Abstract
We present a case of a 40–year old woman diagnosed with a four–place spontaneous paraganglioma–pheochromocytoma syndrome, which was treated surgically. The presence of the succinate dehydrogenase complex subunit D (SDHD) mutation that causes the pheochromocytoma was confirmed but no mutations in the family members were found. After the excision of the paragangliomas located in the areas of the division of carotid arteries, and mediastinum, as well as a tumor on the left site of the celiac trunk, the patient remains asymptomatic and is regularly followed–up.
Keywords: paraganglioma, pheochromocytoma, SDHD mutation
CASE REPORT
A 40–year old female patient presented with lymphadenopathy of the neck with suspicion of malignancy. Thorough diagnostics were engaged in the internal medicine clinic. The computed tomography revealed the following: abnormal masses in the area of the division of the carotid arteries; an abnormal lesion of 40 mm in diameter in the chest, located subcarinally; and tumors of 20 mm in diameter in the left adrenal gland and another in the proximity to the celiac trunk. In order to make a differential diagnosis of the lesions found in the computed tomography, a magnetic resonance of the whole body was performed. In the study of the neck, the lesions of paragangliomatic character were found in the area of the divisions of both carotid arteries, of 38 mm in diameter on the left and of 18 mm on the right. Subcarinally, in the posterior mediastinum of the chest, a lesion of 40 mm in diameter was detected in the enhancement which was suggestive of paranganglioma presence. In the abdominal cavity, the adenoma of 20 mm in diameter in the left adrenal gland, as well as the 20 mm lesion located near the celiac trunk in the enhancement, were both suggestive of paranganglioma presence. The positron emission tomography–computed tomography (PET–CT) study revealed metabolically active masses in the area of the division of left carotid artery, subcarinally in the posterior mediastinum and in the area of the celiac trunk. Simultaneously with the diagnostic imaging, biochemical tests were performed. Consecutive measurements of concentration of metoxycatecholamine in 24 h urine sampling revealed results within normal limits. Furthermore, adrenaline and dopamine serum levels, as well as their urine levels were normal in 4 consecutive tests. The only abnormality found was the elevation of noradrenaline serum and urine concentration within three times the upper limits of normal in consecutive tests. As a consequence, the patient was started on low doses of alpha and beta–blockers. Additionally, genetic tests were conducted in order to exclude the presence of mutation within the locus of RET proto–oncogene and succinate dehydrogenase complex subunit D (SDHD), succinate dehydrogenase complex subunit C (SDHC), succinate dehydrogenase complex subunit B (SDHB) and succinate dehydrogenase complex subunit A (SDHA) genes. The tests revealed the existence of mutation in SDHD gene that causes a hereditary form of pheochromocytoma. There was additional genetic testing in the patient's parents conducted and no mutations were found. Furthermore, the tests were carried out in both patients’ daughters and, again, they were negative as far as the mutations mentioned above are concerned. As a result, the mutation present in the patient was proven to be spontaneous.
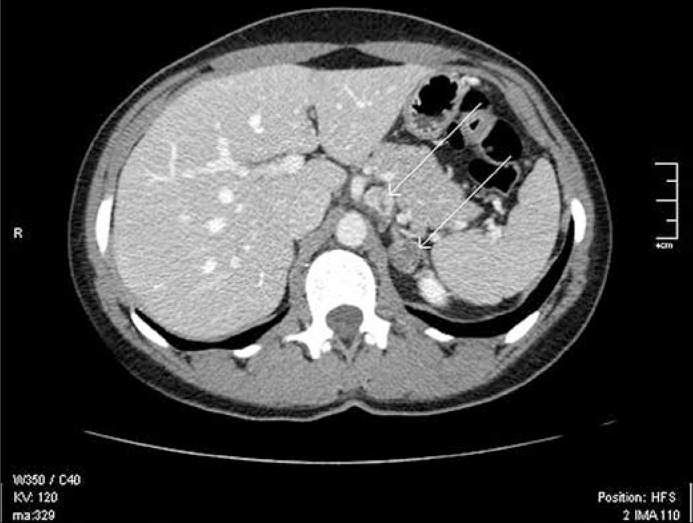
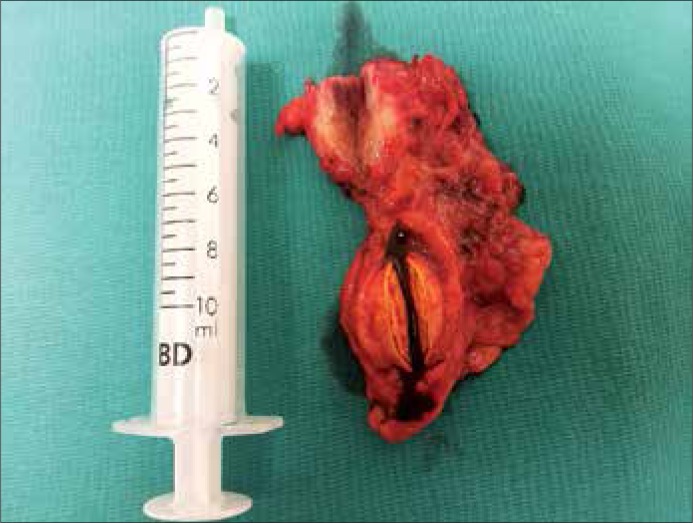
After the completion of diagnostics and interdisciplinary consultations, the patient was qualified for surgical removal of the lesions located close to the carotid arteries. The procedure was performed in two stages; one in the department of vascular surgery in April 2012 and the other in May 2012. Firstly, the larger tumor on the left side was excised then the contralateral, smaller one. No significant intra– and postoperative complications were observed. In both cases, pathological examinations revealed synaptophysin– and chromogranine–positive paragangliomas. In August 2012, the patient was qualified for the surgical removal of the mediastinal tumor. The procedure was conducted via right–sided thoracotomy and the tumor was excised completely with the surrounding tissues. No significant intra– and postoperative complications were observed. In the pathological examination, a pheochromocytoma composed of epitheliod cells, with no signs of atypia and mitotic activity was found. Reactive lymphadenopathy in the specimen was also detected. After the patient had recovered, she complained of persistent paroxysmal hypertension. Due to the compaints, the patient's concentrations of metoxycatecholamines in urine and catecholamines in serum were measured twice. The concentration of metoxycatecholamines in urine in both tests did not reach the upper limit, but remained in close proximity to it. However, in regards to catecholamine concentration in serum, levels of noradrenaline were elevated a few times that of the upper limits of normal, with normal levels of other catecholamines. As a result, the patient was qualified for the surgical excision of the tumor of the adrenal gland and the lesion located next to the celiac trunk. Figure 1 presents the localization of the extra–adrenal lesion in relation to the tumor of the adrenal gland based on the CT scan. In December 2012, a left–sided, open adrenalectomy was performed and the tumor located close to the celiac trunk on its left side was removed en bloc simultaneously. The specimen is presented in Figure 2. No complications in the postoperative period were observed. The level of catecholamines in serum and the level of metoxycatecholamines in 24 daily urine samples were within normal limits in four weeks since adrenalectomy. In the pathological examinations, adenoma was found in the adrenal gland, while the tumor close to celiac trunk was found to be a synaptophysin– and chromogranin–positive pheochromocytoma. Furthermore, in the surrounding tissues, few lesions of ganglioneuroma character were found. At present, the patient is asymptomatic and is regularly followed–up.
Figure 1.
The extraadrenal lesion and its relation to the tumor of the left adrenal gland in CT scan (marked with white arrows).
Figure 2.
The postoperative specimen.
DISCUSSION
Paraganglioma–pheochromocytoma syndromes occur rather rarely, but still constitute a serious diagnostic and treating problem. Due to the overproduction of catecholamines, they induce constitutional symptoms and disturbances, which force the patient to seek diagnosis and treatment. However, according to different sources, in 5 to up to 30% of cases in the absence of overproduction of catecholamine, their course is asymptomatic [1–3]. In both groups, a malignant transformation is possible, and, in the case of asymptomatic pheochromocytoma, there is a risk of a significant delay in the establishment of an adequate diagnosis. The efforts aimed at the assessment of potential malignancy of chromaffin cell tumors in the postural assessment scale for stroke patients (PASS) result in only a suggestion of biological aggressive behavior. The only confirmation of malignant potential is the presence of distant metastases [4]. The distant metastases may occur within 6 to up to even 10 years following adrenalectomy, and the frequency is estimated to be between 5 to 26%. Furthermore, the occurrence of the metastases is more likely in the case of paragangliomas. Their frequency reaches 36% [5, 6]. Based on these grounds, one can judge that in nearly no case after the surgery of pheochromocytoma tumors, irrespective of their location, there is certainty as far as their histological character is concerned. Molecular genetic testing in the case of suspicion of the hereditary paraganglioma–pheochromocytoma syndrome comprises the analysis of the SDHA, SDHB, SDHC and SDHD genes which are four nuclear genes encoding the four subunits of the mitochondrial enzyme succinate dehydrogenase [7, 8]. In our case, the mutation in the SDHD gene proved to be spontaneous as no mutation in family members was found.
The surgical excision of the pathological tissue in the syndrome is connected with a positive outcome with virtually no alternative procedure. On the other hand, the multiplicity of the lesions may be discouraging for the surgeon, as there is no guarantee for radical dissection. Many authors are of the opinion that surgical treatment of multiple lesions is totally justified, and, in the majority of cases, is successful [7]. Based on the 30 years of experience of the authors, paragangliomas may occur in various locations, in which physiologically normal ganglion tissue is located. The most common are presentations along the vertebral column, and aortic and carotid glomus. Furthermore, we have diagnosed paragangliomas located in pericardium and various part of mediastinum, which were excised in our department with a good outcome. Disseminated cancer needs interdisciplinary treatment. Provided that it is possibile, one recommends advanced surgery, radiometabolic procedures, chemotherapy and radiotherapy [9]. In hereditary tumors originated form chromaffin tissue, one should hope that one day gene therapy would be available, as the today results of treatment are far from satisfactory [9, 10].
References
- 1.Karcher KW, Park A, Matthews BD, Rolband G, Sing RF, Heniford BT. Surg Endosc. 2002;16:100–102. doi: 10.1007/s00464-001-8171-1. [DOI] [PubMed] [Google Scholar]
- 2.Kim KH, Chung JS, Kim WT, Oh CK, Chae YB, Yu HS, Ham WS, Choi YD. Clinical experiences of pheochromocytoma in Korea. Yonsei Med J. 2011;1:45–50. doi: 10.3349/ymj.2011.52.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szosland K, Kopff B, Lewiński A. Pheochromocytoma–chromaffin cell tumor. Pol J Endocrinol. 2006;1:54–62. [PubMed] [Google Scholar]
- 4.Sporny S, Musiał J. Markers of malignancy in pheochromocytomas. Pol J Endocrinol. 2005;6:946–951. [PubMed] [Google Scholar]
- 5.Geatti O, Shapiro B, Virgolini L. Late presentation of metastatic pheochromocytoma: a problem case solved by I–131 MIBG scintigraphy. Clin Nucl Med. 1990;15:101–104. doi: 10.1097/00003072-199002000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Szydełko T, Lewandowski J, Panek W, Tupikowski K, Dembowski J, Zdrojowy R. Laparoscopic adrenalectomy – ten–year experience. Centr Eur J Urol. 2012;65:71–74. doi: 10.5173/ceju.2012.02.art3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matro J, Giubellino A, Pacak K. Current and future therapeutic approaches for metastatic pheochromocytoma and paraganglioma: focus on SDHB tumors. Horm Metab Res. 2013;45:147–153. doi: 10.1055/s-0032-1331211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vicha A, Musil Z, Pacak K. Genetics of pheochromocytoma and paraganglioma syndromes: new advances and future treatment options. Curr Opin Endocrinol Diabetes Obes. 2013;20:186–191. doi: 10.1097/MED.0b013e32835fcc45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanabe A, Naruse M, Nomura K, Tsuiki M, Tsumagari A, Ichihara A. Combination chemotherapy with cyclophosphamide, vincristine and decarbazine in patients with malignant pheochromocytoma and paraganglioma. Horm Cancer. 2013;4:103–110. doi: 10.1007/s12672-013-0133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grubbs EG, Rich TA, Ng C, Bhosale PR, Jimenez C, Evans DB, et al. Long–term outcomes of surgical treatment for hereditary pheochromocytoma. J Am Coll Surg. 2013;216:280–289. doi: 10.1016/j.jamcollsurg.2012.10.012. [DOI] [PubMed] [Google Scholar]