Abstract
Background:
Leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5), which is identified as a novel intestinal stem cell marker, is overexpressed in various tumours. In this study, we explore Lgr5 expression in gastric carcinoma and analyse its role in invasion, metastasis, and prognosis in carcinoma.
Methods:
A combination of immunohistochemistry, western blotting, and quantitative reverse transcription–polymerase chain reaction were used to detect mRNA and protein expression levels of Lgr5 and matrix metalloproteinase 2 (MMP2). Small interfering RNA against Lgr5 was designed, synthesised, and transfected into AGS cells. The effects of Lgr5 siRNA on cell invasion were detected by transwell invasion chamber assay and wound healing assay.
Results:
Leucine-rich repeat-containing G-protein-coupled receptor 5 expression was significantly higher in gastric carcinomas than in normal mucosa. Leucine-rich repeat-containing G-protein-coupled receptor 5 expression positively correlated with the depth of invasion, lymph node metastasis, distance of metastasis, and MMP2 expression levels. Multivariate analysis showed that Lgr5 had an independent effect on survival, and that it positively correlated with MMP2. Leucine-rich repeat-containing G-protein-coupled receptor 5 siRNAs inhibited Lgr5 mRNA and protein expression. Transwell assays indicated that these siRNAs resulted in significantly fewer cells migrating through the polycarbonate membrane, and wound healing assay also indicated that siRNAs decreased the migration of cells. Inhibition of Lgr5 resulted in a significant decrease in MMP2 and β-catenin levels compared with those in controls.
Conclusions:
Leucine-rich repeat-containing G-protein-coupled receptor 5 was correlated with invasion and metastasis. Leucine-rich repeat-containing G-protein-coupled receptor 5 inhibition could serve as a novel therapeutic approach.
Keywords: gastric cancer, Lgr5, invasion, metastasis, prognosis
Gastric cancer is one of the most commonly occurring malignant tumours worldwide, with approximately one million patients diagnosed annually (Shah and Kelsen, 2010). Despite advances in surgical techniques, chemotherapy, and radiotherapy, the survival rate of gastric cancer patients remains low because of local invasion and metastasis. These are known to be the major biological characteristics of gastric cancer, and are responsible for a poor prognosis (Samson et al, 2002). However, the detailed molecular mechanisms of this process have not been fully elucidated (Yakata et al, 2007). New markers for cancer prognosis, and therapeutic options targeting invasion and metastasis, are needed to improve gastric cancer therapies.
Leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5), also known as GPR49, is a member of the G-protein-coupled receptor family of proteins. It is also thought to be a target of Wnt signalling (Yamamoto et al, 2003; Van der Flier et al, 2007; Segditsas et al, 2008). The Lgr5 molecule is known to have a large N-terminal extracellular domain containing 18 leucine-rich repeats that interact with extracellular signals via the cytoplasmic G protein and that activate a number of downstream molecules (Fan et al, 2010). Previous studies indicated that Lgr5, a part of the Wnt signalling complex at the membrane level, could enhance Wnt/β-catenin signalling through the following mechanisms: Lgr5 could specifically recruit LRP–Frizzled receptor complex and eventually interfere with the degradation of the crucial signalling molecule β-catenin. Then, more accumulated β-catenin could translocate to the nucleus and regulate the expression of a wide range of target genes (Birchmeier, 2011; de Lau et al, 2011). Barker et al (2007, 2008) recently showed that Lgr5 expression was limited to the crypt base of the small and large intestines, and identified it as a novel intestinal stem cell marker. Leucine-rich repeat-containing G-protein-coupled receptor 5 is overexpressed in hepatocellular carcinoma (Yamamoto et al, 2003), colorectal cancer (McClanahan et al, 2006; Fan et al, 2010; Uchida et al, 2010; Takahashi et al, 2011; Takeda et al, 2011), ovarian cancer (McClanahan et al, 2006), basal cell carcinoma (Tanese et al, 2008), and oesophageal adenocarcinoma (Becker et al, 2010). Leucine-rich repeat-containing G-protein-coupled receptor 5 expression was detected in spheroid cells derived from colorectal cancers (Vermeulen et al, 2008), making it an ideal marker of colorectal cancer stem cells (Takahashi et al, 2011; Takeda et al, 2011). The Lgr5-positive cells were distributed in the invasive tumour front during colorectal cancer progression (Takeda et al, 2011), indicating that Lgr5 might have an important role in invasion and migration. Barker et al (2010) reported that Lgr5 expression was predominantly restricted to the pyloric glands contributing to epithelial self-renewal, and could serve as a unique marker of stem cells in the stomach. Barker et al (2010) also found that transformation of adult Lgr5-positive stem cells could drive tumour formation in the stomach in vivo. However, the expression and function of Lgr5 in gastric cancer has not yet been investigated.
Invasion and metastasis of gastric cancers involves multiple steps. The degradation of extracellular matrix (ECM) is a crucial step (Shim et al, 2007). It is well known that matrix degradation by matrix metalloproteinases (MMPs), such as MMP2 and MMP9, are critical for tumour invasion and metastasis (Deryugina and Quigley, 2006). Matrix metalloproteinase-2 is one of the key enzymes of the invasion and metastasis cascade for gastric cancer (Wu et al, 2006; Shim et al, 2007), because of its ability to degrade type IV collagen of extracellular matrices and basal membranes (Alakus et al, 2008). Furthermore, downregulation of E-cadherin expression could also promote metastasis of gastric cancer (Guo et al, 2013), and abnormal E-cadherin expression may be used as a predictive factor for tumour invasiveness in gastric adenocarcinoma (Anbiaee et al, 2013). To the best of our knowledge, there has been no published study reporting the relationship between Lgr5 and these invasion/metastasis-related factors in gastric cancer.
In this study, we analysed the expression levels of Lgr5 protein and mRNA in gastric cancer using immunohistochemistry, western blotting, and quantitative reverse transcription–polymerase chain reaction (qRT–PCR). We also explored the correlation of Lgr5 expression with clinicopathological features, in particular invasion and metastasis. The relationship between Lgr5 and MMP2 was also examined. We used RNA interference (RNAi) techniques to silence expression of the Lgr5 gene in the AGS gastric cancer cell line, and observed any effects on the invasiveness of these cells.
Materials and methods
Patients and tissue samples
Gastric cancer tissues were obtained from 318 patients who underwent curative surgical resection at the Chinese People's Liberation Army (PLA) General Hospital (Beijing, China) from 1999 to 2004. We randomly selected 80 distal normal gastric tissues from the 318 gastric cancer cases as normal controls. This study was conducted with the approval of the Chinese PLA General Hospital Research ethics committee. Tissues were fixed in formalin and embedded in paraffin. The medical records of patients, and the histopathology of each specimens were reviewed. The ages of patients ranged from 24 to 86 years (median, 65 years; mean, 59.6 years). Using the pathological TNM stages, as revised by the International Union Against Cancer (UICC) in 2009, 63 were classified as stage I, 117 were stage II, 122 were stage III, and 16 were stage IV. Samples of freshly resected gastric carcinoma (n=75) and samples of matched normal mucosal tissue adjacent to the carcinoma (n=75) were collected from patients who were treated at the PLA General Hospital in 2010. Samples were snap-frozen and stored at −80 °C until required.
Immunohistochemistry
Immunohistochemical staining of Lgr5 and MMP2 was conducted according to procedures described previously (Xi et al, 2012). The sections were incubated with a monoclonal rabbit antibody against human Lgr5 (1 : 50 dilution, ab75850; Abcam, Cambridge, MA, USA), and a mouse monoclonal antibody against human MMP2 (1 : 80, ab80737; Abcam) at 4 °C overnight. After washing with PBS, sections were incubated with an appropriate biotinylated secondary antibody (Zymed Laboratories, San Francisco, CA, USA) for 30 min. Peroxidase reactivity was visualised using a 3,3′-diaminobenzidine substrate kit (Zymed Laboratories), and slides were counterstained with haematoxylin. The primary antibody was replaced with PBS for use as a negative control.
Evaluation of immunohistochemistry
Antigen expression was independently scored by two experienced pathologists, who were blinded to all clinical pathology data. For cases where there was a discrepancy, a final score was established by reassessing on a double-headed microscope. For scoring Lgr5 and MMP2 expression, immunohistochemical staining of cells was evaluated according to a score that added a scale of intensity of staining to the proportion of stained cells (Xi et al, 2010). The intensity of staining was scored as follows: 0, no staining; 1+, weak staining; 2+, moderate staining; and 3+, intense staining. The proportion of stained cells was scored as follows: 0, no cells stained; 1+, positive staining in <10% of cells; 2+, positive staining of 10–50% of cells; and 3+, >50% of cells stained positive. The final score was determined by combining the two scores. A score of ⩽1 was considered negative, and a score of 2–6 was considered positive (Wood et al, 2006; Matsubara et al, 2008).
Protein extraction and western blotting
Protein extraction and western blotting were conducted as described previously (Shim et al, 2007). Total proteins from cells and frozen tissue were extracted with a lysis buffer. The concentration of proteins in the supernatant was analysed using the Bradford method (Bio-Rad, Hercules, CA, USA). Protein samples (50 μg per lane) were loaded onto 12% sodium dodecylsulphate gels and electrophoresed, and then transferred to nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ, USA). Membranes were blocked with 5% (w v−1) non-fat milk in TBST (50 mM Tris-HCl (pH 7.6), 150 mM NaCl, 0.1% Tween-20; blocking buffer) at room temperature for 1 h and incubated with a primary antibody diluted in blocking buffer (anti-Lgr5, 1 : 100; anti-MMP2, 1 : 400; anti-β-catenin, 1 : 600, ab2365; anti-MMP9, 1 : 1000, ab52496; anti-β-actin, 1 : 1000, ab8224; anti-GAPDH, 1 : 1500, ab184114 (Abcam); anti-E-cadherin, 1 : 500, sc-71008 (Santa Cruz Biotechnology, Santa Cruz, CA, USA)) overnight at 4 °C. After washing three times with TBST, membranes were incubated with a horseradish peroxidase-conjugated goat anti-rabbit/mouse secondary antibody (1 : 2000; Santa Cruz Biotechnology) for 2 h at room temperature. Antibodies against β-actin and GAPDH were used as internal controls. Bands were visualised using chemiluminescence, and protein bands were quantified with Quantity-One v4.4 software (Bio-Rad).
Cell culture
Five human gastric carcinoma cell lines (MGC-803, SGC-7901, AGS, BGC-823, MKN-45) and a normal human gastric mucosal epithelial cell line (GES-1) were purchased from the American Tissue Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in RPMI 1640 medium (GIBCO-BRL, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (FBS; GIBCO-BRL), 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin at 37 °C/5% CO2.
Transient transfection of Lgr5 siRNAs
An RNAi approach was used to knock down Lgr5 expression in AGS cells that expressed high levels of endogenous Lgr5. The siRNAs were synthesised by GenePharma Company (Shanghai, China), including three siRNA duplexes targeting different regions of Lgr5 mRNA. These were designated Lgr5-homo-409 (sense, 5′-GCAGAAUAAUCAGCUAAGATT-3′ antisense, 5′-UCUUAGCUGAUUAUUCUGCTT-3′), Lgr5-homo-1555 (sense, 5′-GGACGACCUUCAUAAGAAATT-3′ antisense, 5′-UUUCUUAUGAAGGUCGUCCTT-3′), and Lgr5-homo-2664 (sense, 5′-GCUCCAGCAUCACUUAUGATT-3′ antisense, 5′-UCAUAAGUGAUGCUGGAGCTT-3′). The sense and antisense negative controls were: 5′-UUCUCCGAACGUGUCACGUTT-3′ and 5′-ACGUGACACGUUCGGAGAATT-3′ respectively. The GAPDH sense and antisense positive controls were: 5′-GUAUGACAACAGCCUCAAGTT-3′ and 5′-CUUGAGGCUGUUGUCAUACTT-3′, respectively.
The gastric cells overexpressing Lgr5 were cultured in 6-well plates at a density of 5 × 105 ml−1. Cells for transfection were cultured in serum-free medium until 50–70% confluent (approximately 18 h later). Briefly, 4 μl of the siRNA was added to 50 μl of Opti-MEM serum-free medium (Invitrogen, Carlsbad, CA, USA). To another 50 μl of Opti-MEM serum-free medium, we added 2 μl of Lipofectamine 2000 (Invitrogen) and allowed it to incubate for 5 min at room temperature. The two solutions were then mixed and allowed to stand at room temperature for 20 min. The combined solution was added to 6-well plates and incubated at 37 °C/5% CO2 for 6 h. The medium was then replaced with serum-containing medium.
Transwell assays
Following transfections, the invasive potential of gastric cancer cells was assessed by transwell assays. Cell invasion assays were conducted using an Invasion Assay Kit (Chemicon, Millipore, CA, USA) according to the manufacturer's recommended protocols. Briefly, 300 μl of warm serum-free media was added to the interior of the inserts to rehydrate the ECM for 30 min at room temperature. Cells transfected with siRNAs for 48 h were harvested by trypsinisation. Cell suspensions were prepared (5 × 105 cells per ml) in serum-free medium, and added to the inserts within wells (100 μl per chamber). Media containing 10% FBS were added to the lower chambers (500 μl per chamber). Cells were cultured for 24 h, and non-invading cells along with ECM gel from the interior of the inserts were gently removed using a cotton-tipped swab. The membrane was stained using 0.1% crystal violet for 20 min, and washed with PBS. The numbers of cells were determined from five fields of view at × 200 magnification.
Wound healing assay
A total of 2 × 105 cells per well of each group (Blank, Negative, and siRNA-Lgr5) were seeded onto 12-well plates until confluent. The cell monolayer was scraped with a pipette tip to create an artificial wound field. The remaining cells were washed two times with PBS buffer, and supplied with a growth medium containing FBS. Pictures of the wounded area were captured using an inverted microscope (TE2000-E; Nikon, Tokyo, Japan) at 0 and 12 h after scratching and the width of the wounded area at 0 (W0) and 12 h (W12) was measured. The relative migration=(W0−W12)/W0.
Quantitative polymerase chain reaction assays
Total RNA was extracted from cells using an RNeasy Mini Kit (Qiagen, Tokyo, Japan) and reverse transcribed using a cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Assays were conducted on an ABI PRISM 7700 sequence detection system (Applied Biosystems) using SYBR Green PCR Master Mix (Applied Biosystems) at 95 °C for 10 min, followed by 50 cycles at 95 °C for 15 s, and then 60 °C for 1 min. For the quantitation of Lgr5 (GenBank Accession No. NM_003667.2), a 161 bp amplicon was generated using primers 5′-TTTGGACAAGGGAGACCTGGAGAAT-3′ and 5′-GAAAGCCACAGGGCAGTTTAGGAT-3′. For detection and quantitation of MMP2 (NM_001127891), a 160 bp amplicon was generated using primers 5′-AGCATGTCCCTACCGAGTCT-3′ and 5′-AAACAGATGGCAAACACGGC-3′. The primers 5′-AGAAGGCTGGGGCTCATTTG-3′ and 5′-AGGGGCCATCCACAGTCTTC-3′ were used to amplify a 266 bp portion of the GAPDH gene as a control. Relative values of transcripts were calculated using the 2−ΔΔC(T) method (Livak and Schmittgen, 2001). The mRNA expression level of Lgr5 was normalised to that of GAPDH.
Statistical analysis
SPSS v.13.0 (SPSS, Chicago, IL, USA) was used for the statistical analysis. Pearson's χ2-test was used to examine various clinicopathological characteristics with the expression of Lgr5. The Spearman's correlation coefficient test was used to assess the association between the expression of Lgr5 and MMP2. All quantitative data were presented as the mean±s.d. Paired-samples t-test was used to assess the difference of gene or protein expression between gastric cancer and normal mucosas. The Pearson's correlation was used to assess the association between relative expression of Lgr5 and MMP2. Cumulative survival curves were drawn by the Kaplan–Meier method. The difference between the curves was analysed by the log-rank test. Multivariate survival analysis was based on Cox proportional hazard model. Two-tailed Student's t-test was used to compare the number of invasive cells, the relative migration, and the relative expressions of target gene or protein between any two preselected groups. Dunnett's test, a multiple comparison procedure, was used to compare each of a number of groups with a single control. A value of P<0.05 was considered statistically significant. Furthermore, to correct for multiple comparisons a Bonferroni correction was performed. This was achieved by dividing the common P-value border 0.05 by the number of comparisons.
Results
Lgr5 expression and clinicopathological features
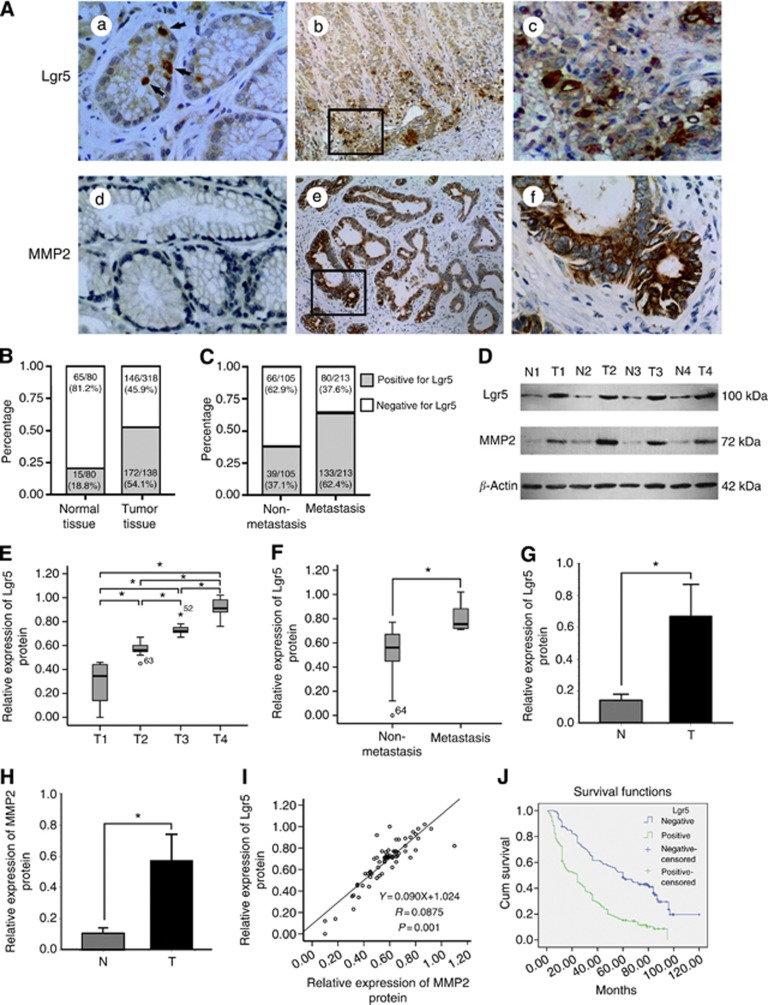
Leucine-rich repeat-containing G-protein-coupled receptor 5 was mainly expressed in the cytoplasm of gastric carcinoma cells (Figure 1A). For the gastric cancer samples, 54.1% (172 out of 318) expressed Lgr5, compared with 18.8% (15 out of 80, P=0.001) in normal mucosal tissues (Figure 1B). Leucine-rich repeat-containing G-protein-coupled receptor 5 was expressed in 33.3% (5 out of 15) of well-differentiated, 37.1% (23 out of 62) of moderately differentiated, and 59.8% (144 out of 241) of poorly differentiated carcinomas (Table 1). The presence of Lgr5 immunoreactivity appeared to correlate with the degree of tumour infiltration. Leucine-rich repeat-containing G-protein-coupled receptor 5 expression was more frequent in advanced T-stage cancers (P=0.001; Table 1). The cancer cells at the invasive front of the tumour exhibited intense staining for the presence of Lgr5 in most cases (Figure 1A). Leucine-rich repeat-containing G-protein-coupled receptor 5 expression was found to correlate with metastasis in regional lymph nodes (P=0.001) and distant (P=0.013) lymph nodes. In addition, the number of samples positive for Lgr5 were significantly greater when metastasis occurred compared with those without metastasis (62.4% vs 37.1% P=0.001; Figure 1C). Considering the TNM stage, an apparent relationship was seen between Lgr5 expression and tumour stage (P=0.001; Table 1).
Figure 1.
Analysis of Lgr5 and MMP2 in human gastric carcinoma and normal tissues. (A) Expression of Lgr5 and MMP2 was determined by immunohistochemical staining. Lgr5 was predominantly expressed in the cytoplasm. (a) Single epithelial cells were Lgr5-positive in normal tissue (arrows). (b and c) Carcinoma cells at the invasive front exhibit strong positive staining (asterisks). (d) Lack of MMP-2 expression in normal tissues. (e and f) MMP-2 staining in the cytoplasm of tumour cells. (a, c, d, and f: × 400 magnification; b and e: × 100 magnification). (B) The distribution of Lgr5 in 318 tumour or 80 normal tissue specimens was analysed. The proportion of Lgr5-positive cells in gastric cancer tissues was much higher than that in normal tissues. (C) The tumour specimens were divided into two groups according to whether cancer patients had tumour metastasis (including lymph node metastasis and distant metastasis). The percentage of specimens positive for Lgr5 were analysed. (D) Representative immunoblots of Lgr5 and MMP2 protein expression in whole tissue extracts from paired samples of gastric cancer tissue (T: tumour) and adjacent normal tissue (N: normal). β-Actin was used as the loading control. (E and F) Box and Whisker plots showing the distribution of Lgr5 proteins, as assessed by western blotting, in gastric cancer tissues according to the degree of tumour infiltration (T stage) and tumour metastasis status. Extremes and outliers are identified by ‘o' and ‘*', respectively. (E) Mean Lgr5 protein levels at T1, T2, T3, and T4. Lgr5 protein expression increased stepwise with T-stage progression (*P=0.001). (F) Leucine-rich repeat-containing G-protein-coupled receptor 5 protein expression was enhanced in metastasising tumours compared with non-metastatic cancer tissue (*P=0.001). (G and H) Comparison of Lgr5 and MMP2 protein expression levels between gastric cancer and normal tissues. Leucine-rich repeat-containing G-protein-coupled receptor 5 and MMP2 protein expression levels were higher in cancer tissues than those in adjacent normal mucosa (*P=0.001). Values are presented as the mean±s.d. (I) Correlation between Lgr5 and MMP2 protein expression levels in gastric cancer were analysed by Pearson's correlation test and linear regression. Each protein was quantified by determining the intensities of the band compared with that of β-actin. There was a positive correlation between Lgr5 and MMP2 levels (r=0.947, *P=0.014). (J) Kaplan–Meier survival curves for survival duration in patients with gastric cancer according to Lgr5 expression levels. Leucine-rich repeat-containing G-protein-coupled receptor 5-positive patients had significantly shorter survival durations than Lgr5-negative patients, as assessed by the log-rank test (P=0.001).
Table 1. Correlation between Lgr5 expression and clinicopathological features in gastric carcinoma.
| |
Lgr5 |
|
|
|---|---|---|---|
| Variable | Positive (%) | Negative (%) | P-value |
|
Gender | |||
| Male | 139 (53.7) | 120 (46.3) | 0.753 |
| Female |
33 (55.9) |
26 (44.1) |
|
|
Age (years) | |||
| n<45 | 13 (40.6) | 19 (59.4) | 0.243 |
| 45⩽n<60 | 107 (56.6) | 82 (43.4) | |
|
n⩾60 |
52 (53.6) |
45 (46.4) |
|
|
Tumour size (cm) | |||
| d<4 | 36 (48.6) | 38 (51.4) | 0.247 |
| 4⩽d<8 | 100 (53.5) | 87 (46.5) | |
|
d⩾8 |
36 (63.2) |
21 (36.8) |
|
|
Histologic differentiation | |||
| Well differentiated | 5 (33.3) | 10 (66.7) | 0.002a |
| Moderately differentiated | 23 (37.1) | 39 (62.9) | |
| Poorly differentiated |
144 (59.8) |
97 (40.2) |
|
|
Depth of invasion | |||
| T1 | 3 (13.6) | 19 (86.4) | 0.001a |
| T2 | 44 (44.4) | 55 (55.6) | |
| T3 | 108 (61.4) | 68 (38.6) | |
| T4 |
17 (81.0) |
4 (19.0) |
|
|
Lymph node metastasis | |||
| N0 | 40 (37.7) | 66 (62.3) | 0.001a |
| N1 | 35 (46.7) | 40 (53.3) | |
| N2 | 29 (64.4) | 16 (35.6) | |
| N3 |
68 (73.9) |
24 (26.1) |
|
|
Distant metastasis | |||
| Negative | 158 (52.3) | 144 (47.7) | 0.013a |
| Positive |
14 (87.5) |
2 (12.5) |
|
|
pTNM stage | |||
| I | 16 (25.4) | 47 (74.6) | 0.001a |
| II | 58 (49.6) | 59 (50.4) | |
| III | 84 (68.9) | 38 (31.1) | |
| IV |
14 (87.5) |
2 (12.5) |
|
|
MMP2 expression | |||
| Positive | 138 (74.6%) | 47 (25.4%) | 0.001a |
| Negative | 34 (25.6%) | 99 (74.4%) | |
Abbreviations: Lgr5=leucine-rich repeat-containing G-protein-coupled receptor 5; MMP-2=matrix metalloproteinase-2; TNM=tumour node metastasis.
A P-value <0.05 was considered statistically significant.
Lgr5 and MMP2 correlation
Matrix metalloproteinase2 expression was observed in 58.2% (185 out of 318) of gastric cancer samples and was mainly seen in the cytoplasm of cells (Figure 1A). Matrix metalloproteinase2 expression correlated with histological differentiation (P=0.015), tumour size (P=0.001), invasive depth (P=0.001), lymph node metastasis (P=0.001), TNM stage (P=0.001), and prognosis (P=0.001). There was also a positive correlation between Lgr5 and MMP2 expression (P=0.001, r=0.485; Table 1).
Association of Lgr5 and MMP2 proteins with tumour invasiveness and metastasis
We used western blotting to investigate Lgr5 and MMP2 protein expression in 75 paired biopsy tissue (Figure 1D). The distribution of the Lgr5 protein in gastric cancers at different T stages was presented (Figure 1E). The mean relative expression level of Lgr5 protein was significantly elevated (all P=0.001) in gastric cancer with respect to the degree of tumour infiltration (T stage): T1, 0.289±0.159 (n=10); T2, 0.571±0.054 (n=16); T3, 0.732±0.038 (n=35); and T4, 0.910±0.075 (n=14). Expression of the Lgr5 protein was increased in metastasising tumours compared with non-metastatic cancer tissue (0.516±0.187 vs 0.795±0.098, P=0.001) (Figure 1F). The mean relative expression levels of Lgr5 protein were 0.672±0.199 for cancerous tissues and 0.135±0.039 for adjacent normal mucosa. The mean relative expression level of the MMP2 protein was 0.568±0.170 for cancer tissues and 0.104±0.033 for adjacent normal mucosa. When compared with normal gastric mucosa, the relative expression levels of the Lgr5 and MMP2 proteins were significantly enhanced (P=0.001) in gastric cancer tissues (Figure 1G and H). There was a positive correlation between Lgr5 and MMP2 expression in gastric cancer tissues (r=0.875, P=0.001; Figure 1I).
Lgr5 expression and patient survival
Survival curves were calculated using the Kaplan–Meier analysis. The postoperative survival time (median, 23.5 months; mean, 31.17±4.18 months) for Lgr5-positive patients was significantly shorter than that for Lgr5-negative patients (median, 55 months; mean, 64.23±6.91 months; log rank=54.45, P=0.001; Figure 1J). Multivariate analysis using the Cox proportional hazards model showed that the expression of Lgr5 was an independent prognostic factor (P=0.001) with a hazard ratio of 1.656 (95% confidence interval: 1.219–2.249). Age, tumour size, lymph node metastasis, TNM stage, and MMP2 expression also appeared to be significant independent prognostic factors (Table 2).
Table 2. Cox regression analysis of prognostic factors in gastric carcinoma.
| |
|
|
|
|
|
95.0% CI for HR |
|
|---|---|---|---|---|---|---|---|
| Prognostic variables | B | s.e. | Wald value | P-value | HR | Lower | Upper |
| Gender | –0.275 | 0.172 | 2.560 | 0.110 | 0.759 | 0.542 | 1.064 |
| Age | 0.297 | 0.117 | 6.429 | 0.011a | 1.345 | 1.070 | 1.692 |
| Size | 0.643 | 0.128 | 25.253 | 0.000a | 1.901 | 1.480 | 2.443 |
| Histologic differentiation | 0.099 | 0.142 | 0.483 | 0.487 | 1.104 | 0.835 | 1.459 |
| Depth of invasion | 0.246 | 0.137 | 3.223 | 0.073 | 1.279 | 0.978 | 1.674 |
| Lymph node metastasis | 0.273 | 0.123 | 4.940 | 0.026a | 1.314 | 1.033 | 1.673 |
| Distant metastasis | 0.695 | 0.385 | 3.251 | 0.071 | 2.004 | 0.941 | 4.266 |
| TNM stage | 0.666 | 0.244 | 7.440 | 0.006a | 1.947 | 1.206 | 3.143 |
| Lgr5 | 0.504 | 0.156 | 10.436 | 0.001a | 1.656 | 1.219 | 2.249 |
| MMP-2 | 0.397 | 0.179 | 4.927 | 0.026a | 1.487 | 1.047 | 2.110 |
Abbreviations: B=partial regression coefficient; CI=confidence interval; HR=hazard ratio; Lgr5=leucine-rich repeat-containing G-protein-coupled receptor 5; MMP-2=matrix metalloproteinase-2; Wald value=statistic for (B/s.e.)2.
Statistically significant.
Expression of Lgr5 and MMP2 in cell lines
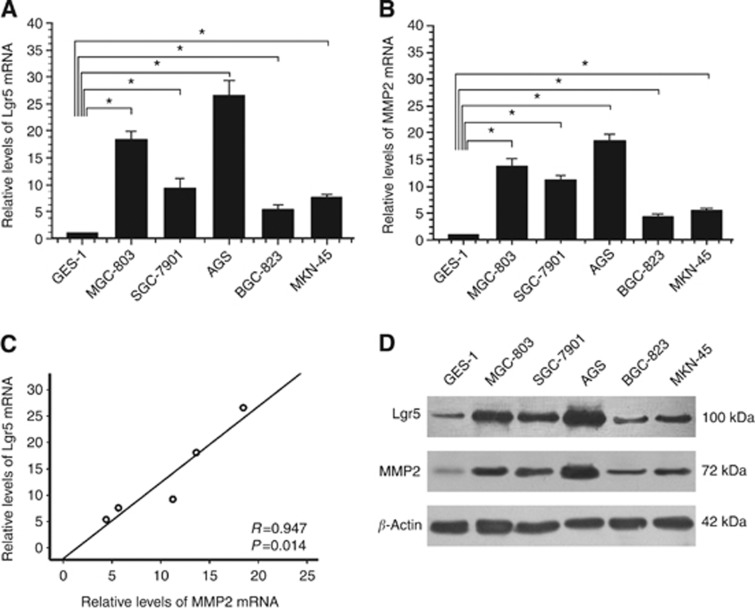
The relative expression levels of Lgr5 and MMP2 mRNAs were significantly higher (all P<0.05) in the gastric cancer cell lines (MGC803, SGC7901, AGS, BGC823, and MKN45) than in the gastric epithelial cell line (GES-1) (Figure 2A and B). There was a positive correlation between the Lgr5 and MMP2 mRNA levels (r=0.947, P=0.014; Figure 2C). Western blot analysis showed that Lgr5 and MMP2 protein levels were significantly higher in the gastric cancer cell lines than in the normal mucosa cell line, corresponding with the qRT–PCR results (Figure 2D). The expression levels of Lgr5 were highest in AGS cells. Therefore, AGS cells were chosen for RNAi experiments.
Figure 2.
Expression of Lgr5 and MMP2 in a gastric epithelial cell line (GES-1) and gastric carcinoma cell lines (MGC803, SGC7901, AGS, BGC823, and MKN45). (A and B) Relative Lgr5 and MMP2 mRNA expression levels were examined by qPCR, and were normalised to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (A) The mean Lgr5 mRNA expression levels were approximately 26-, 18-, 9.2-, 7.6-, and 5.4-fold higher in the MGC803, SGC7901, AGS, BGC823, and MKN45 cell lines, respectively, compared with those in GES-1 cells (*P<0.05). (B) Mean MMP-2 mRNA expression levels were approximately 18-, 13-, 11-, 5.6-, and 4.4-fold higher in the MGC803, SGC7901, AGS, BGC823, and MKN45 cell lines, respectively, compared with the expression levels in the GES-1 cells (*P<0.05). (C) Statistical correlation between Lgr5 and MMP-2 mRNA expression levels in five gastric cancer cell lines was analysed using Pearson's test. There was a positive correlation between Lgr5 and MMP-2 mRNAs (P=0.001). (D) Expression of Lgr5 and MMP2 proteins in a gastric epithelial cell line and five gastric cancer cell lines as determined by western blotting. Leucine-rich repeat-containing G-protein-coupled receptor 5 and MMP-2 protein expression levels were significantly higher in the MGC803, SGC7901, AGS, BGC823, and MKN45 cell lines than in the GES-1 cells. β-Actin was used as the loading control.
siRNAs suppressed Lgr5 expression in gastric cancer cells
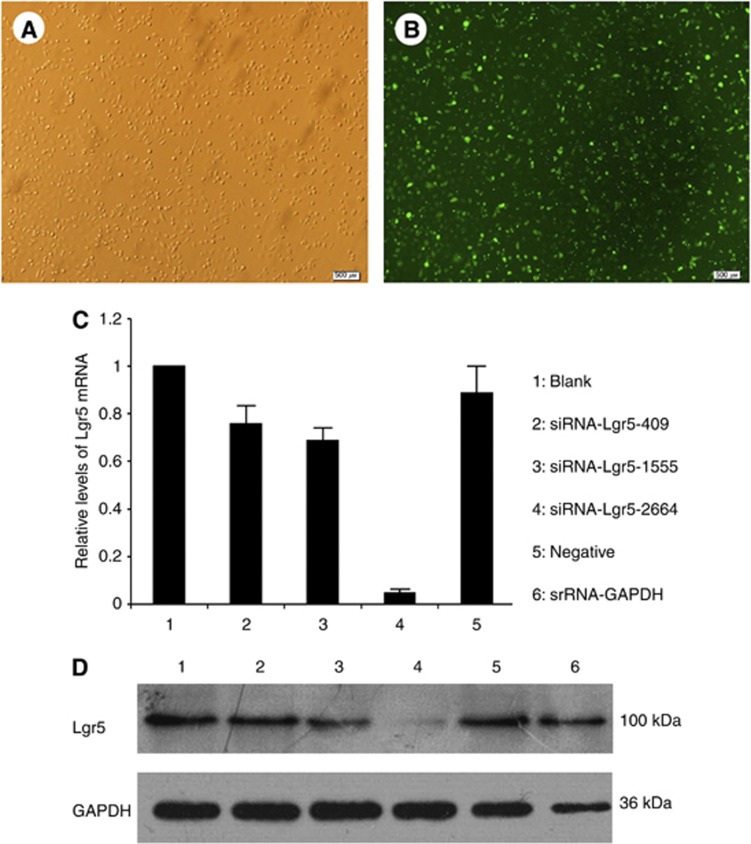
AGS cells were transfected with the negative control FAM-siRNA for 18 h. Once the culture reached 80% confluence, green fluorescence was observed, indicating a transfection efficiency >90% (Figure 3A and B). At 48 h post-transfection, total RNA and protein were extracted from transfected AGS cells for quantitative polymerase chain reaction (qPCR) and western blotting assays. Expression levels of Lgr5 mRNA in different groups (Blank control, 1.00±0; siRNA-Lgr5-409, 0.76±0.08; siRNA-Lgr5-1555, 0.69±0.06; siRNA-Lgr5-2664, 0.05±0.02; and Negative control siRNA, 0.89±0.12) indicated that transfection with these Lgr5 siRNAs resulted in a significant decrease in Lgr5 mRNA. The Lgr5-homo-2664 siRNAs were most efficient in suppressing Lgr5 expression compared with other groups (all P<0.05; Figure 3C). Transfection with the Negative control siRNA did not affect Lgr5 expression. This result was confirmed at the protein level through western blotting (Figure 3D).
Figure 3.
(A and B) Transfection efficiency of negative control FAM-siRNAs in AGS cells. (A) Observation under a regular microscope. (B) Observation of the same field of view using a fluorescence microscope. (A and B: × 200 magnification). (C and D) The effect of different siRNAs on the expression of Lgr5 mRNA and protein. Confluent gastric cancer AGS cells were seeded into 6-well plates, and transfected with different siRNAs (siRNA-Lgr5-409, siRNA-Lgr5-1555, and siRNA-Lgr5-2664) or scramble siRNA (negative control). The untransfected cells served as a blank control. Leucine-rich repeat-containing G-protein-coupled receptor 5 mRNA and protein levels were determined by qPCR and western blotting. Transfection of these Lgr5 siRNAs resulted in a significant decrease in Lgr5 mRNA and protein expression. The Lgr5-homo-2664 exerted the greatest effects in suppressing Lgr5 expression. Triplicate experiments were performed with almost identical results. Expression levels of Lgr5 mRNA are presented as mean±s.d.
Effect of Lgr5 interference on AGS cell invasion and migration
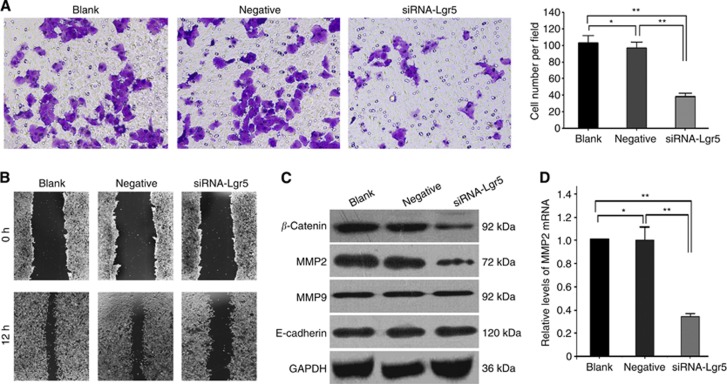
The change in the invasiveness of AGS gastric cancer cells was investigated by Transwell invasion chamber assay. AGS cells were divided into three groups: a non-transfected control group (Blank); a negative-siRNA-transfected control group (Negative); and an Lgr5 siRNA-transfected group (siRNA-Lgr5). Our findings showed that AGS cells transfected with Lgr5 siRNA resulted in significantly fewer cells passing through the polycarbonate membrane of the transwell invasion chamber compared with those in the Blank and Negative groups (P=0.001 and 0.001, respectively). Cell invasiveness was not significantly different between the Blank and Negative groups (P=0.141; Figure 4A).
Figure 4.
(A) Effect of Lgr5 interference on the invasive ability of gastric cancer AGS cells. The invasion of AGS cells in three groups (Blank, Negative, and siRNA-Lgr5) was investigated by transwell invasion assays. Representative images showed that Lgr5 interference significantly reduced cell penetration through the matrigel-coated membrane relative to the other two groups (all P=0.001). (B) Wound healing assay of Lgr5-siRNA-transfected AGS cells. Representative pictures of the wound at 0 and 12 h in different groups. The relative migration of different groups was calculated 12 h after the wound was made. (C) Expression of β-catenin, MMP-2, MMP9, and E-cadherin in Lgr5-silenced AGS cells and control groups at the protein level using western blotting. Leucine-rich repeat-containing G-protein-coupled receptor 5 interference decreased β-catenin, and MMP-2 protein expression levels when compared with the two control groups (all P=0.001). However, the expression of MMP9 and E-cadherin did not change significantly. (D) Leucine-rich repeat-containing G-protein-coupled receptor 5 interference decreased MMP-2 mRNA and protein expression levels when compared with the two control groups (P=0.001). The values presented are the mean±s.d. from three independent experiments. **P=0.001 compared with cells from the Blank or Negative groups.
The wound healing assay was performed to assess the effect of Lgr5 interference on AGS cell migration. In comparison with the Blank (relative migration, 86.5±1.4%) and Negative groups (relative migration, 86.0±2.3%), the silencing of Lgr5 by siRNA decreased the migration of AGS cells (relative migration, 43.9±2.3%) (all P=0.001). There was no difference between the Blank and Negative control groups (P=0.764) (Figure 4B).
MMP2 may be involved in invasion and migration of gastric cancer regulated by Lgr5
Both MMP2 and β-catenin protein expression levels (0.24±0.02 and 0.31±0.02) were significantly lower in the AGS cells transfected with Lgr5 siRNA than that in the Blank (0.73±0.02 and 0.72±0.03) or Negative (0.67±0.03 and 0.70±0.03) groups (all P=0.001). However, Lgr5 siRNA did not alter the expression level of MMP9 and E-cadherin protein (P>0.05) (Figure 4C). Furthermore, qRT–PCR results showed that the expression level of MMP2 mRNA (0.34±0.03) was also decreased in the AGS cells transfected with Lgr5 siRNA compared with Blank (1.00±0.0) or Negative (0.99±0.12) groups. At the mRNA (P=0.912) or protein (P=0.07) level, there was no difference between the Blank and Negative control groups (Figure 4C and D).
Discussion
Leucine-rich repeat-containing G-protein-coupled receptor 5 has been shown to be a stem cell marker of the small intestine, colon, and stomach (Yamamoto et al, 2003; Barker et al, 2007, 2008, 2010; Van der Flier et al, 2007; Segditsas et al, 2008). Reports have demonstrated that overexpression of Lgr5 in different primary tumours, including hepatocellular carcinoma (Yamamoto et al, 2003), colorectal cancer (McClanahan et al, 2006; Fan et al, 2010; Uchida et al, 2010; Takahashi et al, 2011; Takeda et al, 2011), ovarian cancer (McClanahan et al, 2006), basal cell carcinoma (Tanese et al, 2008), and oesophageal adenocarcinoma (Becker et al, 2010), is associated with carcinogenesis and progression. In their recent colorectal cancer study, Takahashi et al (2011)indicated that Lgr5 mRNA expression significantly correlated with metastasis in regional lymph nodes, distant metastasis, and TNM stage.
In the present study, we investigated the expression of Lgr5 in a large sample of gastric cancer tissues with follow-up data using immunohisochemical techniques. Statistical analyses of our data showed that Lgr5 expression was more frequent in cancerous than in normal mucosal tissues. In normal mucosa, only single epithelial cells at the base of the gland displayed Lgr5 staining. This result was in accordance with those from a previous study (Barker et al, 2010). In gastric cancer tissues, it was seen more frequently in advanced gastric cancers. It is suggested that Lgr5 may have an important role in the development and progression of tumours. In addition, patients with Lgr5-positive gastric cancers had poor survival compared with patients who have Lgr5-negative gastric cancers. This implies that Lgr5 might be a specific molecular marker in the prognosis of gastric cancer.
Invasion and metastasis are the most life-threatening aspects of cancer (Liotta and Stetler-Stevenson, 1991; Liotta and Clair, 2000). Gastric cancer usually presents extensive local invasion and early metastasis, especially in regional lymph nodes (Monig et al, 2001). Invasion and metastasis of gastric cancer is a multistep process. Degradation of the ECM and penetration of the basement membrane by MMPs are crucial steps in these processes (Guszczyn and Sobolewski, 2004). Among the MMPs, MMP-2 has been shown to be one of the key enzymes in the invasion and metastasis cascade of gastric cancer (Wu et al, 2006; Shim et al, 2007), because of its ability to degrade type IV collagen, a major component of ECMs and basement membranes (Alakus et al, 2008). In this study, the expression of MMP-2 positively correlated with the depth of tumour infiltration, lymph node metastasis, and TNM stage. These results were in accordance with those from previous studies (Sier et al, 1996; Monig et al, 2001). We also found that a high level of Lgr5 expression in gastric cancer positively correlated with the depth of invasion, lymph node metastasis, distant metastasis, and MMP2 expression. Leucine-rich repeat-containing G-protein-coupled receptor 5 expression exhibited a focal, patchy, or widespread distribution pattern, with increased expression levels at invasive margins. On the basis of these results, we speculated that Lgr5 might have a crucial role in the invasion and metastasis of gastric cancer.
To provide further evidence that Lgr5 contributes to the invasion and metastasis of gastric cancer, we performed a series of experiments in vitro. We used several gastric cancer cell lines (MGC803, SGC7901, AGS, BGC823, and MKN45) and examined Lgr5 mRNA and protein expression levels with qPCR and western blotting techniques, respectively. Leucine-rich repeat-containing G-protein-coupled receptor 5 mRNA and protein levels were highest in AGS cells. Leucine-rich repeat-containing G-protein-coupled receptor 5 siRNAs inhibited Lgr5 mRNA and protein expression in AGS cells. Cell invasiveness and migration were assessed using transwell invasion assays and wound healing assay. The results indicated that AGS cells transfected with Lgr5 siRNAs resulted in significantly fewer cells passing through the polycarbonate membranes. Furthermore, the silencing of Lgr5 by siRNA decreased the migration of AGS cells compared with Blank or Negative groups. These findings suggested that Lgr5 interference could suppress the invasion and migration of AGS cells. This might be related to canonical Wnt (β-catenin) signalling. Recently, some studies have reported that Lgr5 specifically recruits the LRPs–Frizzled receptor complex, which could be activated by extracellular Wnt molecules. This reinforces Wnt signalling following phosphorylation of LRP, and eventually interferes with the degradation of the crucial β-catenin signalling molecule. Consequently, β-catenin accumulates and translocates to the nucleus, together with the Tcf/Lef family of transcription factors, and also induces the expression of a wide range of target genes (Birchmeier, 2011; de Lau et al, 2011), possibly including MMP2. Because of this, the Wnt target Lef1, a member of the Tcf/Lef family, specifically induces MMP2 expression and then promotes cell invasion (Planutiene et al, 2011). In this study, the results showed that the expression of β-catenin and MMP2 was decreased significantly when Lgr5 expression was silenced by siRNA in gastric cancer AGS cells. However, the expression of cancer invasion-related molecules MMP9 and E-cadherin did not change. This indicated that the interference of Lgr5 might affect the activation of Wnt signalling pathway, and might thereby reduce the accumulation and translocation of β-catenin into the nucleus, resulting in reduced expression of MMP2, and might also ultimately inhibit the invasion of gastric cancer. The molecular mechanisms linking Lgr5 and MMP2 need further investigation. As upregulation of Lgr5 is critical for tumour invasion and metastasis, it could be an attractive target for the treatment of gastric cancers. We speculate that antagonists of Lgr5, or human-type neutralising monoclonal antibodies against Lgr5, may be clinically applicable for the prevention and therapy of gastric cancer invasion and metastasis.
In conclusion, Lgr5 expression was commonly upregulated in gastric cancer tissues and was associated with shortened survival time of patients. These findings could help in predicting the clinical outcome of gastric cancer. High levels of Lgr5 expression were associated with the depth of invasion, lymph node metastasis, distant metastasis, and MMP2 expression. Leucine-rich repeat-containing G-protein-coupled receptor 5 siRNA could suppress gastric cancer cell invasion and migration efficiently. Leucine-rich repeat-containing G-protein-coupled receptor 5 regulates invasion of AGS cell lines in vitro by regulating MMP2 expression. Therefore, Lgr5 is responsible for the invasion and metastasis of human gastric cancer. Further investigation is necessary to elucidate Lgr5 functions, and the underlying mechanisms of its regulation. This should provide a better understanding of gastric cancer invasion and metastasis, and will assist in elucidating novel therapeutic strategies against gastric cancer.
Acknowledgments
This work was supported by grants from the National Nature Science Foundation of China (Nos. 81272698, 81101883, and 81172368), a grant from PLA Medical Technology Key Project of Scientific Research in the 12th Five-Year-Plan (No. BWS12J049), a grant from PLA medical and health research fund project (No. 11BJZ17), a grant form the Capital Health Research and Development of Special (No. 2011-5001-01), and a grant from Major Science and Technology Progect of ‘National Significant New Drug Creation' from the Major Science and Technology of China (No. 2011ZX09307-001-05).
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Alakus H, Grass G, Hennecken JK, Bollschweiler E, Schulte C, Drebber U, Baldus SE, Metzger R, Holscher AH, Monig SP. Clinicopathological significance of MMP-2 and its specific inhibitor TIMP-2 in gastric cancer. Histol Histopathol. 2008;23 (8:917–923. doi: 10.14670/HH-23.917. [DOI] [PubMed] [Google Scholar]
- Anbiaee R, Mojir Sheibani K, Torbati P, Jaam H. Abnormal expression of e-cadherin in gastric adenocarcinoma, and its correlation with tumor histopathology and Helicobacter pylori infection. Iran Red Crescent Med J. 2013;15 (3:218–222. doi: 10.5812/ircmj.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6 (1:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Jaks V, Kasper M, Snippert H, Toftgard R, Clevers H. Very long-term self-renewal of small intestine, colon, and hair follicles from cycling Lgr5+ve stem cells. Cold Spring Harb Symp Quant Biol. 2008;73:351–356. doi: 10.1101/sqb.2008.72.003. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449 (7165:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Becker L, Huang Q, Mashimo H. Lgr5, an intestinal stem cell marker, is abnormally expressed in Barrett's esophagus and esophageal adenocarcinoma. Dis Esophagus. 2010;23 (2:168–174. doi: 10.1111/j.1442-2050.2009.00979.x. [DOI] [PubMed] [Google Scholar]
- Birchmeier W. Stem cells: orphan receptors find a home. Nature. 2011;476 (7360:287–288. doi: 10.1038/476287a. [DOI] [PubMed] [Google Scholar]
- de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, Stange DE, van Es JE, Guardavaccaro D, Schasfoort RB, Mohri Y, Nishimori K, Mohammed S, Heck AJ, Clevers H. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476 (7360:293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metast Rev. 2006;25 (1:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- Fan XS, Wu HY, Yu HP, Zhou Q, Zhang YF, Huang Q. Expression of Lgr5 in human colorectal carcinogenesis and its potential correlation with beta-catenin. Int J Colorectal Dis. 2010;25 (5:583–590. doi: 10.1007/s00384-010-0903-z. [DOI] [PubMed] [Google Scholar]
- Guo Y, Yin J, Zha L, Wang Z. Clinicopathological significance of platelet-derived growth factor B, platelet-derived growth factor receptor-beta, and E-cadherin expression in gastric carcinoma. Contemp Oncol (Pozn) 2013;17 (2:150–155. doi: 10.5114/wo.2013.34618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guszczyn T, Sobolewski K. Deregulation of collagen metabolism in human stomach cancer. Pathobiology. 2004;71 (6:308–313. doi: 10.1159/000081726. [DOI] [PubMed] [Google Scholar]
- Liotta LA, Clair T. Cancer. Checkpoint for invasion. Nature. 2000;405 (6784:287–288. doi: 10.1038/35012728. [DOI] [PubMed] [Google Scholar]
- Liotta LA, Stetler-Stevenson WG. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res. 1991;51 (Suppl:5054s–5059s. [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25 (4:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Matsubara J, Yamada Y, Nakajima TE, Kato K, Hamaguchi T, Shirao K, Shimada Y, Shimoda T. Clinical significance of insulin-like growth factor type 1 receptor and epidermal growth factor receptor in patients with advanced gastric cancer. Oncology. 2008;74 (1-2:76–83. doi: 10.1159/000139127. [DOI] [PubMed] [Google Scholar]
- McClanahan T, Koseoglu S, Smith K, Grein J, Gustafson E, Black S, Kirschmeier P, Samatar AA. Identification of overexpression of orphan G protein-coupled receptor GPR49 in human colon and ovarian primary tumors. Cancer Biol Ther. 2006;5 (4:419–426. doi: 10.4161/cbt.5.4.2521. [DOI] [PubMed] [Google Scholar]
- Monig SP, Baldus SE, Hennecken JK, Spiecker DB, Grass G, Schneider PM, Thiele J, Dienes HP, Holscher AH. Expression of MMP-2 is associated with progression and lymph node metastasis of gastric carcinoma. Histopathology. 2001;39 (6:597–602. doi: 10.1046/j.1365-2559.2001.01306.x. [DOI] [PubMed] [Google Scholar]
- Planutiene M, Planutis K, Holcombe RF. Lymphoid enhancer-binding factor 1, a representative of vertebrate-specific Lef1/Tcf1 sub-family, is a Wnt-beta-catenin pathway target gene in human endothelial cells which regulates matrix metalloproteinase-2 expression and promotes endothelial cell invasion. Vasc Cell. 2011;3:28. doi: 10.1186/2045-824X-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson PS, Escovidal LA, Yrastorza SG, Veneracion RG, Nerves MY. Re-study of gastric cancer: analysis of outcome. World J Surg. 2002;26 (4:428–433. doi: 10.1007/s00268-001-0243-9. [DOI] [PubMed] [Google Scholar]
- Segditsas S, Sieber O, Deheragoda M, East P, Rowan A, Jeffery R, Nye E, Clark S, Spencer-Dene B, Stamp G, Poulsom R, Suraweera N, Silver A, Ilyas M, Tomlinson I. Putative direct and indirect Wnt targets identified through consistent gene expression changes in APC-mutant intestinal adenomas from humans and mice. Hum Mol Genet. 2008;17 (24:3864–3875. doi: 10.1093/hmg/ddn286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MA, Kelsen DP. Gastric cancer: a primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J Natl Compr Canc Netw. 2010;8 (4:437–447. doi: 10.6004/jnccn.2010.0033. [DOI] [PubMed] [Google Scholar]
- Shim KN, Jung SA, Joo YH, Yoo K. Clinical significance of tissue levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases in gastric cancer. J Gastroenterol. 2007;42 (2:120–128. doi: 10.1007/s00535-006-1975-y. [DOI] [PubMed] [Google Scholar]
- Sier CF, Kubben FJ, Ganesh S, Heerding MM, Griffioen G, Hanemaaijer R, van Krieken JH, Lamers CB, Verspaget HW. Tissue levels of matrix metalloproteinases MMP-2 and MMP-9 are related to the overall survival of patients with gastric carcinoma. Br J Cancer. 1996;74 (3:413–417. doi: 10.1038/bjc.1996.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Ishii H, Nishida N, Takemasa I, Mizushima T, Ikeda M, Yokobori T, Mimori K, Yamamoto H, Sekimoto M, Doki Y, Mori M. Significance of Lgr5(+ve) cancer stem cells in the colon and rectum. Ann Surg Oncol. 2011;18 (4:1166–1174. doi: 10.1245/s10434-010-1373-9. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kinoshita I, Shimizu Y, Matsuno Y, Shichinohe T, Dosaka-Akita H. Expression of LGR5, an intestinal stem cell marker, during each stage of colorectal tumorigenesis. Anticancer Res. 2011;31 (1:263–270. [PubMed] [Google Scholar]
- Tanese K, Fukuma M, Yamada T, Mori T, Yoshikawa T, Watanabe W, Ishiko A, Amagai M, Nishikawa T, Sakamoto M. G-protein-coupled receptor GPR49 is up-regulated in basal cell carcinoma and promotes cell proliferation and tumor formation. Am J Pathol. 2008;173 (3:835–843. doi: 10.2353/ajpath.2008.071091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida H, Yamazaki K, Fukuma M, Yamada T, Hayashida T, Hasegawa H, Kitajima M, Kitagawa Y, Sakamoto M. Overexpression of leucine-rich repeat-containing G protein-coupled receptor 5 in colorectal cancer. Cancer Sci. 2010;101 (7:1731–1737. doi: 10.1111/j.1349-7006.2010.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M, Van Gijn ME, Suijkerbuijk S, Van de Wetering M, Marra G, Clevers H. The intestinal Wnt/TCF signature. Gastroenterology. 2007;132 (2:628–632. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, Todaro M, de Sousa Mello F, Sprick MR, Kemper K, Perez Alea M, Richel DJ, Stassi G, Medema JP. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci USA. 2008;105 (36:13427–13432. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LD, Calhoun ES, Silliman N, Ptak J, Szabo S, Powell SM, Riggins GJ, Wang TL, Yan H, Gazdar A, Kern SE, Pennacchio L, Kinzler KW, Vogelstein B, Velculescu VE. Somatic mutations of GUCY2F, EPHA3, and NTRK3 in human cancers. Hum Mutat. 2006;27 (10:1060–1061. doi: 10.1002/humu.9452. [DOI] [PubMed] [Google Scholar]
- Wu ZY, Li JH, Zhan WH, He YL. Lymph node micrometastasis and its correlation with MMP-2 expression in gastric carcinoma. World J Gastroenterol. 2006;12 (18:2941–2944. doi: 10.3748/wjg.v12.i18.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi HQ, Wu XS, Wei B, Chen L. Aberrant expression of EphA3 in gastric carcinoma: correlation with tumor angiogenesis and survival. J Gastroenterol. 2012;47 (7:785–794. doi: 10.1007/s00535-012-0549-4. [DOI] [PubMed] [Google Scholar]
- Xi HQ, Zhao P, Han WD. Clinicopathological significance and prognostic value of LRP16 expression in colorectal carcinoma. World J Gastroenterol. 2010;16 (13:1644–1648. doi: 10.3748/wjg.v16.i13.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakata Y, Nakayama T, Yoshizaki A, Kusaba T, Inoue K, Sekine I. Expression of p-STAT3 in human gastric carcinoma: significant correlation in tumour invasion and prognosis. Int J Oncol. 2007;30 (2:437–442. [PubMed] [Google Scholar]
- Yamamoto Y, Sakamoto M, Fujii G, Tsuiji H, Kenetaka K, Asaka M, Hirohashi S. Overexpression of orphan G-protein-coupled receptor, Gpr49, in human hepatocellular carcinomas with beta-catenin mutations. Hepatology. 2003;37 (3:528–533. doi: 10.1053/jhep.2003.50029. [DOI] [PubMed] [Google Scholar]