Abstract
Background
Current methods to identify patients at higher risk for sudden cardiac death, primarily left ventricular ejection fraction (LVEF) ≤35%, miss ∼80% of patients who die suddenly. We tested the hypothesis that patients with elevated QRS scores (index of myocardial scar) and wide QRS-T angles (index abnormal depolarization-repolarization relationship) have high 1-year all-cause mortality and could be further risk-stratified with clinical characteristics.
Methods and Results
We screened all 12-lead ECGs (∼50,000 patients) over 6 months at 2 large hospital systems and analyzed clinical characteristics and 1-year mortality. Patients with ECGs obtained in hospital areas with known high mortality rates were excluded. At one hospital, QRS score ≥5 and QRS-T angle ≥105° identified 8.0% of patients and was associated with an odds ratio (OR) of 2.79 [95% confidence interval 2.10-3.69] for 1-year mortality compared to patients below both ECG thresholds (13.9% vs. 5.5% death rate). LVEF was >35% in 82% of the former group of patients and addition of ECG measures to LVEF increased the discrimination of death risk (p<0.0001). At the second hospital, the OR was 2.42 [1.95-3.01] for 1-year mortality (8.8% vs. 3.8%). Adjustment for patient characteristics eliminated inter-hospital differences. Multivariable adjusted OR combining data from both hospitals was 1.53 [1.28-1.83]. Increasing heart rate and chronic renal impairment further predicted mortality.
Conclusions
Screening hospital ECG databases with QRS scoring and QRS-T angle analysis identifies patients with high 1-year all-cause mortality and predominantly preserved LVEF. This approach may represent a widely-available method to identify patients at increased risk of death.
Keywords: electrocardiography, screening, arrhythmia, fibrosis, death, myocardial scar
Prevention of sudden cardiac death in patients of indeterminate risk represents one of the most challenging issues in contemporary cardiology. Sudden cardiac death accounts for 200,000 to 450,000 deaths in the United States annually.1 However, the current method to identify patients at higher risk, primarily left ventricular ejection fraction (LVEF) ≤35%, misses ∼80% of patients who die suddenly.2 In order to improve survival and reduce morbidity, it is critical to develop better strategies for accurate risk assessment in cases where traditional markers, such as clinical characteristics and LVEF, may be indeterminate. Ideally, initial screening for increased risk would involve simple and inexpensive tests that could be obtained at routine physician office visits and would be reproducible in different medical institutions.
Recent studies have revisited 12-lead electrocardiographic (ECG) markers to stratify patients for risk of arrhythmic sudden cardiac death because, as demonstrated in prior work, large groups of patients at risk for sudden cardiac death have had contact with the medical system, which frequently entails the acquisition of routine 12-lead ECGs.2 Two ECG markers of interest are the QRS score to detect myocardial scar and the spatial QRS-T angle to detect abnormal relationship between depolarization and repolarization.
QRS scoring estimates fibrotic scar by measuring changes in Q-, R-, and S-wave durations, amplitudes and morphologies.3, 4 This approach has been shown to be a strong predictor of appropriate implantable cardioverter-defibrillator (ICD) shocks and cardiovascular mortality.5-7 In the normal heart, the QRS- and T-spatial loops point in the same spatial direction. A wide QRS-T angle indicates that the mean ventricular depolarization and repolarization vectors point in opposite spatial directions and has proved to be a strong predictor of appropriate ICD shocks, sudden death and cardiovascular mortality.8-10 Therefore, the combined use of the QRS score and QRS-T angle could represent a powerful tool to screen large databases of individuals who had contact with the medical system that included the acquisition of a standard 12-lead ECG.
In the present study, we screened the clinical ECG databases of two large hospitals to test the hypothesis that one-year total mortality is high among patients with elevated QRS scores and wide QRS-T angles and that these patients can be further risk-stratified with widely available clinical information.
Methods
The study protocol was approved by the Institutional Review Boards at Johns Hopkins Hospital (JHH) and Hospital of the University of Pennsylvania (HUP). A waiver of informed consent was given for retrospective screening.
Electrocardiographic Screening
All ECGs acquired and stored at JHH (including outpatient clinics) between October 1, 2009, and March 31, 2010, from patients age 21 to 100 years (n=69,088) were exported. Patients with ECGs from hospital areas with high risk of mortality (intensive care units, chemotherapy, radiation-oncology, transplant, catheterization laboratories and inpatient dialysis) were excluded from the study (n=19,829 ECGs from 5,590 patients). After this review, 19,750 eligible ECGs remained (only the most recent ECG from each patient), of which 1,589 (8.0%) had QRS score ≥5 and QRS-T angle ≥105°. A QRS score threshold of ≥5 points was selected prospectively because this level had the highest accuracy for detecting cardiac magnetic resonance late gadolinium enhancement (CMR-LGE) scar in ≥10% of the left ventricle.3 A spatial QRS-T angle threshold of ≥105° was selected prospectively based on a study in a general population.9 Additional ECGs were excluded because they were duplicates or could not be linked to specific patients (n=191) or the patients had pacemakers (n=175), leaving 1,223 patients with QRS score ≥5 and QRS-T angle ≥105° for clinical outcomes analysis at JHH. In addition, a random sample of 1,800 JHH patients not meeting both ECG thresholds was selected as a control group for clinical outcomes analysis, of which 89 ECGs were excluded because they were duplicates, paced or could not be linked to specific patients.
A similar protocol was applied at HUP. After excluding duplicate ECGs from the same patient and patients who had ECGs in high-risk hospital areas, there were 15,544 eligible ECGs at HUP, of which 1,281 (8.2%) had QRS score ≥5 and QRS-T angle ≥105°. As opposed to JHH where a random sample was used as a control group because manual review of medical records was required (described below), at HUP automated medical record screening was performed and thus the control group included all eligible patients.
ECGs were analyzed with 12SL software (GE Healthcare, Wauwatosa, WI, USA) in the Magellan ECG Research Workstation software (GE Healthcare). Vectorcardiograms were reconstructed from 12-lead ECGs using a publicly available transformation matrix.11 The spatial QRS-T angle was calculated based on the difference between the mean QRS- and T-axes.9 Diagnostic statement codes and Q-, R- and S-wave durations and amplitudes were imported into Microsoft Excel (Redmond, WA, USA). ECG confounder (conduction) types for QRS scoring were classified based on diagnostic statement codes, QRS duration and QRS axis, and then QRS scores were calculated (Figure S1, online-only Data Supplement).
Clinical and Mortality Screening
At JHH, the electronic medical records were screened manually to determine the clinical status of all patients with QRS score ≥5 and QRS-T angle ≥105° and the random sample of patients not meeting both of these ECG thresholds. The following parameters were evaluated: age, gender, race, LVEF, history of coronary artery disease, history of myocardial infarction, history of coronary artery bypass graft surgery, history of percutaneous coronary intervention with or without stenting, history of atrial fibrillation, presence of an ICD and presence of a pacemaker. Patients with an estimated glomerular filtration rate <60 ml/min method (based on plasma creatinine level) were classified as having renal impairment.12 At HUP, automated medical record screening (Supplemental Methods) was performed to determine age, gender, race and plasma creatinine levels. Deaths reported in the electronic medical record or identified by searching the SSDMF (Supplemental Methods) were considered if they occurred by December 31, 2010 (1-year median follow-up).
Statistical Analysis
QRS score and QRS-T angle distributions were compared between the two hospitals by histograms. Univariate and multivariable logistic regression was used to compare one-year mortality in each of the following three groups to patients with QRS score <5 and QRS-T angle <105°: 1) QRS score <5 and QRS-T angle ≥105°, 2) QRS score ≥5 and QRS-T angle <105°, and 3) QRS score ≥5 and QRS-T angle ≥105°; separate models were run for each group. Among patients with QRS score ≥5 and QRS-T angle ≥105°, multivariable logistic regression was performed to determine if the different absolute mortality rate between JHH and HUP was explained by baseline characteristics. Logistic regression incorporating random intercepts per hospital was used to calculate univariate and adjusted odds ratios for the combined JHH-HUP population. With JHH patients (where LVEF data was available), receiver operating characteristic (ROC) area under the curve (AUC) analysis was used to compare risk stratification with LVEF alone to LVEF plus QRS score and QRS-T angle. Logistic regression and ROC-AUC analysis was also performed in the population meeting the ECG thresholds with LVEF >35% to determine if the population could be further risk-stratified with demographic and clinical variables contained in Table 1. All statistical analyses were performed using STATA (version 11.1, College Station, TX, USA), with the exception that logistic regression combining the JHH and HUP populations was performed using the LME-4 package for R (version 3.0.0, Vienna, Austria). Two-sided p-values <0.05 were considered significant.
Table 1. Characteristics of Johns Hopkins Hospital Patients.
| Patient characteristics | QRS Score <5 & QRS-T Angle <105° (n=1,406) | QRS Score <5 & QRS-T Angle ≥105° (n=172) | QRS Score ≥5 & QRS-T Angle <105° (n=133) | QRS Score ≥5 & QRS-T Angle ≥105° (n=1,223) |
|---|---|---|---|---|
| Number (%) or median [interquartile range] | ||||
| Age, years | 55 [44-67] | 65 [54-74] | 62 [50-72] | 68 [57-78] |
| Female gender | 700 (49.8%) | 69 (40.1%) | 64 (48.1%) | 470 (38.4%) |
| Race/ethnicity | ||||
| Caucasian | 714 (50.8%) | 89 (51.7%) | 74 (55.6%) | 721 (58.9%) |
| African American | 546 (38.8%) | 72 (41.9%) | 51 (38.3%) | 411 (33.6%) |
| Other | 146 (10.4%) | 11 (6.4%) | 8 (6.0%) | 91 (7.4%) |
| Coronary artery disease | 179 (12.7%) | 55 (31.9%) | 32 (24.1%) | 480 (39.2%) |
| Myocardial infarction | 63 (4.5%) | 23 (13.4%) | 18 (13.5%) | 289 (23.6%) |
| Coronary artery bypass graft | 53 (3.8%) | 27 (15.7%) | 15 (11.3%) | 197 (16.1%) |
| Percutaneous coronary intervention | 89 (6.3%) | 20 (11.6%) | 14 (10.5%) | 191 (15.6%) |
| Atrial fibrillation | 105 (7.5%) | 38 (22.1%) | 20 (15.0%) | 302 (24.7%) |
| Implantable defibrillator | 12 (0.9%) | 5 (2.9%) | 3 (2.3%) | 74 (6.1%) |
| Chronic renal impairment | 195 (13.9%) | 51 (29.6%) | 36 (27.1%) | 360 (29.4%) |
| LVEF (n=523, 108, 133 and 706, respectively) | 60 [55-65] | 55 [45-65] | 60 [50-60] | 55 [40-60] |
| Heart rate, beats per minute | 73 [63-85] | 74 [63-83] | 75 [66-88] | 77 [65-92] |
| QRS score, points | 1 [0-2] | 2 [0-3] | 6 [5-7] | 7 [6-8] |
| QRS-T angle, degrees | 43 [27-61] | 131 [115-144] | 68 [43-84] | 136 [120-151] |
| Deceased after 1 year | 77 (5.5%) | 11 (6.4%) | 17 (12.8%) | 170 (13.9%) |
| Outpatient deceased | 9/665 (1.4%) | 2/81 (2.5%) | 0/48 (0%) | 27/608 (4.4%) |
| Emergency room deceased | 11/343 (3.2%) | 4/39 (10.3%) | 3/36 (8.3%) | 33/229 (14.4%) |
| Inpatient deceased | 57/395 (14.4%) | 5/51 (9.8%) | 14/49 (28.6%) | 110/382 (28.8%) |
ECG location data missing for 8 of 2,934 (0.3%) patients
Results
QRS scores and QRS-T angles were similarly distributed at the two hospitals (Figure S2, online-only Data Supplement). At both JHH (Table 1) and HUP (Table 2), patients with QRS score ≥5 and QRS-T angle ≥105° (compared to patients not meeting both thresholds) were older, less commonly female and more commonly Caucasian. At JHH, the patients meeting the ECG thresholds had higher rates of coronary artery disease (39% vs. 13%), history of myocardial infarction (24% vs. 4.5%), atrial fibrillation (25% vs. 7.5%), chronic renal impairment (29% vs. 14%) and prior cardiac interventions. There was only a 5% difference in LVEF between the patient groups (median 55% vs. 60%).
Table 2. Characteristics of Hospital of the University of Pennsylvania Patients.
| Patient characteristics | QRS Score <5 & QRS-T Angle <105°(n=11,595) | QRS Score <5 & QRS-T Angle ≥105°(n=1,527) | QRS Score ≥5 & QRS-T Angle <105°(n=1,151) | QRS Score ≥5 &QRS-T Angle ≥105°(n=1,281) |
|---|---|---|---|---|
| Number (%) or median [interquartile range] | ||||
| Age, years | 55 [43-65] | 63 [52-75] | 62 [51-71] | 66 [56-76] |
| Female gender | 6,127 (52.8%) | 597 (39.1%) | 591 (51.3%) | 432 (33.7%) |
| Race/ethnicity | ||||
| Caucasian | 6,492 (55.9%) | 756 (49.5%) | 717 (62.3%) | 763 (59.6%) |
| African American | 3,878 (33.4%) | 579 (37.9%) | 321 (27.9%) | 376 (29.4%) |
| Other | 1,225 (10.6%) | 192 (12.6%) | 113 (9.8%) | 142 (11.1%) |
| Chronic renal impairment | 1,661 (14.3%) | 205 (13.4%) | 196 (17.0%) | 142 (11.1%) |
| Heart rate, beats per minute | 72 [63-84] | 73 [63-86] | 73 [63-85] | 74 [64-86] |
| QRS score, points | 1 [0-2] | 2 [1-3] | 6 [5-7] | 7 [6-9] |
| QRS-T angle, degrees | 40 [27-60] | 131 [116-148] | 68 [43-87] | 139 [122-155] |
| Deceased after 1 year | 445 (3.8%) | 124 (8.1%) | 97 (8.4%) | 113 (8.8%) |
| Outpatient deceased | 57/4,247 (1.3%) | 32/719 (4.5%) | 11/447 (2.5%) | 35/683 (5.1%) |
| Emergency room deceased | 145/3,310 (4.4%) | 34/317 (10.7%) | 28/223 (12.6%) | 21/204 (10.3%) |
| Inpatient deceased | 242/4,010 (6.0%) | 57/486 (11.7%) | 58/478 (12.1%) | 56/389 (14.4%) |
ECG location data missing for 41 of 15,554 (0.3%) patients
One-Year Mortality by QRS Score and QRS-T Angle
At 1 year, 13.9% of JHH patients with both high QRS score and wide QRS-T angle had died (Table 1), compared to only 5.5% in patients with both low QRS score and narrow QRS-T angle (OR=2.79 [95% confidence interval 2.10-3.69]). At HUP, the odds ratio (OR=2.42 [1.95-3.01]) was similar to that at JHH, although the absolute mortality numbers of those above vs. below the ECG thresholds were reduced (8.8 % vs. 3.8%) (Table 2). However, when age, gender, chronic renal impairment and heart rate (clinical variables significantly associated with death that were available from both JHH and HUP) were controlled in a multivariable logistic regression model, JHH no longer had significantly higher mortality than HUP (p=0.24, Table S1, online-only Data Supplement).
Figure 1 shows Forest plots of univariate and multivariable adjusted odds ratios for each hospital individually, and with both hospitals combined. When considering the combined data, the three combinations of having QRS score ≥5 and/or QRS-T angle ≥105° were all associated with an increase in mortality in both univariate (OR=2.07, 2.33 and 2.59, respectively) and multivariable models (OR=1.29, 1.72 and 1.53, respectively). Table 3 shows the adjusted odds ratios for age, gender, chronic renal impairment and heart rate from the combined JHH and HUP population for the last multivariable model in Figure 1. Increasing age, male gender, chronic renal impairment and increasing heart rate were all associated with a higher risk of death.
Figure 1.
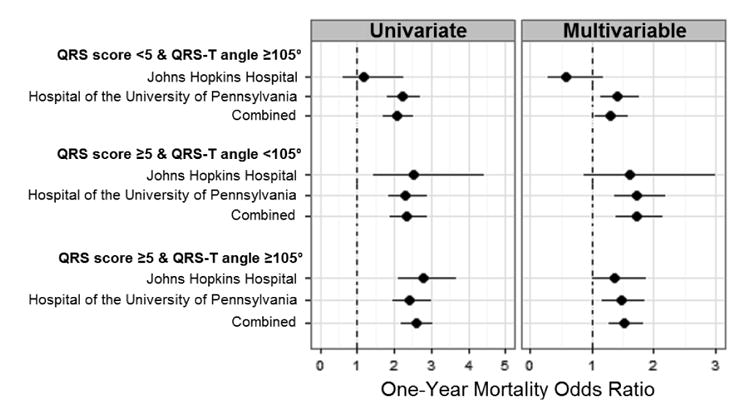
Forest plot of univariate and adjusted odds ratios for combinations of QRS score ≥5 and/or QRS-T angle ≥105° compared to not meeting either threshold. Data are shown for each hospital alone and for the two hospitals combined. Multivariable models are adjusted for age, gender, chronic renal impairment and heart rate. See Table S3 for the exact point estimates and 95% confidence intervals.
Table 3. Adjusted Odds Ratios for One-Year Mortality from Combined JHH and HUP Population.
| Multivariable Adjusted Odds Ratio (95% CI) (n=15,505) | P | ||
|---|---|---|---|
| Age (per 5-year ↑) | 1.21 | 1.18-1.24 | <0.001 |
| Female gender | 0.64 | 0.55-0.75 | <0.001 |
| Chronic renal impairment | 1.92 | 1.62-2.29 | <0.001 |
| Heart rate (per 10-bpm ↑) | 1.45 | 1.39-1.50 | <0.001 |
| QRS Score ≥5 and QRS-T Angle ≥105° | 1.53 | 1.28-1.83 | <0.001 |
This data comes from the same multivariable model as that shown at the bottom of Figure 1.
When grouped by ECG location (outpatient, emergency room or inpatient), the absolute mortality numbers changed (Tables 1 and 2), but the combination of elevated QRS score and wide QRS-T angle remained a predictor of mortality at both institutions.
Added Predictive Value of New Markers
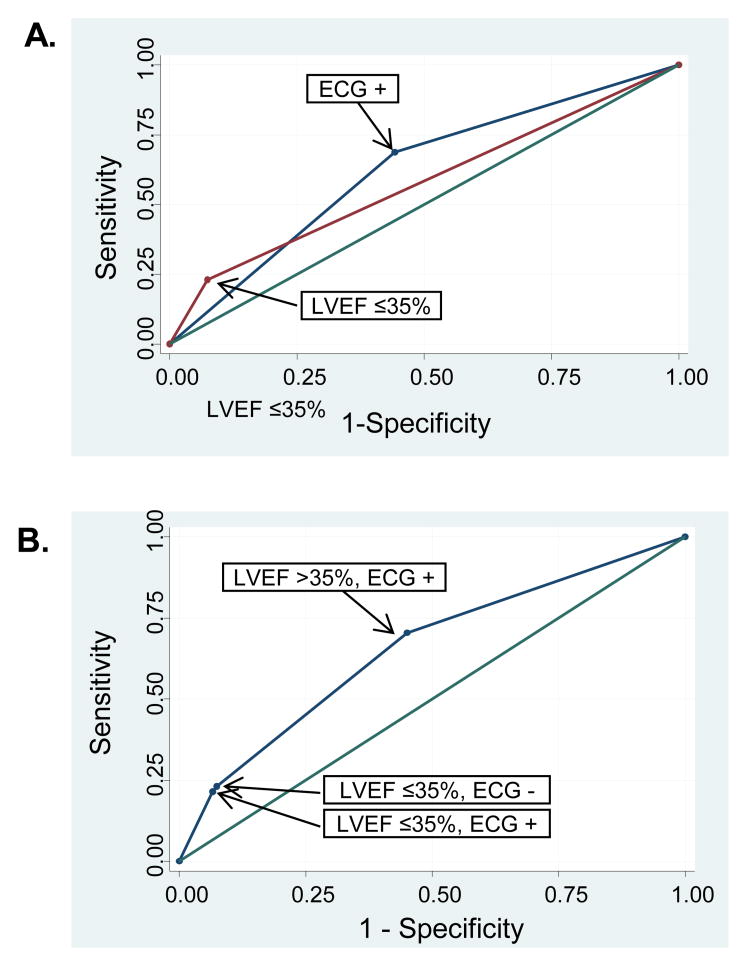
LVEF ≤35% is the current method used to identify patients at increased risk of arrhythmic death. At JHH, LVEF ≤35% was associated with a 22% sensitivity and 93% specificity for identifying patients who died within 1-year (AUC=0.57 [0.55-0.60]) (Figure 2A). Addition of QRS score/QRS-T angle to LVEF increased the AUC to 0.65 [0.62-0.69] (p<0.0001 for comparison to LVEF only) (Figure 2B). The main added value of the new ECG marker was to identify subjects with LVEF >35% who were at increased risk of death (70% sensitivity, 55% specificity) (Figure 2B).
Figure 2.
ROC curves for LVEF ≤35% and ECG (combination of QRS score ≥5 and QRS-T angle ≥105°) to discriminate the risk of death. (A) Individual ROC curves for LVEF ≤35% (AUC=0.57 [0.55-0.60]) and for ECG (AUC=0.62 [0.59-0.65]). (B) ROC curve from the model combining LVEF and ECG (AUC=0.65 [0.62-0.69]). There was an increase in AUC from LVEF alone to LVEF+ECG (p<0.0001). This was driven by an increase in sensitivity for the ECG to detect patients with increased risk of death in patients with LVEF >35%.
The increased sensitivity over LVEF with QRS score and QRS-T angle to identify patients at increased risk of death came at the expense of decreased specificity. Thus, additional risk stratification may be warranted. At JHH, among patients with LVEF >35%, QRS score ≥5 and QRS-T angle ≥105°, significant independent predictors of total mortality included chronic renal impairment, heart rate, further increases in QRS score and age. The model with these variables (Table S2) discriminated the 1-year risk for death with AUC=0.78 [0.74-0.82].
Discussion
This study demonstrates the feasibility of applying a novel strategy to screen entire ECG databases from large hospitals to identify patients at 1-year risk for mortality based on abnormal QRS scores, reflecting the degree of myocardial scar, and on QRS-T angle measurements, indicating abnormal depolarization-repolarization relationship. We performed clinical screening and found that patients with both QRS score ≥5 and QRS-T angle ≥105° had an increased one-year risk of death. This ECG screening strategy may represent an inexpensive and widely-available method to identify patients who are at increased risk of death.
Pathophysiology Underlying the QRS Score and QRS-T angle
In the current study, total mortality was used as the endpoint due to the inability to adjudicate cause of death and thus potential applications for risk-stratifying for arrhythmic death must be validated in future studies. However, there is a pathophysiological basis for the new electrophysiology makers to detect an increased risk of sudden arrhythmic death. Myocardial fibrosis likely represents the most common form of chronic myocardial substrate that predisposes to slowed conduction and re-entrant arrhythmic circuits.13 The presence of fibrosis demonstrated by CMR-LGE can predict ventricular tachyarrhythmias and prognosis.14-16 However, while CMR-LGE analysis of myocardial fibrosis is well established, it is costly and not available for use as a screening tool. In contrast, the 12-lead ECG is inexpensive and universally available and can readily be used to estimate infarct size and myocardial fibrosis caused by cardiomyopathy and predict ventricular tachyarrhythmias.3-5
Multiple methods of repolarization heterogeneity have also been studied; however, in general these methods require prolonged ECG recordings with or without stress on the heart and thus cannot be applied to standard 10-second 12-lead ECGs. In contrast, the spatial QRS-T angle can be measured on the standard 12-lead ECG. A wide spatial QRS-T angle represents discordance in the directions of global depolarization and repolarization vectors and thus reflects myocardial structural disease and/or electrophysiology alterations. Recent reports support the potential value of the QRS-T angle as a predictor of ventricular tachyarrhythmias and general cardiovascular morbidity and mortality in different populations.8-10 In this study, we demonstrate that the combination of QRS-scoring and QRS-T angle analyses is attractive for widespread screening because it can be applied to standard 12-lead ECGs in an automated fashion.
In the current study, patients with both QRS score ≥5 and QRS-T angle ≥105° had a 1-year mortality rate of 13.9% at JHH and 8.8% at HUP. The higher mortality at JHH is likely explained by differences in patient co-morbidities as the odds ratios were similar at both hospitals. When age, gender, chronic renal impairment and heart rate were controlled in a multivariable model, JHH no longer had significantly higher mortality than HUP.
Heart Rate and Chronic Renal Impairment
Among patients identified by both QRS score and QRS-T angle analysis, the most significant predictors of all-cause mortality were increased heart rate and chronic renal impairment. Elevated heart rate is associated more closely with higher rates of sudden death than non-sudden death.17 Heart rate is primarily a marker of autonomic influence on the sinus node, and it is well documented that increased adrenergic activity is pro-arrhythmic while increased vagal tone is cardioprotective.18 In addition, increased heart rate may lead to greater myocardial oxygen consumption and to coronary artery cyclical stretch, which heightens the likelihood of ischemia in patients with coronary artery disease.17
Chronic renal impairment was associated with a significantly increased risk of death within one year. This finding is in line with prior studies showing that mild renal insufficiency in the presence of cardiovascular disease was associated with a significant increase in the risk of sudden cardiac death and of cardiac death.19, 20 The mechanism for increased risk of sudden cardiac death in patients with renal impairment is thought to be multifactorial and may be due to inflammation, development of endocardial and diffuse interstitial fibrosis, and dynamic metabolic changes.19, 20
Further Risk Stratification
A recent Special Report from the American Heart Association on risk stratification for arrhythmic sudden cardiac death indicated that further efforts to stratify risk in patients with mild to moderately depressed LVEF may require the study of 10 to 20 times as many patients as those studied in previous trials to identify the 5-10% of patients whose risk is sufficient to justify ICD implantation.21 In the current study, we demonstrate the ability to screen ECGs from ∼15,000-20,000 patients per hospital and identified ∼8% of patients whose 1-year risk of all-cause mortality was increased and can additionally be discriminated through assessment of resting heart rate and chronic renal impairment. Ultimately, additional hierarchal strategies of risk-stratification among patients identified by ECG screening might be beneficial, including blood biomarkers,22 dynamic markers of electrical instability such as T-wave alternans,23 and myocardial fibrosis assessment by CMR14-16 or other methods.
Limitations
The novel approach used in this study has inherent limitations. The automated QRS scoring algorithm employed slightly modified criteria compared to those previously reported. Minor errors or differences in how patient identification numbers were entered at the time of clinical ECG acquisition resulted in duplication or poor identification of some patients. The patients in this study were a select group coming to a tertiary care hospital or its associated outpatient clinics, and thus the results cannot necessarily be extrapolated outside of this population. Mortality was determined by both electronic medical record and SSDMF for which cause of death is not reported. The high mortality rate found in patients with QRS-score ≥5 and QRS-T angle ≥105° may not only be related to cardiac causes including sudden cardiac death, and likely includes extra-cardiac causes. We tried to control for advanced neoplastic and acute pathological settings associated with higher risk of mortality by excluding patients from oncologic and critical care units. Finally, the SSDMF does not capture all deaths,24 and thus some deaths were likely missed.
Conclusions
Screening of entire health system ECG databases is feasible and may represent an inexpensive and widely available method to identify patients whose 1-year risk of all-cause mortality is elevated. Patients enrolled in this study whose QRS score suggested the presence of myocardial scar and whose wide QRS-T angle indicated an abnormal depolarization-repolarization relationship had a 1-year mortality of 8.8-13.9% (compared to 3.8-5.5% in those not meeting the ECG thresholds) despite preserved or only moderately reduced LVEF. Future work should evaluate the ability of these ECG markers to predict the risk of arrhythmic sudden cardiac death in patients with LVEF >35%, as it was not possible to determine cause of death in this study. The increase in sensitivity for detecting deaths with the ECG markers over LVEF came at a decrease in specificity, and thus further risk-stratification is likely warranted before altering therapy. We demonstrate that elevated heart rate and renal impairment further stratified the identified population with high discriminatory power and may have a role in further risk-stratification.
Supplementary Material
Acknowledgments
We thank Stephen Granite, MS, MBA and Michael Shipway, Loriano Galeotti, PhD and Robbert Zusterzeel, MD for help with data management and analysis. We thank Jeannette Walker, RN and Rosalie Cosgriff for contributions in screening medical records and Jim Clements for contributions to screening ECGs.
Funding Sources: This research was supported in part by the NIH/National Heart, Lung and Blood Institute #P20HL101397 and #R24HL085343, the Leducq Foundation and the French Federation of Cardiology. Johns Hopkins University has a research agreement with GE Healthcare, Inc., which provided technical support only to this project.
Footnotes
Disclaimer: The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services.
Conflict of Interest Disclosures: None
References
- 1.Goldberger JJ, Cain ME, Hohnloser SH, Kadish AH, Knight BP, Lauer MS, Maron BJ, Page RL, Passman RS, Siscovick D, Stevenson WG, Zipes DP. American heart association/american college of cardiology foundation/heart rhythm society scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death. Circulation. 2008;118:1497–1518. [PubMed] [Google Scholar]
- 2.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 3.Strauss DG, Selvester RH, Lima JA, Arheden H, Miller JM, Gerstenblith G, Marban E, Weiss RG, Tomaselli GF, Wagner GS, Wu KC. Ecg quantification of myocardial scar in cardiomyopathy patients with or without conduction defects: Correlation with cardiac magnetic resonance and arrhythmogenesis. Circ Arrhythm Electrophysiol. 2008;1:327–336. doi: 10.1161/CIRCEP.108.798660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strauss DG, Cardoso S, Lima JA, Rochitte CE, Wu KC. Ecg scar quantification correlates with cardiac magnetic resonance scar size and prognostic factors in chagas' disease. Heart. 2011;97:357–361. doi: 10.1136/hrt.2010.210047. [DOI] [PubMed] [Google Scholar]
- 5.Strauss DG, Poole JE, Wagner GS, Selvester RH, Miller JM, Anderson J, Johnson G, McNulty SE, Mark DB, Lee KL, Bardy GH, Wu KC. An ecg index of myocardial scar enhances prediction of defibrillator shocks: An analysis of the sudden cardiac death in heart failure trial. Heart Rhythm. 2011;8:38–45. doi: 10.1016/j.hrthm.2010.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tjandrawidjaja MC, Fu Y, Westerhout CM, Wagner GS, Granger CB, Armstrong PW. Usefulness of the qrs score as a strong prognostic marker in patients discharged after undergoing primary percutaneous coronary intervention for st-segment elevation myocardial infarction. Am J Cardiol. 2011;106:630–634. doi: 10.1016/j.amjcard.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Richardson K, Engel G, Yamazaki T, Chun S, Froelicher VF. Electrocardiographic damage scores and cardiovascular mortality. Am Heart J. 2005;149:458–463. doi: 10.1016/j.ahj.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Borleffs CJ, Scherptong RW, Man SC, van Welsenes GH, Bax JJ, van Erven L, Swenne CA, Schalij MJ. Predicting ventricular arrhythmias in patients with ischemic heart disease: Clinical application of the ecg-derived qrs-t angle. Circ Arrhythm Electrophysiol. 2009;2:548–554. doi: 10.1161/CIRCEP.109.859108. [DOI] [PubMed] [Google Scholar]
- 9.Kardys I, Kors JA, van der Meer IM, Hofman A, van der Kuip DA, Witteman JC. Spatial qrs-t angle predicts cardiac death in a general population. Eur Heart J. 2003;24:1357–1364. doi: 10.1016/s0195-668x(03)00203-3. [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki T, Froelicher VF, Myers J, Chun S, Wang P. Spatial qrs-t angle predicts cardiac death in a clinical population. Heart Rhythm. 2005;2:73–78. doi: 10.1016/j.hrthm.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 11.Kors JA, van Herpen G, Sittig AC, van Bemmel JH. Reconstruction of the frank vectorcardiogram from standard electrocardiographic leads: Diagnostic comparison of different methods. Eur Heart J. 1990;11:1083–1092. doi: 10.1093/oxfordjournals.eurheartj.a059647. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 13.Strauss DG, Wu KC. Imaging myocardial scar and arrhythmic risk prediction--a role for the electrocardiogram? J Electrocardiol. 2009;42:138 e131–138. doi: 10.1016/j.jelectrocard.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt A, Azevedo CF, Cheng A, Gupta SN, Bluemke DA, Foo TK, Gerstenblith G, Weiss RG, Marban E, Tomaselli GF, Lima JA, Wu KC. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–2014. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan AT, Shayne AJ, Brown KA, Gupta SN, Chan CW, Luu TM, Di Carli MF, Reynolds HG, Stevenson WG, Kwong RY. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114:32–39. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]
- 16.Roes SD, Borleffs CJW, van der Geest RJ, Westenberg JJM, Marsan NA, Kaandorp TAM, Reiber JAC, Zeppenfeld K, Lamb HJ, de Roos A, Schalij MJ, Bax JJ. Infarct tissue heterogeneity assessed with contrast-enhanced mri predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circ Cardiovasc Imaging. 2009;2:183–190. doi: 10.1161/CIRCIMAGING.108.826529. [DOI] [PubMed] [Google Scholar]
- 17.Fox KM, Ferrari R. Heart rate: A forgotten link in coronary artery disease? Nat Rev Cardiol. 2011;8:369–379. doi: 10.1038/nrcardio.2011.58. [DOI] [PubMed] [Google Scholar]
- 18.Verrier RL, Tan A. Heart rate, autonomic markers, and cardiac mortality. Heart Rhythm. 2009;6:S68–75. doi: 10.1016/j.hrthm.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pun PH, Smarz TR, Honeycutt EF, Shaw LK, Al-Khatib SM, topton JP. Chronic kidney disease is associated with increased risk of sudden cardiac death among patients with coronary artery disease. Kidney Int. 2009;76:652–658. doi: 10.1038/ki.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shamseddin MK, Parfrey PS. Sudden cardiac death in chronic kidney disease: Epidemiology and prevention. Nat Rev Nephrol. 2011;7:145–154. doi: 10.1038/nrneph.2010.191. [DOI] [PubMed] [Google Scholar]
- 21.Goldberger JJ, Buxton AE, Cain M, Costantini O, Exner DV, Knight BP, Lloyd-Jones D, Kadish AH, Lee B, Moss A, Myerburg R, Olgin J, Passman R, Rosenbaum D, Stevenson W, Zareba W, Zipes DP. Risk stratification for arrhythmic sudden cardiac death: Identifying the roadblocks. Circulation. 2011;123:2423–2430. doi: 10.1161/CIRCULATIONAHA.110.959734. [DOI] [PubMed] [Google Scholar]
- 22.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 23.Verrier RL, Klingenheben T, Malik M, El-Sherif N, Exner DV, Hohnloser SH, Ikeda T, Martinez JP, Narayan SM, Nieminen T, Rosenbaum DS. Microvolt t-wave alternans testing has a role in arrhythmia risk stratification. J Am Coll Cardiol. 2012;59:1572–1573. doi: 10.1016/j.jacc.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Hermansen SW, Leitzmann MF, Schatzkin A. The impact on national death index ascertainment of limiting submissions to social security administration death master file matches in epidemiologic studies of mortality. Am J Epidemiol. 2009;169:901–908. doi: 10.1093/aje/kwn404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.