553711. [DOI] [PubMed] [Google Scholar]
- 29.Ryan AS, Treuth MS, Rubin MA, Miller JP, Nicklas BJ, Landis DM, et al. Effects of strength training on bone mineral density: hormonal and bone turnover relationships. Journal of applied physiology. 1994;77(4):1678–84. doi: 10.1152/jappl.1994.77.4.1678. [DOI] [PubMed] [Google Scholar]
- 30.Snow CM, Shaw JM, Winters KM, Witzke KA. Long-term exercise using weighted vests prevents hip bone loss in postmenopausal women. The journals of gerontology Series A, Biological sciences and medical sciences. 2000;55(9):M489–91. doi: 10.1093/gerona/55.9.m489. [DOI] [PubMed] [Google Scholar]
- 31.Stadelmann VA, Bonnet N, Pioletti DP. Combined effects of zoledronate and mechanical stimulation on bone adaptation in an axially loaded mouse tibia. Clinical biomechanics. 2011;26(1):101–5. doi: 10.1016/j.clinbiomech.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 32.Swift JM, Gasier HG, Swift SN, Wiggs MP, Hogan HA, Fluckey JD, et al. Increased training loads do not magnify cancellous bone gains with rodent jump resistance exercise. Journal of applied physiology. 2010;109(6):1600–7. doi: 10.1152/japplphysiol.00596.2010. [DOI] [PubMed] [Google Scholar]
- 33.Swift JM, Nilsson MI, Hogan HA, Sumner LR, Bloomfield SA. Simulated resistance training during hindlimb unloading abolishes disuse bone loss and maintains muscle strength. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25(3):564–74. doi: 10.1359/jbmr.090811. [DOI] [PubMed] [Google Scholar]
- 34.Umemura Y, Ishiko T, Yamauchi T, Kurono M, Mashiko S. Five jumps per day increase bone mass and breaking force in rats. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1997;12(9):1480–5. doi: 10.1359/jbmr.1997.12.9.1480. [DOI] [PubMed] [Google Scholar]
- 35.Umemura Y, Nagasawa S, Honda A, Singh R. High-impact exercise frequency per week or day for osteogenic response in rats. Journal of bone and mineral metabolism. 2008;26(5):456–60. doi: 10.1007/s00774-007-0848-7. [DOI] [PubMed] [Google Scholar]
- 36.Welch JM, Turner CH, Devareddy L, Arjmandi BH, Weaver CM. High impact exercise is more beneficial than dietary calcium for building bone strength in the growing rat skeleton. Bone. 2008;42(4):660–8. doi: 10.1016/j.bone.2007.12.220. [DOI] [PubMed] [Google Scholar]
- 37.Welch JM, Weaver CM, Turner CH. Adaptations to free-fall impact are different in the shafts and bone ends of rat forelimbs. Journal of applied physiology. 2004;97(5):1859–65. doi: 10.1152/japplphysiol.00438.2004. [DOI] [PubMed] [Google Scholar]
- 38.Westerlind KC, Fluckey JD, Gordon SE, Kraemer WJ, Farrell PA, Turner RT. Effect of resistance exercise training on cortical and cancellous bone in mature male rats. Journal of applied physiology. 1998;84(2):459–64. doi: 10.1152/jappl.1998.84.2.459. [DOI] [PubMed] [Google Scholar]
- 39.Westerlind KC, Gibson KJ, Malone P, Evans GL, Turner RT. Differential effects of estrogen metabolites on bone and reproductive tissues of ovariectomized rats. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1998;13(6):1023–31. doi: 10.1359/jbmr.1998.13.6.1023. [DOI] [PubMed] [Google Scholar]
- 40.Witzke KA, Snow CM. Effects of plyometric jump training on bone mass in adolescent girls. Medicine and science in sports and exercise. 2000;32(6):1051–7. doi: 10.1097/00005768-200006000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Yarasheski KE, Campbell JA, Kohrt WM. Effect of resistance exercise and growth hormone on bone density in older men. Clinical endocrinology. 1997;47(2):223–9. doi: 10.1046/j.1365-2265.1997.2461060.x. [DOI] [PubMed] [Google Scholar]