Abstract
BACKGROUND
Finasteride and dutasteride were developed originally as 5α-reductase inhibitors to block the conversion of testosterone to dihydrotestosterone (DHT). These drugs may possess off-target effects on the androgen receptor (AR) due to their structural similarity to DHT.
METHODS
A total of 4 human prostate cancer cell models were examined: LNCaP (T877A mutant AR), 22Rv1 (H874Y mutant AR), LAPC4 (wild type AR) and VCaP (wild type AR). Cells were cultured in 10% charcoal-stripped fetal bovine serum, either with or without DHT added to the medium. AR activity was evaluated using the ARE-luciferase assay or the expression of AR regulated genes.
RESULTS
Dutasteride was more potent than finasteride in interfering with DHT-stimulated AR signaling. Disruption of AR function was accompanied by decreased cell growth. Cells that rely on DHT for protection against death were particularly vulnerable to dutasteride. Different prostate cancer cell models exhibited different sensitivities to dutasteride and finasteride. LNCaP was most sensitive, LAPC4 and VCaP were intermediate, while 22Rv1 was least sensitive. Regardless of the AR genotype, if AR was transfected into drug-sensitive cells, AR was inhibited by drug treatment; and if AR was transfected into drug-resistant cells, AR was not inhibited.
CONCLUSIONS
The direct inhibitory effect of dutasteride or finasteride on AR signaling is cell line specific. Mutations in the ligand binding domain of AR do not appear to play a significant role in influencing the AR antagonistic effect of these drugs. Subcellular constituent is an important factor in determining the drug effect on AR function.
Keywords: finasteride, dutasteride, antiandrogen, androgen receptor signaling, prostate cancer
INTRODUCTION
A functional androgen receptor (AR) is critical to the growth of prostate cancer cells. Testosterone, the major circulating androgen in the body, is converted to dihydrotestosterone (DHT) by steroid 5α-reductases upon uptake into tissues, including the prostate. Compared to testosterone, DHT is the preferred ligand for the AR by virtue of a slower dissociation rate [1]. Blocking the conversion of testosterone to DHT using 5α-reductase inhibitors, such as finasteride or dutasteride, has been considered promising for prostate cancer prevention. Finasteride inhibits primarily the type 2 5α-reductase, whereas dutasteride inhibits both the type 1 and type 2 isoenzymes. Finasteride and dutasteride reduced the frequency of a diagnosis of prostate cancer in the Prostate Cancer Prevention Trial (PCPT) and Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial, respectively [2, 3].
Finasteride and dutasteride may possess off-target effects on proteins that bind androgen, such as the AR, due to their structural similarity to testosterone and DHT. We have reported previously that finasteride interferes with DHT binding to AR, reduces DHT-stimulated AR activity, and depresses DHT-stimulated growth of LNCaP cells [4]. These findings suggest that this AR inhibition by finasteride is independent of its ability to inhibit 5α-reductase. LNCaP cells contain the T877A mutant AR. Would wild type AR or other AR mutants respond similarly to finasteride? How is dutasteride different from finasteride in this respect? Is there a biological consequence to the effect of finasteride or dutasteride on AR signaling? And finally, do cell type characteristics play an important role in modulating the sensitivity to finasteride or dutasteride? These issues were addressed in the present study.
A total of 4 AR-positive human prostate cancer cell lines were examined: LNCaP, 22Rv1, LAPC-4 and VCaP. These 4 cell lines all respond to DHT stimulation of AR activity, although they vary in the sensitivity of the response. LNCaP and 22Rv1 carry the T877A and H874Y mutations in the ligand binding domain of AR, respectively, while LAPC-4 and VCaP carry wild type AR [5, 6]. Cells were cultured in 10% charcoal-stripped fetal bovine serum, either with or without DHT added to the medium. The confounding effect of dutasteride or finasteride as a 5α-reductase inhibitor is not a factor in these experiments because exogenous DHT was used as the ligand to activate the AR. To address the questions posed in the previous paragraph, a number of readouts were included in the design: ARE-luciferase assay, expression level of AR-regulated genes as determined using qRT-PCR, and measurements of cell proliferation and cell death. Additionally, the effect of dutasteride or finasteride was assessed in the AR-null DU145 cells, which were transfected with various AR isoforms. Experiments also were designed to investigate the importance of cell line specificity in determining the responsiveness of AR to inhibition by dutasteride or finasteride.
MATERIALS AND METHODS
Cell Lines and Reagents
LNCaP, 22Rv1, VCaP and DU145 human prostate cancer cell lines were purchased from American Type Culture Collection (Manassas, VA). LAPC-4 was provided by Dr. Charles Sawyer at Memorial Sloan-Kettering Cancer Center, New York, NY. DHT and finasteride were purchased from Sigma-Aldrich (St. Louis, MO). Dutasteride was obtained from GlaxoSmith Kline (Clifton, NJ). RPMI 1640 and DMEM media were purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) and charcoal-dextran stripped FBS were purchased from Atlanta Biologicals (Lawrenceville, GA) and Thermo Scientific/Hyclone (Logan, UT), respectively.
Cell Culture Treatment
LNCaP, 22Rv1 and LAPC-4 cells were maintained in RPMI 1640 medium supplemented with 10% FBS. Culture dishes for LAPC-4 cells were coated with poly D-lysine (Sigma-Aldrich). VCaP cells were maintained in DMEM supplemented with 10% FBS. All cell lines were pre-cultured for 24 hr in their respective media containing 10% charcoal-stripped FBS before treatment. DHT (dissolved in ethanol), dutasteride or finasteride (the latter two dissolved in DMSO) was added to the culture and continued for various lengths of time depending on the experimental design. Untreated control cultures contained either 0.1% ethanol or 0.1% DMSO to match the concentration of the solvent present in the treated cultures. Cells were incubated at 37°C in an atmosphere of 5% CO2 and air.
ARE-Luciferase Reporter Assay
Cells were transfected with pGL3-ARE-luc as described previously [4], and replated in a medium containing 10% charcoal-stripped FBS at 50,000 cells per well in 24-well plates for 24 hr before treatment with DHT, dutasteride, finasteride, DHT + dutasteride, or DHT + finasteride. Cells were harvested in Reporter Lysis Buffer (Promega, Madison, WI) after 24 hr of treatment. Luciferase activity was determined using the Promega Luciferase 1000 Assay System on a Clarity Luminescence Microplate Reader (BioTek Instruments, Winooski, VT). The protein concentration of the cell lysate was determined using the Coomassie Plus Protein Assay Reagent (VWR, Atlanta, GA). The relative light unit (RLU) of the luciferase activity reading was normalized against the amount of protein in the lysate. In some cases, the RLU/protein value was further normalized against AR abundance. The latter was determined from densitometry reading of AR specific band on Western blots.
Western Blotting and Antibodies
The methods for sample harvesting and processing, SDS-PAGE, and gel blotting were described previously [7]. Antibodies to AR and GAPDH were purchased from BD Pharmigen (San Jose, CA) and Millipore (Billerica, MA), respectively.
RNA Isolation and qRT-PCR
The procedures of RNA isolation and real time qRT-PCR were described in detail previously [8]. The primers for prostate specific antigen (PSA), kallikrein-related peptidase 2 (KLK2), acetyl CoA acetyltransferase 2 (ACAT2), fatty acid synthase (FASN), transmembrane protease serine 2 (TMPRSS2) and NK3 homeobox 1 (NKX3-1) were synthesized by Integrated DNA Technologies (Coralville, IA). The PCR primer sequences used were: FASN, Forward-5-GCTCCAGCCTCGCTCTC-3′, Reverse- 5′-TCTCCGACTCTG GCAGCT T-3′; ACAT2, Forward- 5′-TGGGCAGAATCCTGTTAGACA-3′, Reverse- 5′-TGCAAGGCACACAGCTTTTAG-3′; NKX3-1, Forward- 5′-GTACCTGTCAGCCCCTGA AC-3′, Reverse- 5′-GGAGAGCTGCTTTCGCTTAG-3′; PSA, Forward-5′-GCATGG GATGGGGATGAAGTAAG-3′, Reverse- 5′-CATCAAATCTGAGGGTTGTCTGGA-3′; KLK2, Forward- 5′-GCTGCCCATTGCCTAAAGAAG-3′, Reverse- 5′-TGGGAA GCTGTGGCTGACA-3′; TMPRSS2, Forward-5′-CTGCCAAGGTGCTTCTCATT-3′, Reverse- 5′-CTGTCACCCTGGCAAGAATC-3′. Quantitative RT-PCR reactions were set up with the use of the SYBR Green qPCR Supermix and the Applied Biosystems 7900HT Fast RTPCR System (Foster City, CA). The relative expression of each gene was calculated using the -δδCt method and analyzed statistically as described previously [8].
AR Transfection
Wild type AR was over-expressed in AR-null DU145 cells using pSG5-AR (wt) provided by Dr. Chawnshang Chang [9]. AR T877A was over-expressed using pSG5-AR (LN) provided by Dr. Shutsung Liao [10]. AR H874Y was over-expressed using pCMV-AR (H874Y) provided by Dr. Frank French [11]. Mutations in the various AR genotypes were confirmed by DNA sequencing. The pGL3-Basic plasmid was purchased from Promega. DU145 cells were transfected with each of the AR plasmids using Lipofectamine 2000 from Invitrogen, after which the cells were replated in RPMI 1640 medium containing 10% charcoal-stripped FBS for 24 hr before treatment.
Additional AR transfection experiments involved over-expressing AR H874Y in LNCaP cells, and AR T877A in 22Rv1 cells.
Cell Viability Assay with Trypan Blue Staining
Cells were trypsinized and suspended in phosphate buffered saline and kept on ice. An equal volume of trypan blue solution was added to the cell suspension. Cells were counted under a microscope on a hemacytometer. Clear cells were counted as live cells, while blue colored cells were counted as dead cells. Each counting experiment was repeated 3 times.
RESULTS
Effect of Dutasteride on DHT-Stimulated ARE-Luciferase Activity in Different Prostate Cancer Cell Lines
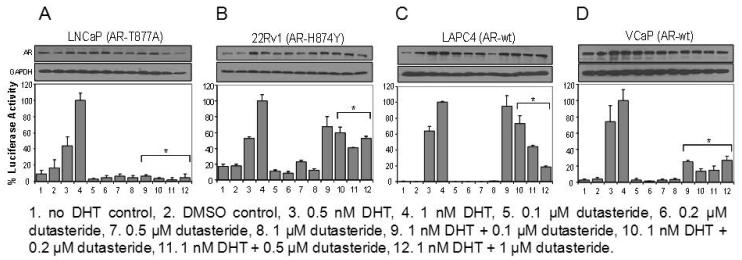
Cells were harvested at 24 hr after various treatments: DHT alone, dutasteride alone, or DHT + dutasteride. Samples also were generated for the determination of AR protein level by Western blotting. The luciferase data were normalized against total protein as well as the relative abundance of AR (Fig.1, bottom). The results for each cell line were expressed as percentages of the value observed in cells treated with 1 nM DHT (set as 100%) in order to facilitate comparisons among the 4 cell lines that have different sensitivity to DHT stimulation of ARE-luciferase activity. The 1 nM DHT data was chosen as the 100% standard because this DHT concentration yielded the maximal ARE-luciferase stimulation. The average fold of increase induced by 1 nM DHT in LAPC-4, LNCaP, VCaP and 22Rv1 cells was about 1,500, 40, 30 and 5, respectively (data not shown). Plotting the luciferase data as RLU values for each cell line would tend to distort the overall picture since the scale of measurement would have been stretched over too wide a range for proper interpretation.
Fig. 1.
Effect of dutasteride on DHT-stimulated ARE-luciferase activity in different prostate cancer cell lines. (A) LNCaP cells. (B) 22Rv1 cells. (C) LAPC4 cells. (D) VCaP cells. The luciferase activity observed in cells treated with 1 nM DHT was set as 100% (column 4). *P<0.05 compared to the 1 nM DHT value. The AR Western blot data are shown above each panel.
Two observations are common to all 4 cell models. First, DHT produced a dose-dependent stimulatory effect up to 1 nM (columns 1-4). Second, dutasteride by itself, at a concentration of 0.1, 0.2, 0.5 or 1 μM, did not increase ARE-luciferase activity above baseline value (columns 5-8), which suggested that dutasteride is not an AR agonist. The response to dutasteride in the presence of 1 nM DHT differed among the 4 cell lines. LNCaP cells were the most sensitive (Fig. 1A). The ARE-luciferase activity was reduced to 10% or less of the 1 nM DHT value over the dose range of 0.1-1 μM dutasteride (columns 9-12). The other end of the sensitivity spectrum was represented by 22Rv1 cells, which were much more resistant to dutasteride (Fig. 1B). A modest depression of ARE-luciferase activity by 40-60% was seen upon treatment with various doses of dutasteride. LAPC-4 cells showed a text-book example of a graded response to escalating doses of dutasteride (Fig. 1C, columns 9-12). Like LNCaP cells, VCaP cells also were sensitive to dutasteride (Fig. 1D). As little as 0.1 μM dutasteride was sufficient to depress the DHT-stimulated ARE-luciferase activity by ~70%. More dutasteride did not enhance the inhibitory effect.
Western blotting results showed that high concentrations of dutasteride appeared to cause a modest decrease of AR protein, an effect that was more pronounced in LNCaP cells (Fig. 1, top). As noted earlier in this subsection, the ARE-luciferase data were normalized against changes of AR protein level in order to eliminate such a potential confounding factor. These experiments demonstrate that different cell lines have different sensitivities to dutasteride inhibition of AR signaling.
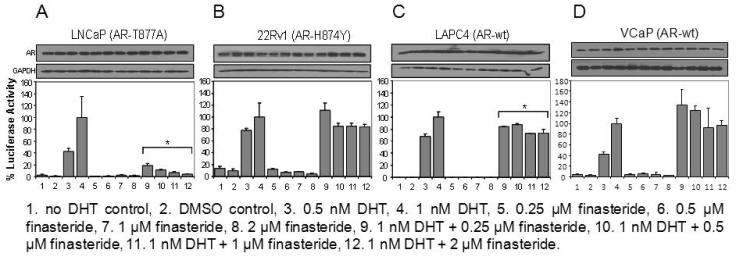
Effect of Finasteride on DHT-Stimulated ARE-Luciferase Activity in Different Prostate Cancer Cell Lines
A parallel set of experiments of identical design was carried out using finasteride (Fig. 2). LNCaP cells were very sensitive to finasteride inhibition of DHT-stimulated ARE-luciferase activity (Fig. 2A). Much of the activity was abolished by as little as 0.25 μM finasteride. In contrast, 22Rv1 cells responded poorly to finasteride (Fig. 2B). The small decreases observed with different doses of finasteride were not statistically significant. In LAPC-4 cells, finasteride caused a modest decrease of ARE-luciferase activity, although a dose-dependent effect was not apparent (Fig. 2C). Like 22Rv1 cells, VCaP cells also showed little evidence of responsiveness to finasteride (Fig. 2D). Finasteride by itself did not increase ARE-luciferase activity above baseline value in any of the 4 cell lines.
Fig. 2.
Effect of finasteride on DHT-stimulated ARE-luciferase activity in different prostate cancer cell lines. (A) LNCaP cells. (B) 22Rv1 cells. (C) LAPC4 cells. (D) VCaP cells. The luciferase activity observed in cells treated with 1 nM DHT was set as 100% (column 4). *P<0.05 compared to the 1 nM DHT value. The AR Western blot data are shown above each panel.
It may be timely at this juncture to take stock of the similarities and differences between the dutasteride and finasteride results. Neither drug is an agonist of AR. Both are capable of inhibiting DHT-stimulated AR activity, although dutasteride is more potent than finasteride in this respect. The last statement is supported by the data from 22Rv1, LAPC-4 and VCaP cells. The magnitude of AR activity inhibition by dutasteride is generally greater than that by finasteride. LNCaP is exquisitely sensitive to both dutasteride and finasteride such that ~90% of DHT-stimulated AR activity is negated by very low concentrations of either drug. In summary, these cell models offer an opportunity to explore how cell-specific characteristics may affect sensitivity to dutasteride or finasteride and their capacity to interfere with AR signaling.
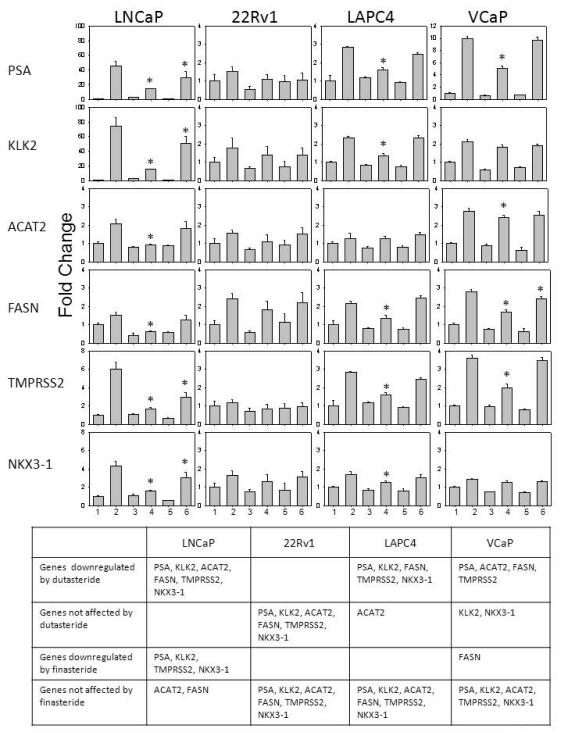
Effect of Dutasteride or Finasteride on Expression of AR-Regulated Genes
Since the ARE-luciferase assay is a measure of total AR activity that may lack physiological relevance, the expression of AR-regulated genes was quantified using RT-PCR after dutasteride or finasteride treatment (Fig. 3). The panel of genes included PSA, KLK2, ACAT2, FASN, TMPRSS2 and NKX3-1. Cells were harvested at 24 hr after various treatments: (i) untreated control, i.e., no DHT, (ii) DHT alone, (iii) dutasteride alone, (iv) DHT + dutasteride, (v) finasteride alone, and (vi) DHT + finasteride. The treatments were numbered in the same order as columns 1-6 in Figure 3. The gene expression data were presented as fold of change relative to the untreated control (the scale may vary from panel to panel). This method of presentation highlighted the up-regulation by DHT (column 2 vs column 1) and the ability of dutasteride (column 4) or finasteride (column 6) to reverse the DHT effect. The table below the bar graphs summarized the results in 4 categories for each cell model: (i) genes downregulated by dutasteride, (ii) genes not affected by dutasteride, (iii) genes downregulated by finasteride, and (iv) genes not affected by finasteride. The summary was based on statistical analysis of the bar graph data. Dutasteride was more effective than finasteride in down-regulating the expression of AR target genes. LNCaP was most sensitive to these 2 drugs, while 22Rv1 was least sensitive.
Fig. 3.
Effect of dutasteride or finasteride on the expression of AR-regulated genes, as determined using real time RT-PCR. Column 1, untreated control, i.e., no DHT. Column 2, 1 nM DHT. Column 3, 0.5 μM dutasteride. Column 4, 1 nM DHT + 0.5 μM dutasteride. Column 5, 1 μM finasteride. Column 6, 1 nM DHT + 1 μM finasteride. The data are expressed as fold of change relative to the untreated control. *P<0.05 compared to the DHT value.
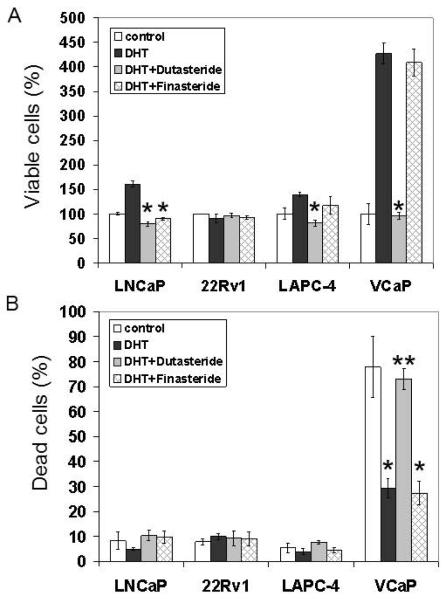
Effect of Dutasteride or Finasteride on Cell Growth and Cell Death
Cell growth was determined by counting viable cells to evaluate the biological consequence of dutasteride or finasteride treatment (Fig. 4A). The day 3 cell growth data were presented as percentages of the untreated control, which was set as 100%. DHT stimulated growth of LNCaP cells; both dutasteride and finasteride reduced this stimulatory effect to the untreated control value. In contrast, 22Rv1 cells were unresponsive to any of these treatments. In LAPC-4 cells, dutasteride but not finasteride blocked DHT-stimulated growth. Of the 4 cell lines, VCaP was the least tolerant of a charcoal-stripped serum condition. As a result, when DHT was added back to the medium, there was a marked increase of growth. Dutasteride completely reversed the stimulation by DHT, whereas finasteride was ineffective.
Fig. 4.
Effect of dutasteride of finasteride on cell growth and cell death. Cells were treated with 1nM DHT, 1 nM DHT + 0.5 μM dutasteride, or 1 nM DHT + 1 μM finasteride. (A) The day 3 viable cell data are expressed as percentages of the untreated control. (B) The day 3 dead cell data are expressed as percentages of the untreated control. * P<0.05 compared to the untreated control. **P<0.05 compared to the DHT value.
The proportion of dead cells also was assessed by trypan blue staining in the same experiment (Fig. 4B). The data were expressed as percentages of the total population. Dead cells accounted for <10% of the total population in LNCaP, 22Rv1 and LAPC4 cultures, even without DHT in the medium. Treatment with DHT had minimal impact on cell death protection in these 3 models. Neither dutasteride nor finasteride induced more cell death. VCaP cells fared poorly in the absence of DHT; after 3 days, close to 80% of the population became non-viable without DHT support. Repletion with DHT decreased markedly the proportion of dead cells, which suggests that the role of DHT in VCaP cells is mainly pro-survival. Dutasteride, but not finasteride, counteracted this pro-survival effect of DHT in VCaP cells.
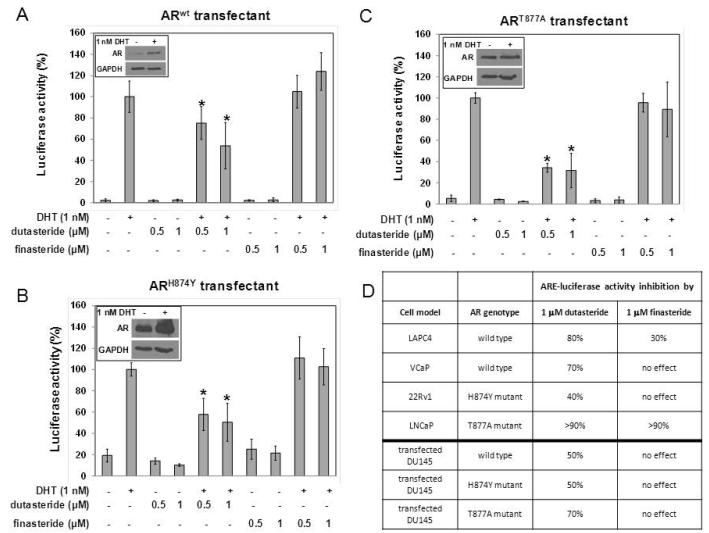
Effect of Dutasteride or Finasteride on ARE-Luciferase Activity in DU145 Cells Transfected with Different AR Genotypes
The data showed that dutasteride caused a more severe disruption of the AR signal than finasteride, although sensitivity to each drug varied among the 4 cell lines. Since different AR genotypes are present in the various cell lines, differences in sensitivity may be due to a cell type-specific effect or an AR genotype-specific effect. To address this question, AR-null DU145 prostate cancer cells were transfected transiently to express different AR isoforms, treated with DHT in the presence of either dutasteride or finasteride for 24 h, and assayed for ARE-luciferase activity (Fig. 5). Dutasteride treatment decreased ARE-luciferase activity in cells transfected with either wild type AR, H874Y mutant AR, or T877A mutant AR. In contrast, finasteride was ineffective in all 3 AR transfectants. The transfected AR Western blot data were shown in the insert of panels A, B and C. In order to highlight the divergence between the DU145 data and the AR-containing cell line data of Figures 1 and 2, a synopsis of the results obtained from the same experimental condition was recapitulated in table form in panel D. The AR-containing cell line data differed from the DU145 data when the same AR genotype was introduced into DU145 cells. The most striking example was provided by the transfected DU145/T877A AR model and the LNCaP/T877A AR cell line. Finasteride inhibited ARE-luciferase activity by >90% in the LNCaP/T877A AR cell line, but had no effect on the transfected DU145/T877A AR model. Likewise, both dutasteride and finasteride inhibited the LAPC-4/wild type AR cell line more than the DU145/wild type AR model. These findings suggest that cell type variation trumps AR genotype as a determinant of responsiveness to dutasteride and finasteride.
Fig. 5.
Effect of dutasteride or finasteride on DHT-stimulated ARE-luciferase activity in DU145 cells transfected with different AR genotypes. (A) wild type AR. (B) H874Y mutant AR. (C) T877A mutant AR. *P<0.05 compared to DHT value, which was set as 100%. (D) Summary of the results from parental cells carrying different AR genotypes versus DU145 cells transfected with different AR genotypes.
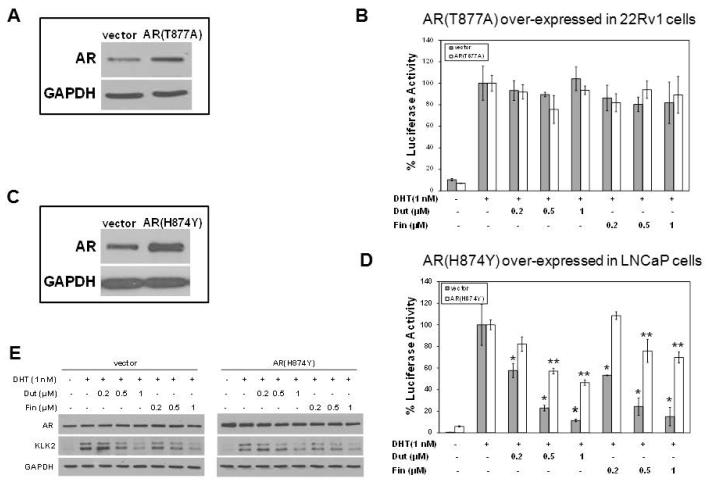
Cell Line Specificity and AR Responsiveness to Finasteride or Dutasteride
Additional experiments were carried out to determine whether cell line specificity is an important determinant in the response of AR to dutasteride or finasteride. The design involved over-expressing an exogenous AR from the most sensitive cell line in the least sensitive cell line, and vice versa. Among the four AR-positive cell models, LNCaP was the most sensitive to both drugs, whereas 22Rv1 was the least sensitive. First, AR (T877A) from LNCaP was over-expressed in 22Rv1 cells. The over-expression of AR (T877A) was confirmed using Western blotting (Fig. 6A). Neither dutasteride nor finasteride had a significant effect on DHT-stimulated luciferase activity either in control cells transfected with the empty vector or in cells over-expressing AR (T877A) (Fig. 6B). Next, AR (H874Y) from 22Rv1 was over-expressed in LNCaP. Western blotting showed the over-expressed AR (H874) was far more abundant than the endogenous AR (T877A) in the transfected LNCaP cells (Fig. 6C). DHT-stimulated luciferase activity of control cells was reduced by dutasteride or finasteride. However, DHT-stimulated luciferase activity also was reduced by either drug in AR (H874Y) over-expressing cells (Fig. 6D), although the magnitude of inhibition was not as great as observed in the empty vector-transfected control cells; this effect contrasted to the response of AR (H874Y) in its parental 22Rv1 cells transfected under exactly the same conditions (Fig. 6B). Treatment with drugs did not alter significantly the amount of AR protein in either empty vector-transfected control or AR (H874Y) transfected cells (Fig. 6E). Western blotting also showed that expression of AR-regulated KLK2 was reduced similarly by drug treatment regardless of the over-expressed AR (H874Y) in LNCaP cells. Responsiveness of an exogenous AR to either dutasteride or finasteride was influenced by the host cells into which it was introduced.
Fig. 6.
Cell line specificity and AR responsiveness to dutasteride or finasteride. (A) Western blotting of AR in empty vector-transfected 22Rv1 cells or AR (T877A)-transfected 22Rv1 cells. (B) Effect of dutasteride or finasteride on DHT-stimulated ARE-luciferase activity in AR (T877A) over-expressing 22Rv1 cells. (C) Western blotting of AR in empty vector-transfected LNCaP cells or AR (H874Y)-transfected LNCaP cells. (D) Effect of dutasteride or finasteride on DHT-stimulated ARE-luciferase activity in AR (H874Y) over-expressing LNCaP cells. * P<0.05 compared to DHT value of empty vector-transfected cells, which was set as 100%. **P<0.05 compared to DHT value of AR(H874Y)-transfected cells, which was set as 100%. (E) Western blots of AR and KLK2 in AR (H874Y) over-expressing LNCaP cells treated with dutasteride or finasteride.
DISCUSSION
There are three major conclusions from this study. First, different prostate cancer cell lines exhibit different sensitivities to dutasteride or finasteride inhibition of AR signaling. LNCaP and 22Rv1 are the most and least sensitive, respectively, while LAPC4 and VCaP are intermediate. Second, mutations in the ligand binding domain of AR do not appear to play a significant role in influencing the AR antagonistic effect of either drug. Disruption of AR signaling may have biological relevance since it is accompanied by decreased cell growth. Third, subcellular constituent is a key determinant of AR responsiveness to both drugs. This statement is supported by the results obtained from over-expressing various AR isoforms in either drug-sensitive or –resistant cells. Regardless of the AR genotype, if AR was transfected into drug-sensitive cells, AR was inhibited by drug treatment; and if AR was transfected into drug-resistant cells, AR was not inhibited.
AR (T877A) or AR (H874Y) were first identified in LNCaP cells [12] or in bone metastasis [13] and human prostate cancer xenograft CWR22 [11], respectively. The mutations have been known to alter AR transcriptional function. For example, the AR mutants are activated by antiandrogen hydroxyflutamide [11], although the mutants remain sensitive to inhibition by bicalutamide [14]. At least 159 AR mutations have been identified in human prostate cancer [15]. Some reports have noted that the frequency of AR mutation tends to increase in advanced/metastatic prostate cancer or after androgen deprivation therapy [16-20]. However, the data were obtained generally from studies involving small sample size. Nonetheless, it is safe to say that the frequency of AR mutation is relatively low across all clinical stages. Our finding suggests that the direct inhibitory effect of dutasteride or finasteride on AR may not be determined by the AR genotype. Therefore, these drugs are unlikely to be associated with the appearance of AR mutation which may be linked, albeit weakly, to disease progression.
A question that begs for an answer is whether the diverse response of the different cell lines extends to treatment with other AR antagonists, such as bicalutamide or MDV3100. To date, no information is available in which a comprehensive study was designed to examine systematically the response to several drugs in the same cell line, or the efficacy of a particular AR antagonist in a panel of cell lines (with the exception of the present study). There are, however, a handful of publications in which either a single drug was tested simultaneously in two different cell lines, or two different drugs in the same cell line [4, 21-26]. The evidence is thus not robust enough to draw any conclusion regarding the issue of whether the lack of sensitivity to AR antagonists falls under the general multidrug resistance phenomenon. Our poor understanding of this important subject would argue for further research into factors which may affect the efficacy of antagonists.
When LNCaP AR was over-expressed in 22Rv1 cells, the AR-over-expressing 22Rv1 remained resistant to dutasteride or finasteride. In contrast, when 22Rv1 AR was over-expressed in LNCaP cells, the AR-over-expressing LNCaP cells remained sensitive to dutasteride or finasteride. This cell line-dependent response of exogenous AR to the drugs was different from observed when AR was over-expressed to study the response to bicalutamide. When wild type AR was over-expressed in LNCaP or LAPC-4 cells, the growth of the AR-over-expressing cells became resistant to bicalutamide, and bicalutamide became an agonist to induce the expression of PSA [27]. Similar resistance to bicalutamide was observed when LNCaP AR or wild type AR was over-expressed in LNCaP and LAPC-4 cells [28]. Collectively, these results suggest different mechanisms govern the interaction with and response to dutasteride or finasteride, compared to bicalutamide.
A primary mode of action of dutasteride and finasteride in blocking the AR signal can be attributed to competitive inhibition of DHT binding to AR. Both drugs are structural analogs of DHT, although dutasteride has a bulkier side chain substitution at the C17 position than finasteride [29]. This chemical feature may account for the greater advantage of dutasteride as a DHT competitor. The IC50 of dutasteride and finasteride is ~1.5 and 3.8 μM, respectively, when R1881 (a synthetic androgen) is used as the ligand [30]. The stronger inhibitory effect of dutasteride also may be due to its higher bioavailability. In men, the circulating half-life of finasteride is 6-8 h, which is considerably shorter than the half-life of 3 days or more for dutasteride [31]. The finding suggests that dutasteride is metabolized slower systematically than finasteride. The prostate cancer cell lines used in this study also may have a low capacity for metabolizing dutasteride. In comparison to finasteride, more dutasteride may be retained intracellularly for a given period of time.
DHT binding to AR is known to be modulated by chaperone proteins, such as Prx1, Hsp90 and Hsp27 [32-34]. In a previous study, we found that lowering the level of Prx1 in prostate cancer cells by gene knockdown decreased substantially the affinity of DHT-AR interaction [32]. Cells with a poor source of these chaperone proteins may be more susceptible to dutasteride or finasteride because DHT can be displaced more easily from the AR. In addition to AR chaperones, post-translational modification of AR also may affect responsiveness to finasteride or dutasteride. At least 5 different post-translational modifications have been described, which include phosphorylation, acetylation, methylation, sumoylation and ubiquitination [35]. Little is known about the relationship between ligand binding and AR modifications. Different prostate cancer cell types may have different capacity and ways to modify AR. These changes could affect responsiveness to finasteride or dutasteride inhibition of AR activity.
Another factor that might affect sensitivity is the ability of cells to retain DHT. Of the 4 AR containing cell lines studied, LNCaP is the most sensitive, and that may be due to an exceptionally high UDP-glucuronosyl transferase level [36, 37]. This phase II detoxification enzyme attaches a glucuronide group to DHT, which facilitates export of DHT from cells [38]. A diminished availability of intracellular DHT would tend to enhance the efficacy of dutasteride or finasteride as an AR antagonist. The role of UDP-glucuronosyl transferase as a contributing factor in determining sensitivity to dutasteride or finasteride as an AR antagonist requires further investigation. Future research needs to be directed to investigating the intracellular factors which could increase the efficacy of dutasteride or finasteride as a direct AR antagonist. The information could be useful in identifying patients who may benefit more from treatment with these drugs.
CONCLUSIONS
The direct inhibitory effect of dutasteride or finasteride on AR signaling is cell line specific. Mutations in the ligand binding domain of AR do not appear to play a significant role in influencing the AR antagonistic effect of these drugs. Subcellular constituent is an important factor in determining the drug effect on AR function.
ACKNOWLEDGMENTS
This work was supported by NCI/NIH P01CA126804 (to C. Ip) and P01CA77739 (to J.L. Mohler), and an NCI Cancer Center Support Grant P30CA16056 to Roswell Park Cancer Institute.
Grant sponsors: National Cancer Institute, P01CA126804 and P01CA77739, and a National Cancer Institute Cancer Center Support Grant P30CA16056.
Footnotes
Conflict of Interest: There are no conflicts of interest or financial disclosures to report.
REFERENCES
- 1.Mohler JL, Titus MA, Wilson EM. Potential prostate cancer drug target: Bioactivation of androstanediol by conversion to dihydrotestosterone. Clin.Cancer Res. 2011;17(18):5844–5849. doi: 10.1158/1078-0432.CCR-11-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, Pettaway CA, Tammela TL, Teloken C, Tindall DJ, Somerville MC, Wilson TH, Fowler IL, Rittmaster RS. Effect of dutasteride on the risk of prostate cancer. N.Eng.J.Med. 2010;362(13):1192–1202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 3.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes D, Atkins JN, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman CA. The influence of finasteride on the development of prostate cancer. N.Eng.J.Med. 2003;349(3):215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y, Chhipa RR, Zhang H, Ip C. The antiandrogenic effect of finasteride against a mutant androgen receptor. Cancer Biol.Ther. 2011;11(10):902–909. doi: 10.4161/cbt.11.10.15187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sobel RE, Sadar MD. Cell lines used in prostate cancer research: A compendium of old and new lines - Part 1. J.Urol. 2005;173(2):342–359. doi: 10.1097/01.ju.0000141580.30910.57. [DOI] [PubMed] [Google Scholar]
- 6.Sobel RE, Sadar MD. Cell lines used in prostate cancer research: A compendium of old and new lines - Part 2. J.Urol. 2005;173(2):360–372. doi: 10.1097/01.ju.0000149989.01263.dc. [DOI] [PubMed] [Google Scholar]
- 7.Wu Y, Fabritius M, Ip C. Chemotherapeutic sensitization by endoplasmic reticulum stress: Increasing the efficacy of taxane against prostate cancer. Cancer Biol.Ther. 2009;8(2):146–152. doi: 10.4161/cbt.8.2.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng J, Wu Y, Mohler JL, Ip C. The transcriptomics of de novo androgen biosynthesis in prostate cancer cells following androgen reduction. Cancer Biol.Ther. 2010;9(12):1033–1042. doi: 10.4161/cbt.9.12.11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeh S, Chang C. Cloning and characterization of a specific coactivator, ARA 70, for the androgen receptor in human prostate cancer cells Proc. Natl.Acad.Sci.USA. 1996;93(11):5517–5521. doi: 10.1073/pnas.93.11.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kokontis J, Ito K, Hipakka RA, Liao S. Expression and function of normal and LNCaP androgen receptors in androgen-insensitive human prostatic cancer cells. Altered hormone and antihormone specificity in gene transactivation. Receptor. 1991;1(4):271–279. [PubMed] [Google Scholar]
- 11.Tan J, Sharief Y, Hamil KG, Gregory CW, Zang DY, Sar M, Gumerlock PH, deVere White RW, Pretlow TG, Harris SE, Wilson EM, Mohler JL, French FS. Dehydroepiandrosterone activates mutant androgen receptors expressed in the androgen-dependent human prostate cancer xenograft CWR22 and LNCaP cells. Mol.Endocrinol. 1997;11(4):450–459. doi: 10.1210/mend.11.4.9906. [DOI] [PubMed] [Google Scholar]
- 12.Veldscholte J, Ris-Stalpers C, Kuiper GG, Jenster G, Berrevoets C, Claassen E, van Rooij HC, Trapman J, Brinkmann AO, Mulder E. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem.Biophys.Res.Commun. 1990;173(2):534–540. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- 13.Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, Keer HN, Balk SP. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N.Eng.J.Med. 1995;332(21):1393–1398. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 14.Fenton MA, Shuster TD, Fertig AM, Taplin ME, Kolvenbag G, Bubley GJ, Balk SP. Functional characterization of mutant androgen receptors from androgen-independent prostat cancer. Clin.Cancer Res. 1997;3(8):1383–1388. [PubMed] [Google Scholar]
- 15.Gottlieb B, Beitel LK, Nadarajah A, Paliouras M, Trifiro M. The androgen receptor gene mutations database: 2012 update. Hum.Mutat. 2012;33(5):887–894. doi: 10.1002/humu.22046. [DOI] [PubMed] [Google Scholar]
- 16.Balk SP. Androgen receptor as a target in androgen-independent prostate cancer. Urology. 2002;60(Suppl 3A):132–139. doi: 10.1016/s0090-4295(02)01593-5. [DOI] [PubMed] [Google Scholar]
- 17.Bergerat JP, C,raline J. Pleiotropic functional properties of androgen receptor mutants in prostate cncer. Hum.Mutat. 2009;30(2):145–157. doi: 10.1002/humu.20848. [DOI] [PubMed] [Google Scholar]
- 18.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocrine Rev. 2004;25(2):276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 19.Steinkamp MP, O’Mahony OA, Brogley M, Rehman H, LaPensee EW, Dhanasekaran S, Hofer MD, Keufer R, Chinnaiyan A, Rubin MA, Pienta KJ, Robins DM. Treatment-dependent androgen receptor mutations in prostate cancer exploit multiple mechanisms to evade therapy. Cancer Res. 2009;69(10):4434–4442. doi: 10.1158/0008-5472.CAN-08-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan X, Balk SP. Mechanisms mediating androgen receptor reactivation after castration. Urol.Oncol. 2009;27(1):36–41. doi: 10.1016/j.urolonc.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrieu T, Bertolini R, Nichols SE, Setoud R, Frey FJ, Baker ME, Frey BM. A novel steroidal antiandrogen targeting wild type and mutant androgen receptors. Biochem.Pharmacol. 2012;82(11):1651–1662. doi: 10.1016/j.bcp.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Arnold JT, Liu X, Allen JD, Le H, McFann KK, Blackman MR. Androgen receptor or estrogen receptor-beta blockade alters DHEA-, DHT-, and E(2)-induced proliferation and PSA production in human prostate cancer cells. Prostate. 2007;67(11):1152–1162. doi: 10.1002/pros.20585. [DOI] [PubMed] [Google Scholar]
- 23.Cherian MT, Wilson EM, Shapiro DJ. A competitive inhibitor that reduces recruitment of androgen receptor to androgen-responsive genes. J.Biol.Chem. 2012;287(28):23368–23380. doi: 10.1074/jbc.M112.344671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makkonen H, Kauhanen M, Jaaskelainen T, Palvimo JJ. Androgen receptor amplification is reflected in the transcriptional responses of vertebral-cancer of the prostate cells. Mol.Cell.Endocrinol. 2011;331(1):57–65. doi: 10.1016/j.mce.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 25.McCourt C, Maxwell P, Mazzucchelli R, Montironi R, Scareplli M, Salto-Tellez M, O’Sullivan JM, Longley DB, Waugh DJ. Elevation of c-FLIP in castrate-resistant prostate cancer antagonizes therapeutic response to androgen receptor-targeted therapy. Clin.Cancer Res. 2012;18(14):3822–3833. doi: 10.1158/1078-0432.CCR-11-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seaton A, Scullin P, Maxwell PJ, Wilson C, Pettigrew J, Gallagher R, O’Sullivan JM, Johnston PG, Waugh DJ. Interleukin-8 signaling promotes androgen-independent proliferation of prostate cancer cells via induction of androgen receptor expression and activation. Carcinogenesis. 2008;29(6):1148–1156. doi: 10.1093/carcin/bgn109. [DOI] [PubMed] [Google Scholar]
- 27.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nature Medicine. 2004;10(9):33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 28.Sun C, Xu LL, Nageswararao C, Davis LD, Segawa T, Dobi A, McLeod DG, Srivastava S. Androgen receptor mutation (T877A) promotes prostate cancer cell growth and cell survival. Oncogene. 2006;25(28):3905–3913. doi: 10.1038/sj.onc.1209424. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt LJ, Tindall DJ. Steroid 5 alpha-reductase inhibitors targeting BPH and prostate cancer. J.Steroid Biochem.Mol.Biol. 2011;125(1-2):32–38. doi: 10.1016/j.jsbmb.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Lazier CB, Thomas LN, Douglas RC, Vessey JP, Rittmaster RS. Dutasteride, the dual 5α-reductase inhibitor, inhibits androgen action and promotes cell death in the LNCaP prostate cancer cell line. Prostate. 2004;58(2):130–144. doi: 10.1002/pros.10340. [DOI] [PubMed] [Google Scholar]
- 31.Xu Y, Dalrymple SL, Becker RE, Denmeade SR, Isaacs JT. Pharmacologic basis for the enhanced efficacy of dutasteride against prostatic cancers. Clin.Cancer Res. 2006;12(13):4072–4079. doi: 10.1158/1078-0432.CCR-06-0184. [DOI] [PubMed] [Google Scholar]
- 32.Chhipa RR, Lee KS, Onate S, Wu Y, Ip C. Prx1 enhances androgen receptor function in prostate cancer cells by increasing receptor affinity to dihydrotestosterone. Mol.Cancer Res. 2009;7(9):1543–1552. doi: 10.1158/1541-7786.MCR-08-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang Y, Fliss AE, Robins DM, Caplan AJ. Hsp90 regulates androgen receptor hormone binding affinity in vivo. J.Biol.Chem. 1996;271(45):28697–28702. doi: 10.1074/jbc.271.45.28697. [DOI] [PubMed] [Google Scholar]
- 34.Zoubeidi A, Zardan A, Beraldi E, Fazli L, Sowery R, Rennie P, Nelson C, Gleave M. Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer Res. 2007;67(21):10455–10465. doi: 10.1158/0008-5472.CAN-07-2057. [DOI] [PubMed] [Google Scholar]
- 35.Gioeli D, Paschal BM. Post-translational modification of the androgen receptor. Mol.Cell.Endocrinol. 2012;352(1-2):70–78. doi: 10.1016/j.mce.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Chang GTG, Blok LJ, Steenbeek M, Veldscholte J, van Weerden WM, van Steenbrugge GJ, Brinkmann AO. Differentially expressed genes in androgen-dependent and - independent prostate carcinomas. Cancer Res. 1997;57(18):4075–4081. [PubMed] [Google Scholar]
- 37.Guillemettte C, Hum DW, B,langer A. Evidence for a role of glucuronosyltransferase in the regulation of androgen action in the human prostatic cancer cell line LNCaP. J.Steroid Biochem.Mol.Biol. 1996;57(3-4):225–231. doi: 10.1016/0960-0760(95)00258-8. [DOI] [PubMed] [Google Scholar]
- 38.Barbier O, Belanger A. Inactivation of androgens by UDP-glucuronosyltransferases in the human prostate. Best Practice Res.Clin.Endocrinol.Metab. 2008;22(2):259–270. doi: 10.1016/j.beem.2008.01.001. [DOI] [PubMed] [Google Scholar]