Abstract
Introduction
Renal cell carcinoma (RCC) is a widespread oncourological disease with a tendency towards a slow increase of incidence. In the recent decade, there has been development of numerous effective drugs targeted at different molecules that play a dominant role in RCC carcinogenesis. Understanding of RCC carcinogenesis confirms the key role of angiogenesis in maintaining the viability of renal tumours and their metastases.
Material and methods
We aimed to systemize numerous medicines, used to inhibit the angiogenesis in patients with advanced RCC according to their targets, and to analyze their efficacy.
Results
There are roughly four main mechanisms of action of the targeted drugs:
Blockade of circulating extracellular VEGF molecules.
The selective blockade of tyrosine kinase receptors’ domains.
The simultaneous blockage of the tyrosine kinase receptors’ domains and intracellular tyrosine kinases.
The blockade of mammalian target of rapamycin (mTOR) which is responsible for support of vital functions of cancer cells.
In addition to FDA officially approved drugs, numerous experimental agents have been synthesized, which are currently on initial stages of clinical studies in RCC treatment.
Conclusions
The results of the currently used targeted drugs demonstrate perspectives of metastatic RCC conservative treatment, that are able to prolong cancer–specific survival in previously doomed patients for up to 29 months. The development of schedules for sequential treatment or combination targeted therapy remains a current challenge. The quality of life is an important factor that influences remedy choice. The advantages and disadvantages of neoadjuvant and adjuvant targeted therapy are currently being intensively discussed.
Keywords: metastatic renal cell carcinoma, targeted therapy, survival, side–effects
Kidney cancer or renal cell carcinoma (RCC) is a widespread oncourological disease with a tendency towards morbidity progression. There appears to be an increase in the incidence of all stages of RCC over the past few decades. At the time of RCC diagnosis, metastatic lesions are present in 20–30% of patients (pts.) [1]. Also, in 20–30% of patients with preoperative absence of metastases, after nephrectomies metastatic lesions appear or local recurrence of disease take place [2].
Surgical treatment of kidney tumours is the only effective treatment modality. Until the 21st century, the efficacy of drug therapy for disseminated RCC (interferon–α, interleukins, hormonal therapy and chemotherapy) was insignificant or absent, and the median overall survival (OS) in metastatic disease rarely exceeded 6–8 months [3]. It was only in the recent decade, after deeper understanding of the peculiarities of initiation and development of tumour progression in RCC, that it became possible to approve more efficacious drugs targeted on the key molecules involved in the RCC carcinogenesis.
Features and key molecules of RCC carcinogenesis
It is clear that after initiation of tumour growth the cancerogenesis of RCC occurs via two inseparable pathways: proliferation, enabling the Raf/MEK/ERK–kinase pathway, and the vascularisation pathway, maintaining reactions mediated by growth factors. The role of Von Hippel–Lindau (VHL) gene, hypoxia–inducible factor–1–alpha (HIF1–alpha) and mammalian target of rapamycin (mTOR) in the processes of cell proliferation and angiogenesis is indisputable [4]. Radiation causes oxidative stress & gene mutations, thus the role of chronic exposure to low doses of ionizing radiation in RCC genesis has been proved too [5].
To grow beyond 1–2 mm in diameter, the tumour requires the emergence and further development of its own blood vessels. This complex process, managed by growth factors (vascular endothelial growth factor (VEGF), platelet–derived growth factor (PDGF), etc.) is known as “neoangiogenesis”. Under normal conditions there is a dynamic equilibrium in the body (angiogenic balance) between activators and inhibitors of this process of new vessel formation, neoangiogenesis. The synthesis of activators and inhibitors of angiogenesis is managed by pro–angiogenic and anti–angiogenic genes. In case of a natural necessity of a vascular net (trauma, surgeries, burns etc.), the pro–angiogenesis genes are activated, the concentration of activators of angiogenesis increases, launching the mechanisms of new vessel formation for renewing tissues trophism. Upon reaching the required effect, the anti–angiogenesis genes are activated; the inhibitors of angiogenesis are synthesized, which impedes vascular growth. The latter mechanism may be displayed as the so–called ‘angiogenic switch’ [6].
The process of angiogenesis is controlled by the Von Hippel–Lindau gene. In most cases of clear–cell renal cancer inactivation or mutations of this gene are observed. Inactivation of the VHL gene causes premalignant renal cysts. Further conversion of the latter into carcinomas requires additional gene mutations in the body [7].
Up to 90% of all sporadic ccRCCs (clear–cell renal cell carcinomas) demonstrate HIF1–alpha stabilization and tissue accumulation because of a consequence of VHL loss or its inactivation [8].
Mammalian target of rapamycin (mTOR) is an intracellular enzyme, activating the kinases, responsible for support of vital functions of cancer cells. It exists in the form of two signal complexes: mTOR Complex 1 (mTORC1), that is involved in the intracellular synthesis of proteins and mTOR Complex 2 (mTORC2), that participates in the formation of the cytoskeleton (cellular framework stabilizing the cellular cytoplasm). There is activation of protein kinases Cα (PKCα) and AKT/PKB with the participation of mTOR, thereby increasing the synthesis of biologically active proteins HIF1–alpha and cyclin D. The molecules of mTOR are activated under the influence of tyrosine kinase receptor extracellular domain stimulation by growth factors, due to inhibition of genes, suppressing tumour growth and the direct action of oncogens. Overproduction of HIF1–alpha, in turn, causes overexpression of VEGF and PDGF with subsequent activation of angiogenesis. Due to the influence of mTOR on the synthesis of HIF1–alpha, inhibition of mTOR was selected as one of the directions of targeted therapy [9].
An important contribution to the studies of the mechanisms of cancerogenesis was made by the studies of structure, varieties, and functions of tyrosine kinases and their receptors, the activation of which allows execution of the normal and abnormal intracellular effects of ligands.
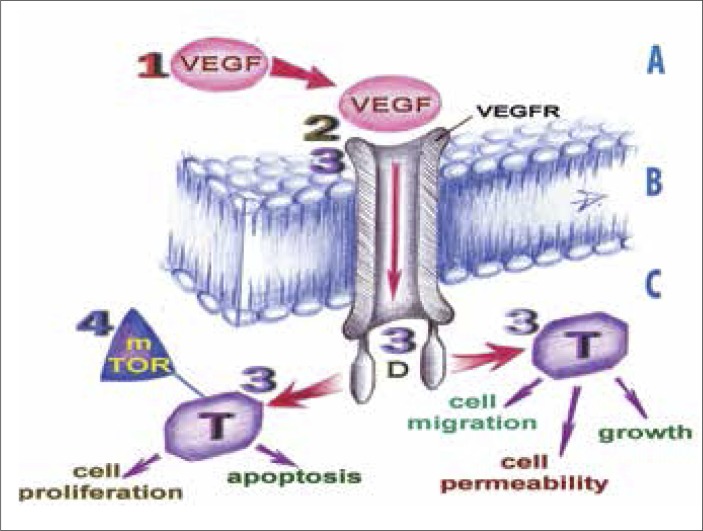
Understanding of RCC carcinogenesis (especially its clear–cell forms) confirms the key position of angiogenesis that is ruled by the HIF1–alpha/VEGF&PDGF pathway, in sustaining the viability of the cells of this type of renal tumours and their metastases [4, 10]. Clarification of the structure and function of all components of the mechanism behind tumour vascularisation stimulated the search for the pharmacological agents able to block the process of vascularisation on different levels. The directed anti–tumour therapy employing such agents began to be referred to as “targeted therapy”. We aimed to systemize numerous drugs, used to inhibit the angiogenesis in patients with RCC according to their targets and analyze their efficacy. We propose the original scheme with four main mechanisms of action of RCC–targeted therapy, which are shown in Figure 1.
Figure 1.
Targets of RCC–targeted therapy.
1, 2, 3, 4 – the targets for the relevant groups of targeted drugs; A – extracellular environment; B – cytoplasmic membrane of an endothelial cell; C – intracellular environment; D – intracellular domain of tyrosine kinase receptors; mTOR – mammalian target of rapamycin; T – tyrosine kinases; VEGF – vascular endothelial growth factor molecule; VEGFR – the tyrosine kinase receptor to VEGF
By blocking the key target molecules on these four levels, targeted drugs are able to stop new blood vessels formation as well as tumour cells’ proliferation and migration. As a consequence the tumour is not supplied with nutritional substrates, so tumour shrinkage occurs. Also tumour cell proliferation ceases.
TARGET 1. The first approach of RCC–targeted therapy (TT) is blockade of circulating extracellular VEGF molecules. The mechanism of action of such agents as bevacizumab (Bev), aflibercept and lenalidomide is based on this principle. The following phenomena occur due to VEGF inhibition:
arrest of angiogenesis;
regression of VEGF–dependent vessels (tumourous vessels and normal capillaries);
- reversal of VEGF–induced effects, such as:
- reversal of tumourous vessels;
- decrease in blood flow through the tumour, therefore decreased influx of oxygen and nutrients into the tumour;
- tumour shrinkage as a consequence.
Usually, neoangiogenesis resumes in average 3 weeks after the discontinuation of VEGF inhibitors [11].
Bevacizumab (“Avastin”, Roche) is an IgG1–monoclonal antibody, capable of the detection and binding of all VEGF isoforms. This results in inability of transmission of the specific signal through the transmembrane tyrosine kinase receptor, due to isolation of the circulating molecules of the receptor's ligand. Classical data Yang J. et al. 2003, demonstrates statistically significant increase of progression–free survival (PFS) in a group of patients receiving the drug at a dose of 10 mg/kg I.V. once in 2 weeks, up to 4.8 months (compared to 2.5 months in the placebo group). There was a low level of objective response rate (ORR), only in 10% of patients [12]. The above indices are substantially increased whenever the therapy is appended by interferon 2α (IFN–2α). Multicentre randomized studies of combination mRCC therapy (Bev + IFN–2α) have demonstrated the prolongation of PFS up to 10.2–13.5 months and increase of ORR to 30% [13]. The above combination of drugs is a first–line therapy in patients with disseminated clear–cell renal cancer in Europe and the U.S. Single–agent bevacizumab has an acceptable level of toxicity and moderate disease–stabilizing activity in selected patients with metastatic RCC (mRCC) who have failed prior therapy by VEGFR tyrosine kinases and mTOR inhibitors. The median PFS of bevacizumab therapy was 4.4 months (95% CI, 2.8–9.6 months), and the median OS was 19.4 months (95% CI, 9.9–NR months) respectively. For subjects treated with prior VEGF and mTOR inhibitors, median PFS and OS were 4.4 and 13.2 months, respectively. Grade 3 to 4 toxicities included fatigue (29%), dehydration (24%), failure to thrive (10%), constipation (10%), and muscle weakness (10%) [14].
Aflibercept (“VEGF Trap”, Regeneron/Sanofi Aventis) is a soluble protein consisting of segments of the extracellular domains of VEGFR–1 and VEGFR–2. The product functions as a “trap” for the molecules of VEGF. After administration, aflibercept is accumulated in the extracellular medium of vascular endothelium, where it selectively binds VEGF molecules in a manner similar to that of bevacizumab, thus preventing VEGF from binding with the extracellular domains of the tyrosine kinase receptors and therefore stopping angiogenesis. The clinical trials of aflibercept efficacy in the treatment of patients with different cancers including RCC are currently ongoing [15].
The same VEGF–targeted group includes Lenalidomide (“Revlimid”, Celgene Corp.), a thalidomide derivative, which possesses anti–angiogenic and immunomodulating effect. The immunomodulating action of lenalidomide is due to the impact on T–cell immunity. Lenalidomide therapy in patients with mRCC, ensure the median of PFS 6 months and the median OS – 17 months [16]. In another study, 39% of patients had a stabilization of their cancer for at least three months but median OS had not yet been reached at 13.5 months of follow–up. Major side effects included fatigue, skin problems, and low levels of immune cells [17].
AMG 386 (Amgen Inc.). Taking into consideration the direct influence of HIF1–alpha proteins on the VEGF synthesis and therefore of angiogenesis activation, there is a search for the possibilities to block the HIF1α–mechanism, thereby decreasing VEGF concentrations. The experimental drug AMG 386 (Amgen Inc.) is a neutralizing polypeptide, created as a substance able to disrupt the connections of TIE–2 receptors with angiopoietin 1/2, thereby arresting the pro–angiogenic effects of HIF1–alpha and as a consequence VEGF. As Phase I clinical trials has shown, AMG 386 manifests moderate anti–angiogenic activity [18].
Girentuximab (WX–G250, “Rencarex”, Wilex). Another HIF1α–targeted drug, a chimeric monoclonal antibody targeted at carbonic anhydrase IX (CA–IXMN/G250) cell surface receptor, which is hyper–expressed in RCC cells and is HIF1–alpha–dependent. When the drug is taken by the patients with clear–cell mRCC, the OS is 15 months [19]. In combination therapy with WX–G250 + IL–2 the OS achieves 22 months [20].
TARGET 2. The second approach of targeted therapy is the selective blockade of domains of tyrosine kinase receptors: VEGFR, PDGFR and epidermal growth factor's receptor (EGFR). This mechanism of action is typical for such drugs as pazopanib, erlotinib, axitinib, semaxanib, tivozanib, ramucirumab etc.
Pazopanib (“Votrient”, GSK) is a selective inhibitor of VEGFR–1;–2;–3 and PDGFR receptors. PFS = 9.2 months, OS = 21.1 months, ORR = 30%. Administration of the drug produces tumour shrinkage (TS) by 30% compared to baseline [21]. In October 2009 pazopanib was approved by FDA as a first–line therapy agent for clear–cell mRCC.
The final overall survival results of a randomized, double–blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma, demonstrate that the difference in final OS between pazopanib– and placebo–treated patients was not statistically significant (22.9 vs. 20.5 months, respectively; hazard ratio [HR] = 0.91; 95% confidence interval [CI], 0.71–1.16; one–sided P = 0.224). Although no significant difference in OS was observed, extensive crossover from placebo to pazopanib confounded the final OS analysis. Post–hoc analyses adjusting for crossover suggest OS benefit with pazopanib treatment for mRCC patients [22]. In our opinion, such a long OS in placebo–treated patients (20.5 months) in comparison with results of similar investigations presented in our article may be caused only by a special selection of patients. Thus, the preliminary conclusion of this study about equal survival in pazopanib & placebo groups may be a topic for discussion.
Erlotinib (“Tarceva”, OSI Pharmaceutical/Genentech/Roche) is a selective inhibitor of extracellular domains of epidermal growth factor receptors (EGFR). The drug is also able to bind the VEGFR receptors. PFS = 11.0 months, in 60% patients OS = 18 months [23].
Axitinib (“Inlyta”, Pfizer) is a selective inhibitor of extracellular domains of VEGFR–1;–2;–3; PDGFR; RTKs; cKIT receptors. PFS = 15.7 months, OS = 29.9 months [24]. Data from the trial AGILE 1046 presented at the 2012 ASCO annual meeting demonstrated a median PFS of more than one year, and an overall response rate of 40.2% in the treatment arm of the study [25]. Thus, axitinib was granted FDA approval in January 2012 to treat advanced RCC in patients who failed to respond to prior therapy.
Semaxanib (SU–5416, SUGEN) is a VEGFR–2 blocker. In combination with IFNα, semaxanib demonstrates that ORR = 50%, OS = 10.0 months [26].
Tivozanib (AV–951, AVEO Pharmaceuticals), an inhibitor of all three types of VEGF–1;–2;–3 receptors, initially demonstrated promising significant improvement in PFS and ORR compared with sunitinib in patients who had received no prior systemic therapy for metastatic RCC [27]. However, in 2013 the FDA has rejected a new drug application for tivozanib for the treatment of advanced RCC, recommending an additional clinical trial to address concerns over existing clinical data. At the time of the OS analysis, presented at the ASCO Genitourinary Symposium, mortality rates were 45.4% in the tivozanib group and 39.3% in the sorafenib group, corresponding to a stratified hazard ratio of 1.245 (95% CI, 0.954–1.624; P = .105) trending in favor of sorafenib. The median OS was 28.8 months in the tivozanib arm and 29.3 months in the sorafenib arm [28].
Ramucirumab (IMC–1121B; ImClone Systems/Eli Lilly) is a fully human, high–affinity monoclonal antibody to the extracellular domain of VEGFR–2. Its binding prevents VEGF binding to receptor. Patients with progressive disease or intolerance to either sorafenib, sunitinib, or both were IV administered 8 mg/kg ramucirumab biweekly and received tumour assessments every six weeks. A total of 40 patients were enrolled and 39 were treated. Grade 1–2 headache (23% of patients), Grade 1–3 fatigue (18% of patients) and Grade 1 nausea (13% of patients) were the most common therapy–related adverse effects. Nineteen patients (49%) had stable disease that lasted for more than 5 months; preliminary median progression–free survival was 6 months [29].
TARGET 3. The third approach of TT is simultaneous blockage of the domains of tyrosine kinase receptors and intracellular tyrosine kinases.
Sorafenib (“Nexavar”, Bayer) is an inhibitor of multikinases. It is proved that the drug inhibits the intracellular kinases (B–Raf, mutant B–Raf, C–Raf) and the receptors, located on the surface of cells (KIT, FLT–3, RET, VEGFR1–3 and PDGFR–beta). In 2006 Ratain M. et al. published the results of sorafenib efficacy in the therapy of patients with mRCC resistant to cytokine therapy. After 12 weeks of therapy, tumour regression was documented in 40% of patients; stabilization of the disease was documented in 29% of patients. By week 24 of therapy there was a longer progression–free survival in the group of sorafenib compared to placebo (50% and 18%, respectively). PFS was 23 and 6 weeks, respectively [30]. In December 2005, sorafenib was approved by FDA and recommended as therapy for advanced RCC.
Sunitinib (“Sutent”, Pfizer) is an inhibitor of multikinases, blocking the VEGFR, PDGFR, KIT, RET, CSF–1R and FLT–3 receptors. In 2008, Robert Figlin have presented the final stage results of this drug studies: ORR = 39–47%; PFS = 11 months; OS = 26 months. In 2006 the drug was approved by FDA, as a first–line therapy for clear–cell mRCC [31]. Tissue markers expression, such as CA9, CD31, CD34 and VEGFR1/2 in the primary tumours of metastatic ccRCC patients might serve as predictors of a good response to sunitinib treatment [32]. ORR to sunitinib is higher, and metastatic sites were few in patients with low immature blood vessel ratio (IBVR). Immature vessels are defined as vessels that stain only with CD31 endothelial cells. Thus, IBVR should be considered as a very useful prognostic factor to make an estimate in sunitinib treatment in patients with mRCC [33].
Acquired resistance to sunitinib in human renal cell carcinoma cells is mediated by constitutive activation of signal transduction pathways associated with tumour cell proliferation. The maintained phosphorylation of several types of protein kinases during the treatment with sunitinib may be involved in the acquisition of resistant phenotype in RCC cells to this agent; therefore, it would be worthy to further investigate the significance of agents inactivating either protein kinases, such as LY294002, as possible candidates for overcoming resistance to sunitinib in patients with RCC [34].
Sunitinib and sorafenib can be safely given to patients with renal insufficiency, providing there is adequate monitoring of renal function. For those patients developing an increase in blood creatinine levels, dose modifications may be required in order to allow continuation of therapy [35].
In 2009, Tim Eisen presented the results of a Stage II clinical study of a novel multi–targeted drug regorafenib (BAY 73–4506,“Stivarga”, Bayer). Like the above drugs, regorafenib is targeted at angiogenesis due to the blockade of VEGFR1–3, TIE–2, PDGFR–beta, and cell proliferation by inhibiting the oncogenic RAF, RET and c–KIT tyrosine kinases. By the time of presentation, 81% of patients were found to have stabilization (50%) or regression (31%) of the disease. PFS = 8.3 months [36]. However, serious drug–related adverse events were registered in 35% of all patients, including hand and foot skin reaction (33%), diarrhoea (10%), renal failure (10%), fatigue (8%), hypertension (6%), cardiac ischaemia or infarction (4%), hypomagnesaemia (2%), and pain in the chest or thorax (2%) [37].
Another experimental multi–targeted drug cediranib (AZD 2171; “Recentin”, AstraZeneca) demonstrated antitumour activity in various forms of cancer, including RCC, in preclinical studies. In a preliminary report of a single–arm phase II study of cediranib in treatment–naive RCC, 12 out of 32 evaluable patients (38%) had a partial response, and 15 patients (47%) had stabilization of disease, yielding a benefit rate of 85%. In a randomized, double–blind phase II trial, 71 patients with RCC were randomized 3:1 to cediranib or placebo. At 12 weeks, the investigators noted a highly significant difference in mean percentage change in tumour size between the study and control groups (–20% vs. +19%, P <0.0001). Of the 18 patients in the placebo arm, 14 crossed over to cediranib; of these 14 patients, 10 had tumour reduction. In the cediranib arm, 34% achieved a partial response, and 47% had stabilization of disease [38].
Nintedanib (BIBF 1120, “Vargatef”, Boehringer Ingelheim), is a triple tyrosine kinase inhibitor of VEGFR–1–3, PDGFR–α/β, and FGFR–1–3, as well as RET and Flt3, and demonstrated similar efficacy to sunitinib (PFS (median: 8.44 vs. 8.38 mo; hazard ratio: 1.16; 95% CI: 0.71–1.89; p = 0.56); OS (median: 20.37 vs. 21.22 mo; p = 0.63)). It possessed a manageable safety profile, including a lower incidence of dermatologic adverse effects vs. sunitinib. In addition, nintedanib was not associated with QT prolongation (grade ≥3 adverse effects occurred in 47% of nintedanib–treated patients and 56% of sunitinib–treated) [39].
TARGET 4. The fourth approach of TT is the blockade of mTOR.
Temsirolimus (“Torisel”, Wyeth Pharmaceuticals) is a specific blocker of mTORC1. Administration of temsirolimus causes arrest of mTORC1 synthesis, decreases levels of VEGF and PDGF, thereby halting angiogenesis and tumour progression. It has been proven that the efficacy and safety of temsirolimus monotherapy is higher than in combination with IFNα or bevacizumab. PFS in monotherapy of non–clear–cell forms of mRCC is 3.8 months, OS = 10.9 months [40]. The benefit of temsirolimus compared to IFN–α was significant in the group of patients with non–clear–cell histology. In this population, the median OS was 11.6 months with temsirolimus and 4.3 months with IFN–α (HR 0.49; 95% CI: 0.29–0.85 months); median PFS, based on independent assessment, was 7.0 months with temsirolimus and 1.8 months with IFN–α (HR 0.38; 95% CI: 0.23–0.62 months) [41]. Temsirolimus has been registered by FDA and EMEA in 2007; this is a first–line agent in therapy of non–clear–cell forms of mRCC and RCC with high degree of risk for disease progression.
Recent investigations confirm that activation of the signal transduction pathway via mTORC2, but not via mTORC1, may play an important role in the acquisition of resistance to temsirolimus in RCC, through the constitutive activation of AKT and MARK even after treatment with temsirolimus [42].
Everolimus (“Afinitor”, Novartis) is a rapamycin derivative, a drug with immunosuppressive and anti–angiogenic actions, specifically targeted at the blockage of mTORC1. In 2008 Motzer R. et al. published the results of a Phase III clinical trial of everolimus efficacy in patients with mRCC, in whom the administration of sorafenib or sunitinib failed to demonstrate clinical improvement, or there was renewal of tumour progression after prior improvement. ORR = 26%, PFS = 4.0 months as opposed to 1.9 months in the placebo group [43]. In 2009 the drug was registered by FDA and EMEA and recommended for the treatment of mRCC in cases of low efficacy of sorafenib and sunitinib, as well as in loss of the therapeutic effect thereof. In 2010, Casciano R. et al. in 2010 reported on the results of an indirect comparative efficacy study of everolimus and sorafenib in the therapy of sunitinib–refractory patients. The study included 98 patients; 46 of these received everolimus and 52 received sorafenib. In the group of everolimus, PFS was equal to 40.8 weeks, OS = 78 weeks; in the group of sorafenib PFS =17.7 weeks and OS = 32 weeks [44].
Everolimus is effective in the treatment of large angiomiolypomas (AML) in patients with subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC). This treatment demonstrates efficacy in reducing AML lesion volume in patients with SEGA associated with TSC who also presented with AML. AML downsizing >50% were seen only in everolimus–treated patients (56.5%, 78.3%, 80%) compared with placebo–treated patients (0% at each time point) at week 12, 24, and 48, respectively. It may offer a pharmacological treatment option for patients with TSC and concomitant AML and SEGA [45].
In 2010 ASCO developed the algorithm for assigning targeted drugs depending on the histological type of RCC and the risk of disease progression according to Motzer R., 1999 [46, 47]. By generalizing the recommendations of ASCO and the results of recently finished and ongoing studies, which have demonstrated a positive clinical effect, modern approaches to drug therapy of RCC can be streamlined as follows (see Table 1).
Table 1.
The algorithm of sequential targeted drugs administration in RCC treatment
| Hystological type of RCC | 1st line therapy | 2nd line therapy | 3rd line therapy | |
|---|---|---|---|---|
| Clear–cell type | Low and medium risk of progression | Bev + IFNα Sunitinib Pazopanib Cytokines* |
Everolimus (after TKI) Sorafenib (after CT) Pazopanib (after CT) Sunitinib*(after CT) Axitinib (after TKI) |
Sorafenib #
Sorafenib + rIL–21 # Sunitinib # Axitinib # |
| High risk of progression | Temsirolimus Sunitinib* |
|||
| Non–clear–cell type | Temsirolimus | Sorafenib*
Sunitinib* |
Moderate drug activity
The research is ongoing; Bev – bevacizumab; rIL – recombinant interleukin; TKI – tyrosine kinase inhibitors; CT – cytokine therapy
In addition to the above drugs, a number of experimental agents have been synthesized, which are currently in initial stages of efficacy studies in RCC.
Studies on the efficacy of OSI–027 and AZD8055, both novel mTOR inhibitors, have been initiated. The action of these drugs is simultaneously directed on the blockade of both mTORC1 and mTORC2 complexes [48]. The same mechanism of action is also appropriate to a new class of mTOR inhibitors, the TORKinhibs, which have been developed recently. Their specific association on the ATP–site of the mTOR molecule allows a more comprehensive inhibition of the pathway and their superiority over rapalogs has been reported recently in a pre–clinical trials. The pre–clinical trial revealed a superiority of the novel dual mTOR inhibitor INK128 vs. temsirolimus and placebo, to control RCC Tissue Slice Graft growth rate: INK128 was significantly superior to placebo in the three arms containing 20, 12 and 9 mice, respectively (p = 0.012, 0.005, 0.01). INK128 was significantly superior to temsirolimus in the first cohort (p = 0.012), superior but not significant in another cohort (p = 0.77), and not compared to temsirolimus in the third cohort [49].
Bortezomib (PS–341, “Velcade”, Millenium Pharmaceuticals Inc.) is a highly selective reversible inhibitor of 26–S proteasome, a modified boric acid. As a consequence of inhibition of the proteolytic effects of the proteasome there is an inactivation of the NF–kB oncogenic protein, inhibition of proteolysis, decrease in quantity of anti–apoptosis factors, the molecules of inflammation mediators and the molecules of cellular adhesion, as well as cytokines that facilitate tumour growth. The influence of the product on the course of RCC is an object of ongoing research. The combination of bortezomib and pan–deacetylase inhibitor panobinostat can induce drastic apoptosis and anable to inhibit renal cancer growth synergistically. This combination would kill cancer cells effectively by inhibiting the degradation of oncogenic proteins and thereby inducing endoplasmic reticulum stress and ubiquitinated protein accumulation [50].
There is currently ongoing research concerning the efficacy of a combination of sunitinib and an autologous tumour vaccine AGS–003 (Argos Therapeutics), developed from tumourous cells of an individual patient. AGS–003 is a highly specific product consisting of dendritic cells, tumour antigen and CD40, the molecule stimulating immune response. In March 2010, Theodora Logan presented the results of an efficacy study of a vaccine in patients with metastatic RCC, diagnosed a few months after nephrectomies for localized RCC. Given that most subjects were patients with medium or high risk of disease progression, the results obtained can be considered quite encouraging. ORR = 40%, whereas PFS in the group of AGS–003 was 5.6 months, compared to the known indices of PFS after application of IFNα in similar cases of 5.1 months in moderate risk patients and 2.5 months in high risk patients [51].
DISCUSSION
The accumulated experience of using anti–angiogenic drugs in RCC treatment demonstrates that neoangiogenesis and tumour progression resume on the average in 10–12 months of monotherapy, in spite of continued TT [11]. However, there is no cross resistance of tumourous cells to representatives of various groups of target drugs. Therefore the development of schedules of sequential treatment or combination TT remains a current challenge that allows an extended CSS in some RCC patients up to 25–29 months [52]: Now there are numerous officially approved and experimental targeted drugs available. Aside from survival terms, the patient's quality of life is an important factor that influences drug choice. As noted by Dr. Motzer, pazopanib and sunitinib are similarly effective in first line treatment of metastatic renal cell carcinoma. For both drugs, the median progression–free survival by the treating physician's assessment was slightly more than 10 months. An interim analysis found median overall survival was 28.4 months with pazopanib versus 29.3 months with sunitinib (HR = 0.908; p = 0.275). Final survival data are expected. Independent review found that objective response rate was 31% with pazopanib versus 25% with sunitinib (p = 0.032). In terms of health–related quality of life, 11 out of 14 domains favoured pazopanib over sunitinib. Both drugs resulted in side effects, but fatigue and skin sores, occurred with less frequency for pazopanib than with sunitinib, the researchers found. The quality of life questionnaires were in favor of pazopanib over sunitinib, and suggested improved tolerability for pazopanib over sunitinib, Dr. Motzer sumarized [53]. Thus, tolerability should be considered as an important factor influencing the choice of the targeted drug.
There are two current options for initiation of targeted therapy: before surgery or in the postoperative period. Neoadjuvant administration of TT facilitates reducton in the size of tumour and its metastases, which facilitates subsequent removal. Also, in a pronounced effect of TT, a revision of planned surgical tactics is possible, with inclination towards organ–sparing surgery. Such therapeutic tactics probably could produce reduction of microscopic metastatic foci and prevent intraoperative dissemination of the disease as a result of angiogenesis blockade.
Administration of TT 12.2 weeks before surgery reduces kidney tumour size by 10–30% [54]. Neodjuvant sunitinib use in 12 patients with locally advanced or centrally located tumours for 4 weeks, followed by a washout phase of 2 weeks before nephron–sparing surgery, decreased the primary tumour size with a mean reduction in maximum diameter of 21% [55].
Patients with renal cell carcinoma and imperative indication for renal sparing options may benefit from tumour downsizing by targeted therapies. Especially patients with tumours in the range of 5–7 cm are of interest, because a reduced size may make them eligible for ablative techniques. Smaller tumour size (<5 cm) was related to more effective shrinkage, with median downsizing of 34% (from – 46% to +11%). In the second group (5–7 cm) median downsizing was 11% (from – 55% to +16%). In this group 8/22 (37%) reduced into a range of 2.3–4.7 cm in which ablative techniques are feasible and nephron–sparing surgery may benefit from the reduced size. In tumors sized 7–10 cm, median downsizing was 14% (from – 39% to +2%). In the group with tumours >10 cm median downsizing was 9% (from – 31 to +8%) [56].
According to Karakiewicz P.I. et al., 2008, it is possible to downstage inferior vena cava thrombi with targeted drugs administration [57]. In another retrospective study, Cost N.G. et al., 2011, the effect of neoadjuvant therapy on vena cava tumour thrombi was also evaluated: 12 patients received sunitinib, 9 received bevacizumab, 3 received temsirolimus, and one patient received sorafenib. Opposite to previous research, analysis revealed minimal effect on the tumour thrombus level and failed to demonstrate a significant impact on the surgical approach. Interestingly, only the administration of sunitinib showed measurable thrombus regression [58].
A substantial disadvantage of the above tactics is disease progression in cases of low TT efficacy with subsequent expansion of the volume of surgical intervention. In a certain number of patients adverse actions of targeted drugs (hypertension, vomiting, diarrhoea, changes in blood rheology) deteriorate the course of the postoperative period, complicate wound healing, and may cause haemorrhages and thromboembolism [59].
Discussion regarding neoadjuvant TT is ongoing. The research of Jakub Kłącz et al, 2013, demonstrates that there is no scientific proof that neoadjuvant targeted therapy may significantly reduce the stage and size of a tumour in large renal tumours. At the same time authors consider that the removal of the tumour in disseminated disease is only justified in patients with a condition suitable for targeted adjuvant treatment [60].
The advantages of adjuvant TT are: reduction of cancer intoxication immediately after tumour removal, a possibility for detecting the histological type of the tumour and the degree of nuclear atypia, which allows informed selection of the relevant target drug and predicting its efficacy. Surgery and the postoperative period are not complicated by possible adverse effects of TT. However, immediate TT is impossible in the above tactics since, taking into consideration the possible adverse effects of the drugs, TT can be started only after postoperative wounds heal.
Currently there is no unanimous understanding concerning the timing of TT and surgical treatments for RCC. The therapeutic tactics and drug choice should be individual in each case. Undoubtedly, in cases when the patients present with hematuria, significant tumour intoxication and high risk for surgical complications against the background of TT, the treatment should be started with surgical intervention, followed by pharmacological therapy.
Due to the enhanced immune response against the background of AGS–003 administration, it was logical to expect the favourable efficacy of adjuvant combination therapy using anti–angiogenic drugs. Adjuvant treatment with an AGS–003 + sunitinib combination after nephrectomies in 22 patients with mRCC demonstrates an ORR of 81%, and PFS – 12.5 months [61].
Adjuvant therapy by girentuximab in 286 patients with locally advanced RCC improved a median of 5 years disease–free survival by over 50%, compared to a placebo group (73.6 vs. 51.2 months) [62].
Due to a high price in the majority of targeted drugs, it is actually a problem of cost–saving treatment. A cost–minimization analysis of bevacizumab + IFNα or sunitinib alone in mRCC treatment was performed, focusing on survival terms and direct medical costs only (drugs, administration and management of adverse events). The analysis compared the cost of bevacizumab (10 mg/kg) plus IFNα (9, 6 or 3 million IU [MIU]) versus sunitinib (50 mg) as first–line therapies for advanced or metastatic clear–cell RCC. The efficacy profiles of bevacizumab + IFNα or sunitinib alone have been shown (indirectly) to be similar in patients with RCC; indeed, median PFS with either treatment is in the 10– to 11–month range. Assuming a PFS of 11 months for these options, bevacizumab + IFNα (9 MIU) would be a lower cost strategy (cost savings of €2,052 per patient) than sunitinib. The cost advantages for bevacizumab would increase in parallel with a reduction in IFNα dosing; for example, with IFNα 6 MIU the corresponding cost savings would be €4,185, and with 3 MIU the cost advantage would be €6,320 per patient. Thus, bevacizumab + IFNα cost–saving alternative to sunitinib as the first–line treatment of metastatic RCC [63].
Thus, the results of using targeted therapy demonstrate the promising prospects of conservative treatment for metastatic RCC. Under the influence of TT, patients previously considered to be hopelessly ill demonstrated clinically significant reversal of the disease with an improved quality of life. A number of unresolved issues have to be conceded, such as the ability of certain tumours to adapt to TT, insignificant progress in the treatment of non–clear–cell RCC, and the high costs of drugs, which limits their wide application. However, taking into consideration the substantial progress in the studies of RCC cancerogenesis, it is obvious that growth factors, their receptors, tyrosine kinases, HIF, mTOR as well as the processes regulated by these substances, will continue to be viewed as principal targets of pharmacological therapy for renal cell carcinoma.
CONCLUSIONS
The following general regularities concerning selection of targeted therapy and predicting its efficacy can be formulated:
Targeted therapy does not exclude surgical treatments in RCC, but rather appends them, whenever removal of the primary tumour and/or metastatic foci is feasible. There are studies indicating the expediency of neoadjuvant and adjuvant TT.
In most cases targeted drugs are effective in the treatment of clear–cell mRCC. Only temsirolimus and partially sorafenib/sunitinib demonstrate their efficacy in the treatment of non–clear–cell forms. Not all patients with RCC have a positive therapeutic response to targeted drugs. Drug–related adverse events are common among the patients receiving TT, with serious adverse events forcing the rejection and stoppage of targeted drug utilization.
Usually, after 10–12 months of monotherapy, despite continued administration of VEGF–targeted drugs, angiogenesis and tumour progression resume. That is why the schedules of sequential administration of anti–angiogenic drugs of various directions, as well as options of combination TT are currently under development.
In the era of individualized medicine, every RCC patient should be completely informed and given the option to choose a targeted drug. Tolerability is an important factor influencing the drug choice.
Presented scheme of RCC–targeted therapy enables the classification of numerous drugs that are used in kidney cancer treatment, helps to imagine cell–localization of the key targets, and contributes to further understanding the ways of angiogenesis inhibition.
References
- 1.Konethy BR, Williams RD. Renal Parenchymal Neoplasm in Smith's General Urology. 17th Edition. 2008. p. 330. [Google Scholar]
- 2.Athar U, Gentile TC. Treatment options for metastatic renal cell carcinoma: a review. Can J Urol. 2008;15:3954–3966. [PubMed] [Google Scholar]
- 3.Bukowski RM. Cytokine therapy for metastatic renal cell carcinoma. Semin Urol Oncol. 2001;19:148–154. [PubMed] [Google Scholar]
- 4.Rini B, Small E. Biology and Clinical Development of Vascular Endothelial Growth Factor–Targeted Therapy in Renal Cell Carcinoma. J Clin Oncol. 2005;23:1028–1043. doi: 10.1200/JCO.2005.01.186. [DOI] [PubMed] [Google Scholar]
- 5.Richardson DB, Hamra G. Ionizing radiation and kidney cancer among Japanese atomic bomb survivors. Radiat Res. 2010;173:837–842. doi: 10.1667/RR2096.1. [DOI] [PubMed] [Google Scholar]
- 6.Abdollahi A, Schwager C, Kleeff J, Esposito I, Domhan S, Peschke P, et al. Transcriptional network governing the angiogenic switch in human pancreatic cancer. Proc Natl Acad Sci USA. 2007;104:12890–12895. doi: 10.1073/pnas.0705505104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaelin W. The Von Hippel–Lindau Tumour Supressor Gene and Kidney Cancer. Clin Can Res. 2004;10:6290S. doi: 10.1158/1078-0432.CCR-sup-040025. [DOI] [PubMed] [Google Scholar]
- 8.Brauch H, Weirich G, Brieger J, Glavac D, Rödl H, Eichinger M, et al. VHL alterations in human clear–cell renal cell carcinoma: association with advanced tumour stage and a novel hot spot mutation. Cancer Res. 2000;60:1942–1948. [PubMed] [Google Scholar]
- 9.Wullschleger S, Loewith R, Hall M. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Folkman J. Tumour angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 11.Vosseler S, Mirancea N, Bohlen P, Mueller MM, Fusenig NE. Withdrawal of anti–VEGF therapy may result in vessel regrowth. Cancer Res. 2005;65:1294–1305. doi: 10.1158/0008-5472.CAN-03-3986. [DOI] [PubMed] [Google Scholar]
- 12.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti–vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, et al. AVOREN Trial investigators. Bevacizumab plus interferon alfa–2a for treatment of metastatic renal cell carcinoma: a randomised, double–blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 14.Turnbull JD, Cobert J, Jaffe T, Harrison MR, George DJ, Armstrong AJ. Activity of Single–Agent Bevacizumab in Patients With Metastatic Renal Cell Carcinoma Previously Treated With Vascular Endothelial Growth Factor Tyrosine Kinase Inhibitors. Clin Genitourin Can. 2013;11:45–50. doi: 10.1016/j.clgc.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Teng LS, Jin KT, He KF, Zhang J, Wang HH, Cao J. Clinical Applications of VEGF–Trap (Aflibercept) in Cancer Treatment. J Chin Med Assoc. 2010;73:449–456. doi: 10.1016/S1726-4901(10)70097-6. [DOI] [PubMed] [Google Scholar]
- 16.Amato RJ, Hernandez–McClain J, Saxena S, Khan M. Lenalidomide Therapy for Metastatic Renal Cell Carcinoma. Am J Clin Oncol. 2008;31:244–249. doi: 10.1097/COC.0b013e31815e451f. [DOI] [PubMed] [Google Scholar]
- 17.Choueiri TK, Dreicer R, Rini BI, Elson P, Garcia JA, Thakkar SG, et al. Lenalidomide Therapy for Metastatic Renal Cell Carcinoma Phase II study of lenalidomide in patients with metastatic renal cell carcinoma. Cancer. 2006;107:2609–2616. doi: 10.1002/cncr.22290. [DOI] [PubMed] [Google Scholar]
- 18.Rosen LS, Hong D, Chap L, Kurzrock R, Garcia A, Rasmussen E. First–in–human study of AMG 386, a selective angiopoetin1/2–neutralizing peptibody, in adult patients with advanced solid tumours. J Clin Oncol (Meeting Abstracts) 2007;20:3522. Abstr. [Google Scholar]
- 19.Bleumer I, Knuth A, Oosterwijk E, Hofmann R, Varga Z, Lamers C, et al. A phase II trial of chimeric monoclonal antibody WX–G250 for advanced renal cell carcinoma patients. Br J Cancer. 2004;90:985–990. doi: 10.1038/sj.bjc.6601617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bleumer I, Oosterwijk E, Oosterwijk–Wakka JC, Voller MC, Melchior S, Warnaar SO, et al. A clinical trial with chimeric monoclonal antibody WX–G250 and low dose interleukin–2 pulsing scheme for advanced renal cell carcinoma. J Urol. 2006;175:57–62. doi: 10.1016/S0022-5347(05)00040-6. [DOI] [PubMed] [Google Scholar]
- 21.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized Phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 22.Sternberg CN, Hawkins RE, Wagstaff J, Salman P, Mardiak J, Barrios CH, et al. A randomised, double–blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: final overall survival results and safety update. Eur J Cancer. 2013;49:1287–1296. doi: 10.1016/j.ejca.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Hainsworth JD, Sosman JA, Spigel DR, Edwards DL, Baughman C, Greco A. Treatment of metastatic renal cell carcinoma with a combination of bevacizumab and erlotinib. J Clin Oncol. 2005;23:7889–7896. doi: 10.1200/JCO.2005.01.8234. [DOI] [PubMed] [Google Scholar]
- 24.Rixe O, Bukowski RM, Michaelson MD, Wilding G, Hudes GR, Bolte O, et al. Axitinib treatment in patients with cytokine–refractory metastatic renal–cell cancer: a phase II study. Lancet Oncol. 2007;8:975–984. doi: 10.1016/S1470-2045(07)70285-1. [DOI] [PubMed] [Google Scholar]
- 25.Rini BI, Grünwald V, Fishman MN, Melichar B, Ueda T, Karlov PA, et al. Axitinib for first–line metastatic renal cell carcinoma (mRCC): Overall efficacy and pharmacokinetic analyses from a randomized phase II study. J Clin Oncol. 2012;30:4503. Abstr. [Google Scholar]
- 26.Lara PN, Jr, Quinn DI, Margolin K, Meyers FJ, Longmate J, Frankel P, et al. SU5416 plus interferon alpha in advanced renal cell carcinoma: a phase II California Cancer Consortium Study with biological and imaging correlates of angiogenesis inhibition. Clin Cancer Res. 2003;9:4772–4781. [PubMed] [Google Scholar]
- 27.Sternberg CN, Eisen T, Tomczak P, Strahs AL, Esteves B, Berkenblit A, et al. Tivozanib in patients treatment–naive for metastatic renal cell carcinoma: A subset analysis of the phase III TIVO–1 study. J Clin Oncol. 2013;31(Suppl):4513. Abstr. [Google Scholar]
- 28.Motzer RJ, Eisen T, Hutson TE, Szczylik C, Krygowski M, Strahs A, et al. Overall survival in patients from a phase III study of tivozanib hydrochloride versus sorafenib in patients with renal cell carcinoma. J Clin Oncol. 2013;31(Suppl):350. Abstr. [Google Scholar]
- 29.Garcia JA, Hudes GR, Choueiri TK, Stadler WM, Wood LS, Bhatia S, et al. Phase II study of IMC–1121B in patients with metastatic renal cancer (mRCC) following VEGFR–2 tyrosine kinase inhibitor (TKI) therapy (IMCL CP12–0605/ NCT00515697); Am Soc Clin Oncol Annual Meeting; 2010. p. 326. [Google Scholar]
- 30.Ratain MJ, Eisen T, Stadler WM, Flaherty KT, Kaye SB, Rosner GL, et al. Phase II placebo–controlled randomized dicountinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:2505–2512. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 31.Figlin R, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Négrier S, et al. Overall survival with sunitinib versus interferon (IFN)–alfa as first–line treatment of metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2008;26(Suppl):5024. Abstr. [Google Scholar]
- 32.Dornbusch J, Meinhardt M, Erdmann K, Zacharis A, Zastrow S, Füssel S, et al. Potential predictive markers for a response to sunitinib and their association with survival of patients with metastatic renal cell carcinoma. Eur Urol Suppl. 2013;12:e984. doi: 10.1371/journal.pone.0076386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim BH, Kim CI, Ha JY, Park CH, Kim KH, Choe MS, et al. Relationship between sunitinib effect and immature blood vessel ratio in metastatic renal cell cancer. Eur Urol Suppl. 2013;12:e986. [Google Scholar]
- 34.Miyake H, Sakai I, Fujisawa M. Acquired resistance to sunitinib in human renal cell carcinoma cells is mediated by constitutive activation of signal transduction pathways associated with tumour cell proliferation. Eur Urol Suppl. 2013;12:e983. doi: 10.1111/j.1464-410X.2012.11655.x. [DOI] [PubMed] [Google Scholar]
- 35.Khan G, Golshayan A, Elson P, Wood L, Garsia J, Bukowski R, et al. Sunitinib and sorafenib in metastatic renal cell carcinoma patients with renal insufficiency. Ann Oncol. 2010;21:1618–1622. doi: 10.1093/annonc/mdp603. [DOI] [PubMed] [Google Scholar]
- 36.Eisen T, Joensuu H, Nathan PD, Harper PG, Wojtukiewicz MZ, Nicholson S, et al. Phase II study of BAY 73–4506, a multikinase inhibitor, in previously untreated patients with metastatic or unresectable renal cell cancer. J Clin Oncol. 2009;27:5033–5033. [Google Scholar]
- 37.Eisen T, Joensuu H, Nathan PD, Harper PG, Wojtukiewicz MZ, Nicholson S, et al. Regorafenib for patients with previously untreated metastatic or unresectable renal–cell carcinoma: a single–group phase 2 trial. Lancet Oncol. 2012;13:1055–1062. doi: 10.1016/S1470-2045(12)70364-9. [DOI] [PubMed] [Google Scholar]
- 38.Mulders P, Hawkins R, Nathan P, De Jong I, Osanto S, Porfiri E, et al. Final results of a phase II randomised study of cediranib (RECENTIN) in patients with advanced renal cell carcinoma (RCC) Eur J Cancer. 2009;21:49LBA. doi: 10.1016/j.ejca.2011.12.022. Abstr. [DOI] [PubMed] [Google Scholar]
- 39.Eisen T, Shparyk Y, Jones R, MacLeod NJ, Temple G, Finnigan H, et al. Phase II efficacy and safety study of nintedanib versus sunitinib in previously untreated renal cell carcinoma (RCC) patients. J Clin Oncol. 2013;31(Suppl):4506. Abstr. [Google Scholar]
- 40.Escudier B, Negrier S, Gravis G. “Can the combination of temsirolimus and bevacizumab improve the treatment of metastatic renal cell carcinoma (mRCC)?”. J Clin Oncol. 2010;28(15 Suppl):4516. Results of the randomized TORAVA phase II trial / ASCO Annual Meeting, June 4–8, McCormic Place, Chicago, Illinois, USA, Abstr. [Google Scholar]
- 41.Dutcher JP, de Souza P, McDermott D, Figlin RA, Berkenblit A, Thiele A, et al. Effect of temsirolimus versus interferon–alpha on outcome of patients with advanced renal cell carcinoma of different tumour histology. Med Oncol. 2009;26:202–209. doi: 10.1007/s12032-009-9177-0. [DOI] [PubMed] [Google Scholar]
- 42.Harada K. Acquired resistance to temsirolimus in human renal cell carcinoma cells is mediated by constitutive activation of signal transduction pathways through mammalian target of rapamycin complex–2. Eur Urol Suppl. 2013;12:e985. doi: 10.1038/bjc.2013.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Motzer R, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double–blind, randomized, placebo–controlled phase III trial. The Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 44.Casciano R, Malangone E, Sherman S, Baladi J, Kay AC, Kim D, et al. An indirect comparison of everolimus and sorafenib therapy in sunitinib–refractory mRCC patients; ASCO Annual Meeting; 2010. p. 4611. Abstr. [DOI] [PubMed] [Google Scholar]
- 45.Jozwiak S, Belousova E, Kingswood C, Frost M, Kuperman R, Bebin M, et al. The effect of everolimus on renal angiomyolipoma in patients with tuberous sclerosis complex being treated for subependymal giant cell astrocytoma. Eur Urol Suppl. 2012;11:e74. [Google Scholar]
- 46.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. I Clin Oncol. 1999;17:2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 47.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Renal Cell Carcinoma, v.1.2010. (Available at http://www.nccn.org) [DOI] [PubMed]
- 48.Sparks CA, Guertin DA. Targetting mTOR: prospects for mTOR complex 2 inhibitors in cancer therapy. Oncogene. 2010;29:3733–3744. doi: 10.1038/onc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ingels A, Thong A, Saar M, Valta MP, Nolley R, Santos J, et al. Pre–clinical trial of a new dual mTOR inhibitor: INK128 for renal cell carcinoma. Eur Urol Suppl. 2013;12:e97. [Google Scholar]
- 50.Sato A, Asano T, Ito K, Asano T. Panobinostat and bortezomib inhibit renal cancer growth in vitro and in vivo by inducing endoplasmic reticulum stress and ubiquitinated protein accumulation synergistically. Eur Urol Suppl. 2013;12:e976. [Google Scholar]
- 51.Logan T. A phase I/II study of AGS–003, a personalized immunotherapeutic evaluated in newly diagnosed metastatic renal cell carcinoma (mRCC) subjects; ASCO Genitourinary Cancers Symposium; 2010. p. 379. Abstr. [Google Scholar]
- 52.Escudier B, Goupil M.G, Massard C, Fizazi K. Sequential therapy in renal cell carcinoma. Cancer. 2009;115(Suppl):2321–2326. doi: 10.1002/cncr.24241. [DOI] [PubMed] [Google Scholar]
- 53.Motzer RJ, Hutson TE, Reeves J, Hawkins R, Guo J, Nathan P, et al. Randomized, open–label, phase III trial of pazopanib versus sunitinib in first–line treatment of patients with metastatic renal cell carcinoma (mRCC): results of the COMPARZ trial. Ann Oncol. 2012:238 PR. [Google Scholar]
- 54.Kondo T, Hashimoto Y, Kobayashi H, Iizuka J, Nishikawa T, Nakano M, et al. Presurgical Targeted Therapy with Tyrosine Inhibitor for Advanced Renal Cell Carcinoma: Clinical Results and Histopathological Therapeutics Effects. Jpn J Clin Oncol. 2010;40:1173–1179. doi: 10.1093/jjco/hyq150. [DOI] [PubMed] [Google Scholar]
- 55.Silberstein JL, Millard F, Mehrazin R, Kopp R, Bazzi W, DiBlasio CJ, et al. Feasibility and efficacy of neoadjuvant sunitinib before nephron–sparing surgery. BJU Int. 2010;106:1270–1276. doi: 10.1111/j.1464-410X.2010.09357.x. [DOI] [PubMed] [Google Scholar]
- 56.Kroon BK, De Bruin R, Prevoo W, Horenblas S, Bex A. Primary tumour downsizing in renal cell carcinoma is more prominent in smaller tumours and may enable nephron sparing strategies. Eur Urol Suppl. 2012;11:e78. [Google Scholar]
- 57.Karakiewicz PI, Suardi N, Jeldres C, Audet P, Ghosn P, Patard JJ, et al. Neoadjuvant sutent induction therapy may effectively down–stage renal cell carcinoma atrial thrombi. Eur Urol. 2008;53:845–848. doi: 10.1016/j.eururo.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 58.Cost NG, Delacroix SE, Sleeper JP, Smith PJ, Youssef RF, Chapin BF, et al. The impact of targeted molecular therapies on the level of renal cell carcinoma vena caval tumour thrombus. Eur Urol. 2011;59:912–918. doi: 10.1016/j.eururo.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 59.Jacqmin D. Timing of cytoreductive nephrectomy prior or after molecular targeted therapy? // 7th Meeting of EAU Section of Oncological Urology (ESOU); 2010. p. 53. [Google Scholar]
- 60.Kłącz J, Matuszewski M, Michajłowski J, Zachalski W, Markuszewski M, Krajka K. There is no place for targeted therapy neoadjuvant treatment in Polish Health System – An analysis of radical nephrectomies in patients with large kidney tumours Cent. Eur J Urol. 2013;66:31–35. doi: 10.5173/ceju.2013.01.art9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amin A, Dudek A, Logan T, Lance RS, Holzbeierlein JM, Williams WL, et al. A Phase II Study Testing the Safety and Activity of AGS–003 as an Immunotherapeutic in Subjects with Newly Diagnosed Advanced Stage Renal Cell Carcinoma (RCC) in Combination with Sunitinib. J Clin Oncol. 2010;(28 Suppl):4588. Abstr. [Google Scholar]
- 62.Belldegrun AS, Chamie K, Kloepfer P, Fall B, Bevan P, Störkel S, et al. ARISER: A randomized double blind phase III study to evaluate adjuvant cG250 treatment versus placebo in patients with high–risk ccRCC – Results and implications for adjuvant clinical trials. J Clin Oncol. 2013;(31 Suppl):4507. Abstr. [Google Scholar]
- 63.Ravasio R, Ortega C, Sabbatini R, Porta C. Bevacizumab plus interferon–α versus sunitinib for first–line treatment of renal cell carcinoma in Italy: a cost–minimization analysis. Clin Drug Investig. 2011;31:507–517. doi: 10.2165/11590230-000000000-00000. [DOI] [PubMed] [Google Scholar]