INTRODUCTION
The decision regarding whether to initiate treatment with an SSRI for patients with depression is a common and important question for both patients and clinicians. Multiple clinical efficacy trials have demonstrated superiority of antidepressants to placebo.[1] Recently there has been increased debate about the effectiveness of antidepressants especially among patients with mild to moderate depression.[2–4] Parker in “Antidepressants on trial: how valid is the evidence?” discusses the concerns about using pharmaceutical registration efficacy trials to answer this question.[5] He underscores the exclusion of patients with documented medical and psychiatric co-morbidity, and the method of recruiting patients as important limitations in the generalizability of these studies. He also discusses the potential impact of heterogeneity within depressed populations which include patients with more classic biologic forms of depression such as melancholia and patients with more acute and situationally related depressive symptoms. Identification of subgroups more likely to reach remission or otherwise benefit from treatment is a critical clinical question. The aim of the Pharmacogenomic Research Network Antidepressant Medication Pharmacogenomic Study (PGRN-AMPS) is to identify subgroups of patients most likely to benefit from SSRIs.
The PGRN-AMPS was designed to assess clinical outcomes of patients with major depressive disorder (MDD) treated with either citalopram or escitalopram, and to investigate genetic factors associated with these outcomes. This report describes the clinical outcomes following treatment in 529 subjects and compares these treatment outcomes with those achieved in the first phase of the Sequence Treatment Alternatives to Relieve Depression Study (STAR*D). As in the STAR*D study,[6] the primary outcome measures in the PGRN-AMPS were remission defined as a Quick Inventory of Depressive Symptomatology Clinician Rated (QIDS-C16)[7] score of 5 or less and response defined as at least a 50% reduction in the QIDS-C16 score from baseline. However, unlike the STAR*D study, DNA and plasma were collected for all subjects and drug serum levels were monitored over the course of treatment. Blood drug levels were examined to assess medication adherence. The availability of DNA samples made it possible to verify self-reported gender and ethnicity which has rarely been done in depression efficacy and effectiveness studies.
While STAR*D was designed to be an effectiveness trial, data from this study have been used to address pharmacogenomic questions.[8, 9] In contrast, the PGRN-AMPS was designed to be a pharmacogenomic study and was conducted by a single research team within an integrated health care system.
MATERIALS AND METHOD
Subjects
This report focuses on the treatment outcomes of 529 participants in the PGRN-AMPS. Subjects with nonpsychotic MDD between 18 and 84 years of age were recruited from the inpatient and outpatient practices of the Department of Psychiatry and Psychology at Mayo Clinic in Rochester, Minnesota. Subjects with MDD could also be referred to the study by their primary care physician at Mayo Clinic or their clinician at a Mayo Clinic Health System facility in Austin, Minnesota. Participants were offered an eight-week course of treatment with either citalopram or escitalopram. All risks and benefits of the study were discussed with participants who gave written informed consent before entering the study. The Institutional Review Board of Mayo Clinic approved and monitored the protocol.
Subjects with medical contraindications to citalopram or escitalopram treatment and those who previously failed to respond to an adequate course of treatment with citalopram or escitalopram were excluded. Subjects with schizophrenia, schizoaffective disorder, bipolar I disorder or active substance abuse were excluded. Subjects who were actively suicidal were also excluded. No potential study subjects currently taking an antidepressant, antipsychotic or mood stabilizing medication were enrolled in the study. Pregnancy was an exlusionary criterion.
Diagnostic and Outcome Measures
At the baseline visit, a clinical research coordinator completed modules A, B, and D of the Structured Clinical Interview for DSM-IV (SCID) [10] to screen for mania, bipolar disorder, psychosis and major depression. The 17-item Hamilton Depression Rating Scale (HAMD-17)[11] and the QIDS-C16[7] were also completed to assess the severity of depression. A score ≥14 on the HAMD-17 at baseline was required for enrollment into the study. The Patient Reported Inventory of Side Effects (PRISE)[12] was administered to document symptoms prior to initiation of treatment for depression.
The HAMD-17 was administered by experienced clinical research coordinators certified to have a high rate of inter-rater reliability and level of procedural integrity. Quality monitoring was conducted quarterly in order to minimize rater drift.
Intervention
After the baseline evaluation, subjects were treated with citalopram or escitalopram for eight weeks. The initial plan was for all subjects to receive escitalopram. However, because a long-term guarantee of free medication could not be made and, given the differential cost of the two medications at the time of the study, some referring physicians preferred for their patients to receive citalopram. If the referring physician did not have a preference regarding study medication, the decision was made by a research clinician after a discussion with the subject. Participants were started on 20 mg/day of citalopram or 10 mg/day of escitalopram unless a lower dose was clinically indicated.
Face-to-face follow-up was scheduled for four and eight weeks after beginning the study medication. In addition, subjects were contacted by phone one and two weeks after the initiation of treatment to document side effects. Side effects were evaluated at each follow-up visit using the PRISE[13] and the Frequency and Intensity of Side Effect Rating/Global Rating of Side Effect Burden (FISER/GRSEB).[14] Participants were evaluated at each visit by a research clinician who reviewed the HAMD-17, QIDS-C16, Quick Inventory of Depressive Symptoms-Self Report (QIDS-SR)[15] and FISER/GRSEB. If the QIDS-C16 score was ≤5, the dose was maintained. If the QIDS-C16 score was between 6 and 8, the clinician would evaluate the subject and either maintain or increase the dose. If the QIDS-C16 score was ≥9, the dose was increased unless there was a contraindication due to intolerable side effects or patients’ refusal. The scheduled dose increase at the four-week visit was to 40 mg/day for citalopram or 20 mg/day for escitalopram.
Statistical Analysis
To facilitate comparisons with the STAR*D study, three subsets of subjects in the PGRN-AMPS were considered: (1) the entire “Intent to Treat” subset consisting of the 529 participants enrolled at the time of these analyses, (2) a subset of 463 “Eligible for Analysis” subjects who had at least one return visit after baseline, and (3) a subset of 398 white, non-Hispanic “Protocol Adherent” subjects who completed eight weeks of treatment and were compliant as demonstrated by having adequate blood drug levels
Our analysis focused on outcomes based on QIDS-C16 scores. Remission and response were defined based on the QIDS-C16 last visit score. Remission was defined as a QIDS-C16 score of 5 or less, while response was defined as at least a 50% reduction in the QIDS-C16 score from baseline. Clinical improvement was defined as a decrease of 5 or more points on the QIDS-C16. Association of remission with demographic and baseline clinical factors was assessed using logistic regression, and odds ratio estimates with confidence intervals were calculated. Association of baseline QIDS-C16 scores with quantitative treatment outcomes (i.e. absolute or percent change in QIDS-C16 score) was assessed using Spearman rank correlation.
Demographic characteristics of the PGRN-AMPS Eligible for Analysis subset were compared to the Eligible for Analysis STAR*D subset using likelihood ratio tests from logistic regression. Baseline QIDS-C16 scores were compared between groups using a t-test, and remission and response rates were compared using chi-square tests.
RESULTS
PGRN-AMPS Demographic and Clinical Characteristics and Comparison to STAR*D
The mean (SD) age of the Eligible for Analysis subset was 39.9 (13.8) years. The median length of the current episode was 7.1 months (lower quartile 2.4, upper quartile 24.0) and 97% of subjects described themselves as white. The PGRN-AMPS subjects and STAR*D subjects, had similar demographic characteristics except the PGRN-AMPS sample was younger, more likely to be married and employed, and to have completed college. (Additional information on clinical characteristics is in Supplement Table 1)
Treatment Outcomes in the PGRN-AMPS
Treatment outcomes were examined for the three subsets within the PGRN-AMPS. The Intent to Treat subset (n=529) had a remission rate of 40.0% and response rate of 56.7%. The Eligible for Analysis subset (n=463) had a remission rate of 45.8%, and a response rate of 64.8%. The Protocol Adherent subset (n=398) had a remission rate of 49.7% and a response rate of 68.8%. By definition, the Protocol Adherent subset remained in the study for eight weeks of supervised treatment and had a documented medication serum level. Fifteen patients in the Eligible for Analysis subset (12 that completed eight weeks and three that completed four weeks) had low serum levels of study drug.
There were no statistically significant differences in remission or response rates between subjects taking citalopram versus escitalopram in any of the three subsets. However, it is not possible to generalize this finding given the non-random assignment to the drug groups
Association of Remission with Demographic and Clinical Characteristics
PGRN-AMPS subjects who were currently employed were more likely to experience remission than those who were either unemployed or retired (p=0.003). Additionally, a history of attempted suicide was associated with lower likelihood of remission (p=0.04). Gender, marital status, level of education, family history, age group, age of onset, and the length of current depressive episode were not associated with likelihood of remission (Supplement Table 2).
Almost all patients in the Eligible for Analysis subset had improved depression severity after initiating treatment and very few patients had increased depression severity. Baseline depression severity predicted absolute decrease in depression severity and percent severity improvement (Table 1), with patients with higher baseline QIDS-C16 scores having greater absolute and percent decrease in QIDS-C16 during the course of treatment (p-value for Spearman correlation between baseline QIDS-C16 and change in QIDS-C16 < 0.001, p-value for Spearman correlation between baseline QIDS-C16 and percent change in QIDS-C16 = 0.021). However, even the patients with mild baseline depression symptoms had almost a 50% average decrease in depressive symptoms (Table 1). Remission rates were lower among subjects with greater depression severity at baseline (Table 1), with baseline QIDS-C16 score being a significant predictor of remission (OR=0.91, p<0.001).
Table 1.
Clinical outcomes by baseline severity defined based on Quick Inventory of Depressive Symptomology-Clinician Rated (QIDS-C16)
| Mean Baseline score |
Average change in QIDS-C16a |
Average Percent change in QIDS-C16a |
% Remission | |
|---|---|---|---|---|
| Very severe symptoms(QIDS-C16≥21) | 21.71 | 13.34 | 61.51% | 40.00% |
| Severe symptoms (16≤QIDS-C16≤20) | 17.53 | 10.06 | 57.16% | 38.37% |
| Moderate symptoms(11≤QIDS-C16≤15) | 13.19 | 7.11 | 53.82% | 48.15% |
| Mild symptoms(6≤QIDS-C16≤10) | 9.25 | 4.58 | 49.35% | 70.00% |
Change in QIDS-C16 from baseline to last visit
Alternative Method of Measuring Treatment Outcomes
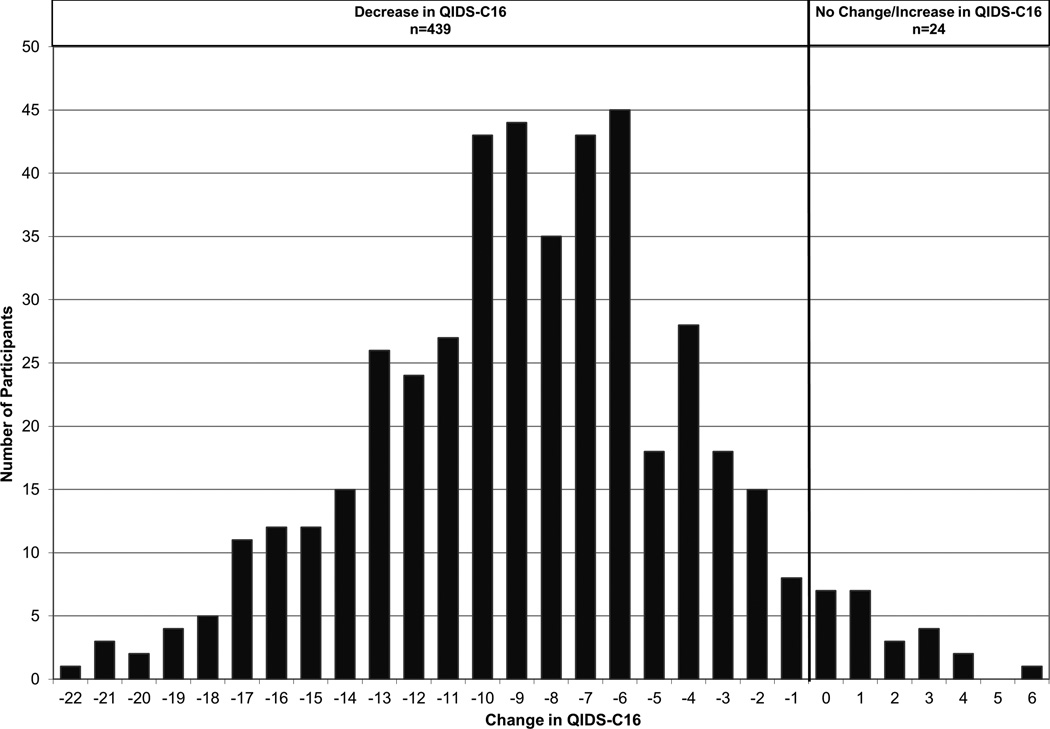
Figure 1 presents the absolute change in QIDS-C16 scores over the eight weeks of treatment for the Eligible for Analysis subset. At entry into the study, 7.6% of subjects had very severe symptoms and 83.8% had moderate to severe symptoms. At the eight week visit, only one subject still had very severe symptoms and 16.6% had moderate to severe symptoms. The mean (SD) QIDS-C16 scores in the Eligible for Analysis subset were 15.1 (3.4), 8.5 (4.4), 6.3 (4.1) at baseline, 4, and 8 weeks, respectively. The mean (SD) HAMD-17 scores were 22.3 (5.1), 12.2 (6.8), 8.9 (5.9) at the three time points. Both QIDS-C16 and HAMD-17 scores decreased significantly from baseline to 4 and 8 weeks (all p< 0.001). In the Eligible for Analysis subset, 79.9% of the subjects achieved an improvement in their QIDS-C16 score of more than 5 points.
Figure 1.
Change in QIDS-C16 Scores of the PGRN-AMPS Eligible for Analysis Subset (N=463)
Adverse Events and Side Effects at Week Four
Side effect frequency, intensity and burden are summarized in Table 2. Side effects for the Protocol Adherent subset were similar to those of the Eligible for Analysis subset. There were no statistically significant differences in frequency, intensity, or burden of side effects between citalopram and escitalopram within the PGRN-AMPS subsets (results not shown).
Table 2.
Comparison of Side Effects of the PGRN-AMPS Eligible for Analysis Subset Who Received Citalopram (N=143) and STAR*D Subset Who Received Citalopram (N=1,483) at the Four Week Visi
| PGRN-AMPS | STAR*D | ||||
|---|---|---|---|---|---|
| N | % | N | % | P | |
| Side Effect Frequency | 0.005 | ||||
| None | 69 | 48.30 | 533 | 35.90 | |
| 10%–25% of the time | 43 | 30.10 | 534 | 36.00 | |
| 50%–75% of the time | 23 | 16.10 | 267 | 18.00 | |
| 90%–100% of the time | 8 | 5.60 | 149 | 10.00 | |
| Side Effect Intensity | 0.001 | ||||
| None | 68 | 47.60 | 526 | 35.50 | |
| Trivial-mild | 45 | 31.50 | 513 | 34.60 | |
| Moderate-marked | 30 | 21.00 | 365 | 24.60 | |
| Severe-intolerable | 0 | 0.00 | 79 | 5.30 | |
| Side Effect Burden | <0.0001 | ||||
| No impairment | 87 | 60.80 | 662 | 44.60 | |
| Minimal-mild impairment | 45 | 31.50 | 608 | 41.00 | |
| Moderate-marked impairment | 11 | 7.70 | 181 | 12.20 | |
| Severe impairment-unable to function | 0 | 0.00 | 32 | 2.20 | |
A Comparison of Outcomes with STAR*D
The PGRN-AMPS Eligible for Analysis subset had a remission rate of 45.8%, which was not significantly different from the STAR*D Eligible for Analysis subset remission rate of 41.1% (p=0.07). However, the PGRN-AMPS Eligible for Analysis subset response rate was 64.8%, which was significantly higher than the STAR*D Eligible for Analysis subset response rate of 54.9% (p<0.0001). The mean baseline QIDS-C16 score was 16.4 for the STAR*D Eligible for Analysis subset and 15.1 for the PGRN-AMPS Eligible for Analysis subset (p<0.001). After accounting for demographic factors (marital status, employment, years of school, age group and white race), the response and remission rates were not significantly different between the two studies (p=0.096 for response, p=0.56 for remission).
The PGRN-AMPS Eligible for Analysis subjects who received citalopram reported a lower frequency of side effects and less severe side effects than the STAR*D Eligible for Analysis subset at four weeks (Table 2). These differences remained statistically significant after adjustment for demographic and baseline clinical covariates.
DISCUSSION
Most patients in the PGRN-AMPS showed improvement in their depressive symptoms over the course of eight weeks of treatment. Subjects in the Eligible for Analysis subset achieved a remission rate of 45.8%, while subjects in the Protocol Adherent subset achieved a remission rate of 49.7% after eight weeks of treatment. Of the 54.2% subjects in the Eligible for Analysis subset who did not achieve remission, many improved. Although studies designed to establish efficacy generally do not focus on partial responders, partial response is an important consideration for estimating the overall burden of an illness, and evaluating various degrees of improvement provides insight into the continued widespread use of antidepressant medications. If a decrease of 5 points or more is chosen to represent a clinically helpful improvement, 79.9% of the subset would be classified as having achieved this level of improvement. Some evidence to support the perspective that this level of improvement was of benefit was that virtually all subjects who achieved this level of improvement chose to continue to take their antidepressant medication after the completion of the eight week study.
The selection of treatment with citalopram versus escitalopram was not random. To replicate the STAR*D study and optimize generalizability there was no placebo control group. Additionally, there was limited information collected about psychiatric and medical co-morbidities prior to enrollment into the study making it impossible to investigate the effects of these factors on outcome.
Analyses comparing the PGRN-AMPS and the STAR*D study used the Eligible for Analysis subsets derived from the two studies. After accounting for demographic variables, the response rates of the two studies were not significantly different. However, the comparison of outcomes between the studies is also limited by methodological differences such as duration of treatment, study physician’s specialty, and number of visits.
The PGRN-AMPS subjects reported a lower frequency of side effects and a lesser severity of side effects than the STAR*D subjects. One possible explanation for this difference is that no subject in the PGRN-AMPS received more than 40 mg of citalopram, while 15.2% of subjects in the STAR*D study received a daily dose of more than 40 mg.
Patients contemplating initiating an SSRI to treat their MDD can anticipate a high probability of symptom improvement (79.9%) with a low probability that their symptoms will become worse. Patients with lower baseline severity have a higher probability of achieving remission. The probability of having moderate or severe burden of side effects is low (7.8%). An important objective for future research will be to develop methodologies to identify patients who are most likely to respond to antidepressant treatment.
Supplementary Material
Acknowledgements
We thank the staff of the Mayo Clinic Rochester Department of Psychiatry and Psychology for their effort in recruiting the patients to the Mayo PGRN-AMPS. The authors also wish to thank the STAR*D investigators for both their effort and generosity in sharing the clinical data and DNA samples. Forest Pharmaceuticals, Inc. provided the Lexapro samples for the study. Except providing study medication, Forest Pharmaceuticals had no role in the study financing, design, or data analyses.
Source of Funding
David A. Mrazek M.D. F.R.C. Psych has received grant support from the National Institutes of Health grants U19 GM61388 (The Pharmacogenomics Research Network) and U54RR 024150 (Mayo Clinic Center for Translational Science Activities). Dr Mrazek has an interest in intellectual property that has been licensed from the Mayo Clinic by AssureRx, which is a clinical decision support company.
David J. Katzelnick M.D. has equity ownership in Principal Healthcare Technology Systems Inc. Victor M. Karpyak M.D. Ph.D. has received grant support from the National Institutes of Health grant U19 GM61388 (The Pharmacogenomics Research Network), NIH/NIAAA grant P20 1P20AA017830-01 (The Mayo Clinic Center for Individualized Treatment of Alcohol Dependence).
Mark D. Williams M.D. was paid by the Interstate Postgraduate Medical Association for presenting updates to Family Practice on several topics including depression. Dr. Williams also was paid by the Institute for Clinical Systems Improvement for assisting in training primary care providers and others on treatment models for depression. Dr. Williams was paid for his participation in peer review presentations at the Neuroscience Education Institute on several topics including depression.
Richard Weinshilboum M.D. has no competing interests but has received grant support from the National Institutes of Health grants R01 GM28157, U19 GM61388 (The Pharmacogenomics Research Network), U01 HG05137, R01 CA138461.
This research was supported, in part, by NIH grants RO1 GM28157, U19 GM61388 (The Pharmacogenomics Research Network), U01 HG005137, R01 CA138461, P20 1P20AA017830-01 (The Mayo Clinic Center for Individualized Treatment of Alcohol Dependence), and a PhRMA Foundation Center of Excellence in Clinical Pharmacology Award. This publication was supported by NIH/NCRR/NCATS CTSA Grant Number UL1 RR024150. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
This paper has not been previously presented at a meeting.
Conflicts of Interest
For the remaining authors there were no conflicts of interest or source funding declared.
This research has been reviewed by the Mayo Clinic Conflict of interest Review Board and is being conducted in compliance with Mayo Clinic Conflict of Interest Policies and in accordance with the Declaration of Helsinki.
References
- 1.National Guideline C. Practice guideline for the treatment of patients with major depressive disorder. [Date Accessed 1/31/2013]; Available from: http://www.guidelines.gov/content.aspx?id=24158.
- 2.Kirsch I, Deacon BJ, Huedo-Medina TB, et al. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS medicine. 2008;5:e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303:47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hicks P, Hicks XP. Depression severity and effect of antidepressant medications. JAMA. 2010;303:1598. doi: 10.1001/jama.2010.508. author reply 1599. [DOI] [PubMed] [Google Scholar]
- 5.Parker G. Antidepressants on trial: how valid is the evidence? Brit J Psychiat. 2009;194:1–3. doi: 10.1192/bjp.bp.108.054767. [DOI] [PubMed] [Google Scholar]
- 6.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 7.Rush AJ, Bernstein IH, Trivedi MH, et al. An evaluation of the quick inventory of depressive symptomatology and the hamilton rating scale for depression: a sequenced treatment alternatives to relieve depression trial report. Biol Psychiatry. 2006;59:493–501. doi: 10.1016/j.biopsych.2005.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mrazek DA, Biernacka JM, O'Kane DJ, et al. CYP2C19 variation and citalopram response. Pharmacogenet Genomics. 2011;21:1–9. doi: 10.1097/fpc.0b013e328340bc5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mrazek DA, Rush AJ, Biernacka JM, et al. SLC6A4 variation and citalopram response. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:341–351. doi: 10.1002/ajmg.b.30816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.First M, Spitzer R, Williams J, et al. Structured clinical interview for DSM-IV (SCID) Washington, DC: American Psychiatric Association; 1995. [Google Scholar]
- 11.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wisniewski SR, Stegman D, Trivedi M, et al. Methods of testing feasibility for sequenced treatment alternatives to relieve depression (STAR*D) J Psychiatr Res. 2004;38:241–248. doi: 10.1016/j.jpsychires.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Rush AJ, Fava M, Wisniewski SR, et al. Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clin Trials. 2004;25:119–142. doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 14.Wisniewski SR, Rush AJ, Balasubramani GK, et al. Self-rated global measure of the frequency, intensity, and burden of side effects. J Psychiatr Pract. 2006;12:71–79. doi: 10.1097/00131746-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.