Abstract
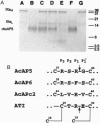
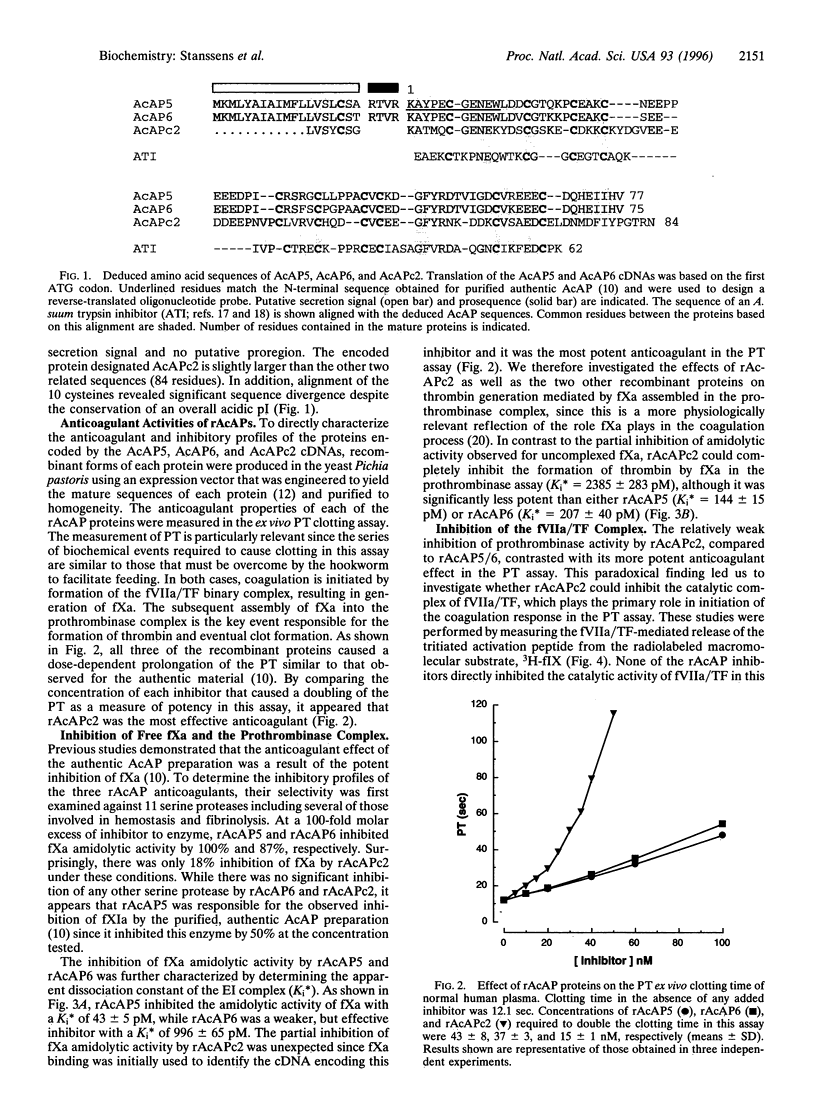
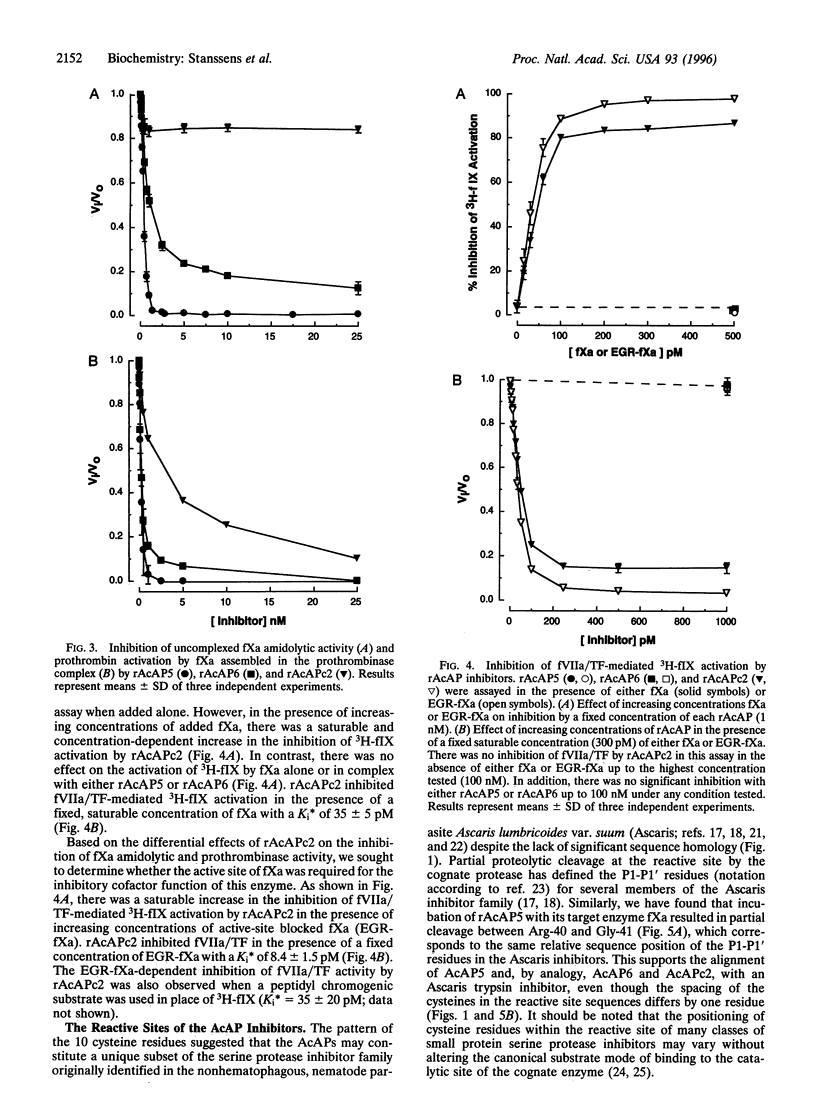
Hookworms are hematophagous nematodes that infect a wide range of mammalian hosts, including humans. There has been speculation for nearly a century as to the identity of the anticoagulant substances) used by these organisms to subvert host hemostasis. Using molecular cloning, we describe a family of potent small protein (75-84 amino acids) anticoagulants from the hookworm Ancylostoma caninum termed AcAP (A. caninum anticoagulant protein). Two recombinant AcAP members (AcAP5 and AcAP6) directly inhibited the catalytic activity of blood coagulation factor Xa (fXa), while a third form (AcAPc2) predominantly inhibited the catalytic activity of a complex composed of blood coagulation factor VIIa and tissue factor (fVIIa/TF). The inhibition of fVIIa/TF was by a unique mechanism that required the initial formation of a binary complex of the inhibitor with fXa at a site on the enzyme that is distinct from the catalytic center (exo-site). The sequence of AcAPc2 as well as the utilization of an exo-site on fXa distinguishes this inhibitor from the mammalian anticoagulant TFPI (tissue factor pathway inhibitor), which is functionally equivalent with respect to fXa-dependent inhibition of fIIa/TF. The relative sequence positions of the reactive site residues determined for AcAP5 with the homologous regions in AcAP6 and AcAPc2 as well as the pattern of 10 cysteine residues present in each of the inhibitors suggest that the AcAPs are distantly related to the family of small protein serine protease inhibitors found in the nonhematophagous nematode Ascaris lumbricoides var. suum.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bode W., Huber R. Proteinase-protein inhibitor interaction. Biomed Biochim Acta. 1991;50(4-6):437–446. [PubMed] [Google Scholar]
- Broze G. J., Jr, Girard T. J., Novotny W. F. Regulation of coagulation by a multivalent Kunitz-type inhibitor. Biochemistry. 1990 Aug 21;29(33):7539–7546. doi: 10.1021/bi00485a001. [DOI] [PubMed] [Google Scholar]
- Broze G. J., Jr, Warren L. A., Novotny W. F., Higuchi D. A., Girard J. J., Miletich J. P. The lipoprotein-associated coagulation inhibitor that inhibits the factor VII-tissue factor complex also inhibits factor Xa: insight into its possible mechanism of action. Blood. 1988 Feb;71(2):335–343. [PubMed] [Google Scholar]
- Butenas S., Ribarik N., Mann K. G. Synthetic substrates for human factor VIIa and factor VIIa-tissue factor. Biochemistry. 1993 Jul 6;32(26):6531–6538. doi: 10.1021/bi00077a006. [DOI] [PubMed] [Google Scholar]
- Cappello M., Clyne L. P., McPhedran P., Hotez P. J. Ancylostoma factor Xa inhibitor: partial purification and its identification as a major hookworm-derived anticoagulant in vitro. J Infect Dis. 1993 Jun;167(6):1474–1477. doi: 10.1093/infdis/167.6.1474. [DOI] [PubMed] [Google Scholar]
- Cappello M., Vlasuk G. P., Bergum P. W., Huang S., Hotez P. J. Ancylostoma caninum anticoagulant peptide: a hookworm-derived inhibitor of human coagulation factor Xa. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):6152–6156. doi: 10.1073/pnas.92.13.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregg J. M., Barringer K. J., Hessler A. Y., Madden K. R. Pichia pastoris as a host system for transformations. Mol Cell Biol. 1985 Dec;5(12):3376–3385. doi: 10.1128/mcb.5.12.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie C., Thornberry N. A., Bull H. G., Sardana M., Friedman P. A., Jacobs J. W., Simpson E. Antistasin, a leech-derived inhibitor of factor Xa. Kinetic analysis of enzyme inhibition and identification of the reactive site. J Biol Chem. 1989 Oct 5;264(28):16694–16699. [PubMed] [Google Scholar]
- Eiff J. A. Nature of an anticoagulant from the cephalic glands of ancylostoma caninum. J Parasitol. 1966 Oct;52(5):833–843. [PubMed] [Google Scholar]
- Goodman R. B., Martzen M. R., Peanasky R. J. Trypsin inhibitors from Ascaris: the reactive P1 site of the inhibitors (a correction) and location of the inhibitors and host trypsin in cross-sections of Ascaris. Acta Biochim Pol. 1983;30(2):233–244. [PubMed] [Google Scholar]
- Grasberger B. L., Clore G. M., Gronenborn A. M. High-resolution structure of Ascaris trypsin inhibitor in solution: direct evidence for a pH-induced conformational transition in the reactive site. Structure. 1994 Jul 15;2(7):669–678. doi: 10.1016/s0969-2126(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Huang K., Strynadka N. C., Bernard V. D., Peanasky R. J., James M. N. The molecular structure of the complex of Ascaris chymotrypsin/elastase inhibitor with porcine elastase. Structure. 1994 Jul 15;2(7):679–689. doi: 10.1016/s0969-2126(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Huang Z. F., Wun T. C., Broze G. J., Jr Kinetics of factor Xa inhibition by tissue factor pathway inhibitor. J Biol Chem. 1993 Dec 25;268(36):26950–26955. [PubMed] [Google Scholar]
- Jespers L. S., Messens J. H., De Keyser A., Eeckhout D., Van den Brande I., Gansemans Y. G., Lauwereys M. J., Vlasuk G. P., Stanssens P. E. Surface expression and ligand-based selection of cDNAs fused to filamentous phage gene VI. Biotechnology (N Y) 1995 Apr;13(4):378–382. doi: 10.1038/nbt0495-378. [DOI] [PubMed] [Google Scholar]
- Laroche Y., Storme V., De Meutter J., Messens J., Lauwereys M. High-level secretion and very efficient isotopic labeling of tick anticoagulant peptide (TAP) expressed in the methylotrophic yeast, Pichia pastoris. Biotechnology (N Y) 1994 Nov;12(11):1119–1124. doi: 10.1038/nbt1194-1119. [DOI] [PubMed] [Google Scholar]
- Laskowski M., Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- Liu L. W., Vu T. K., Esmon C. T., Coughlin S. R. The region of the thrombin receptor resembling hirudin binds to thrombin and alters enzyme specificity. J Biol Chem. 1991 Sep 15;266(26):16977–16980. [PubMed] [Google Scholar]
- MARKWARDT F. Die Isolierung und chemische Charakterisierung des Hirudins. Hoppe Seylers Z Physiol Chem. 1957;308(2-4):147–156. [PubMed] [Google Scholar]
- Mann K. G., Krishnaswamy S., Lawson J. H. Surface-dependent hemostasis. Semin Hematol. 1992 Jul;29(3):213–226. [PubMed] [Google Scholar]
- Morrison J. F. Kinetics of the reversible inhibition of enzyme-catalysed reactions by tight-binding inhibitors. Biochim Biophys Acta. 1969;185(2):269–286. doi: 10.1016/0005-2744(69)90420-3. [DOI] [PubMed] [Google Scholar]
- Naski M. C., Fenton J. W., 2nd, Maraganore J. M., Olson S. T., Shafer J. A. The COOH-terminal domain of hirudin. An exosite-directed competitive inhibitor of the action of alpha-thrombin on fibrinogen. J Biol Chem. 1990 Aug 15;265(23):13484–13489. [PubMed] [Google Scholar]
- Nutt E., Gasic T., Rodkey J., Gasic G. J., Jacobs J. W., Friedman P. A., Simpson E. The amino acid sequence of antistasin. A potent inhibitor of factor Xa reveals a repeated internal structure. J Biol Chem. 1988 Jul 25;263(21):10162–10167. [PubMed] [Google Scholar]
- Ruf W., Miles D. J., Rehemtulla A., Edgington T. S. Mutational analysis of receptor and cofactor function of tissue factor. Methods Enzymol. 1993;222:209–224. doi: 10.1016/0076-6879(93)22015-8. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Seymour J. L., Lindquist R. N., Dennis M. S., Moffat B., Yansura D., Reilly D., Wessinger M. E., Lazarus R. A. Ecotin is a potent anticoagulant and reversible tight-binding inhibitor of factor Xa. Biochemistry. 1994 Apr 5;33(13):3949–3958. doi: 10.1021/bi00179a022. [DOI] [PubMed] [Google Scholar]
- Spellman G. G., Jr, Nossel H. L. Anticoagulant activity of dog hookworm. Am J Physiol. 1971 Apr;220(4):922–927. doi: 10.1152/ajplegacy.1971.220.4.922. [DOI] [PubMed] [Google Scholar]
- Usharani P., Warn-Cramer B. J., Kasper C. K., Bajaj S. P. Characterization of three abnormal factor IX variants (Bm Lake Elsinore, Long Beach, and Los Angeles) of hemophilia-B. Evidence for defects affecting the latent catalytic site. J Clin Invest. 1985 Jan;75(1):76–83. doi: 10.1172/JCI111700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasuk G. P. Structural and functional characterization of tick anticoagulant peptide (TAP): a potent and selective inhibitor of blood coagulation factor Xa. Thromb Haemost. 1993 Jul 1;70(1):212–216. [PubMed] [Google Scholar]
- Waxman L., Smith D. E., Arcuri K. E., Vlasuk G. P. Tick anticoagulant peptide (TAP) is a novel inhibitor of blood coagulation factor Xa. Science. 1990 May 4;248(4955):593–596. doi: 10.1126/science.2333510. [DOI] [PubMed] [Google Scholar]