Abstract
Purpose:
18F-FAMT as an amino-acid tracer for positron emission tomography (PET) is useful for detecting human neoplasms. 18F-FAMT is accumulated in tumour cells solely via L-type amino-acid transporter 1 (LAT1). This study was conducted to investigate the biological significance of 18F-FAMT uptake in patients with oesophageal cancer.
Methods:
From April 2008 to December 2011, 42 patients with oesophageal cancer underwent both 18F-FAMT PET/CT and 18F-FDG PET/CT before surgical treatment. The immunohistochemical analysis of LAT1, CD98, Ki-67, CD34, p53, p-Akt and p-mTOR was performed on the primary lesions. In vitro experiments were performed to examine the mechanism of 18F-FAMT uptake.
Results:
High uptake of 18F-FAMT was significantly associated with advanced stage, lymph node metastasis and the expression of LAT1, CD98, Ki-67 and CD34. LAT1 expression yielded a statistically significant correlation with CD98 expression, cell proliferation, angiogenesis and glucose metabolism. In vitro experiments revealed that 18F-FAMT was specifically transported by LAT1.
Conclusions:
The uptake of 18F-FAMT within tumour cells is determined by the LAT1 expression and correlated with cell proliferation and angiogenesis in oesophageal cancer. The present experiments also confirmed the presence of LAT1 as an underlying mechanism of 18F-FAMT accumulation.
Keywords: 18F-FAMT, PET, LAT1, oesophageal cancer, BCH, biology
Tumour cells have an increased demand for nutrients such as glucose and amino acids. Cancer cells enhance the uptake of glucose and amino acids via increased expression of their transporters. As the imaging modality of glucose uptake within tumour cells, positron emission tomography (PET) with 2-[18F]-fluoro-2-deoxy-D-glucose (18F-FDG) has been widely used for the diagnosis of malignant lesions(Vansteenkiste et al, 1999). 18F-FDG PET is based on glucose metabolism, and the overexpression of glucose transporter 1 (Glut1) has been shown to be significantly correlated with 18F-FDG uptake in human cancers (Kaira et al, 2010a). On the other hand, amino-acid transporter systems have an important role in the regulation of cellular proliferation, and an isoform L-type amino-acid transporter 1 (LAT1) is widely expressed in various primary human cancers and cell lines (Kanai et al, 1998; Yanagida et al, 2001; Kobayashi et al, 2005; Nawashiro et al, 2006; Nakanishi et al, 2007; Kaira et al, 2008a; Sakata et al, 2009; Ichinose et al, 2011; Kaira et al, 2011a, 2011b; Furuya et al, 2012; Kaira et al, 2012). To detect the malignant lesions, we have developed L-[3-18F]-α–methyltyrosine (18F-FAMT) as an amino-acid PET tracer for tumour imaging and confirmed its potential usefulness in the detection of various neoplasms (Tomiyoshi et al, 1997). 18F-FAMT is specific to neoplasms, whereas 18F-FDG is taken up by non-tumour cells such as inflammation or granulation (Kaira et al, 2007a). Recent experimental study confirmed that 18F-FAMT is selective to LAT1 and is tumour-specific (Wiriyasermkul et al, 2012).18F-FAMT PET has been shown to be useful for the cancer diagnosis of non-small cell lung cancer (NSCLC; Kaira et al, 2007b), oesophageal squamous cell carcinoma (SCC; Sohda et al, 2010), oral SCC (Miyashita et al, 2010; Nobusawa et al, 2013) and multiple myeloma (Isoda et al, 2012).The uptake of 18F-FAMT has been significantly correlated with the expression of LAT1 in patients with NSCLC; however, little is known about the correlation in other human neoplasms.
Previous studies demonstrated that the expression level of LAT1 is a significant factor for worse prognosis in human cancers such as NSCLC (Kaira et al, 2008a), urological cancer (Nakanishi et al, 2007), glioma (Nawashiro et al, 2006), prostatic cancer (Sakata et al, 2009), gastric cancer (Ichinose et al, 2011) and pancreatic cancer (Kaira et al, 2012). The heavy chain of 4F2 cell surface antigen (CD98) is essential for the functional expression of LAT1 in the plasma membrane (Kanai et al, 1998; Kaira et al, 2011a, 2012). LAT1 provides cancer cells with amino acids that are necessary not only for protein synthesis but also for the stimulation of tumour cell growth via mammalian targeting of rapamycin (mTOR; Imai et al, 2010; Kaira et al, 2011a). Moreover, LAT1 expression yielded a significant correlation with CD98, cell proliferation (Ki-67 labeling index), the cell cycle regulator p53 and angiogenesis in cancer specimens (Kaira et al, 2011a, 2012). As a potential targeting therapy for LAT1, 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH), an inhibitor of system L amino-acid transporter, was investigated using tumour cell lines (Imai et al, 2010). The results of these studies suggest that the inhibition of LAT1 could be effective for various types of cancer.
Oesophageal cancer is a common cancer with a dismal prognosis in patients with advanced stages. To improve the prognosis of patients, clinical markers for predicting therapeutic response as well as effective therapy should be established; however, we have not established biomarker that closely correlates with the prognosis and treatment response (Wieder et al, 2004). Previously, we reported that 18F-FAMT-PET is useful for the diagnosis of lymph node metastasis in oesophageal cancer (Sohda et al, 2010). However, many factors can influence the extent of 18F-FAMT accumulation within tumour cells; thus, it remains unclear about the underlying mechanisms for 18F-FAMT uptake. Clarifying a relationship between 18F-FAMT uptake and the molecular markers including LAT1 may lead to better understanding for the rationale use of 18F-FAMT PET in patients.
On the basis of these backgrounds, we conducted a study to confirm the relationship between LAT1 expression and 18F-FAMT uptake in patients with oesophageal cancer. In addition, LAT1 expression was compared with CD98, Ki-67 labelling index, cell cycle regulator (p53) and angiogenic markers such as microvessel density (MVD) determined by the CD34 and mTOR signaling pathway. In vitro experiments were also performed to investigate the basic mechanism of 18F-FAMT uptake.
Materials and methods
Patients
The current retrospective study included a consecutive series of patients with pathologically confirmed oesophageal squamous cell carcinoma who underwent surgical resection from April 2008 to December 2011. All patients underwent pretreatment work-up with conventional imaging studies. 18F-FAMT-PET and 18F-FDG-PET were also performed according to the study protocol approved by the institutional review board after providing written informed consent. Patients were excluded from the study if they received previous chemotherapy or radiotherapy before surgical resection and had any concomitant malignancy or heart disease. Therefore, a total of 42 patients were analysed in the study. Tumour stage and disease grade were assigned according to the 6th edition of the tumour, node, metastasis system classification of the International Union Against Cancer. Resectability was determined by the conventional staging methods, which included CT of the neck, chest and abdomen; 18F-FDG-PET; endoscopic ultrasonography; and oesophagography.
PET studies and data analysis
18F-FAMT was synthesised in our cyclotron facility according to the method developed by Tomiyoshi et al (1997). The radiochemical yield of 18F-FAMT was ∼20%, and radiochemical purity was ∼99%. 18F-FDG was also produced in our facility as described previously (Oriuchi et al, 1996). The patients fasted for at least 6 h before the PET studies, which were performed using a PET/CT scanner (Discovery STE, GE Healthcare, Pewaukee, WI, USA) with a 700-mm field of view. Three-dimensional data acquisition was initiated 50 min after the injection of 5 MBq kg−1 of 18F-FAMT or 5 MBq kg−1 of 18F-FDG (Inoue et al, 1999a). We acquired 4–10 bed positions (3-min acquisition per bed position) according to the range of the imaging. Attenuation-corrected transverse images obtained with 18F-FAMT and 18F-FDG were reconstructed with the ordered-subset expectation maximisation algorithm into 128 × 128 matrices with a slice thickness of 3.27 mm. All 18F-FDG and 18F-FAMT PET images were independently reviewed by two experienced physicians. Uptake that was either moderate or intense was defined as a positive finding on visual interpretation, and either the absence of uptake or uptake that was less than that in normal mediastinum was defined as a negative finding. Discrepant results were resolved by consensus review.
For the semiquantitative analysis, functional images of the standardised uptake value (SUV) were reconstructed using attenuation-corrected transaxial images, the injected dose of 18F-FAMT or18F-FDG (MBq), the patient's body weight (g) and the cross-calibration factor between PET and dose calibrator (Inoue et al, 1999b). SUV (MBq g−1) of the lesion was defined by the region of interest (ROI). A circular ROI of ∼1 cm in diameter was manually drawn on the SUV images over the primary tumour. When the tumour was >1 cm in diameter or the shape of the tumour was irregular or multifocal, a ROI was drawn over the area corresponding to the maximal tracer uptake. ROI analysis was conducted by a nuclear physician with the aid of corresponding CT scans. The maximal SUV in the ROI (SUVmax) was used as a representative uptake value for the statistical analyses.
Immunohistochemical staining
LAT1 expression was determined with immunohistochemical staining with an LAT1 antibody (2 mg ml−1, anti-human monoclonal mouse antibody, 4A2, provided by Dr H Endou (J-Pharma, Tokyo, Japan), 1 : 3200 dilution). The production and characterisation of the LAT1 antibody has been described previously(Sakata et al, 2009). CD98 was detected using an affinity-purified rabbit polyclonal antibody (Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA, 1 : 100 dilution) raised against a peptide mapping to the carboxy terminus of human CD98. The detailed protocol for immunostaining has been published elsewhere (Kaira et al, 2010a, 2011a, 2012). The following antibodies were used: a mouse monoclonal antibody against CD34 (Nichirei, Tokyo, Japan, 1 : 800 dilution); a mouse monoclonal antibody against Ki-67 (Dako, Glostrup, Denmark, 1 : 40 dilution); a mouse monoclonal antibody against p53 (D07; Dako, 1 : 50 dilution); a rabbit polyclonal antibody against GLUT1 (AB15309, Abcam, Tokyo, Japan, 1 : 200 dilution); a mouse monoclonal antibody against EGFR (Novovastra laboratories Ltd., Newcastle, UK, 1 : 100 dilution); a rabbit polyclonal antibody against phospho-Akt (Abcam, 1 : 200 dilution); a rabbit monoclonal antibody against phospho-mTOR (Cell Signaling Technology, Beverly, MA, USA, 1 : 80 dilution). The expression of Glut1 and EGFR was considered positive if distinct membrane staining was present and that of p-Akt and p-mTOR were considered positive if membranous and/or cytoplasmic staining was present. For LAT1, CD98, p-Akt, p-mTOR, Glut1 and EGFR, a semiquantitative scoring method was used as follows: (1) ⩽10% of tumour area stained; (2) 11–25% stained; (3) 26–50% stained; (4) 51–75% stained; and (5) ⩾76% stained. The tumours in which stained tumour cells were scored as 3 or 4 were defined as positive expression. Moreover, the tumours with scoring of 4 or 5 were defined as high expression.
The number of CD34-positive vessels was counted in four selected hotspots in a × 400 field (0.26 mm2 field area). MVD was defined as the mean microvessel count per 0.26-mm2 field area. The median numbers of CD34-positive vessels were evaluated, and the tumours in which stained tumour cells exceeded the median value were defined as high-expression tumours. For p53, microscopic examination of the nuclear reaction product was performed and scored. A positive p53 expression was defined as expression in more than 10% of the tumour cells. For Ki-67, a highly cellular area of the immunostained sections was evaluated. All epithelial cells with nuclear staining of any intensity were defined as high-expression cells. Approximately 1000 nuclei were counted on each slide. Proliferative activity was assessed as the percentage of Ki-67-stained nuclei (Ki-67 labeling index) in the sample. The median value of the Ki-67 labeling index was evaluated, and the tumours exceeding the median value were defined as high-expression tumours. The sections were assessed using light microscopy in a blinded manner by at least two of the authors.
Cell culture
Human oesophageal cancer cell lines, KYSE30 (JCRB0188) and KYSE150 (JCRB1095), were purchased from the Health Science Research Resources Bank (Osaka, Japan; Shimada et al, 1992) and routinely maintained in Dulbecco's modified Eagle's medium (Wako Pure Chemical Industries, Osaka, Japan) containing 10% heat-inactivated fetal bovine serum (AusGeneX, Loganholme, Queensland, Australia), penicillin (100 units ml−1), streptomycin (100 μg ml−1) and L-glutamine (2 mM) at 37 °C in 5% CO2 and 95% air.
Immunoblotting
Cells were dissolved in sample buffer (25% glycerin, 1% SDS, 62.5 mM Tris-Cl, 10 mM dithiothreitol) and incubated at 65 °C (LAT1) or 95 °C (CD98 and β-actin) for 15 min. Aliquots of samples containing 40 μg of protein were analysed by 10% SDS–polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane. Blots were incubated at 4 °C overnight in 10 mM Tris–HCl, 100 mM NaCl, 0.1% Tween 20, pH 7.5 (TBST), with 5% skim milk and then with rabbit anti-LAT1 C-terminus antibody (1 : 5000 dilution; Morimoto et al, 2008), rabbit anti-LAT1 N-terminus antibody (1 : 5000 dilution; Morimoto et al, 2008), rabbit anti-CD98 antibody (Santa Cruz Biotechnology, 1 : 200 dilution) or rabbit anti-actin antibody (Cell Signaling Technology, 1 : 1000 dilution) at 4 °C overnight. After having been washed with TBST, the blots were incubated with goat horseradish peroxidase-conjugated anti-rabbit IgG antibody (Cell Signaling Technology,1 : 20 000 dilution) for 1.5 h at room temperature. The blots were further washed with TBST, and specific proteins were visualised by using enhanced chemiluminescence western blotting detection reagents (GE Healthcare).
Cellular uptake studies
Cells (1.0 × 105 cells per well) were plated in the 24-well plates and incubated in the growth medium for 24 h. After the incubation, the cells were washed three times with sodium-free Hunk's balanced salt solution (Na+-free HBSS; 137 mM choline chloride, 5.3 mM KC1, 1.3 mM CaCl2, 0.49 mM MgCl2, 0.41 mM MgSO4, 0.35 mM K2HPO4, 0.44 mM KH2PO4, 4.2 mM KHCO3, 5.6 mM D-glucose (pH 7.4)) and then incubated in Na+-free HBSS for 10 min. For the 18F-FAMT uptake, cells were incubated with 100 kBq (1.5 μM) 18F-FAMT at 37 °C in Na+-free HBSS for 1 min. For inhibition assay of 18F-FAMT uptake, cells were incubated in Na+-free HBSS containing 100 kBq (1.5 μM) 18F-FAMT and BCH (NARD Institute, Hyogo, Japan) with various concentration (1, 3, 10, 30, 100, 300, 1000, 3000 μM) for 1 min. After the incubation, cells were washed three times with ice-cold Na+-free HBSS and then lysed with 500 μl of 0.1 M NaOH. The radioactivity in the cell lysate was measured by a well-type γ-counter (ARC-7001, Aloka, Tokyo, Japan).
Expression of LAT mRNA in human oesophageal cancer cells
Total RNA was isolated using a NucleoSpin RNA II kit (Macherey-Nagel, Düren, Germany). The first-strand complement DNA was synthesised from 0.5 μg of total RNA with PrimeScript Reverse Transcriptase (Takara Bio, Shiga, Japan). The sequences of specific primers were shown in Supplementary Table S1 (online only). The real-time PCR analysis was performed by first incubating each complement DNA sample with the primers (0.5 μM each) and Thunderbird SYBR qPCR Mix (Toyobo, Osaka, Japan). Amplification was carried out for 40 cycles (95 °C for 15 s, 60 °C for 30 s) with Piko-Real thermal cycler (Thermo Fisher Scientific, Waltham, MA, USA). The data were analysed according to 2−ΔΔCt method (internal control: β-actin, calibrator: LAT1).
Statistical analysis
Probability values of <0.05 indicated a statistically significant difference. Fisher's exact test was used to examine the association of two categorical variables. The correlation between different variables was analysed using the nonparametric Spearman's rank test. Statistical analysis was performed using the GraphPad Prism 4 software (Graph Pad Software, San Diego, CA, USA) and JMP 8 (SAS, Institute Inc, Cary, NC, USA) for Windows.
Results
PET imaging
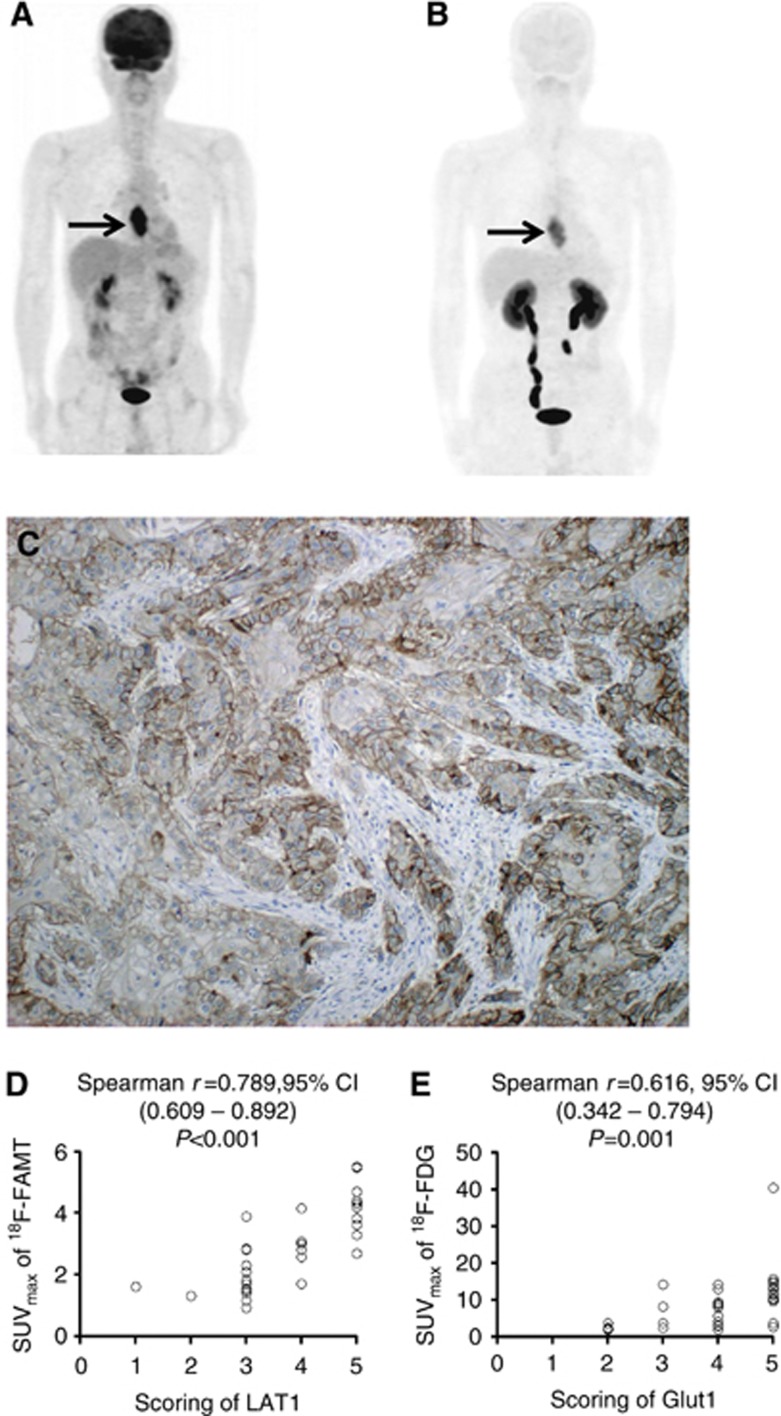
Visual interpretation of the PET images revealed that the primary tumour was visualised by 18F-FAMT-PET in 34 patients (80.9%) and 18F-FDG-PET in 36 patients (85.7%). The sensitivity for detecting primary tumour was not significantly different between them (P=0.771). Representative images of 18F-FDG-PET and 18F-FAMT-PET are shown in Figure 1.
Figure 1.
18F-FDG PET/CT (maximum intensity projection image and transaxial section (A) and 18F-FAMT PET/CT (maximum intensity projection image and transaxial section; (B) of a 56-year-old man with oesophageal cancer. 18F-FDG PET/CT imaging shows high uptake of 18F-FDG by the primary Tumour (SUVmax, 14.2; arrows). 18F-FAMT PET/CT imaging shows high uptake of 18F-FAMT by the primary tumour (SUVmax, 4.2; arrows). The score of LAT1 immunostaining of the surgically resected primary tumour was 4, and its immunostaining pattern was membranous (C).18F-FAMT uptake is significantly correlated with LAT1 score (D). 18F-FDG uptake is significantly correlated with Glut1 score (E).
The median values of SUVmax by18F-FAMT and 18F-FDG uptake in primary tumours were 2.3 (0.9–5.5) and 7.9 (1.9–40.4), respectively, demonstrating a significant difference (P<0.001). A median value of 2.3 in 18F-FAMT-PET was used as the cutoff SUVmax in the following analyses and the SUVmax more than 2.3 was defined as high uptake, whereas the cutoff value of 7.9 in 18F-FDG-PET was used and more than 7.9 was defined as high uptake.
Immunohistochemical analysis
The immunohistochemical analysis was performed on the 42 primary lesions with oesophageal cancer. LAT1 expression was detected in carcinoma cells in the tumour tissues and was localised predominantly on the plasma membrane (Figure 1C). All positive cells revealed strong membranous LAT1 expression. Cytoplasmic staining was rarely observed. A positive LAT1 and CD98 expression was recognised in 85.7% (36 out of 42) and 80.9% (34 out of 42) of cases, respectively (P=0.771). Thirty-three (91.6%) of the 36 patients with positive LAT1 expression overlapped the positive expression of CD98 (33 out of 34, 97.1%). High LAT1 and CD98 expression (score of 4 or 5) was 42.8% (18 out of 42) and 52.3% (22 out of 42) of cases, respectively. All patients (100%) with high LAT1 expression overlapped the expression of CD98. The average LAT1 and CD98 expression scores were 3.5±1.1 and 3.5±1.2 on a scale of 1–5, respectively.
The median number of CD34 was 18 (range 5–36), and the value of 18 was chosen as a cutoff point. The median value for the Ki-67 labelling index was 40% (range, 10–80), and the value of 40% was chosen as a cutoff point. Positive p53 expression was recognised in 71.4% (30 out of 42) of cases. A high expression of Glut1, EGFR, p-Akt and p-mTOR was 85.7% (36 out of 42), 71.4% (30 out of 42), 35.7% (15 out of 42) and 21.4% (9 out of 42), respectively.
Relationship between 18F-FAMT uptake and different variables
The result of the statistical correlation according to 18F-FAMT uptake is listed in Table 1. The patients with high 18F-FAMT uptake were observed in 47.6% (20 out of 42). High 18F-FAMT uptake was significantly associated with lymph node metastasis, disease staging and the expression of LAT1, CD98, Glut1, Ki-67 and CD34.
Table 1. Demographics according to 18F-FAMT uptake.
| Variables | High 18F-FAMT (n=20) | Low 18F-FAMT (n=22) | P-value |
|---|---|---|---|
| Age (years, ⩽65/>65) |
11/9 |
10/12 |
0.75 |
| Sex, M/F |
18/2 |
19/3 |
>0.999 |
| Tumour size (mm, ⩽50/>50) |
8/12 |
13/9 |
0.354 |
| Differentiation, WD-MD/PD |
14/6 |
17/5 |
0.729 |
| Lymph meta, yes/no |
16/4 |
10/12 |
0.028 |
| Staging, I+II/III+IV |
6/14 |
18/4 |
0.001 |
| Lymphatic invasion, yes/no |
18/2 |
15/7 |
0.134 |
| Vascular invasion, yes/no |
17/3 |
13/9 |
0.091 |
|
LAT1 | |||
| High/low | 17/3 | 1/21 | <0.001 |
| Positive/negative |
20/0 |
16/6 |
0.022 |
|
CD98 | |||
| High/low | 18/2 | 4/18 | <0.001 |
| Positive/negative |
20/0 |
14/8 |
0.004 |
| Glut1, Score 4–5/1–3 |
18/2 |
11/11 |
0.007 |
| Ki-67, high/low |
15/5 |
6/16 |
<0.001 |
| CD34, high/low |
13/7 |
5/17 |
<0.001 |
| EGFR, high/low |
15/5 |
15/7 |
0.738 |
| p-Akt, high/low |
6/14 |
9/13 |
0.531 |
| p-mTOR, high/low |
2/18 |
7/15 |
0.134 |
| p53, positive/negative | 14/6 | 16/6 | >0.999 |
Abbreviations: EGFR=epidermal growth receptor factor; 18F-FAMT=L-[3-18F]-α–methyltyrosine; Glut1=glucose transporter 1; LAT1=L-type amino acid transporter 1; M/F=male/female; p-mTOR=phospho-mammalian targeting of rapamycin. The bold entries show statistically significant difference.
Correlation between 18F-FAMT uptake and different variables
The different biomarkers were correlated with the 34 primary sites that are visualised by both 18F-FAMT-PET and 18F-FDG PET (Table 2). 18F-FAMT uptake was significantly correlated with LAT1, CD98, Glut1, Ki-67 and CD34 (Table 2). The uptake of 18F-FAMT was closely associated with the expression of LAT1. On the other hand, the 34 primary sites that are visualised by 18F-FDG uptake also yielded a significant correlation with LAT1, CD98, Glut1, Ki-67 and CD34 (Table 3). In all patients (n=42), moreover, LAT1 expression was statistically correlated with the expression of CD98, Glut1, Ki-67 and CD34 (Table 4). Figures 1D and E showed the correlation between scoring of LAT1 and SUVmax by 18F-FAMT and between scoring of Glut1 and SUVmax by 18F-FDG.
Table 2. Correlation according to 18F-FAMT uptake (n=34).
| Spearman r | 95% CI | P-value | |
|---|---|---|---|
| LAT1 |
0.789 |
0.609–0.892 |
<0.001 |
| CD98 |
0.765 |
0.567–0.878 |
<0.001 |
| Glut1 |
0.628 |
0.359–0.800 |
<0.001 |
| Ki-67 |
0.529 |
0.222–0.740 |
0.001 |
| CD34 |
0.429 |
0.096–0.676 |
0.011 |
| EGFR |
0.264 |
−0.092–0.560 |
0.131 |
| p-Akt |
−0.229 |
−0.583–0.128 |
0.191 |
| p-mTOR | −0.121 | −0.449–0.237 | 0.469 |
Abbreviations: 95% CI=95% confidence interval; EGFR=epidermal growth receptor factor; 18F-FAMT=L-[3-18F]-α–methyltyrosine; Glut1=glucose transporter 1; LAT1=L-type amino-acid transporter 1; p-mTOR=phospho-mammalian targeting of rapamycin. The bold entries show statistically significant difference.
Table 3. Correlation according to 18F-FDG uptake (n=34).
| Spearman r | 95% CI | P-value | |
|---|---|---|---|
| LAT1 |
0.664 |
0.411–0.822 |
<0.001 |
| CD98 |
0.734 |
0.519–0.862 |
<0.001 |
| Glut1 |
0.616 |
0.341–0.794 |
<0.001 |
| Ki-67 |
0.399 |
0.061–0.656 |
0.019 |
| CD34 |
0.357 |
−0.057–0.568 |
0.036 |
| EGFR |
0.197 |
−0.161–0.501 |
0.263 |
| p-Akt |
−0.093 |
−0.427–0.267 |
0.599 |
| p-mTOR | −0.159 | −0.181–0.0.199 | 0.367 |
Abbreviations: 95% CI=95% confidence interval; EGFR=epidermal growth receptor factor; 18F-FAMT=L-[3-18F]-α–methyltyrosine; Glut1=glucose transporter 1; LAT1=L-type amino-acid transporter 1; p-mTOR=phospho-mammalian targeting of rapamycin. The bold entries show statistically significant difference.
Table 4. Correlation according to LAT1 expression (n=42).
| Spearman r | 95% CI | P-value | |
|---|---|---|---|
| CD98 |
0.843 |
0.727–0.917 |
<0.001 |
| Glut1 |
0.677 |
0.463–0.817 |
<0.001 |
| Ki-67 |
0.427 |
0.132–0.652 |
0.005 |
| CD34 |
0.632 |
0.386–0.783 |
<0.001 |
| EGFR |
0.248 |
−0.068–0.521 |
0.112 |
| p-Akt |
–0.086 |
−0.388–0.232 |
0.587 |
| p-mTOR | 0.057 | −0.463–0.187 | 0.716 |
Abbreviations: 95% CI=95% confidence interval; EGFR=epidermal growth receptor factor; 18F-FAMT=L-[3-18F]-α–methyltyrosine; Glut1=glucose transporter 1; LAT1=L-type amino-acid transporter 1; p-mTOR=phospho-mammalian targeting of rapamycin. The bold entries show statistically significant difference.
18F-FAMT was taken up by human oesophageal cancer cell depending on the expression level of LAT1
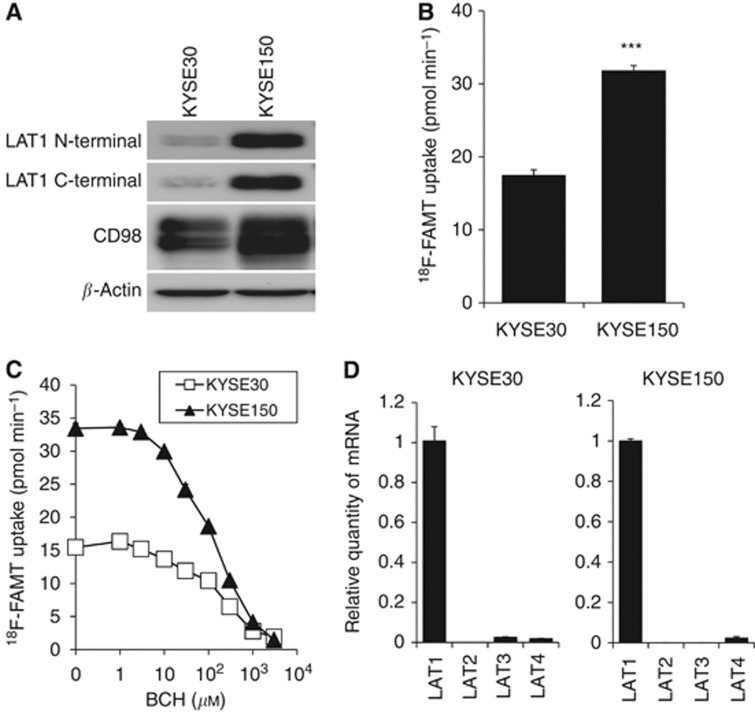
As shown in Figure 2A, both LAT1 and CD98 were expressed in oesophageal cancer cell lines; however, the expression level of both proteins in KYSE150 was higher than that in KYSE30, especially for the expression of LAT1. Cellular uptake of 18F-FAMT in KYSE150 cells was significantly higher than that in KYSE30 cells consistent with the expression levels of LAT1 and CD98 (Figure 2B). 18F-FAMT uptake was inhibited by the treatment with BCH concentration-dependently in both cell lines (Figure 2C). Expression profile of LAT1-4 mRNA in KYSE30 and KYSE150 showed that the expression of LAT1 was extremely higher than the other LATs (Figure 2D). These results indicate that 18F-FAMT is taken up through LAT1 and the cellular uptake of 18F-FAMT depends on the expression level of LAT1 in oesophageal cancer cells.
Figure 2.
LAT1 expression and18F-FAMT uptake in human oesophageal cancer. (A) The expression of LAT1 and CD98 in two human oesophageal cancer cell lines, KYSE30 and KYSE150. β-Actin was detected as an internal control. (B) 18F-FAMT uptake in KYSE150 was significantly higher than that in KYSE30 (P<0.001). (C) BCH inhibits 18F-FAMT uptake concentration-dependently in both KYSE30 and KYSE150 (n=4). Ordinate shows 18F-FAMT uptake. (D) Expression of LAT1, LAT2, LAT3 and LAT4 mRNA in KYSE30 and KYSE150 (n=4). Ordinate shows relative quantity of mRNA calibrated by the amount of LAT1 mRNA.
Discussion
This is the first study to investigate the underlying mechanism of 18F-FAMT uptake in oesophageal cancer. The clinical studies showed that the 18F-FAMT uptake was closely correlated with the expression of LAT1, and the high uptake was significantly associated with advanced stage and lymph node metastasis. Pathological analyses revealed significant correlation with CD98, Ki-67 and CD34 as well. LAT1 expression was significantly correlated with CD98, cell proliferation, angiogenesis and glucose metabolism; however, neither LAT1 expression nor 18F-FAMT accumulation was significantly associated with the mTOR signaling pathway in patients with oesophageal cancer. In vitro study identified that the uptake of 18F-FAMT was determined by the expression of LAT1.
Recent studies have described that a LAT1 expression has a crucial role in the tumour progression and metastatic process of various human cancers (Kaira et al, 2008a, 2008b, 2012). The level of LAT1 expression varies according to histological types of cancer; however, the detailed mechanism remains unclear. In the previous reports, expression of LAT1 was significantly higher in patients with SCC than those with adenocarcinoma (AC). The positive rate in pulmonary SCC was 91% however, those in pulmonary AC, pancreatic cancer, breast cancer, gastric cancer and prostate cancer ranged from 22 from 52.6% (Kaira et al, 2008a, 2012; Sakata et al, 2009; Ichinose et al, 2011; Furuya et al, 2012). In the current study, the positive rate was 85.7%, and the LAT1 expression was closely associated with cell proliferation, angiogenesis, glucose metabolism and CD98 expression in oesophageal cancer. Although no statistically significant correlation was observed between LAT1 expression and the mTOR signaling pathway, further study with a large sample size is required to investigate whether the activation of mTOR is essential for the overexpression of LAT1 in oesophageal cancer.
In oesophageal cancer, we also identified that the LAT1 expression was an important mechanism of 18F-FAMT uptake within tumour cells. LAT1 is specific to neoplasm, whereas LAT2 is highly expressed in non-neoplastic cells. As 18F-FAMT PET visualises LAT1 expression within the tumour, it would be an imaging tool to predict disease stage and metastatic involvement in patients with oesophageal cancer. Recently, we described that the uptake of 18F-FAMT was identified as a significant prognostic indicator (Kaira et al, 2009) and 18F-FAMT PET could be a useful procedure for the monitoring of chemotherapy in lung cancer (Kaira et al, 2010b). Further study is required to evaluate the clinical significance of 18F-FAMT PET as a prognostic marker and treatment monitoring in patients with oesophageal cancer.
Considering 18F-FAMT PET as an imaging modality, the lower uptake of 18F-FAMT is a limitation. In this study, the uptake of 18F-FAMT was significantly lower than that of 18F-FDG. Previous studies also demonstrated the same results; however, little is known about the detailed mechanism to explain this finding. The lower uptake leads to poorer delineation of tumours especially in small tumours. In fact the detection rate for tumours was lower for 18F-FAMT (81%) compared with 18F-FDG (86%), even if the difference did not reach statistical significance. In a recent report, 18F-FDG PET demonstrated the sensitivity of 95% (70 of 74 patients) in the primary tumour and has a higher accuracy for the detection of advanced oesophageal disease compared with conventional modalities such as computed tomography (Flamen et al, 2000). A higher sensitivity is accompanied by the lower specificity in general and vice versa.
In conclusion, the accumulation of 18F-FAMT is determined by the presence of LAT1 expression, which has a significant correlation with CD98 expression, cell proliferation and angiogenesis shown by the present in vitro studies. A high uptake of 18F-FAMT had a close relationship with advanced stage and lymph node metastases. Therefore, inhibiting LAT1 function may provide a new and effective therapeutic target of oesophageal cancer.
Acknowledgments
This study was supported by a grant from the Advanced Research for Medical Products Mining Program of the National Institute of Biomedical Innovation.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Furuya M, Horiguchi J, Nakajima H, Kanai Y, Oyama T. Correlation of L-type amino acid transporter 1 and CD98 expression with triple negative breast cancer prognosis. Cancer Sci. 2012;103:382–389. doi: 10.1111/j.1349-7006.2011.02151.x. [DOI] [PubMed] [Google Scholar]
- Flamen P, Lerut A, Van Cutsem E, De Wever W, Peeters M, Stroobants S, Dupont P, Bormans G, Hiele M, De Leyn P, Van Raemdonck D, Coosemans W, Ectors N, Haustermans K, Mortelmans L. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol. 2000;18:3202–3210. doi: 10.1200/JCO.2000.18.18.3202. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Mikami T, Yoshida T, Igawa I, Tsuruta T, Nakada N, Anzai N, Suzuki Y, Endou H, Okayasu I. High expression of L-type amino-acid transporter 1 (LAT1) in gastric carcinomas: comparison with non-cancerous lesions. Pathol Int. 2011;61:281–289. doi: 10.1111/j.1440-1827.2011.02650.x. [DOI] [PubMed] [Google Scholar]
- Imai H, Kaira K, Oriuchi N, Shimizu K, Tominaga H, Yanagitani N, Sunaga N, Ishizuka T, Nagamori S, Promchan K, Nakajima T, Yamamoto N, Mori M, Kanai Y. Inhibition of L-type amino acid transporter 1 has antitumor activity in non-small cell lung cancer. Anticancer Res. 2010;30:4819–4828. [PubMed] [Google Scholar]
- Inoue T, Shibasaki T, Oriuchi N, Aoyagi K, Tomiyoshi K, Amano S, Mikuni M, Ida I, Aoki J, Endo K. 18F α-methyl tyrosine PET studies in patients with brain tumors. J Nucl Med. 1999;40:399–405. [PubMed] [Google Scholar]
- Inoue T, Oriuchi N, Kunio M, Tomiyoshi K, Tomaru Y, Aoyagi K, Amano S, Suzuki H, Aoki J, Sato T, Endo K. Accuracy of standardized uptake value (SUV) measured by simultaneous emission and transmission scanning in PET oncology. Nucl Med Commun. 1999;20:849–857. doi: 10.1097/00006231-199909000-00012. [DOI] [PubMed] [Google Scholar]
- Isoda A, Higuchi T, Nakano S, Arisaka Y, Kaira K, Kamio T, Mawatari M, Matsumoto M, Sawamura M, Tsushima Y. (18)F-FAMT in patients with multiple myeloma: clinical utility compared to (18)F-FDG. Ann Nucl Med. 2012;26:811–816. doi: 10.1007/s12149-012-0645-9. [DOI] [PubMed] [Google Scholar]
- Kaira K, Endo M, Abe M, Nakagawa K, Ohde Y, Okumura T, Takahashi T, Murakami H, Tsuya A, Nakamura Y, Naito T, Hayashi I, Serizawa M, Koh Y, Hanaoka H, Tominaga H, Oriuchi N, Kondo H, Nakajima T, Yamamoto N. Biologic correlation of 2-[18F]-fluoro-2-deoxy-D-glucose uptake on positron emission tomography in thymic epithelial tumors. J Clin Oncol. 2010;28:3746–3753. doi: 10.1200/JCO.2009.27.4662. [DOI] [PubMed] [Google Scholar]
- Kaira K, Sunose Y, Arakawa K, Ogawa T, Sunaga N, Shimizu K, Tominaga H, Oriuchi N, Itoh H, Nagamori S, Kanai Y, Segawa A, Furuya M, Mori M, Oyama T, Takeyoshi I. Prognostic significance of L-type amino-acid transporter 1 expression in surgically resected pancreatic cancer. Br J Cancer. 2012;107:632–638. doi: 10.1038/bjc.2012.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Tanaka S, Ishizuka T, Kanai Y, Endou H, Nakajima T, Mori M. Prognostic significance of L-type amino acid transporter 1 expression in resectable stage I-III nonsmall cell lung cancer. Br J Cancer. 2008;98:742–748. doi: 10.1038/sj.bjc.6604235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Tanaka S, Ishizuka T, Kanai Y, Endou H, Nakajima T, Mori M. L-type amino acid transporter 1 and CD98 expression in primary and metastatic sites of human neoplasms. Cancer Sci. 2008;99:2380–2386. doi: 10.1111/j.1349-7006.2008.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaira K, Oriuchi N, Otani Y, Yanagitani N, Sunaga N, Hisada T, Ishizuka T, Endo K, Mori M. Diagnostic usefulness of fluorine−18-α-methyltyrosine positron emission tomography in combination with 18F-fluorodeoxyglucose in sarcoidosis patients. Chest. 2007;131:1019–1027. doi: 10.1378/chest.06-2160. [DOI] [PubMed] [Google Scholar]
- Kaira K, Oriuchi N, Otani Y, Shimizu K, Tanaka S, Imai H, Yanagitani N, Sunaga N, Hisada T, Ishizuka T, Dobashi K, Kanai Y, Endou H, Nakajima T, Endo K, Mori M. Fluorine-18-alpha-methyltyrosine positron emission tomography for diagnosis and staging of lung cancer: a clinicopathologic study. Clin Cancer Res. 2007;13:6369–6378. doi: 10.1158/1078-0432.CCR-07-1294. [DOI] [PubMed] [Google Scholar]
- Kaira K, Oriuchi N, Takahashi T, Nakagawa K, Ohde Y, Okumura T, Murakami H, Shukuya T, Kenmotsu H, Naito T, Kanai Y, Endo M, Kondo H, Nakajima T, Yamamoto N. LAT1 expression is closely associated with hypoxic markers and mTOR in resected non-small cell lung cancer. Am J Transl Res. 2011;3:468–478. [PMC free article] [PubMed] [Google Scholar]
- Kaira K, Oriuchi N, Takahashi T, Nakagawa K, Ohde Y, Okumura T, Murakami H, Shukuya T, Kenmotsu H, Naito T, Kanai Y, Endo M, Kondo H, Nakajima T, Yamamoto N. L-type amino acid transporter 1 (LAT1) expression in malignant pleural mesothelioma. Anticancer Res. 2011;31:4075–4082. [PubMed] [Google Scholar]
- Kaira K, Oriuchi N, Yanagitani N, Sunaga N, Ishizuka T, Mori M, Endo K. Assessment of therapy response in lung cancer with 18F-α-methyl tyrosine PET. AJR Am J Roentgenol. 2010;195:1204–1211. doi: 10.2214/AJR.09.4167. [DOI] [PubMed] [Google Scholar]
- Kaira K, Oriuchi N, Shimizu K, Tominaga H, Yanagitani N, Sunaga N, Ishizuka T, Kanai Y, Mori M, Endo K. 18F-FMT uptake seen within primary cancer on PET helps predict outcome of non-small cell lung cancer. J Nucl Med. 2009;50:1770–1776. doi: 10.2967/jnumed.109.066837. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem2. 1998;73:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Ishii Y, Takayama T. Expression of L-type amino acid transporter 1 (LAT1) in esophageal carcinoma. J Surg Oncol. 2005;90:233–238. doi: 10.1002/jso.20257. [DOI] [PubMed] [Google Scholar]
- Miyashita G, Higuchi T, Oriuchi N, Arisaka Y, Hanaoka H, Tominaga H, Morita S, Miyakubo M, Ishikita T, Nakasone Y, Negishi A, Yokoo S, Endo K. 18F-FAMT uptake correlates with tumor proliferative activity in oral squamous cell carcinoma: comparative study with 18F-FDG PET and immunohistochemistry. Ann Nucl Med. 2010;24:579–584. doi: 10.1007/s12149-010-0398-2. [DOI] [PubMed] [Google Scholar]
- Morimoto E, Kanai Y, Kim do K, Chairoungdua A, Choi HW, Wempe MF, Anzai N, Endou H. Establishment and characterization of mammalian cell lines stably expressing human L-type amino acid transporters. J Pharmacol Sci. 2008;108:505–516. doi: 10.1254/jphs.08232fp. [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Ogata S, Matsuo H, Kanai Y, Endou H, Hiroi S, Tominaga S, Aida S, Kasamatsu H, Kawai T. Expression of LAT1 predicts risk of progression of transitional cell carcinoma of the upper urinary tract. Virchows Arch. 2007;451:681–690. doi: 10.1007/s00428-007-0457-9. [DOI] [PubMed] [Google Scholar]
- Nawashiro H, Otani N, Shinomiya N, Fukui S, Ooigawa H, Shima K, Matsuo H, Kanai Y, Endou H. L-type amino acid transporter 1as a potential molecular target in human astrocytic tumors. Int J Cancer. 2006;119:484–492. doi: 10.1002/ijc.21866. [DOI] [PubMed] [Google Scholar]
- Nobusawa A, Kim A, Kaira K, Miyashita G, Negishi A, Oriuchi N, Higuchi T, Tsushima Y, Kanai Y, Yokoo S, Oyama T. Diagnostic usefulness of 18F-FAMT PET and L-type amino acid transporter 1 (LAT1) expression in oral squamous cell carcinoma. Eur J Nucl Med Mol Imag. 2013;40:1692–1700. doi: 10.1007/s00259-013-2477-9. [DOI] [PubMed] [Google Scholar]
- Oriuchi N, Tomiyoshi K, Inoue T, Ahmad K, Sarwar M, Tokunaga M, Suzuki H, Watanabe N, Hirano T, Horikoshi S, Shibasaki T, Tamura M, Endo K. Independent thallium-201 accumulation and fluorine-18-fluorodeoxyglucose metabolism in glioma. J Nucl Med. 1996;37:457–462. [PubMed] [Google Scholar]
- Sakata T, Ferdous G, Tsuruta T, Satoh T, Baba S, Muto T, Ueno A, Kanai Y, Endou H, Okayasu I. L-type amino acid transporter 1as a novel biomarker for high-grade malignancy in prostate cancer. Pathol Int. 2009;59:7–18. doi: 10.1111/j.1440-1827.2008.02319.x. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Imamura M, Wagata T, Yamaguchi N, Tobe T. Characterization of twenty one newly established esophageal cancer cell lines. Cancer. 1992;69:277–284. doi: 10.1002/1097-0142(19920115)69:2<277::aid-cncr2820690202>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Sohda M, Kato H, Suzuki S, Tanaka N, Sano A, Sakai M, Inose T, Nakajima M, Miyazaki T, Fukuchi M, Oriuchi N, Endo K, Kuwano H. 18F-FAMT-PET is useful for the diagnosis of lymph node metastasis in operable esophageal squamous cell carcinoma. Ann Surg Oncol. 2010;17:3181–3186. doi: 10.1245/s10434-010-1177-y. [DOI] [PubMed] [Google Scholar]
- Tomiyoshi K, Amed K, Muhammad S, Higuchi T, Inoue T, Endo K, Yang D. Synthesis of new fluorine-18 labeled amino acid radiopharmaceutical: L-F-alpha-methyl tyrosine using separation and purification system. Nucl Med Commun. 1997;18:169–175. doi: 10.1097/00006231-199702000-00013. [DOI] [PubMed] [Google Scholar]
- Vansteenkiste JF, Stroobants SG, Dupont PJ, De Leyn PR, Verbeken EK, Deneffe GJ, Mortelmans LA, Demedts MG. Prognostic importance of the standardized uptake value on 18F-fluoro-2-deoxy-glucose-positron emission tomography in non-small cell lung cancer: an analysis of 125 cases. J Clin Oncol. 1999;17:3201–3206. doi: 10.1200/JCO.1999.17.10.3201. [DOI] [PubMed] [Google Scholar]
- Wieder HA, Brücher BL, Zimmermann F, Becker K, Lordick F, Beer A, Schwaiger M, Fink U, Siewert JR, Stein HJ, Weber WA. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol. 2004;22:900–908. doi: 10.1200/JCO.2004.07.122. [DOI] [PubMed] [Google Scholar]
- Wiriyasermkul P, Nagamori S, Tominaga H, Oriuchi N, Kaira K, Nakao H, Kitashoji T, Ohgaki R, Tanaka H, Endou H, Endo K, Sakurai H, Kanai Y. Transport of 3-Fluoro-L-α-methyl-tyrosine by tumor-upregulated L-type amino acid transporter 1: a cause of the tumor uptake in PET. J Nucl Med. 2012;53:1253–1261. doi: 10.2967/jnumed.112.103069. [DOI] [PubMed] [Google Scholar]
- Yanagida O, Kanai Y, Chairoungdua A, Kim DK, Segawa H, Nii T, Cha SH, Matsuo H, Fukushima J, Fukasawa Y, Tani Y, Taketani Y, Uchino H, Kim JY, Inatomi J, Okayasu I, Miyamoto K, Takeda E, Goya T, Endou H. Human L-type amino acid transporter 1 (LAT 1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta. 2001;1514:291–302. doi: 10.1016/s0005-2736(01)00384-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.