Abstract
Background
Tagged cardiac magnetic resonance (CMR) provides detailed information on regional myocardial function and mechanical behavior. T1 mapping by CMR allows non-invasive quantification of myocardial extracellular expansion (ECE) which has been related to interstitial fibrosis in previous clinical and sub-clinical studies. We assessed gender associated differences in the relation of ECE to LV remodeling and myocardial systolic and diastolic deformation in a large community based multi-ethnic population.
Methods and Results
Mid-ventricular mid-wall peak circumferential shortening and early diastolic strain rate (EDSR); LV torsion and torsional recoil rate were determined using CMR tagging. Mid ventricular short axis T1 maps were acquired in the same examination pre and post-contrast injection using Modified Look-Locker Inversion Recovery sequence (MOLLI). Multivariable linear regression (B= estimated regression coefficient) was used to adjust for risk factors and sub-clinical disease measures. Of 1230 participants, 114 participants had visible myocardial scar by late gadolinium enhancement. Participants without visible myocardial scar (n=1116) had no previous history of clinical events. In the latter group, multivariable linear regression demonstrated that lower post-contrast T1 times, reflecting greater ECE were associated with lower circumferential shortening (B=−0.1, p=0.0001), lower end diastolic volume index (LVEDVi) (B=0.6, p=0.0001) and lower LV end diastolic mass index (LVMi) (B=0.4, p=0.0001). In addition, lower post-contrast T1 times were associated with lower EDSR (B=0.01, p=0.03) in women only; and lower LV torsion (B=0.005, p=0.03) a lower LV ejection fraction (B=0.2, p=0.01) in men only.
Conclusions
Greater ECE is associated with reduced LVEDVi and LVMi in a large multi-ethnic population without history of previous cardiovascular events. In addition, greater ECE is associated with reduced circumferential shortening, lower EDSR, and a preserved ejection fraction in women; while in men, greater ECE is associated with greater LV dysfunction manifested as reduced circumferential shortening, reduced LV Torsion and reduced ejection fraction.
Keywords: interstitial myocardial fibrosis, circumferential strain, LV torsion, T1 mapping, tagging
Cardiac remodeling refers to structural and functional cardiac alterations in response to pathogenic processes and cardiovascular risk factors.1 Previous animal and human studies have shown that in patients with cardiomyopathies of various etiologies, myocardial fibrosis is associated with abnormal cardiac remodeling accompanied by increased ventricular wall stress and stiffness leading to mechanical dysfunction and symptomatic heart failure.2–6 Importantly, the alterations of left ventricular (LV) structure and function associated with LV remodeling and heart failure are gender dependant 7, 8, and represent a major cause of morbidity and mortality in the US. 9 In this context, interstitial fibrosis is a common histological feature underlying LV remodeling and heart failure due to various disease processes. 10
Cardiovascular magnetic resonance (CMR) has emerged as a noninvasive imaging method to assess myocardial structure and function with great level of accuracy and reproducibility.11 LV ejection fraction is used as a global measure of left ventricular performance but it does not take into consideration incipient alterations of myocardial contractile behavior which are commonly seen early in several cardiovascular disorders. CMR tissue tagging provides precise quantification of incipient myocardial dysfunction through the assessment of myocardial strain and torsion.12 Harmonic Phase (HARP) analysis of tagged MRI images is currently the most widely used method for analysis of tagged images particularly in population studies, with good inter and intra observer agreement. 13
The evaluation of fibrosis by MRI is best assessed after injection of gadolinium contrast agents that are employed to reduce the T1 relaxation time of myocardial tissue, generating specific differences of regional signal intensity. 10, 14 In the absence of confounding conditions such as myocardial edema due to inflammation or amyloidosis, myocardial extracellular expansion (ECE) results from accumulation of excess collagen in the interstitium. Although late gadolinium enhanced (LGE) CMR allows for the assessment of macroscopic replacement myocardial fibrosis, it is limited for the evaluation of diffuse interstitial fibrosis.
T1 myocardial mapping using the Modified Look-Locker Inversion Recovery (MOLLI) sequence with high spatial resolution enables direct myocardial signal quantification, characterization of myocardial tissue composition and assessment of interstitial fibrosis with good reproducibility. 15, 16 Altered T1 values have been shown to be associated with diffuse myocardial fibrosis in ischemic and non ischemic cardiomyopathies.5 These gadolinium contrast based changes in myocardial T1 times correlate with histological evidence of fibrosis even in the absence of CMR LGE defined scar. 5
However, it is not clear how ECE relates to LV remodeling and affects myocardial mechanical behavior and cardiac performance among individuals without history of previous cardiovascular events. No studies have yet been performed to determine the relationship between interstitial myocardial fibrosis measured by T1 myocardial mapping and myocardial systolic and diastolic function in a large population study using contrast enhanced CMR and myocardial tagging. In the current study, we aim to determine the relationship between interstitial myocardial fibrosis and deformation relative to LV remodeling, in a large multi-ethnic cohort. We also aim to determine whether gender associated alterations in LV structure and function parallel gender related differences in interstitial myocardial fibrosis in a multi-ethnic population free of heart disease.
Methods
Study Population
MESA is a prospective, population based, epidemiological study started in the year 2000 to investigate the prevalence and progression of subclinical cardiovascular disease in a multi-ethnic cohort (Caucasian, African-American, Hispanic, Chinese). The study protocol was approved by Institutional Review Board of participating institutions. The characteristics of subjects enrolled in MESA have been described previously.17 In this ancillary study, after obtaining informed consent, 1230 participants had simultaneous contrast enhanced CMR and tagging during the period of 2010 to 2012 at five different centers: Wake Forest University, Winston Salem, NC; Johns Hopkins University, Baltimore, MD; University of Minnesota, Minneapolis; Northwestern University, Chicago, IL and UCLA, Los Angeles, CA, USA.
CMR Imaging
Cardiac MR Images were obtained with 1.5 T MR Systems (Avanto, Siemens Medical Solutions, Germany). Intravenous infusion of gadolinium contrast (gadopentate dimeglumine, Bayer healthcare pharmaceuticals, New Jersey, USA) was performed at 0.15mmol/kg bolus dose. After acquisition of standard scout image, two and four chamber cine images were acquired. Short axis cine images were then obtained with retrospective gating from above the mitral valve plane to the LV apex. Fifteen minute gadolinium enhancement imaging was acquired in three long-axis slices and a stack of short-axis slices using a gradient recalled echo phase-sensitive inversion recovery sequence. LV structural measures (LV mass and volumes) and LV ejection fraction were measured using commercially available software (CIM v6.2, Auckland, New Zealand).18 LV mass and LV end diastolic volumes were indexed to body surface area (LVMi and LVEDVi). Papillary muscles were excluded from LV mass calculations and included in LV end diastolic volume calculations.
T1 Mapping
In T1 myocardial mapping, after a preparation pulse sequence, signal recovery from each voxel is sampled during multiple measurements. The associated T1 relaxation time is calculated for each pixel and a parametric image is reconstructed, which is referred to as the T1 map. Standard four-chamber long-axis and midcavity short-axis section orientations were used for MOLLI T1 mapping. The MOLLI single slice T1 determinations were performed pre-contrast, 12 min and 25 min post-contrast injection using three inversion-recovery pulses. We acquired a set of 11 consecutive source images at mid-ventricle within one breath hold (17 heart beats), allowing for the reconstruction of one parametric T1 map. 16 All source images have identical voxel sizes, image position and phase of cardiac cycle except for different effective inversion times (TI). Scan parameters for MOLLI were: Field of view 360 × 360 mm2, flip angle 35°, repetition time (TR) 3.9ms, Echo time (TE) 1.95ms, matrix size 256 × 192, slice thickness 8mm.
MOLLI data was processed offline using the MASS research software (MASS V2010-EXP, Leiden University Medical Center, Leiden, The Netherlands) with Levenberg-Marquardt fitting algorithm 16. Left ventricular endocardial and epicardial borders were traced semi-automatically on all phases in each sequence to extract the mean T1 values. Care was taken to exclude epicardial structures and blood pool from contours. Intra and inter reader agreement of myocardial T1 times was excellent, with Intra-class correlation coefficient (ICC) ranging from 0.98 to 0.99. 19 Precontrast T1 times were negatively correlated with 12 and 25 minute post-contrast T1 times (r= −0.084 and −0.055, p<0.05 respectively) while 12 minute post-contrast T1 time was significantly correlated with 25 minute post-contrast T1 time (r=0.905, p<0.05)
CMR Tagging
Tagged images were acquired using a segmented k-space; electrocardiogram gated fast low angle shot (FLASH) pulse sequence. Three tagged short-axis slices were obtained (base to apex) with two orthogonally oriented parallel striped tags (0 ° and 90 °) using spatial modulation of magnetization (SPAMM). Parameters for tagged images were: field of view 360 × 360 mm2, slice thickness 10mm, Echo time 2.5ms, flips angle 10, matrix size 256 × 128, 9 phase encoding views per segment, spatial resolution 1.4 × 2.8 × 10 mm3, temporal resolution 25ms, tag spacing 7mm. Tagged short-axis slices were analyzed by HARP software (Diagnosoft, Palo Alto, CA) 20. Average peak mid-ventricular mid-wall circumferential shortening (Ecc, %) and early diastolic strain rate (EDSR, s); LV torsion (with and without adjusting for epicardial radius), torsional recoil rate (TRR °/cm/ms) and LV Torsion to endocardial circumferential shortening ratio (TSR)21 were determined. Ecc is a negative number and more negative values indicate greater circumferential shortening. Average peak Ecc was calculated by averaging the corresponding mid-ventricular peak segmental strain values from the 6 segments of the AHA 16-segment model. 22 LV Torsion (rad) was defined as defined by Russel et al (φ-apex * r-apex - φ-base * r-base)/(distance between apical and basal slices); where φ is rotation and r is radius. 23 Intra- and interobserver reproducibility of Ecc and LV Torsion was excellent with and ICC ranging from 0.8 to 0.9. 24
Statistical Analysis
All continuous variables are presented as mean ± standard deviation and categorical variables as frequencies and percentages. Comparisons between the participants with and without visible scar were performed using t-tests and chi-square tests for continuous variables and categorical variables respectively. Multivariable linear regression analyses (B= Estimated regression coefficient) were performed to evaluate the effect of covariates including demographic and traditional risk factors. ECM expansion was assessed using pre and post-contrast times (12 and 25 min) separately as independent variables. Model 1 reflected (Univariate) unadjusted relations of myocardial fibrosis to measures of LV structure (LV mass, LV End diastolic volume) and function (LV EF, Ecc, EDSR, LV torsion, TRR and TSR). Model 2 was adjusted for age, gender, ethnicity, body surface area, heart rate, Glomerular filtration rate (GFR), systolic and diastolic blood pressure, anti hypertensive medication use, history of diabetes mellitus and current smoking status. Model 3 was adjusted for LV mass and end diastolic volume in addition to model 2 covariates. 8 participants had missing covariates data. Because of the design of study, the missing covariates were assumed to be missing completely at random. These missing participants were excluded for the models (model 2 and 3) they were missing. As there was a significant gender interaction of association of post contrast T1 times with EDSR, LV Torsion, TSR and LV ejection, gender specific analysis was repeated. A two-tailed p value of <0.05 was considered statistically significant. All statistical analyses were performed using STATA v11.0 (Stata Corp LP, College Station, TX, USA)
Results
MESA Participant Characteristics
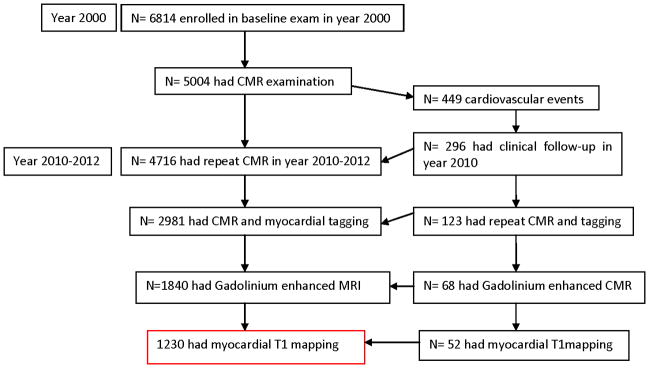
MESA is a prospective, population based, epidemiological study started in the year 2000 to investigate the prevalence and progression of subclinical cardiovascular disease in a multi-ethnic cohort. Most participants who had an interim event during the MESA follow-up did not consent for a gadolinium enhanced CMR examination in this current study (n=381) (Figure 1). The participants who had an interim cardiovascular event diagnosed elsewhere, but did not have a visible myocardial scar by LGE were excluded from the study (n=3). The purpose of this study design is to compare participants without any history of clinical cardiovascular events, with patients, who have evidence of symptomatic or asymptomatic visible myocardial replacement scar.
Figure 1. Participant enrollment for the current study.
Baseline demographics, clinical and CMR parameters of the study participants are shown in Table 1. Of the 1230 participants, 114 (85.2% men) had visible myocardial scar as detected by delayed gadolinium enhancement images. Of these 114 participants with LGE defined myocardial scar, 18 had known myocardial infarction, 31 had history of unstable angina, and 11 had Congestive heart failure, 30 had percutaneous coronary intervention and 13 participants had coronary artery bypass graft (CABG). Participants with visible myocardial scar were older (71.5 vs. 67.2 yrs, p=0.0001), had significantly higher body surface area (1.9m2 vs. 1.8m2 p=0.0001) and lower GFR (80.6 vs. 84.7 mL/min/1.73m2, p= 0.02) compared to the group without visible scar. There were no significant differences in heart rate, systolic or diastolic blood pressure between the two groups. A higher percentage of participants in the group with visible scar had history of diabetes (20.9% vs. 13.8%, p=0.001), were on antihypertensive medication (64.3% vs. 48.8%, p=0.002) and were current smokers (13.2% vs. 6.8%, p=0.01) compared to the group without LGE defined scar.
Table 1.
Baseline demographics: Mean (standard deviation)
| No scar (1116) | Scar (114) | |
|---|---|---|
| Age, yrs | 67.2(8.7) | 71.5 (9.3) † |
| Men, n (%) | 533 (47.8%) | 98 (85.2%) † |
| Ethnicity, n (%) | ||
| White Caucasian | 579 (51.9%) | 66(57.4%) |
| Chinese American | 129 (11.6%) | 5 (4.3%) |
| African American | 250 (22.4%) | 31 (27.0%) |
| Hispanic | 158 (14.3%) | 13 (11.3%) |
| Heart rate (beats/min) | 66.3 (10.8) | 64.4 (10.7) |
| Body surface area (m2) | 1.8 (0.2) | 1.9 (0.2) † |
| SBP (mmHg) | 121.1 (18.8) | 123.6 (18.3) |
| DBP (mmHg) | 68.3 (9.6) | 69.2 (10.4) |
| GFR (mL/min/1.73m2) | 84.7 (18.8) | 80.6 (17.3) † |
| Diabetes Mellitus, n (%) | 153 (13.8%) | 24 (20.9%) † |
| Anti hypertensive medication, n (%) | 545 (48.8%) | 74 (64.3%) † |
| Current Smoker, n (%) | 76 (6.8%) | 15 (13.2%) † |
| LV Ejection Fraction % | 62.2 (6.6) | 56.8 (9.7) † |
| LVEDVi (mL/m2) | 65.4 (12.8) | 70.0 (19.8) † |
| LVMi (g/m2) | 64.8 (12.1) | 78.2 (15.8) † |
| Pre Contrast T1, ms | 975.8 (42.8) | 985.5 (41.4) † |
| Post contrast T1 @12 min (ms) | 454.6 (40.1) | 444.2 (39.7) † |
| Post Contrast T1@25 min (ms) | 518.3 (40.5) | 508.4 (42.4) † |
| Ecc (%) | −15.5 (3.2) | −13.6 (3.3) † |
| EDSR (1/s) | 1.04 (0.4) | 0.86 (0.4) † |
| LV Torsion (rad) | 8.7 (2.04) | 8.9 (2.04) |
| TRR (°/cm/ms) | −23.2 (8.8) | −20.1 (8.1) † |
| TSR | −0.56 (0.16) | −0.65 (0.19) † |
SBP=Systoli c blood pressure, DBP= Diastolic blood pressure, GFR= Glomerular filtration rate, LVEDVi=LV End diastolic volume index, LVMi= LV mass index, Ecc=Circumferential strain, EDSR= Early diastolic strain rate, TRR= Torsional recoil rate, LV Torsion (rad) =LV Torsion adjusted for epicardial radius, TSR= Torsion to endocardial shortening ratio.
Two tailed p value < 0.05.
LV structure and function in participants without LGE defined myocardial scar
In participants without LGE defined myocardial scar (n=1116), lower 12 minute and 25 minute post-contrast T1 times (greater ECE) were associated with lower circumferential shortening, but without any change in LV ejection fraction in univariate regression analyses. Moreover, lower post-contrast T1 times were associated with both lower LVEDVi and LVMi (Table 2). After adjustment for traditional cardiovascular risk factors, the associations of lower post-contrast T1 times were significant with lower circumferential shortening (B= −0.111, SE=0.02, p<0.001); lower LV ejection fraction (B=0.135, SE=0.05, p<0.05), lower LVEDVi (B=0.623, SE=0.09, p<0.05) and LVMi (B=0.496, SE=0.07, p<0.05) (Table 2). Moreover, the associations of post contrast T1 measures with lower circumferential shortening persisted even after adjustment for measures of LV remodeling such as LV end diastolic mass and volume. However, post contrast T1 times were associated with an unaltered LV Torsion to endocardial circumferential shortening ratio (TSR) in all the models (B=−0.0002, SE=0.0001, p=0.6 for model 3).
Table 2.
Association of post contrast T1 times with LV structural and functional measures in participants without LGE scar: Estimated Regression coefficient (standard error in parenthesis)
| 12 min | 25 min | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| R2 | Model 1 | Model 2 | Model 3 | R2 | Model 1 | Model 2 | Model 3 | |
| Ecc | 0.27 | −0.082 (0.02) ** | −0.111 (0.02) ** | −0.101 (0.02)** | 0.28 | −0.095 (0.02) ** | −0.122 (0.02) ** | −0.125 (0.02)** |
| EDSR | 0.13 | 0.004 (0.003) | 0.006 (0.003)* | 0.007 (0.003)** | 0.13 | 0.003 (0.003) | −0.003 (0.003) | 0.004 (0.003) |
| Torsion | 0.12 | −0.001 (0.001) | 0.001 (0.001) | 0.002 (0.001) | 0.11 | −0.001(0.001) | 0.001 (0.001) | 0.002 (0.001) |
| TRR | 0.11 | −0.032 (0.06) | −0.101 (0.07) | −0.151 (0.07)* | 0.11 | −0.006 (0.06) | −0.066 (0.07) | −0.113 (0.07) |
| LV EF | 0.12 | −0.007 (0.04) | 0.135 (0.05) * | - | 0.12 | −0.037 (0.04) | 0.094 (0.05)^ | - |
| EDVi | 0.22 | 0.813 (0.09) ** | 0.623 (0.09) ** | - | 0.21 | 0.748 (0.09) ** | 0.512 (0.09) ** | - |
| LVMi | 0.48 | 0.993 (0.08) ** | 0.496 (0.07) ** | - | 0.48 | 0.997 (0.08) ** | 0.509 (0.07) ** | - |
=p<0.001,
=p<0.05, p=0.06
The Coefficient represents the change in LV measures per 10ms change in post contrast T1 time, with adjustments for multiple variables.
Model 1: Unadjusted
Model 2: Adjusted for age, gender, race, BSA, GFR, heart rate, SBP, DBP, Anti hypertensive medication use, History of Diabetes, Smoking status.
Model 3: Adjusted for age, gender, race, BSA, GFR, heart rate, SBP, DBP, Anti hypertensive medication use, History of Diabetes, Smoking status, LV end diastolic mass and volume.
R2: R-squared values for model 3 (for model 2 if model3 is not available)
EDSR= Early diastolic strain rate, LV Torsion =LV Torsion adjusted for epicardial radius, TRR= Torsional recoil rate, BSA=Body Surface Area, LV EF=LV Ejection fraction, LVEDVi=LV End diastolic volume index, LVMi= LV mass index, SBP= Systolic blood pressure, DBP= Diastolic blood pressure, GFR= Glomerular Filtration rate.
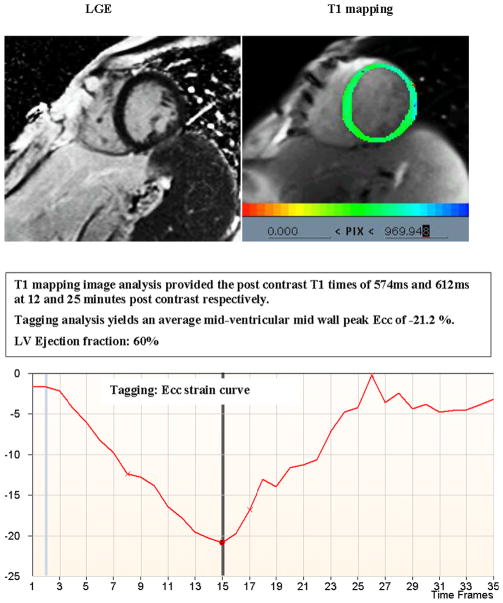
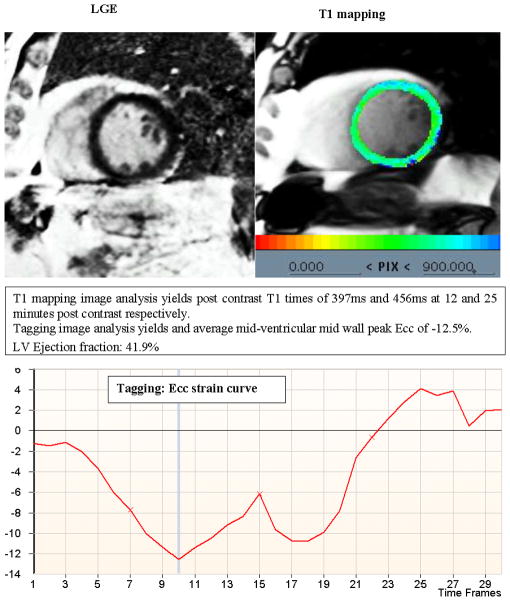
Figure 2 demonstrates the T1 mapping results and corresponding CMR tagging images from a participant with no evidence of extracellular expansion (ECE) and normal circumferential strain reflected by more negative Ecc. Conversely, Figure 3 demonstrates a similar dataset from a participant with shorter post-contrast T1 time indicating greater ECE, in the absence of LGE defined myocardial scar, and less negative Ecc indicating lower levels of systolic myocardial deformation.
Figure 2. Delayed Gadolinium enhanced image (LGE), T1 mapping parametric image and corresponding CMR tagging strain curve from a participant without LGE defined myocardial scar and no evidence of extracellular expansion.
A normal circumferential strain is reflected by more negative Ecc.
Figure 3. Delayed Gadolinium enhanced image (LGE), T1 mapping parametric image and corresponding CMR tagging strain curve from a participant without LGE defined myocardial scar with evidence of extracellular expansion.
Shorter post-contrast T1 time indicates greater ECE in the absence of LGE defined myocardial scar and a less negative Ecc indicates lower levels of systolic myocardial deformation.
The relationships for pre-contrast T1 times were however different than those found for post-contrast T1 times. Greater pre-contrast T1 time was associated with greater as opposed to lesser circumferential shortening (Supplemental Table 1). After adjustment for traditional cardiovascular risk factors, pre-contrast T1 associations remained significant for greater circumferential shortening (B=−0.082, SE=0.02, p<0.001). LV ejection fraction, LVMi and LVEDVi were unrelated to pre-contrast T1 times after adjustment for traditional cardiovascular risk factors. The associations of greater pre-contrast T1 times with greater Ecc (B=−0.074, SE=0.01, p<0.001) persisted after adjusting for LV end diastolic mass and volume.
Sex specific changes in LV structure and function in participants without LGE defined myocardial scar
In participants without LGE defined myocardial scar, compared to men, women had greater LVEF (64.1±6.2 vs. 60.1±6.5 in men, p=0.0001), greater circumferential shortening (−16.06±3.2 vs. −15.04±3.1 in men, p=0.0001), higher diastolic function (0.11±0.04 vs. 0.09±0.03 in men, p=0.0001), greater LV torsion (4.6±1.2 vs. 3.8±1.1 in men, p=0.0001) greater LV torsion (rad) adjusted for epicardial radius (9.1±1.9 vs. 8.3±2.1 in men, p value=0.0001), greater TRR (−25.1±9.2 vs. −21.1±7.9 in men, p=0.0001), greater TSR (0.57±0.17 vs. −0.54±0.15 in men (p=0.003), lower LVMi (58.1±8.6 vs. 72.1±11.1 in men, p=0.0001, lower LVEDVi (62.2±10.7 vs. 68.8±13.8 in men, p=0.0001)
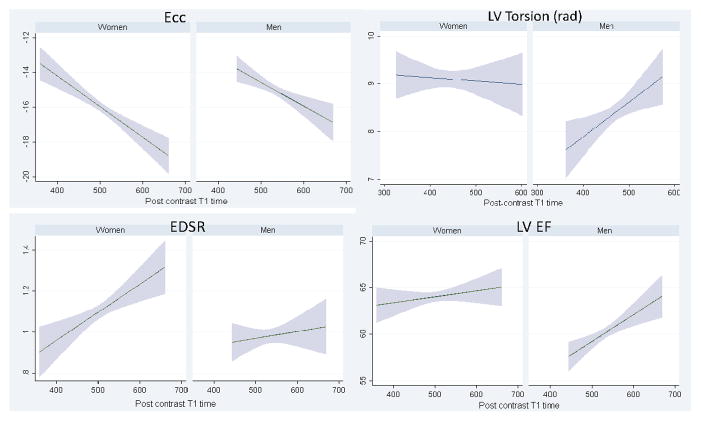
Multivariable analyses demonstrated sex specific differences in the associations between indices of ECE and measures of myocardial deformation among MESA participants without clinical events or visible myocardial scars (Table 3). In men, lower 12 min and 25 min post-contrast T1 times (greater ECE) were associated with lower circumferential shortening (B=−0.096, SE=0.03, p<0.05), lower LV Torsion (rad) (B=0.005, SE=0.002, p<0.05) and lower LV ejection fraction (B=0.248, SE=0.08, p<0.05) after adjustment for traditional risk factors. In women, lower 12 min and 25 min post-contrast T1 times were associated with lower circumferential shortening (B=−0.132, SE=0.03, p<0.05) and lower EDSR (B=0.009, SE=0.004, p<0.05) but LV ejection fraction remained unchanged after adjustment for traditional risk factors (Figure 4). Without adjustment for epicardial radius, lower post-contrast T1 times were associated with increased LV torsion in women (B=−0.002, SE=0.001, p<0.05 in model 2) while unchanged in men. Also, lower post-contrast T1 times were associated with lower LVEDVi and LVMi in both men and women. Moreover, after further adjustment for LV end diastolic mass and volume, the associations of lower 12 min and 25 min post-contrast T1 times remained significant for lower circumferential shortening in both men and women. In addition, lower post-contrast T1 time was associated with lower EDSR in women and lower LV Torsion (rad) in men. However, there was no significant association between TSR and post contrast T1 times (B=−0.0007, p=0.3 and B=0.0001, p=0.8 for men and women respectively in model 3)
Table 3.
Gender specific changes in LV structure and function with post-contrast T1 times in participants without LGE scar: Estimated Regression Coefficient (Standard Error in parenthesis).
| 12 min post | 25 min post | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Women | ||||||||
|
| ||||||||
| R2 | Model 1 | Model 2 | Model 3 | R2 | Model 1 | Model 2 | Model 3 | |
| Ecc | 0.25 | −0.151 (0.03) ** | −0.132 (0.03)** | −0.126 (0.03) ** | 0.26 | −0.175 (0.03) ** | −0.155 (0.03) ** | −0.154 (0.03) ** |
| EDSR | 0.11 | 0.013 (0.004)** | 0.009 (0.004)* | 0.013 (0.004)* | 0.11 | 0.013 (0.004)* | 0.009 (0.004)* | 0.01 (0.0004)* |
| Torsion | 0.15 | −0.0007 (0.002) | −0.0005 (0.002) | 0.0009 (0.002) | 0.15 | −0.001 (0.002) | 0.0009 (0.002) | 0.0002 (0.002) |
| TRR | 0.09 | −0.277 (0.09)* | −0.088 (0.1) | −0.212 (0.1) | 0.09 | −0.221 (0.09) * | −0.035 (0.1) | −0.146 (0.1) |
| LV EF | 0.02 | 0.116 (0.06) | 0.086 (0.06) | - | 0.02 | 0.064 (0.06) | 0.028 (0.07) | - |
| EDVi | 0.21 | 0.575 (0.1) ** | 0.683 (0.1) ** | - | 0.20 | 0.585 (0.1)** | 0.642 (0.1) ** | - |
| LVMi | 0.28 | 0.410 (0.08)** | 0.544 (0.08) ** | - | 0.28 | 0.417 (0.08)** | 0.532 (0.08) ** | - |
| Men | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| R2 | Model 1 | Model 2 | Model 3 | R2 | Model 1 | Model 2 | Model 3 | |
| Ecc | 0.28 | −0.138 (0.04) * | −0.096 (0.03) * | −0.076 (0.03) * | 0.29 | −0.136 (0.03)** | −0.089 (0.03)* | −0.09 (0.03)* |
| EDSR | 0.14 | 0.003 (0.004) | −0.005 (0.005) | −0.0025 (0.005) | 0.14 | 0.003 (0.004) | −0.005 (0.005) | −0.003 (0.005) |
| Torsion | 0.11 | 0.007 (0.02)** | 0.005 (0.002)* | 0.005 (0.002)** | 0.11 | 0.007 (0.002) ** | 0.006 (0.002) * | 0.006 (0.002)* |
| TRR | 0.05 | −0.194 (0.1) ^ | −0.056 (0.1) | −0.073 (0.1) | 0.05 | −0.202 (0.1) * | 0.048 (0.1) | −0.072 (0.1) |
| LV EF | 0.08 | 0.313 (0.08) ** | 0.248 (0.08)* | - | 0.08 | 0.287 (0.08) * | 0.223 (0.08) * | - |
| EDVi | 0.15 | 0.608 (0.1)* | 0.581 (0.17)** | - | 0.14 | 0.411 (0.1)* | 0.379 (0.1)* | - |
| LVMi | 0.20 | 0.439 (0.14)* | 0.376 (0.1) * | - | 0.21 | 0.466 (0.1)* | 0.461 (0.1) * | - |
=p<0.05,
=p<0.001,
p=0.06
The Coefficient represents the change in LV measures per 10 ms change in post contrast T1 time, with adjustments for multiple variables. R2: R-squared values for model 3 (for model 2 if model3 is not available)
Model 1: Unadjusted
Model 2: Multivariate linear regression adjusted for age, race, BSA, GFR, heart rate, SBP, DBP, Anti hypertensive medication use, History of Diabetes, Smoking status.
Model 3: Adjusted for age, race, BSA, GFR, heart rate, SBP, DBP, Anti hypertensive medication use, History of Diabetes, Smoking status, LV end diastolic mass and volume.
EDSR= Early diastolic strain rate, LV Torsion =LV Torsion adjusted for epicardial radius, TRR= Torsional recoil rate, BSA=body surface area, LVEF=Ejection fraction, LVEDVi=LV End diastolic volume index, LVMi = LV mass index, SBP= Systolic blood pressure, DBP= Diastolic blood pressure, GFR= Glomerular Filtration rate.
Figure 4. Sex specific differences in association between myocardial extracellular expansion represented by post contrast T1 time and LV Ecc, LV Torsion, EDSR and LV Ejection fraction in the group without LGE defined scar.
EDSR= Early diastolic strain rate. Figure demonstrates that with greater ECE (shorter post contrast T1 times), men have greater LV dysfunction manifested as decreased LV ejection fraction compared to women, despite of lower circumferential shortening in both genders. Women have unchanged LV Torsion (rad) compared to men, but have more diastolic dysfunction as measured by lower EDSR with shorter post contrast T1 times. All these relationships are statistically significant (p<0.05).
Greater pre-contrast T1 time however was associated with greater circumferential shortening in women only (B=−0.105, SE=0.02, p<0.05) (Supplemental Table 2). The associations of higher pre-contrast T1 time with greater circumferential strain in women remained significant after adjustment for measures of LV remodeling including LV end diastolic mass and end diastolic volume.
Myocardial fibrosis and deformation in MESA participants with visible LGE defined replacement scar
Participants with visible scar had higher LVMi (78.2 vs. 64.8 g/m2, p=0.0001), LVEDVi (70 vs. 65.4 mL/m2, p=0.01) and significantly lower ejection fraction (56.8 vs. 62.2%, p=0.0001) compared to the group with no visible myocardial scar (Table 1). Also, they had higher pre-contrast T1 times (985.5ms vs. 975.8, p =0.01) and lower 12 min (444.2ms vs. 454.6 ms, p=0.008) and 25 min (508.4ms vs. 518.3ms, p=0.01) post-contrast T1, less myocardial deformation as measured by lower circumferential shortening (−13.6 vs. −15.5%, p=0.0001) when compared to participants without visible myocardial scar. In addition, they had lower diastolic function as measured by lower early diastolic strain rate (0.86 s− 1 vs. 1.04 s− 1, p=0.0003) and torsional recoil rate (−20.1 vs. −23.2 °/cm/s, p=0.03); higher TSR (−0.65±0.19 vs. −0.56±0.16) when compared to participants without visible myocardial scar.
Moreover, among participants with LGE defined visible scar, greater ECE indexed as lower 12 minute post-contrast T1 was associated with lower circumferential shortening (Figure 5) and lower LV ejection fraction (B=−0.011 and 0.04; SE= 0.008 and 0.02 respectively, p<0.05). Greater ECE indexed as shorter 12 and 25 minute post contrast T1 times were also associated with lower LVMi (B=0.085 and 0.086; SE= 0.03 and 0.03 respectively, p<0.05) in an unadjusted univariate analysis (given relatively small sample size). Greater precontrast T1 time was associated with lower ejection fraction (B= −0.77, p<0.05).
Figure 5. T1 mapping and corresponding CMR tagging from a participant with LGE defined scar.
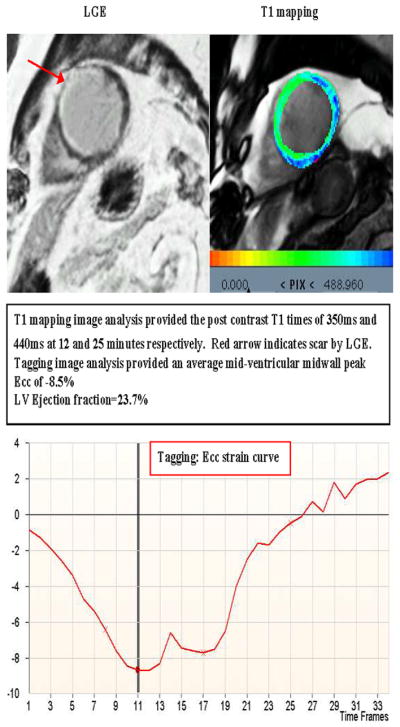
Red arrow indicates myocardial scar.
Discussion
This study demonstrates marked fibrosis-associated differences in LV remodeling in a large multi-ethnic population cohort. We found that a greater degree of myocardial extracellular expansion (ECE) as measured by post-contrast T1 time was associated with reduced circumferential shortening, reduced LV Torsion (rad) and reduced ejection fraction in MESA participants without LGE defined scar. Furthermore, in this population, increasing ECE was associated with a marked decrease in LV mass and end-diastolic volume. The association of ECE with altered myocardial deformation indices persisted after adjustment for traditional cardiovascular risk factors and measures of LV remodeling such as LV mass and LV end-diastolic volume.
An explanation for the decrease in LV mass in association with ECE is offered by animal and human histomorphometric studies which have demonstrated an age-related increase in the number of collagen fibers and a decrease in the absolute number of myocytes despite concomitant hypertrophy of the remaining myocytes. 25–27 In MESA participants, as there were no identified confounding conditions such as myocardial edema or amyloidosis etc.; myocardial ECE appears to result from the accumulation of excess collagen fibers in the interstitium. 28 The increased myocardial collagen deposition associated with decreased LVMi, may contribute to reduced LVEDVi and impaired myocardial contractility. The accumulation of fibrotic tissue may influence LV systolic performance in two ways. Firstly, cardiomyocyte apoptosis results in decreased number of myocytes leading to impaired systolic contraction, secondly, cardiomyocyte hypertrophy results in altered calcium metabolism leading to LV dysfunction. 29
With greater fibrosis, both men and women have lower circumferential shortening, whereas lower early diastolic strain rate was noted only in women and lower LV Torsion (rad) and lower ejection fraction was noted only in men. Therefore, gender differences appear to exist in the cardiac adaptation to ECE, with men developing greater global LV dysfunction than women even though the circumferential shortening was lower in both genders. Such differences could be related to differences in LV architecture, neurohumoral factors and/or exposure to cardiovascular risk factors, the latter developing at an earlier age in men. 30, 31 Previous studies have demonstrated gender differences in cardiac remodeling with women having higher LV torsion than men. 7 Reduced circumferential shortening and reduced LV torsion were described previously in patients with systolic heart failure whereas in diastolic dysfunction, torsion can be normal which can contribute to the preservation of LV ejection fraction.32
Previous papers have used different definitions for LV Torsion.7, 33 In the current study, LV Torsion was calculated with and without adjustment for epicardial radius. Our interpretation of results is that there is significant sex related difference in LV torsion by both methods. LV Torsion adjusted for epicardial radius decreases with increasing ECE in men, while it remains unchanged among women. Without adjustment for epicardial radius, LV torsion increases with fibrosis in women and is unchanged in men. We suspect that adjustment for cardiac (body) size underlies the difference in the direction of relationships although the sex effect on torsion-fibrosis relationship remains.
With greater ECE, greater diastolic dysfunction as measured by lower early diastolic strain rate with a preserved ejection fraction was noted in women only. Previous studies have demonstrated that female sex is a dominant risk factor for development of heart failure with preserved ejection fraction (HFpEF).31 HFpEF is a result of alterations is ventricular-vascular coupling leading to diastolic and systolic dysfunction. The accumulation of excess collagen affects the viscoelasticity of myocardium compromising the cardiac muscle fiber shortening; and during diastole, limiting torsional recoil and ventricular suction. Furthermore, the presence of myofibroblasts may have an additional effect on contractile tonus, thus aggravating diastolic dysfunction.34 In univariate analyses, greater ECE was associated with greater torsional recoil rate whereas in multivariate analysis, the relationship between greater ECE and torsional recoil rate lost statistical significance. Previous smaller clinical investigations have demonstrated similar findings of unchanged torsional recoil rate in diabetic patients with diastolic dysfunction and normal ejection fraction.35
In our study, increased ECE was associated with an unaltered Torsion to endocardial circumferential shortening ratio. Aging is associated with increased interstitial myocardial fibrosis and previous studies have suggested a predominance of subendocardial involvement.33, 36 On the other hand, recent animal and human studies have shown that there is a significant increase in collagen fibers content of LV myocardium with age and that perimysial and endomysial collagen fibers increase in number and thickness suggesting uniform myocardial involvement.37–39 This might explain the reason for unaltered TSR in association with diffuse interstitial myocardial fibrosis.
In our study, presence of LGE defined myocardial scar was accompanied by changes in post-contrast T1 times suggesting greater ECE not detected by delayed enhancement CMR. MESA participants with LGE defined scar also had greater global and regional myocardial dysfunction than those without visible myocardial scar. Previous studies have shown lower post contrast T1 times in heart failure patients, even in areas remote from those with delayed gadolinium enhancement. In those studies, reduced systolic performance was also detected in non-infarcted areas suggesting the presence of diffuse interstitial myocardial fibrosis and pathological remodeling in corresponding areas. 5
Fibrosis-associated alterations of LV structure and function in this study differed between T1 times measured before or after contrast administration. Among MESA participants without LGE defined myocardial scar or history of previous cardiovascular event; greater ECE as measured by lower post contrast T1 times was associated with decreased circumferential shortening, while higher pre-contrast T1 times were associated with greater circumferential shortening. Pre-contrast T1 times can comprise myocardial signal from both the intracellular and extracellular spaces. In healthy individuals, pre-contrast T1 times may reflect factors other than extracellular interstitial fibrosis. However, as the ventricle remodels in response to overt myocardial damage, pre-contrast T1 time may be more influenced by extracellular fibrosis as documented in previous clinical studies. 40, 41 On the other hand, several previous studies have documented consistent correlations between post-contrast T1 times and diffuse interstitial myocardial fibrosis. 5, 42 In this study, T1 times measured at 12 and 25 minutes after contrast administration were more consistent indices of greater ECE.
The equilibrium contrast-CMR (EQ-CMR) technique is another non-invasive method described by Flett et al. to assess diffuse myocardial fibrosis.43 In EQ-CMR, a bolus of contrast administration is followed by a continuous infusion to achieve contrast equilibrium. Although EQ-CMR has been validated against histologically derived collagen volume fraction, the technique requires continuous infusion of gadolinium contrast with longer scanning time. Using MOLLI sequence however, the images are obtained in a single breath-hold and the image acquisition is therefore faster.
Clinical Implications
Gadolinium enhanced T1 mapping techniques can detect and quantify diffuse myocardial interstitial fibrosis. The current study demonstrates a relationship between contrast-enhanced myocardial T1 time and myocardial mechanical behavior as well as ventricular remodeling among multi-ethnic individuals without history of previous cardiovascular events. The current study also demonstrates sex specific differences in diffuse interstitial fibrosis associated with cardiac remodeling. Contrast-enhanced T1 mapping techniques may eventually assist in monitoring the effectiveness of therapy aimed at regression of myocardial fibrosis and altering ventricular remodeling.44, 45
Limitations
Since the participants in our sample were between ages 52–92 years, it is not possible to generalize these results to younger adults. The analysis of the association between myocardial fibrosis and measures of myocardial deformation were cross-sectional across a large cohort of individuals and future longitudinal investigations using serial cardiac MRI examinations are needed to assess the possibility of cohort effect involving the associations of myocardial fibrosis with LV function and progressive LV remodeling. Furthermore, T1 times are nonspecific measures of gadolinium distribution and other processes that result in expansion of the extracellular space beyond fibrosis may also result in lower post contrast T1 times. In our protocol, the MOLLI sequence was obtained only at the mid-ventricular level, and hence the extracellular expansion in other regions of the heart could not be assessed. Recent studies have used the extracellular volume fraction (ECV) or fibrosis index as a marker of extracellular expansion. The ECV fraction incorporates precontrast and post-contrast T1 times adjusted for hematocrit, which was not available for all the participants, precluding ECV fraction estimation.
Conclusion
Increased interstitial myocardial fibrosis indexed as increased extracellular expansion (ECE) is characterized by a distinct pattern of altered myocardial deformation in the general population. In MESA participants without prior history of clinical events and without CMR LGE defined scar, greater ECE is associated with lower LV end diastolic mass and volumes. ECE is also associated with reduced circumferential shortening independent of LV mass and end diastolic volume. However, this relationship is gender dependent. In women, ECE is also associated with greater diastolic dysfunction and preserved LV ejection fraction; while in men, ECE is associated with greater global LV dysfunction manifested as lower LV torsion and lower ejection fraction. Finally, in participants with LGE defined myocardial scar, greater ECE is associated with reduced circumferential shortening and reduced ejection fraction.
Supplementary Material
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Sources of Funding
This research was supported by contracts N01-HC-95159 through N01-HC-95168 from the National Heart, Lung, and Blood Institute, grants UL1-RR-024156 and UL1-RR-025005 from National Center for Research Resources (NCRR) and the NIH intramural research program.
Footnotes
Disclosures
None.
References
- 1.Gjesdal O, Bluemke DA, Lima JA. Cardiac remodeling at the population level--risk factors, screening, and outcomes. Nature reviews. 2011;8:673–685. doi: 10.1038/nrcardio.2011.154. [DOI] [PubMed] [Google Scholar]
- 2.Conrad CH, Brooks WW, Hayes JA, Sen S, Robinson KG, Bing OH. Myocardial fibrosis and stiffness with hypertrophy and heart failure in the spontaneously hypertensive rat. Circulation. 1995;91:161–170. doi: 10.1161/01.cir.91.1.161. [DOI] [PubMed] [Google Scholar]
- 3.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. Journal of the American College of Cardiology. 2006;48:1977–1985. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 4.Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111:2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 5.Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta SN, Kaye DM, Taylor AJ. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. Journal of the American College of Cardiology. 2008;52:1574–1580. doi: 10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 6.Alter P, Rupp H, Adams P, Stoll F, Figiel JH, Klose KJ, Rominger MB, Maisch B. Occurrence of late gadolinium enhancement is associated with increased left ventricular wall stress and mass in patients with non-ischaemic dilated cardiomyopathy. European journal of heart failure. 2011;13:937–944. doi: 10.1093/eurjhf/hfr082. [DOI] [PubMed] [Google Scholar]
- 7.Yoneyama K, Gjesdal O, Choi EY, Wu CO, Hundley WG, Gomes AS, Liu CY, McClelland RL, Bluemke DA, Lima JA. Age, Gender and Hypertension-Related Remodeling Influences Left Ventricular Torsion Assessed by Tagged Cardiac Magnetic Resonance in Asymptomatic Individuals: The Multi-Ethnic Study of Atherosclerosis. Circulation. 2012;126:2481–2490. doi: 10.1161/CIRCULATIONAHA.112.093146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan V, Fenning A, Levick SP, Loch D, Chunduri P, Iyer A, Teo YL, Hoey A, Wilson K, Burstow D, Brown L. Cardiovascular changes during maturation and ageing in male and female spontaneously hypertensive rats. Journal of cardiovascular pharmacology. 2011;57:469–478. doi: 10.1097/FJC.0b013e3182102c3b. [DOI] [PubMed] [Google Scholar]
- 9.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. Journal of the American College of Cardiology. 2011;57:891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pennell DJ, Sechtem UP, Higgins CB, Manning WJ, Pohost GM, Rademakers FE, van Rossum AC, Shaw LJ, Yucel EK. Clinical indications for cardiovascular magnetic resonance (CMR): Consensus Panel report. European heart journal. 2004;25:1940–1965. doi: 10.1016/j.ehj.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 12.Gotte MJ, Germans T, Russel IK, Zwanenburg JJ, Marcus JT, van Rossum AC, van Veldhuisen DJ. Myocardial strain and torsion quantified by cardiovascular magnetic resonance tissue tagging: studies in normal and impaired left ventricular function. Journal of the American College of Cardiology. 2006;48:2002–2011. doi: 10.1016/j.jacc.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 13.Castillo E, Osman NF, Rosen BD, El-Shehaby I, Pan L, Jerosch-Herold M, Lai S, Bluemke DA, Lima JA. Quantitative assessment of regional myocardial function with MR-tagging in a multi-center study: interobserver and intraobserver agreement of fast strain analysis with Harmonic Phase (HARP) MRI. J Cardiovasc Magn Reson. 2005;7:783–791. doi: 10.1080/10976640500295417. [DOI] [PubMed] [Google Scholar]
- 14.Judd RM, Atalay MK, Rottman GA, Zerhouni EA. Effects of myocardial water exchange on T1 enhancement during bolus administration of MR contrast agents. Magn Reson Med. 1995;33:215–223. doi: 10.1002/mrm.1910330211. [DOI] [PubMed] [Google Scholar]
- 15.Messroghli DR, Plein S, Higgins DM, Walters K, Jones TR, Ridgway JP, Sivananthan MU. Human myocardium: single-breath-hold MR T1 mapping with high spatial resolution--reproducibility study. Radiology. 2006;238:1004–1012. doi: 10.1148/radiol.2382041903. [DOI] [PubMed] [Google Scholar]
- 16.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52:141–146. doi: 10.1002/mrm.20110. [DOI] [PubMed] [Google Scholar]
- 17.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. American journal of epidemiology. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 18.Young AA, Cowan BR, Thrupp SF, Hedley WJ, Dell’Italia LJ. Left ventricular mass and volume: fast calculation with guide-point modeling on MR images. Radiology. 2000;216:597–602. doi: 10.1148/radiology.216.2.r00au14597. [DOI] [PubMed] [Google Scholar]
- 19.Kawel N, Nacif M, Zavodni A, Jones J, Liu S, Sibley CT, Bluemke DA. T1 mapping of the myocardium: intra-individual assessment of the effect of field strength, cardiac cycle and variation by myocardial region. J Cardiovasc Magn Reson. 2012;14:27. doi: 10.1186/1532-429X-14-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garot J, Bluemke DA, Osman NF, Rochitte CE, McVeigh ER, Zerhouni EA, Prince JL, Lima JA. Fast determination of regional myocardial strain fields from tagged cardiac images using harmonic phase MRI. Circulation. 2000;101:981–988. doi: 10.1161/01.cir.101.9.981. [DOI] [PubMed] [Google Scholar]
- 21.Bachner-Hinenzon N, Ertracht O, Leitman M, Vered Z, Shimoni S, Beeri R, Binah O, Adam D. Layer-specific strain analysis by speckle tracking echocardiography reveals differences in left ventricular function between rats and humans. American journal of physiology. 2010;299:H664–672. doi: 10.1152/ajpheart.00017.2010. [DOI] [PubMed] [Google Scholar]
- 22.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 23.Russel IK, Tecelao SR, Kuijer JP, Heethaar RM, Marcus JT. Comparison of 2D and 3D calculation of left ventricular torsion as circumferential-longitudinal shear angle using cardiovascular magnetic resonance tagging. J Cardiovasc Magn Reson. 2009;11:8. doi: 10.1186/1532-429X-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donekal S, Ambale-Venkatesh B, Berkowitz S, Wu CO, Choi EY, Fernandes V, Yan R, Harouni AA, Bluemke DA, Lima JA. Inter-study reproducibility of cardiovascular magnetic resonance tagging. J Cardiovasc Magn Reson. 2013;15:37. doi: 10.1186/1532-429X-15-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anversa P, Palackal T, Sonnenblick EH, Olivetti G, Meggs LG, Capasso JM. Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circulation research. 1990;67:871–885. doi: 10.1161/01.res.67.4.871. [DOI] [PubMed] [Google Scholar]
- 26.Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circulation research. 1991;68:1560–1568. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 27.Cheng S, Fernandes VR, Bluemke DA, McClelland RL, Kronmal RA, Lima JA. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2009;2:191–198. doi: 10.1161/CIRCIMAGING.108.819938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83:1849–1865. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
- 29.Diez J, Frohlich ED. A translational approach to hypertensive heart disease. Hypertension. 2010;55:1–8. doi: 10.1161/HYPERTENSIONAHA.109.141887. [DOI] [PubMed] [Google Scholar]
- 30.Hees PS, Fleg JL, Lakatta EG, Shapiro EP. Left ventricular remodeling with age in normal men versus women: novel insights using three-dimensional magnetic resonance imaging. The American journal of cardiology. 2002;90:1231–1236. doi: 10.1016/s0002-9149(02)02840-0. [DOI] [PubMed] [Google Scholar]
- 31.Scantlebury DC, Borlaug BA. Why are women more likely than men to develop heart failure with preserved ejection fraction? Current opinion in cardiology. 2011;26:562–568. doi: 10.1097/HCO.0b013e32834b7faf. [DOI] [PubMed] [Google Scholar]
- 32.Gillebert TC, Van de Veire NR. About left ventricular torsion, sex differences, shear strain, and diastolic heart failure. European heart journal. 2008;29:1215–1217. doi: 10.1093/eurheartj/ehn173. [DOI] [PubMed] [Google Scholar]
- 33.Lumens J, Delhaas T, Arts T, Cowan BR, Young AA. Impaired subendocardial contractile myofiber function in asymptomatic aged humans, as detected using MRI. American journal of physiology. 2006;291:H1573–1579. doi: 10.1152/ajpheart.00074.2006. [DOI] [PubMed] [Google Scholar]
- 34.Burlew BS, Weber KT. Cardiac fibrosis as a cause of diastolic dysfunction. Herz. 2002;27:92–98. doi: 10.1007/s00059-002-2354-y. [DOI] [PubMed] [Google Scholar]
- 35.Fonseca CG, Dissanayake AM, Doughty RN, Whalley GA, Gamble GD, Cowan BR, Occleshaw CJ, Young AA. Three-dimensional assessment of left ventricular systolic strain in patients with type 2 diabetes mellitus, diastolic dysfunction, and normal ejection fraction. The American journal of cardiology. 2004;94:1391–1395. doi: 10.1016/j.amjcard.2004.07.143. [DOI] [PubMed] [Google Scholar]
- 36.Anversa P, Hiler B, Ricci R, Guideri G, Olivetti G. Myocyte cell loss and myocyte hypertrophy in the aging rat heart. Journal of the American College of Cardiology. 1986;8:1441–1448. doi: 10.1016/s0735-1097(86)80321-7. [DOI] [PubMed] [Google Scholar]
- 37.Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Bonnema DD, Zile MR. Age-dependent alterations in fibrillar collagen content and myocardial diastolic function: role of SPARC in post-synthetic procollagen processing. American journal of physiology. 2010;298:H614–622. doi: 10.1152/ajpheart.00474.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biernacka A, Frangogiannis NG. Aging and Cardiac Fibrosis. Aging and disease. 2011;2:158–173. [PMC free article] [PubMed] [Google Scholar]
- 39.Gazoti Debessa CR, Mesiano Maifrino LB, Rodrigues de Souza R. Age related changes of the collagen network of the human heart. Mechanisms of ageing and development. 2001;122:1049–1058. doi: 10.1016/s0047-6374(01)00238-x. [DOI] [PubMed] [Google Scholar]
- 40.Lima JA. The promise of myocardial fibrosis assessment by t1 mapping. Jacc. 2013;6:485–487. doi: 10.1016/j.jcmg.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Puntmann VO, Voigt T, Chen Z, Mayr M, Karim R, Rhode K, Pastor A, Carr-White G, Razavi R, Schaeffter T, Nagel E. Native t1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. Jacc. 2013;6:475–484. doi: 10.1016/j.jcmg.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 42.Ng AC, Auger D, Delgado V, van Elderen SG, Bertini M, Siebelink HM, van der Geest RJ, Bonetti C, van der Velde ET, de Roos A, Smit JW, Leung DY, Bax JJ, Lamb HJ. Association between diffuse myocardial fibrosis by cardiac magnetic resonance contrast-enhanced T(1) mapping and subclinical myocardial dysfunction in diabetic patients: a pilot study. Circ Cardiovasc Imaging. 2012;5:51–59. doi: 10.1161/CIRCIMAGING.111.965608. [DOI] [PubMed] [Google Scholar]
- 43.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 44.Diez J, Querejeta R, Lopez B, Gonzalez A, Larman M, Martinez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–2517. doi: 10.1161/01.cir.0000017264.66561.3d. [DOI] [PubMed] [Google Scholar]
- 45.Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102:1388–1393. doi: 10.1161/01.cir.102.12.1388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.