Abstract
Temporopolar cortex plays a crucial role in the pathogenesis of temporal lobe epilepsy and subserves important cognitive functions. Because of its shape and position in the middle cranial fossa, complete electrode coverage of the temporal pole (TP) is difficult to achieve using existing devices. We designed a novel TP electrode array that conforms to the surface of temporopolar cortex and achieves dense electrode coverage of this important brain region. A multi-pronged electrode array was designed that can be placed over the surface of the TP using a straightforward insertion technique. Twelve patients with medically intractable epilepsy were implanted with the TP electrode array for purposes of seizure localization. Select patients underwent cognitive mapping by electrocorticographic (ECoG) recording from the TP during a naming task. Use of the array resulted in excellent TP electrode coverage in all patients. High quality ECoG data were consistently obtained for purposes of delineating seizure activity and functional mapping. During a naming task, significant increases in ECoG power were observed within localized subregions of the TP. One patient developed a transient neurological deficit thought to be related to the mass effect of multiple intracranial recording arrays, including the TP array. This deficit resolved following removal of all electrodes. The TP electrode array overcomes limitations of existing devices and enables clinicians and researchers to obtain optimal multi-site recordings from this important brain region.
Keywords: anterior temporal lobectomy, cortico-amygdalohippocampectomy, anterior temporal lobe, epilepsy, intracranial recording, seizures, language mapping
Introduction
Epilepsy is one of the most common neurological disorders and is estimated to affect more than 1% of the population (Hauser et al., 1993). Of people with epilepsy, approximately one-fifth have medically intractable seizures and many of these patients are subsequently found to have a localizable seizure focus amenable to surgical resection (Begley et al., 1994, Engel, 1994, Rosenow and Luders, 2001). When performing resection surgery for medically refractory epilepsy it is critically important to accurately localize epileptic foci and regions of eloquent cortex (Elger and Burr, 1994). Noninvasive methods such as positron emission tomography and scalp electroencephalography (EEG) provide useful preoperative localization information but lack the spatial resolution, or capacity to capture ictal events, required to precisely identify sites of seizure onsets in many patients with epilepsy (Elger and Burr, 1994). The highest degree of localization accuracy is achieved with the use of intracranial recording methods (electrocorticography, ECoG). A wide range of techniques and devices are employed for this purpose, including surface recording arrays and depth electrodes (Wyler et al., 1984, Spencer, 1981). One of the most important brain regions to consider within the context of epilepsy resection surgery is the temporal pole (TP), corresponding roughly to Brodmann area 38 (Brodmann, 1909). Temporopolar cortex is frequently implicated in the pathogenesis of temporal lobe epilepsy, and standard surgical techniques used to resect mesial temporal lobe seizure foci often involve resecting TP tissue (Chabardes et al., 2005, Ding et al., 2009, Kahane et al., 2002, Pail et al., 2012). Accurately defining the ictal and interictal involvement of the TP cortex is therefore important for epileptic focus localization.
Electrocorticography of the Temporal Pole
The purpose of this manuscript is to describe a novel ECoG array that provides dense and reproducible coverage of the TP cortex for seizure localization and cognitive mapping. Reproducible dense ECoG coverage of the TP is nearly impossible to achieve using standard strip and grid ECoG arrays. Because the TP has the shape of a half-sphere, a standard electrode array embedded within a flat continuous silicon sheet will not conform smoothly to TP cortical surface. Multiple strip electrodes can be implanted over the TP cortex, but due to limited visualization of TP cortex during surgery, the final positions of the strip electrode recording contacts cannot be determined at the time of implantation. Also, if multiple strip electrodes are manually inserted towards the TP using standard technique, the spatial distribution of the collective array is not controlled, resulting in gaps in coverage. The modified array described in this report addresses these design considerations by taking advantage of the attributes of individual strip electrodes while at the same time circumventing the spatial control limitations described above.
Cognitive Mapping
Lesion studies provide clear evidence that the anterior temporal lobe is important to a variety of cognitive functions including naming, recognition, and social function (Milner, 2003, Tranel, 2009). Studies in temporal lobe epilepsy patients suggest that the language dominant TP may play a specific role in proper noun naming and this may have quality of life implications for patients who undergo cortico-amygdalohippocampectomy (CAH) (Yucus and Tranel, 2007, Griffin and Tranel, 2007). Although there is strong evidence for eloquent semantic memory function in the anterior temporal lobe, standard techniques for identifying functional cortex lack the sensitivity to detect it. Functional mapping of the TP with magnetic resonance imaging (MRI) techniques is challenging due to the signal dropout caused by susceptibility artifact (Devlin et al., 2000). With limited exposure of the TP from a standard temporal craniotomy, electrocortical stimulation techniques are not possible without ECoG array placement. Placement of ECoG arrays allow physiologic mapping or electrocortical stimulation mapping, but standard electrode arrays do not provide dense or consistent coverage of the TP neocortex.
Methods
Patients and Clinical Data
Twelve patients with medically intractable epilepsy were included in this study (11 males, 1 female, age 19 – 51; see Table 1). The patients underwent placement of the TP recording array as part of ECoG monitoring for seizure localization; all patients were treated between July 2010 and May 2013. The patients were selected for intracranial EEG seizure focus localization as part of the standard clinical management protocol of the Iowa Comprehensive Epilepsy Program (ICEP) at the University of Iowa. Intracranial monitoring candidates were patients with medically intractable epilepsy who were likely to have a surgically resectable seizure focus but required more refined localization of this focus than was possible using non-invasive methods. Each patient underwent a standard craniotomy procedure for placement of electrodes. Preoperative 3T brain MRI and postoperative high-density head computed tomography (CT) were performed for precise localization of cortical electrodes. During stage II monitoring, ictal and interictal ECoG activity was interpreted by an ICEP epileptologist. Each patient subsequently underwent re-operation for electrode removal and in most cases resection of the identified epileptic focus. Electrode implantation and seizure focus resection were all performed by the same surgical team (M.H. and H.K.).
Table 1.
Clinical characteristics of patients with TP grid monitoring
| Subject | Age sex |
Seizure localization |
TP involvement |
Mesial temporal sclerosis |
Associated pathology | TP electrode laterality |
Surgical procedure |
TP resection |
Postoperative complications | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| S01 | 49 M |
L mesial temporal | early propagation | L | - | L | L ATL | + | Mild aphasia | Seizure-free at 1 year |
| S02 | 26 M |
L mesial temporal | early propagation and focus for subclinical seizures | L | Cortical dysplasia of temporal cortex, hippocampus, amygdala | L | L ATL | + | Temporary aphasia, R cerebellar hemorrhage | Seizure-free at 1 year |
| S03 | 41 M |
R mesial temporal + R ventral frontal | early propagation | R | PTE, cortical dysplasia in amygdala | R | R ATL + lesionectomy | + | Subdural CSF collection | Seizure-free at 10 mo |
| S04 | 34 M |
L mesial temporal, less R mesial temporal | early propagation | L | - | L | L ATL | + | None | Seizure-free at 9 mo |
| S05 | 29 M |
R mesial temporal | - | R | - | L | R ATL | - | None | Seizure-free at 2 year |
| S06 | 51 F |
bilateral mesial temporal | late propagation | L | PTE, R diffuse white matter change | L | none | - | None | No resection |
| S07 | 29 M |
R mesial temporal + TP | early propagation | - | R mesial temporal Dysembryoplastic neuroepithelial tumor |
R | R ATL + lesionectomy | + | R parietal IPH | 2 seizures in 2 years, both due to medical noncompliance |
| S08 | 28 M |
L mesial temporal + TP | focus | - | L mesial temporal cortical dysplasia | L | L ATL +lesionectomy | + | None | Seizure-free at 6 wks |
| S09 | 23 M |
R ventral temporal | late propagation | - | R ventral temporal Ganglioglioma |
R | R ATL + lesionectomy | + | None | Seizure-free at 1 yr |
| S10 | 50 M |
L lateral temporal | - | - | L temporal cortical dysplasia | L | L posterior temporal lesionectomy | - | None | Seizure-free at 2 mo |
| S11 | 19 M |
L lateral temporal | late propagation | - | L atrium periventricular nodular heterotopia, L temporal cortical dysplasia, | L | L posterior temporal lesionectomy | - | None | No follow up |
| S12 | 33 M |
R frontal | - | - | PTE, R frontal contusion | R | R frontal lobectomy | - | None | Seizure-free at 1yr |
Abbreviation key: M = male, F = female, R = right, L = left, TP = temporal pole, PTE = post-traumatic epilepsy, ATL = anterior temporal lobectomy, CSF = cerebrospinal fluid, IPH= intraparenchymal hemorrhage, yr = year, mo = month
We performed a retrospective review of each patient in this series using a protocol approved by the University of Iowa Institutional Review Board. Clinical documents were included for review including inpatient and outpatient notes, imaging studies, intraoperative photographs, ECoG interpretations, and pathology reports. Surgical complications were noted from the medical records.
Electrode Design and Surgical Technique
As noted above, a standard electrode array embedded within a flat continuous silicon sheet will not conform smoothly to the cortical surface of the TP due to its curved shape. Another device design consideration relates to the required electrode insertion technique. The TP is not exposed at the time of surgery and cannot be directly visualized. This necessitates that a malleable recording array be advanced within the subdural space from a starting position on the exposed anterior lateral temporal lobe, forward onto the TP without visual feedback. Thin strip electrodes can be safely inserted using this method, but the final positions of the strip electrode recording contacts cannot be determined during surgery. Also, if multiple strip electrodes are manually inserted towards the TP using the standard technique, the spatial distribution of the collective array is not controlled, resulting in gaps in coverage. The modified array described in this report addresses these design considerations by taking advantage of the attributes of individual strip electrodes while at the same time circumventing the spatial control limitations described above. This is achieved by adopted a ‘multi-prong’ design whereby a sheet electrode array is modified to create multiple thin strip electrodes that remain connected along one boarder of the sheet.
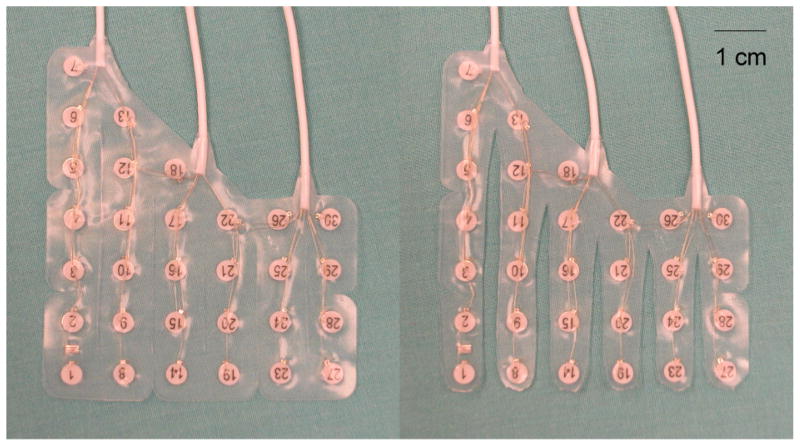
Each strip is sufficiently malleable to contour itself along the curvature of the TP and the points of mechanical fusion at the array edge insure that even spacing is achieved between the parallel strips. This results in dense, predictable electrode coverage of the TP. This device design concept was implemented by modifying a commercially available grid array (Ad-Tech Medical Instrument, Racine, WI). Two grid sizes were developed, a 23-contact grid and a 30-contact grid, to accommodate different TP sizes. The grids consist of 5 or 6 columns with the first 2 or 3 columns tapered such that they are composed of columns of 6-5-4-4-4 or 7-6-5-4-4-4 contacts for 23-contacts or 30-contacts grids, respectively (Fig. 1). Each grid is cut into separate parallel columns, sparing the portion of the grid closest to the proximal contacts in order to retain spatial alignment. Each column is trimmed as it tapers off to reduce its mass. There are two versions of each 23- and 30-contact grid: one for left hemisphere cases and the other for right hemisphere cases. The left hemisphere version is made such that the exposed surface of contacts faces the cortical surface when the longest edge of the array is placed superiorly. For the right hemisphere version, electrode contacts are exposed on the reverse side. Each electrode grid is embedded in silastic, with a 1 cm inter-electrode distance and 2.3 mm electrode diameter exposed to the brain surface.
Figure 1.
30-contact TP grid before and after cutting columns (left and right panel, respectively).
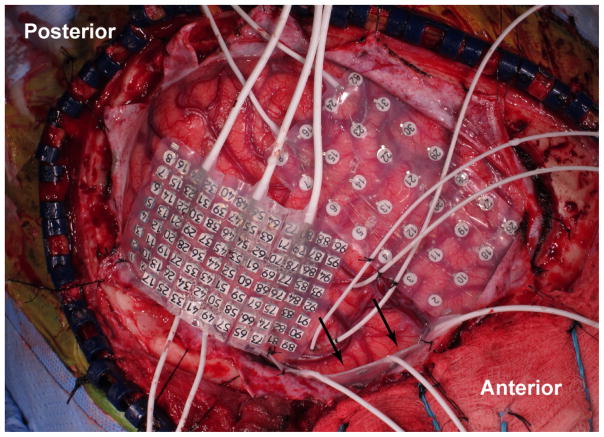
The TP electrode grid is inserted after the brain surface is exposed through a frontotemporal craniotomy. In the course of performing the craniotomy it is important to expose the middle cranial fossa (MCF) as anterior and inferior as possible. Once the frontotemporal neocortex is visualized, placement of the TP electrode grid is initiated. Irrigation is applied to the grid to lubricate the electrode surface prior to implantation. Tapered separation of each electrode column of the electrode grid increases the malleability and facilitates flexible coverage of the TP surface without compressing the cortex. The long edge of the array is held parallel to the inferior surface of the sphenoid wing. The array is designed such that following insertion the strip forming the short edge of the array is positioned on the floor of the MCF oriented roughly perpendicular to the long axis of the temporal lobe. In order to insert the array, the tips of each column are placed between the temporal lobe and the dura mater of the MCF. Then the grid is carefully advanced medial and anterior into the MCF. As it is advanced, the electrode grid follows the inferior and anterior curvature of the MCF to line the TP. Since cranial exposure does not permit direct visualization of the TP, care must be taken while advancing the grid to avoid exerting forces on unseen adhesions, bridging veins, or mass lesions. The grid is not advanced any further if any resistance is encountered as this may represent contact with a bridging vein. If subdural bleeding is encountered, it is managed by gently irrigating the subdural space until the bleeding stops. Once the grid is in place, the majority of electrode contacts are not visible to the surgeon (Fig. 2).
Figure 2.
Intraoperative photograph depicting placement of a TP electrode grid (indicated with arrows).
Imaging Coregistration
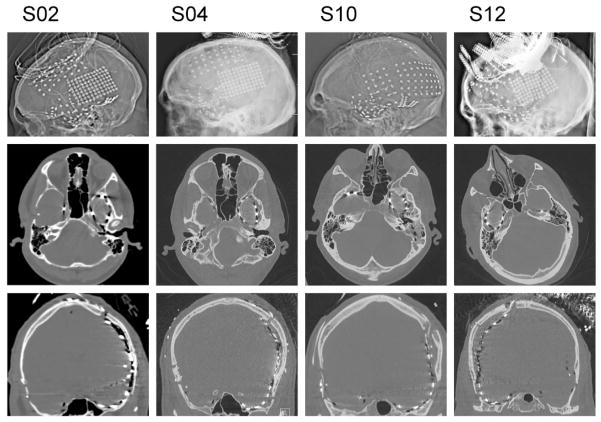
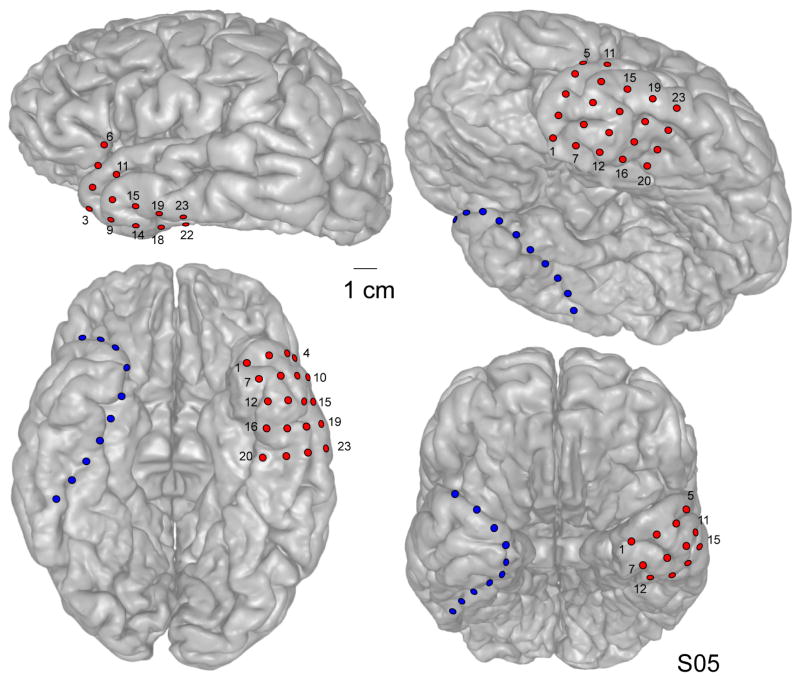
All patients underwent postoperative imaging (skull films and high density CT) to document the location of all electrode contacts. Examples of postoperative head X-ray/CT images from four patients are shown in Figure 3. For each patient, electrode localization over the TP was confirmed using a well-established CT – MRI co-registration protocol (Brugge et al., 2008, Greenlee et al., 2011, Kovach et al., 2011). An example of such a reconstruction from one patient is shown in Figure 4. The precise position of each subdural electrode was determined by co-registering thin-cut (1 mm) post-implantation CT scans with pre-implantation T1-weighted 3T brain MRI using Analyze software (Biomedical Imaging Resource, Mayo Clinic) (Brugge et al., 2008). In this way, electrode locations were mapped on a three-dimensional surface rendering of the structural MR image of each patient’s brain.
Figure 3.
X-ray/CT imaging of four subjects (left to right) implanted with the TP grid.
Figure 4.
CT – MRI fusion images from a single patient (S05) showing location of implanted electrode contacts. Different electrode arrays are shown in different colors (TP grid and anteromedial strip contacts are shown in red and blue, respectively).
Cognitive Mapping
Resection of the left TP often results in some degree of anomia for familiar people and places (Griffin and Tranel, 2007). Additionally, lesion studies and positron emission tomography functional imaging have also demonstrated a critical role for the left TP for naming famous people and famous places (Damasio et al., 1996, Grabowski et al., 2001, Griffin and Tranel, 2007, Tranel, 2009). One of the objectives in developing the TP grid was to provide a technical means of studying the functional properties of this cortical region. This information could be used in formulating a resection plan, and for neuroscience research purposes. Once electrodes are in place, functional mapping can be accomplished using a variety of complementary methods including electrical stimulation disruption techniques and local field potential recordings. By using appropriately designed experimental protocols and contemporary electrophysiological analytic methods, investigators have used local field potential recordings in neurosurgery patients to characterize the functional properties of many different human brain regions (Crone et al., 1998, Kawasaki et al., 2001, Miller et al., 2007, Miller et al., 2011, Quiroga et al., 2005). We performed a series of experiments to determine whether this same strategy could be applied to study the functional organization of the TP.
Patients participated in a simple naming task in which they were presented with a picture of a famous person, landmark, or tool on a computer monitor 1 m from the patient at the bedside. Each patient was presented with at least 50 unique pictures of a specific famous person (actor or politician, e.g. George W. Bush, Brad Pitt) or specific famous landmark (e.g., Eiffel Tower, Space Needle). Also, each patient was shown at least 50 unique pictures of non-specific tools (e.g. scissors, forks). Since many patients with left temporal lobe have preoperative naming deficits, each patient was tested on an informal version of the Iowa Famous Faces Test and the Iowa Famous Landmark Test to assess preoperative language function (Tranel, 2006, Tranel, 2009). After implantation, patients were only tested on famous people or landmarks that they were able to name prior to implantation. Pictures were presented for 1 s each. There was a 1 s wait period followed by a visual cue to state the name of the picture. Trials were randomized within each block. The patient’s vocal response triggered the presentation of the next picture. ECoG signals were acquired continuously during the naming task (TDT RZ2 processor, Tucker-Davis Technologies, Alachua, FL). Recorded data were digitized, filtered, and stored for offline analysis (2034.5 Hz sampling rate, 0.7– 800 Hz band pass filter). A subgaleal electrode was used as reference. Continuous data were filtered to remove 60 Hz line noise and its harmonics, downsampled to 1000 Hz for computational efficiency and segmented offline into 5-second epochs, from −1 s to 4 s relative to the onset of each picture. ECoG recordings obtained during naming people, landmarks, and tools were averaged within each category and analyzed in the time-frequency plane using multitaper spectral estimation (Thomson, 1982). Relative power change during naming compared to a prestimulus baseline (between 500 and 300 ms prior to onset of picture) was calculated and plotted.
Results
A total of twelve patients underwent placement of the TP electrode grid. In each patient, electrodes were placed for localization of medically intractable epilepsy based on the recommendations of the ICEP Clinical Review Board. Six patients had mesial temporal sclerosis. Five had cortical dysplasia, three had post-traumatic epilepsy (PTE), and two were diagnosed with neoplasms (ganglioglioma, and dysembryonic neuroepithelial tumor). The TP grid fit well over the convexity of the TP in all patients. Postoperatively placement of the TP grid was confirmed using skull X-ray/CT imaging (see Fig. 3). As Figure 3 demonstrates in four patients, placement of the TP electrode array was consistent and reproducible across all patients. There was no evidence of significant variance in electrode coverage across patients. Coregistration of CT and MRI demonstrates the position of the electrodes over the surface of the TP (see Fig. 4).
Seizure Localization
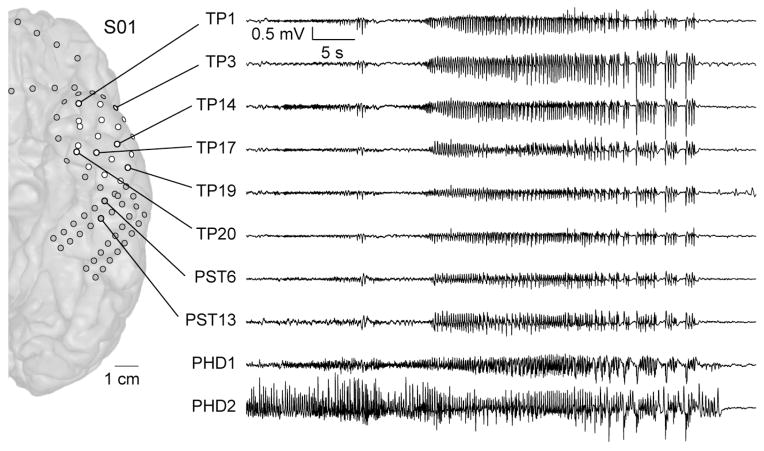
Signal quality obtained from the TP electrode array was comparable with other standard intracranial electrode arrays (Fig 5). A primary seizure focus was localized in all twelve patients. The TP cortex covered by the TP electrodes was found to be involved in ictal seizure activity in 9 patients. In one of those 9 patients, TP was included in the seizure onset zone (S08). This patient had left mesial temporal cortical dysplasia. In four patients with mesial temporal sclerosis (MTS), ictal events started in the mesial temporal lobe and quickly propagated to the TP within a few seconds. In 3 patients (S06, S09, S11), ictal activity propagated to the TP during the later stages of seizure events. One of these 3 patients had MTS, however, the remaining 2 patients did not have mesial temporal pathology. Therefore, in 5 out of 6 patients with MTS, seizures rapidly propagated to the TP very early in the course of the seizure.
Figure 5.
ECoG trace demonstrating spread of ictal activity from the posterior hippocampus to the TP. PHD, posterior hippocampal depth; TP, temporal pole; PST, posterior subtemporal. TP contacts are shown in white; other subdural contacts are shown in grey.
A seizure focus resection was performed in all but one patient who had bilateral mesial temporal lobe seizure foci (S06). In 7 patients who had involvement of TP at ictal onset or in the early propagation stage, TP was resected along with mesial temporal structures.
Clinical outcomes at most recent follow up are summarized in Table 1. Of the eleven patients who underwent resection, nine are currently seizure free, one patient had two seizures, and one patient has not yet been evaluated in follow up. The resection patient (S07) who is not seizure free to-date had two breakthrough seizures when he was not compliant with his medication regimen. Otherwise, this patient has been seizure free.
Significant complications occurred in three patients. In one of these patients (S02), resection surgery was deferred for a second procedure because the patient developed word-finding difficulties following electrode implantation that necessitated electrode removal. The etiology of this finding is unclear since the patient had seizures postoperatively and may have suffered postictal aphasia. Therefore, the aphasia in this patient cannot be directly linked with implantation of the TP array. This patient was also noted to have subarachnoid blood layered over the superior surface of the cerebellum following surgery, and the etiology of this finding is unclear. That patient recovered his neurological functions fully within four weeks of electrode removal surgery and no complications were encountered during his subsequent resection surgery.
Patient S03 developed mild lethargy 12 days after electrode implantation surgery. Initially, this was presumed to reflect a post-ictal state. The day prior to electrode removal and resection surgery, an MRI demonstrated a 1.6 cm subdural fluid collection with 4 mm of midline shift. This subdural fluid collection was holohemispheric, not localized to the temporal lobe, and therefore not felt to be related to the TP array. The subdural fluid collection was removed at the time of resection surgery performed the following day. The patient was neurologically normal following surgery.
Patient S07 developed a right posterior temporal intraparenchymal hemorrhage (3×3×3 cm) after electrode removal and anterior temporal lobectomy. This hemorrhage was not associated with any detectable neurological deficit. This was managed conservatively and monitored with serial CT imaging. The cerebral hemorrhage was superficial and remote from the TP, likely related to injury to a cortical vein during dural opening and not related to the TP array. Additionally, patient S01 had mild self-limited aphasia after electrode implantation, which could possibly be related to implantation of the TP electrode. This resolved spontaneously and the patient successfully participated in stimulation mapping of language prior to electrode removal and left anterior temporal lobectomy.
Resection surgery was not performed for patient S06. Intracranial recordings demonstrated a primary seizure focus in the left anterior mesial temporal lobe, her language dominant hemisphere. After reconsidering the risks and benefits of resection surgery, this patient elected not to proceed with resection surgery as was planned, and therefore the electrodes were removed without resecting the seizure focus. Patient S10 had no ictal events during his three weeks of recording. He did have frequent interictal spikes and subclinical gamma bursts in the posterior ventral and lateral temporal cortex around his cortical dysplasia. The decision was made to resect the region of temporal lobe cortical dysplasia delineated on the preoperative studies and the area with frequent interictal spikes. In this case there were less frequent interictal spikes in the TP and the TP was not removed.
Cognitive Mapping
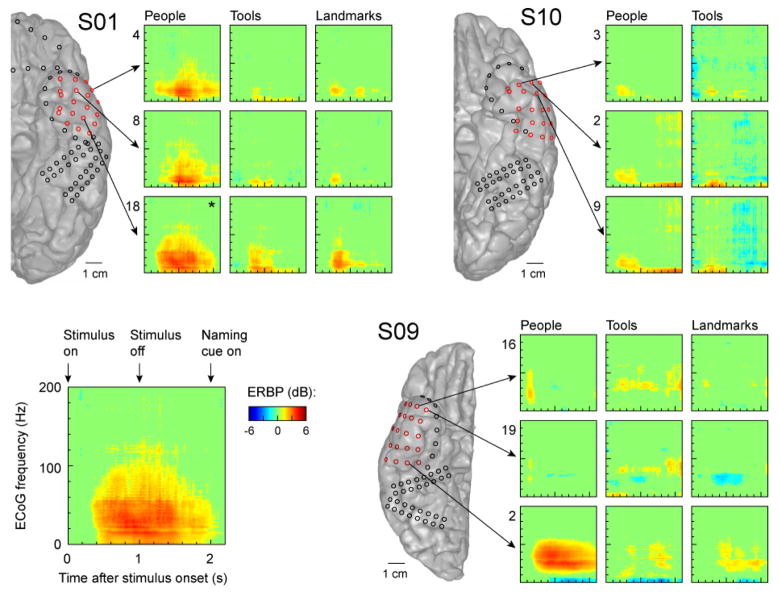
A pilot cognitive mapping study was performed in a subset of patients who underwent implantation of the TP array (see S01, S09, S10 in Fig 5). Naming people resulted in dramatic increases in relatively low frequency ECoG power (below 50 Hz) at select sites over the left TP (Fig. 6). Naming landmarks and tools also resulted in an increase in power in low frequencies at certain TP locations, but the magnitude of the responses was markedly attenuated compared to the response observed with naming people. Naming tools resulted in only minimal low frequency power increases. There was minimal change in power in the right TP when naming people, landmarks, and tools. An exception to this is a single electrode (#2) on the posterior aspect of the right-side implanted TP array in S09, which demonstrated a robust power change below 50Hz only when naming people. Naming famous people resulted in the most robust activation responses in the left TP.
Figure 6.
Spectrotemporal analysis of cortical activity recorded from the TP during the naming task. Data from three subjects (S01, S10, S09) are shown. Exemplary response to images of famous people recorded from contact #22 in subject S01 (marked by an asterisk) is shown in detail in the bottom left panel.
Discussion
Temporal Pole Electrocorticography
Temporopolar cortex is frequently involved in the pathogenesis of temporal lobe seizures (Chabardes et al., 2005). The TP also plays a crucial functional role in both social (Olson et al., 2007) and language (Tranel, 2006, Tranel, 2009) functions. For these reasons it is important to have a safe, effective, and standardized method for mapping this region of cortex. This is difficult to accomplish using conventional non-invasive methods or intracranial recording devices because of the bony anatomy of the MCF, the contoured surface of the TP, and the fact that this surface is not directly visualized during a standard craniotomy procedure. In this report we describe a device and method for placing an optimally spaced array of electrodes over this important brain region.
Conventional surface recording arrays consist of multi-contact strip electrodes or rectangular shaped grid devices. Neither configuration is well suited to provide dense or consistent TP coverage. Flat rectangular grids are unable conform to the curved, half-domed shape of the TP without buckling or compressing the underlying cortex. Without substantial modification, rectangular electrode arrays simply will not work and introduce safety concerns related to mass effect and cortical injury. Strip electrodes are malleable and can be safely introduced into the subdural space in the vicinity of the TP, but the resulting location cannot be directly visualized during surgery. This limitation prevents placement of multiple strip electrodes with pre-determined even spacing, resulting uneven coverage of the targeted cortex. Therefore, existing strip and grid electrode are inadequate to provide safe, dense, and consistent coverage of the TP cortex.
The TP array described in this report was designed to exploit the positive attributes of strip electrodes while at the same time overcoming the problem of limited positional control. This was achieved by modifying existing grid electrodes to create multi-pronged arrays that remain connected at one boarder. The array was further modified by making the prongs of the array different lengths to optimize coverage of the TP without exceeding the size limitations of the TP cortex or the MCF.
The TP electrode array provides information about the ictal and interictal electrical abnormalities on the TP neocortex. It is our practice to perform a temporopolar disconnection or full CAH for all patients with localizable temporal lobe epilepsy. In this setting, the TP array provides precise localization of the epileptic focus in the TP and is particularly helpful for identifying anterobasal or anterolateral epileptiform abnormalities. Though we have not yet applied the TP array in a case of anterior temporal cortical dysgenesis, it would be very helpful in that setting. At centers performing selective amygdalohippocampectomy (selAH), it is conceivable that chronic ECoG recordings with the TP array could identify patients who would benefit most from cortical resection in addition to selAH. Interictal spiking from the lateral temporal neocortex increase after transcortical selAH, making post-resective intraoperative recordings of the lateral neocortex difficult to interpret in terms of the need for further cortical resection (Cendes et al., 1993).
In this series of twelve patients we observed that the method for inserting the device over the TP is technically straightforward, and the resulting position of the electrodes achieves dense and consistent spatial coverage. The results of clinical ECoG recordings, and cognitive mapping experiments demonstrated the capacity of this device to capture ictal electrophysiological data as well as localize cortical activity evoked by naming tasks. Although the anterior temporal coverage achieved by the TP array in the presented patients was consistent, there are possible challenges in obtaining consistent coverage across all patients. Mesial temporal mass lesions could potentially prevent complete placement of the TP array. Also, it may not be possible to place the TP array in patients with especially prominent basotemporal bridging veins.
We observed one transient complication that may have been related to use of the device. A 26-year-old patient underwent left temporal lobe implantation of multiple strip, and grid electrodes, including the TP array. Following surgery he was noted to be aphasic and this persisted until electrode removal. This patient had several seizures in the postoperative period and it is possible that postictal effects contributed to the aphasia. However, in the absence of post-operative imaging evidence of supratentorial hemorrhage or continuous ongoing seizures, it was presumed that this neurological deficit was caused by, or contributed to by the cumulative mass effect of the multiple electrode arrays. The patient was returned to surgery for removal of all electrodes and he subsequently recovered all of his language functions. With electrode coverage of a significant portion of the frontotemporal convexity, it is difficult to directly attribute this patient’s aphasia to the TP array, especially given concurrent electrode coverage over classical eloquent language regions. Later he underwent a left temporal lobe resection and was seizure free without neurological deficits at one-year follow-up.
Ultimately, none of the complications observed in the small cohort presented here appear to be directly attributable to the TP electrode array. One-third (33%) of the patients in this study developed complications, none of which resulted in any permanent neurological deficit. Although none of the complications describe here had permanent effects, it is important to note that a complications rate of 33% is higher than the literature average. We believe this result is spuriously high due to complications unrelated to the TP array occurring within a small sample size. In larger studies, complication rates for invasive subdural ECoG range from 4 – 13% (Albert et al., 2010, Van Gompel et al., 2008, Vale et al., 2013, Arya et al., 2013). A recent meta-analysis suggests a 4% risk of intracranial hemorrhage associated with intracranial recordings (Arya et al., 2013). In our previous series of 46 patients who underwent electrode implantation without the TP array, nearly all patients had extra-axial fluid collections similar to S03, but only four required early removal of electrodes for mass effect (Albert et al., 2010).
Mapping Temporopolar Cognitive Function
The anterior temporal lobes house the neural substrates for eloquent language and memory functions. Damage to the language-dominant (typically left) TP is associated with a specific deficit for naming unique concrete entities, with preservation of knowledge for those specific entities (Tranel, 2009). Damage to non-dominant (typically right) TP is associated with impaired face memory and recognition (Tranel et al., 1997, Milner, 2003). Dysfunction in these cognitive realms after CAH is well established (Milner, 2003, Tranel, 2009, Yucus and Tranel, 2007, Drane et al., 2009, Griffin and Tranel, 2007), and likely has a significant influence on quality of life. Given the impact of medically intractable epilepsy on quality of life, the subtle cognitive deficits associated with the temporal cortex resection in CAH is considered an acceptable tradeoff for a chance at improved seizure control. The ability to localize subregions of eloquent cortex within the anterior temporal lobes could potentially enable tailored CAH or at least improved preoperative neuropsychological risk stratification for patients with temporal lobe epilepsy undergoing surgical resection.
Our recordings from the TP clearly demonstrate event-related spectral perturbations resulting from visual naming of famous people. Similar results are seen for naming tools, which are less robust. Increases in power occurred in the sub-50 Hz range, reflecting increased power in the alpha and beta frequency ranges (7–14 and 15–30 Hz, respectively). Ultimately, our results demonstrate the feasibility of using TP recordings to identify functionally relevant regions of the anterior temporal lobes. In the future, these types of recordings may improve preoperative neuropsychological risk stratification or possibly guide tailored resections of the anterior temporal lobe to preserve neuropsychological function.
Conclusions
In this report we describe a novel electrode array that can be used to record seizure activity and map brain functions of TP cortex. The device is easy to fabricate by modifying existing grid arrays and the technique used to insert the array is practical and efficient. In this initial series, we encountered one transient complication that may have been linked to use of the array. This likely resulted from the cumulative effect of placing multiple intracranial surface recording arrays in a young patient with no age related brain atrophy. Our experience demonstrates that by using this array it will be possible to consistently record electrophysiological patterns of seizure activity, and study normal functions of the TP neocortex with a spatial precision that could not achieved previously.
Acknowledgments
This work was supported through grants by the National Institutes of Health (NIH RO1-DC004290, NIH UL1RR024979), the Hoover Fund, and the Hearing Health Foundation. We wish to thank Tammy Bryant, REEGT, for her assistance with data analysis.
Footnotes
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study.
References
- ALBERT GW, DAHDALEH NS, REDDY C, HANSEN DR, VOGEL TW, KAWASAKI H, HOWARD MA., 3RD Postoperative radiographic findings in patients undergoing intracranial electrode monitoring for medically refractory epilepsy. J Neurosurg. 2010;112:449–54. doi: 10.3171/2009.7.JNS09838. [DOI] [PubMed] [Google Scholar]
- ARYA R, MANGANO FT, HORN PS, HOLLAND KD, ROSE DF, GLAUSER TA. Adverse events related to extraoperative invasive EEG monitoring with subdural grid electrodes: a systematic review and meta-analysis. Epilepsia. 2013;54:828–39. doi: 10.1111/epi.12073. [DOI] [PubMed] [Google Scholar]
- BEGLEY CE, ANNEGERS JF, LAIRSON DR, REYNOLDS TF, HAUSER WA. Cost of Epilepsy in the United-States - a Model-Based on Incidence and Prognosis. Epilepsia. 1994;35:1230–1243. doi: 10.1111/j.1528-1157.1994.tb01794.x. [DOI] [PubMed] [Google Scholar]
- BRODMANN K. Vergleichende localisationslehre der grosshirnrinde in ihren principien dargestellt auf grund des zellenbaues. Leipzig: Barth; 1909. [Google Scholar]
- BRUGGE JF, VOLKOV IO, OYA H, KAWASAKI H, REALE RA, FENOY A, STEINSCHNEIDER M, HOWARD MA. Functional localization of auditory cortical fields of human: Click-train stimulation. Hearing Research. 2008;238:12–24. doi: 10.1016/j.heares.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CENDES F, DUBEAU F, OLIVIER A, CUKIERT A, ANDERMANN E, QUESNEY LF, ANDERMANN F. Increased neocortical spiking and surgical outcome after selective amygdalo-hippocampectomy. Epilepsy Res. 1993;16:195–206. doi: 10.1016/0920-1211(93)90080-q. [DOI] [PubMed] [Google Scholar]
- CHABARDES S, KAHANE P, MINOTTI L, TASSI L, GRAND S, HOFFMANN D, BENABID AL. The temporopolar cortex plays a pivotal role in temporal lobe seizures. Brain. 2005;128:1818–1831. doi: 10.1093/brain/awh512. [DOI] [PubMed] [Google Scholar]
- CRONE NE, MIGLIORETTI DL, GORDON B, LESSER RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis - II. Event-related synchronization in the gamma band. Brain. 1998;121:2301–2315. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- DAMASIO H, GRABOWSKI TJ, TRANEL D, HICHWA RD, DAMASIO AR. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- DEVLIN JT, RUSSELL RP, DAVIS MH, PRICE CJ, WILSON J, MOSS HE, MATTHEWS PM, TYLER LK. Susceptibility-induced loss of signal: Comparing PET and fMRI on a semantic task. Neuroimage. 2000;11:589–600. doi: 10.1006/nimg.2000.0595. [DOI] [PubMed] [Google Scholar]
- DING SL, VAN HOESEN GW, CASSELL MD, POREMBA A. Parcellation of Human Temporal Polar Cortex: A Combined Analysis of Multiple Cytoarchitectonic, Chemoarchitectonic, and Pathological Markers. Journal of Comparative Neurology. 2009;514:595–623. doi: 10.1002/cne.22053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRANE DL, OJEMANN GA, OJEMANN JG, AYLWARD E, SILBERGELD DL, MILLER JW, TRANEL D. Category-specific recognition and naming deficits following resection of a right anterior temporal lobe tumor in a patient with atypical language lateralization. Cortex. 2009;45:630–40. doi: 10.1016/j.cortex.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELGER CE, BURR W. Subdural electrodes. Acta Neurol Scand Suppl. 1994;152:44–50. doi: 10.1111/j.1600-0404.1994.tb05185.x. [DOI] [PubMed] [Google Scholar]
- ENGEL J., JR Epilepsy surgery. Curr Opin Neurol. 1994;7:140–7. doi: 10.1097/00019052-199404000-00010. [DOI] [PubMed] [Google Scholar]
- GRABOWSKI TJ, DAMASIO H, TRANEL D, PONTO LL, HICHWA RD, DAMASIO AR. A role for left temporal pole in the retrieval of words for unique entities. Hum Brain Mapp. 2001;13:199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENLEE JD, JACKSON AW, CHEN F, LARSON CR, OYA H, KAWASAKI H, CHEN H, HOWARD MA., 3RD Human auditory cortical activation during self-vocalization. PLoS One. 2011;6:e14744. doi: 10.1371/journal.pone.0014744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFIN S, TRANEL D. Age of seizure onset, functional reorganization, and neuropsychological outcome in temporal lobectomy. J Clin Exp Neuropsychol. 2007;29:13–24. doi: 10.1080/13803390500263568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUSER WA, ANNEGERS JF, KURLAND LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34:453–68. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- KAHANE P, CHABARDES S, MINOTTI L, HOFFMANN D, BENABID AL, MUNARI C. The role of the temporal pole in the genesis of temporal lobe seizures. Epileptic Disord. 2002;4(Suppl 1):S51–8. [PubMed] [Google Scholar]
- KAWASAKI H, KAUFMAN O, DAMASIO H, DAMASIO AR, GRANNER M, BAKKEN H, HORI T, HOWARD MA, 3RD, ADOLPHS R. Single-neuron responses to emotional visual stimuli recorded in human ventral prefrontal cortex. Nat Neurosci. 2001;4:15–6. doi: 10.1038/82850. [DOI] [PubMed] [Google Scholar]
- KOVACH CK, TSUCHIYA N, KAWASAKI H, OYA H, HOWARD MA, 3RD, ADOLPHS R. Manifestation of ocular-muscle EMG contamination in human intracranial recordings. Neuroimage. 2011;54:213–33. doi: 10.1016/j.neuroimage.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER KJ, ABEL TJ, HEBB AO, OJEMANN JG. Rapid online language mapping with electrocorticography. J Neurosurg Pediatr. 2011;7:482–90. doi: 10.3171/2011.2.PEDS1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER KJ, LEUTHARDT EC, SCHALK G, RAO RP, ANDERSON NR, MORAN DW, MILLER JW, OJEMANN JG. Spectral changes in cortical surface potentials during motor movement. J Neurosci. 2007;27:2424–32. doi: 10.1523/JNEUROSCI.3886-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILNER B. Visual recognition and recall after right temporal-lobe excision in man. Epilepsy Behav. 2003;4:799–812. doi: 10.1016/j.yebeh.2003.08.027. [DOI] [PubMed] [Google Scholar]
- OLSON IR, PLOTZKER A, EZZYAT Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–31. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- PAIL M, MARECEK R, HERMANOVA M, SLANA B, TYRLIKOVA I, KUBA R, BRAZDIL M. The role of voxel-based morphometry in the detection of cortical dysplasia within the temporal pole in patients with intractable mesial temporal lobe epilepsy. Epilepsia. 2012;53:1004–12. doi: 10.1111/j.1528-1167.2012.03456.x. [DOI] [PubMed] [Google Scholar]
- QUIROGA RQ, REDDY L, KREIMAN G, KOCH C, FRIED I. Invariant visual representation by single neurons in the human brain. Nature. 2005;435:1102–7. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- ROSENOW F, LUDERS H. Presurgical evaluation of epilepsy. Brain. 2001;124:1683–700. doi: 10.1093/brain/124.9.1683. [DOI] [PubMed] [Google Scholar]
- SPENCER SS. Depth electroencephalography in selection of refractory epilepsy for surgery. Ann Neurol. 1981;9:207–14. doi: 10.1002/ana.410090302. [DOI] [PubMed] [Google Scholar]
- THOMSON D. Spectral estimation and harmonic analysis. Proceedings of the IEEE. 1982;7:1055–1096. [Google Scholar]
- TRANEL D. Impaired naming of unique landmarks is associated with left temporal polar damage. Neuropsychology. 2006;20:1–10. doi: 10.1037/0894-4105.20.1.1. [DOI] [PubMed] [Google Scholar]
- TRANEL D. The left temporal pole is important for retrieving words for unique concrete entities. Aphasiology. 2009;23:867–884. doi: 10.1080/02687030802586498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRANEL D, DAMASIO H, DAMASIO AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997;35:1319–27. doi: 10.1016/s0028-3932(97)00085-7. [DOI] [PubMed] [Google Scholar]
- VALE FL, POLLOCK G, DIONISIO J, BENBADIS SR, TATUM WO. Outcome and complications of chronically implanted subdural electrodes for the treatment of medically resistant epilepsy. Clin Neurol Neurosurg. 2013;115:985–90. doi: 10.1016/j.clineuro.2012.10.007. [DOI] [PubMed] [Google Scholar]
- VAN GOMPEL JJ, WORRELL GA, BELL ML, PATRICK TA, CASCINO GD, RAFFEL C, MARSH WR, MEYER FB. Intracranial electroencephalography with subdural grid electrodes: techniques, complications, and outcomes. Neurosurgery. 2008;63:498–505. doi: 10.1227/01.NEU.0000324996.37228.F8. discussion 505–6. [DOI] [PubMed] [Google Scholar]
- WYLER AR, OJEMANN GA, LETTICH E, WARD AA., JR Subdural strip electrodes for localizing epileptogenic foci. J Neurosurg. 1984;60:1195–200. doi: 10.3171/jns.1984.60.6.1195. [DOI] [PubMed] [Google Scholar]
- YUCUS CJ, TRANEL D. Preserved proper naming following left anterior temporal lobectomy is associated with early age of seizure onset. Epilepsia. 2007;48:2241–52. doi: 10.1111/j.1528-1167.2007.01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]