Abstract
This report describes the case of a 44-year-old Caucasian woman of Northern European descent with a medical history of pyoderma gangrenosum, chronic abdominal pain and erythema nodosum which required intermittent use of high-dose steroids that failed to improve her symptoms. The patient was initially diagnosed with Crohn's disease and most recently with sclerosing mesenteritis. She presented to the hospital with worsening abdominal pain. She was found to have recurrent painful aphthous oral, genital and perianal ulcers and a clinical diagnosis of Behçet's disease was made. Her hospitalisation was complicated by haemoptysis, and bronchoscopy revealed alveolar haemorrhage. Treatment was initiated with three days of pulse intravenous solumedrol 1 g/day and cyclophosphamide at 700 mg/m2. The case had a favourable outcome despite the initial diagnostic challenges. This report emphasises that systemic diseases, including Behçet's disease, can have variable presentations and can be frequently misdiagnosed.
Background
Behçet's disease (BD) is a chronic relapsing inflammatory disease characterised by orogenital ulcers, cutaneous inflammation and uveitis. Although it may have an acute presentation, its course is usually indolent and chronic. Even though the underlying cause of BD is largely unknown, infectious, autoimmune and genetic aetiologies have been implicated. As with other autoimmune conditions, BD may develop as a result of an aberrant immune response to an agent, perhaps infectious, in a patient who is genetically predisposed to the disease.1 In addition to the typical ocular and mucocutaneous manifestations, BD also affects the gastrointestinal, pulmonary, musculoskeletal and nervous systems. Oshima et al2 reported that more than 40% of patients with BD had gastrointestinal symptoms, including abdominal pain, nausea, anorexia, abdominal distension and diarrhoea. Pulmonary involvement, although rare, represents one of the most severe complications of BD and an important cause of mortality.3 Pulmonary vasculitis can lead to aneurysms, thrombosis or arteriobronchial fistula and it can result in various degrees of bleeding from hemosputum to fatal pulmonary haemorrhage. Besides considerable morbidity, the disease is associated with increased mortality, mainly due to pulmonary as well as large vessel involvement, cerebrovascular disease and bowel perforation.
Case presentation
This report describes the case of a 44-year-old Caucasian woman of Northern European descent with a complicated medical history who presented with worsening abdominal pain and nausea. In 1999, she developed high-grade fever and pyoderma gangrenosum following bilateral reduction mammoplasty that resulted in significant damage to both breasts. Since then, she has had intermittent flu-like symptoms, including low-grade fevers, malaise and fatigue. Five years ago she developed severe generalised abdominal pain and underwent multiple surgeries, including an appendectomy in 2012 that showed focal acute appendicitis with rare non-caseating granulomas, raising concern for Crohn's disease. However, multiple colonoscopies with biopsies and a small bowel evaluation with video capsule endoscopy were normal. She also had two exploratory laparotomies to rule out endometriosis and a cholecystectomy for chronic cholecystitis. In 2010, the patient was involved in a motor vehicle accident in which she sustained superficial injuries and developed a violaceous plaque at a site of injury on the left leg. The skin biopsy showed thickened vessel walls with perivascular lymphocytic and neutrophilic inflammation. Three days after the skin biopsy, she developed clinical signs of pyoderma gangrenosum. The patient has been on steroids intermittently since 1999 for pyoderma gangrenosum flares and abdominal pain. In the past 2 years, she has required continuous high-dose steroids for worsening abdominal pain with recurrence of symptoms when prednisone dose was decreased to less than 40 mg daily. In the past year, she was hospitalised on a monthly basis for abdominal pain and developed many side effects from chronic high-dose steroid therapy, including Cushingoid facies, steroid-induced diabetes mellitus, hypertension, weight gain and proximal muscle weakness. She was briefly trialled on azathioprine but the drug was discontinued due to the development of bilateral pulmonary infiltrates.
At the time of interview, the patient was found to have a large and painful aphthous ulcer on the right lateral border of the tongue (figure 1). She also had a well-demarcated aphthous ulcer in the perianal area as well as several 5 mm, shallow and ulcerating lesions involving the mons pubis and left labium majus (figure 2). The patient reported having similar and recurrent oral and genital lesions in the past. A review of the medical record found that the patient had erythema nodosum on the anterior surfaces of both legs in the previous year. During a recent hospitalisation, the diagnosis of sclerosing mesenteritis was entertained based on small bowel mesenteric infiltrates seen on serial abdominal CT scans. The non-specific inflammation was redemonstrated within the central mesentery on serial CTs as it extended to include the right lower quadrant and left mid-mesentery. The CT images showed interval decreases and increases in inflammatory changes that were worse when the patient was not on steroid therapy (figure 3A,B).
Figure 1.
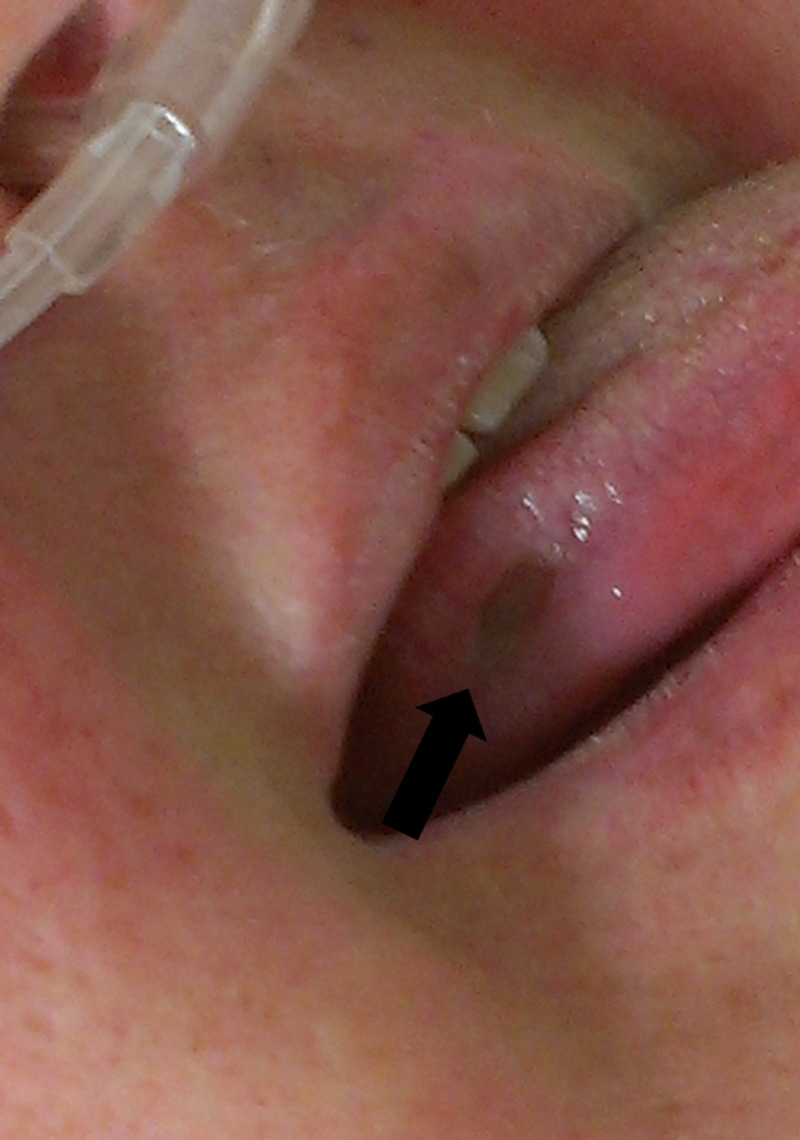
Aphthous ulcer on the right lateral border of the tongue.
Figure 2.
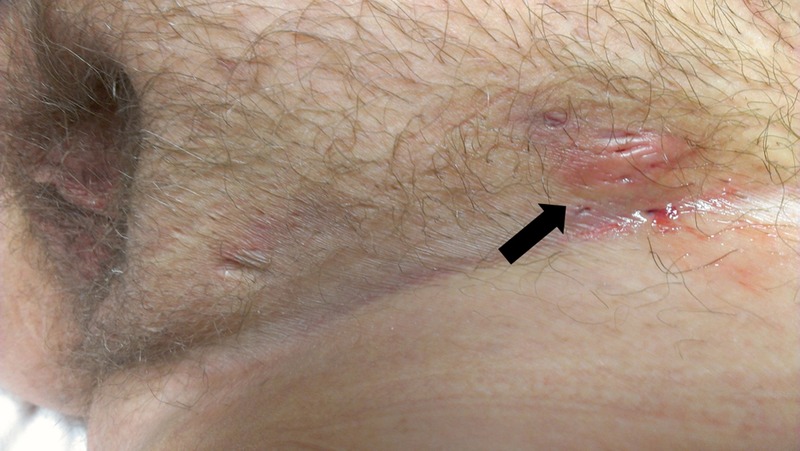
Shallow and ulcerated lesions involving the mons pubis and left labium majus.
Figure 3.
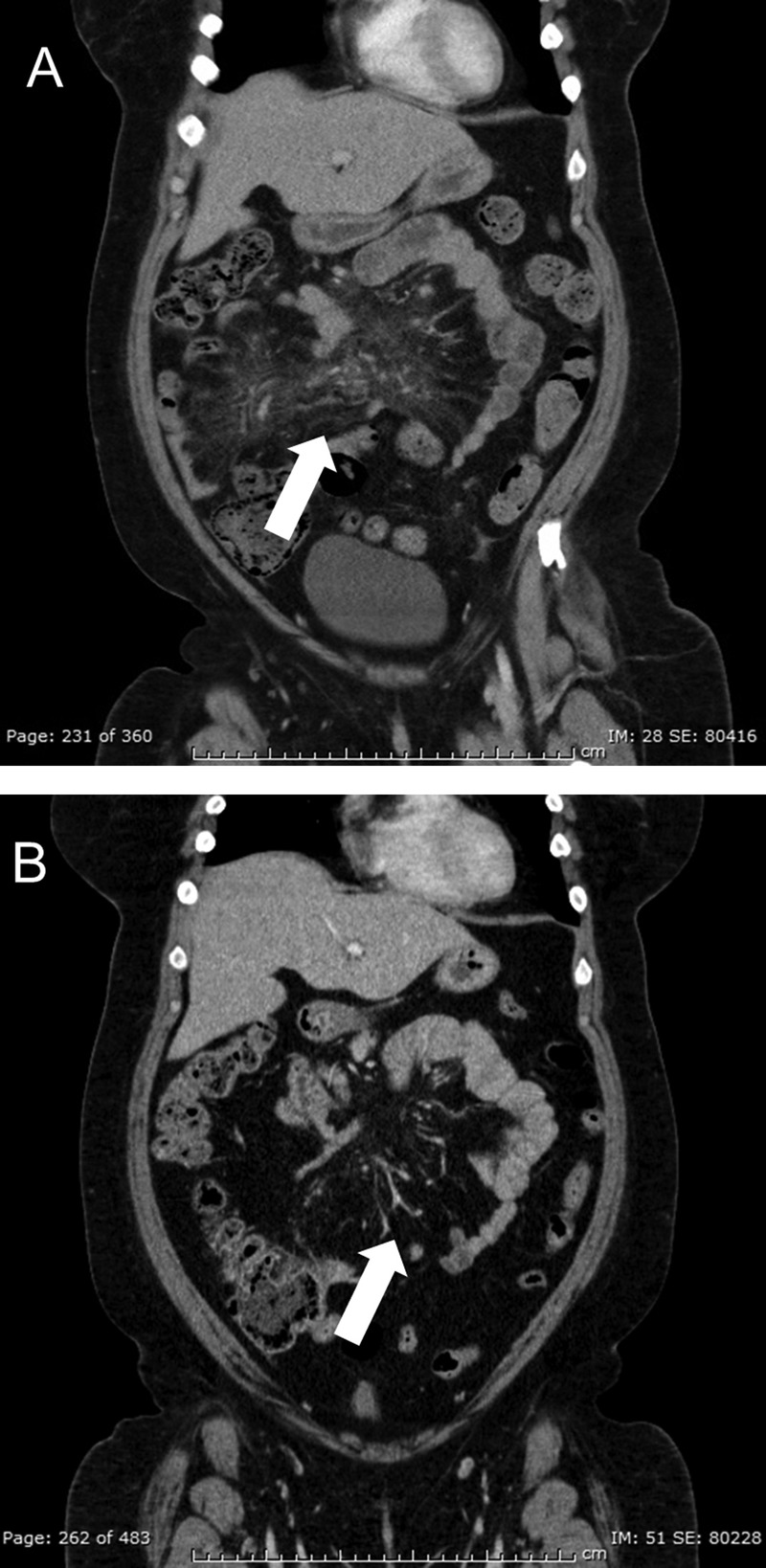
(A) Patient presented with worsening abdominal pain while on prednisone 25 mg daily and CT of the abdomen and pelvis demonstrated mesenteric infiltrates. She was hospitalised and treated with high-dose steroids that were then tapered. (B) CT of the abdomen and pelvis clearly shows an interval improvement in the mesenteric infiltrates 4 weeks after the patient was discharged.
Investigations
Blood tests on admission revealed slight increases in the levels of ESR and CRP, with a normal complete blood count. Echocardiography and blood cultures were negative. Further investigations included negative antinuclear antibody, negative rheumatoid factor, negative myeloperoxidase and negative proteinase 3 antibodies, normal C3 and C4 levels and negative herpes simplex virus culture obtained from the genital lesions. Her hospital course was complicated by shortness of breath, haemoptysis and oxygen desaturation. She underwent a bronchoscopy with bronchoalveolar lavage that revealed alveolar haemorrhage (figure 4A,B). The Diff-Quik and modified Kinyoun, Gomori methenamine silver stains demonstrated numerous neutrophils and 80–90% hemosiderin-laden macrophages. The bronchoalveolar lavage was negative for atypical cells, viral inclusions, acid-fast microorganisms, bacteria, Pneumocystis jirovecii or fungi. CT angiography of the chest, abdomen and pelvis did not show major vessel involvement. A clinical diagnosis of BD was made based on the history of pyoderma gangrenosum, erythema nodosum, alveolar haemorrhage and recurrent orogenital ulcerations using the International Criteria for BD (table 1).
Figure 4.
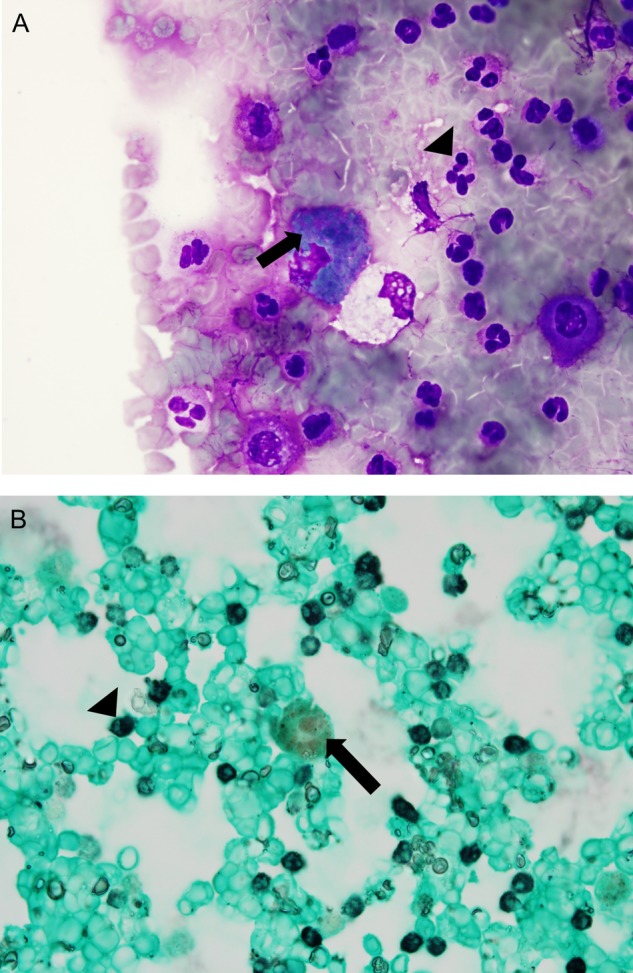
(A) Diff-Quik and Grocott's methenamine silver (GMS)-stained smears (B) of the bronchoalveolar lavage pellet demonstrate numerous neutrophils (arrowheads) and 80–90% hemosiderin-laden macrophages (arrows), concerning for intra-alveolar haemorrhage. Hemosiderin stains blue–green with Diff-Quik and it does not stain with GMS, so it appears with its distinctive natural yellow–brown colour.
Table 1.
International criteria for Behçet's disease—score ≥4 indicates a diagnosis of Behçet's disease
| Clinical manifestations | Score |
|---|---|
| Ocular lesions (retina vasculitis, anterior and posterior uveitis) | 2 |
| Genital aphthosis | 2 |
| Oral aphthosis | 2 |
| Skin lesions (pseudofolliculitis, erythema nodosum, pyoderma gangrenosum) | 1 |
| Neurological manifestations | 1 |
| Vascular manifestations (arterial and large vein thrombosis, phlebitis) | 1 |
| Positive pathergy test (cutaneous hyper-reactivity in response to minor cutaneous trauma) | 1* |
*Pathergy test is optional and if carried out, it can be included in the scoring.
Following the working diagnosis of BD, human lymphocyte antigens B5/B51 were tested and found to be negative. The pathergy test was positive, evidenced by skin hyper-reactivity 48 h following peripherally inserted central catheter line removal and the presence of the tender, indurated and erythematous 5 mm papule 4 weeks later (figure 5). Eye fundoscopic examination was unremarkable.
Figure 5.
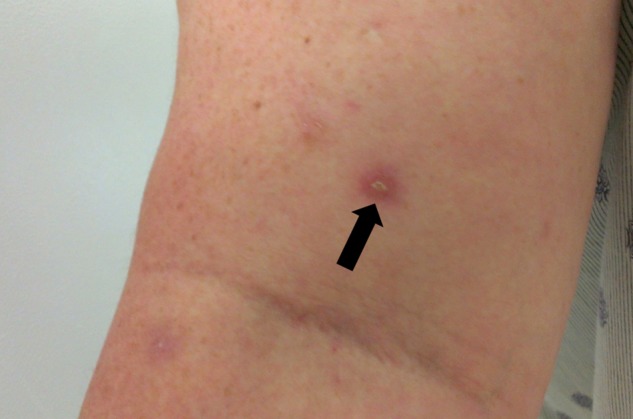
Erythematous, tender and indurated 5 mm papule that occurred 48 h after the removal of a peripherally inserted central catheter line and that was still present 4 weeks later, consistent with a positive pathergy test.
Differential diagnosis
Intestinal manifestations of BD are rare and can often mimic more common abdominal conditions, including inflammatory bowel disease and particularly Crohn's disease. Both diseases tend to occur in younger patients and both have relapsing natures, non-specific gastrointestinal symptoms and similar extraintestinal involvement. The patient in this case was suspected of having Crohn's disease after the resected appendix revealed non-caseating granulomas and focal acute inflammation. However, multiple colonoscopies with biopsy were unremarkable. The patient's relapsing mesentery infiltrates were thought to be due to sclerosing mesenteritis but this diagnosis was excluded given the constellation of other symptoms, including orogenital aphthosis, pyoderma gangrenosum, erythema nodosum, positive pathergy phenomenon and alveolar haemorrhage. The mesentery inflammatory changes and bilateral pneumonia that occurred following azathioprine treatment were likely representations of the vasculitis. Granulomatosis with polyangiitis was also briefly considered in the differential but this diagnosis was rejected based on negative myeloperoxidase and proteinase 3 antibodies, a normal CT of the sinuses and lack of renal compromise.
Treatment
Treatment of BD is generally determined by organ involvement and severity. Initially, the patient was started on azathioprine 250 mg daily and prednisone 40 mg daily with plans to start infliximab after confirming a negative purified protein derivative and negative hepatitis B. The goal was to wean the steroids quickly and safely. Azathioprine has been shown to be helpful for cutaneous manifestations of BD, while infliximab has been beneficial for intestinal BD. After the patient developed alveolar haemorrhage, a shared decision between the patient and the multidisciplinary team was made to begin treatment with 3 days of pulse intravenous solumedrol at 1 g/daily and a course of cyclophosphamide at 700 mg/m2.
Outcome and follow-up
The case had a favourable outcome despite the initial diagnostic challenges. The patient tolerated the first two infusions of cyclophosphamide and she has been able to gradually decrease the prednisone to less than 20 mg daily. Since starting treatment, she has had improvement in her symptoms. The therapeutic plan involves slow tapering of prednisone with the goal to establish symptomatic control without prednisone. The cyclophosphamide will be given as monthly infusions for 6 months before starting the patient on a disease-modifying agent.
Discussion
The diagnosis of BD is based on clinical manifestations as there is no pathognomonic test. The International Criteria for BD was used here because it has increased sensitivity compared with the International Study Group criteria (94.8% vs 85%).4 The patient had a score of 6 based on recurrent orogenital aphthous, positive pathergy test and typical skin lesions (erythema nodosum and pyoderma gangrenosum). She also developed alveolar haemorrhage, a severe complication of BD.
The diagnosis of BD proved to be difficult due to the chronic relapsing nature of this disease. In addition, the patient presented with several different conditions that seemed unrelated but that could be explained by a unifying diagnosis of BD. She first developed major skin reactions and systemic symptoms after breast exploration and after the skin biopsy of the left leg. These included severe erythema, induration, pain at the incision site and high-grade fever. These skin reactions after major trauma point to an underlying vasculitis. In addition, the patient developed erythematous, indurated and tender papules after the removal of peripheral intravenous lines, consistent with a positive pathergy phenomenon. The patient was also found to have focal acute appendicitis with non-caseating granulomas, at which point she was thought to have Crohn's disease. In focal appendicitis, the focus of inflammation is confined to only a few serial sections, polymorphonuclear neutrophils are in a single area and there is an increased lymphocyte and plasma cell response throughout the organ.5 The predominance of lymphocytes and plasma cells in the lamina propria of focal appendicitis may reflect the presence of a specific immune response to a luminal antigen as a cause of appendicitis, as seen in patients with autoimmune disorders, including inflammatory bowel disease and as in this case, BD. In addition, granulomatous endocarditis has been described in a patient with BD.6 And so, it is possible that small-vessel vasculitis of BD led to focal acute appendicitis that may have progressed to granuloma formation.
Treatment of BD has become more effective in recent years because of a better understanding of the disease and the increased availability of therapeutic agents. Despite the increased therapy options, complete remission is rarely achieved. Treatment is generally determined on the organ system involvement and the severity of the disease. Recommendations related to ocular, skin and mucosal lesions are evidence based, but recommendations on neurological, gastrointestinal and vascular manifestations are based on uncontrolled trials and expert opinion. Several controlled studies found a reduction in orogenital ulcer, skin lesions and arthritis with colchicine, thalidomine, azathioprine, cyclosporin A and interferon α2a.7–11 Some studies have indicated that patients with BD have increased levels of tumour necrosis factor α (TNF-α) and TNF-α receptors and so far, three anti-TNF-α agents, infliximab, adalimumab and etanercept, have shown therapeutic benefits for mucocutaneous lesions, arthritis, ocular disease, gastrointestinal involvement and cerebral vasculitis.12–14 Several immunosuppressive regimens have been used in pulmonary BD, including calcineurin inhibitors, cyclophosphamide and azathioprine. A double-blind cross-over study showed that the combination of cyclophosphamide and corticosteroid therapy is beneficial for eye disease and systemic vasculitis, including neurological and arterial aneurysms.15 The European League Against Rheumatism (EULAR) recommends the use of cyclophosphamide and corticosteroids for pulmonary and peripheral arterial aneurysms in BD. For pulmonary aneurysms, EULAR has recommended the continued use of cyclophosphamide for 2 years followed by azathioprine.16 Although BD therapies have become more effective in recent years, the disease is still associated with severe morbidity and significant mortality.
Learning points.
This report emphasises that systemic diseases, including Behçet's disease (BD), can have variable presentations and can be frequently misdiagnosed.
Unexplained severe abdominal pain associated with mesenteric infiltrates may suggest gastrointestinal manifestation of a vasculitis syndrome.
Anti-tumour necrosis factor antibodies, particularly infliximab, have shown to be beneficial in the suppression of intestinal BD symptoms.
The European League Against Rheumatism (EULAR) recommends the use of cyclophosphamide and corticosteroids for pulmonary and peripheral arterial aneurysms in BD.
For pulmonary aneurysms, EULAR has recommended the continued use of cyclophosphamide for 2 years followed by azathioprine.
Acknowledgments
The authors thank Bryan H Schmitt, DO for his contribution to the histopathological diagnosis.
Footnotes
Contributors: EN was involved in the management of the patient, writing of the manuscript, literature research and compiling images. MF was involved in the management of the patient and reviewing the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Direskeneli H. Behçet's disease: infectious aetiology, new autoantigens, and HLA-B51. Ann Rheum Dis 2001;60:996–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oshima Y, Shimizu T, Yokohari R, et al. Clinical studies on Behçet's syndrome. Ann Rheum Dis 1963;22:36–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kural-Seyahi E, Fresko I, Seyahi N, et al. The long-term mortality and morbidity of Behçet syndrome: a 2-decade outcome survey of 387 patients followed at a dedicated center. Medicine (Baltimore) 2003;82:60–76 [DOI] [PubMed] [Google Scholar]
- 4.Davatchi F, Asaad-Khalil S, Calamia KT, et al. The International Criteria for Behçet's Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol 2014;28:338–47 [DOI] [PubMed] [Google Scholar]
- 5.Tsuji M, Puri P, Reen DJ. Characterisation of the local inflammatory response in appendicitis. J Pediatr Gastroenterol Nutr 1993;16:43–8 [DOI] [PubMed] [Google Scholar]
- 6.Huycke EC, Robinowitz M, Cohenn IS, et al. Granulomatous endocarditis with systemic embolism in Behçet's disease. Ann Intern Med 1985;102:791–3 [DOI] [PubMed] [Google Scholar]
- 7.Yurdakul S, Mat C, Tuzun Y, et al. A double-blind trial of colchicine in Behçet's syndrome. Arthritis Rheum 2001;44:2686–92 [DOI] [PubMed] [Google Scholar]
- 8.Hamuryudan V, Mat C, Saip S, et al. Thalidomide in the treatment of the mucocutaneous lesions of the Behçet syndrome. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 1998;128:443–50 [DOI] [PubMed] [Google Scholar]
- 9.Yazici H, Pazarli H, Barnes CG, et al. A controlled trial of azathioprine in Behçet's syndrome. N Engl J Med 1990;322:281–5 [DOI] [PubMed] [Google Scholar]
- 10.Assaad-Khalil SH. Low-dose cyclosporin in Behçet's disease: follow-up controlled study with emphasis on extraocular manifestations and neuro-Behçet's disease. In: O'DuVy JD, Kokmen E. eds. Behçet's disease: basic and clinical aspects. New York: Marcel Dekker, 1991:603–12 [Google Scholar]
- 11.Alpsoy E, Durusoy C, Yilmaz E, et al. Interferon alpha-2a in the treatment of Behçet disease: a randomized, placebo-controlled and double blind study. Arch Dermatol 2002;138:467–71 [DOI] [PubMed] [Google Scholar]
- 12.Song YW, Kang EH. Behçet's disease and genes within the major histocompatibility complex region. Mod Rheumatol 2012;22:178–85 [DOI] [PubMed] [Google Scholar]
- 13.Sfikakis PP, Markomichelakis N, Alpsoy E, et al. Anti-TNF therapy in the management of Behçet's disease—review and basis for recommendations. Rheumatology (Oxford) 2007;46:736–41 [DOI] [PubMed] [Google Scholar]
- 14.Melikoglu M, Fresko I, Mat C, et al. Short-term trial of etanercept in Behçet's disease: a double blind, placebo controlled study. J Rheumatol 2005;32:98–105 [PubMed] [Google Scholar]
- 15.Davatchi F, Shahram F, Chams H, et al. Pulse cyclophosphamide (PCP) for ocular lesions of Behçet's disease: double blind crossover study. Arthritis Rheum 1999;42:S320 [Google Scholar]
- 16.Hatemi G, Silman A, Bang D, et al. EULAR recommendations for the management of Behçet disease. Ann Rheum Dis 2008;67:1656–62 [DOI] [PubMed] [Google Scholar]


