Abstract
Background
Qualitative reporting of home indoor moisture problems predicts respiratory diseases. However, causal agents underlying such qualitative markers remain unknown.
Methods
In the homes of 198 multiple allergic case children and 202 controls in Sweden, we cultivated culturable fungi by directly plating dust, and quantified(1–3, 1–6)-β-D-glucan, and ergosterol in dust samples from the child’s bedroom. We examined the relationship between these fungal agents and degree of parent or inspector reported home indoor dampness, and microbiological laboratory’s mold index. We also compared the concentrations of these agents between multiple allergic cases and healthy controls, as well as IgE-sensitization among cases.
Results
The concentrations of culturable fungal agents were comparable between houses with parent and inspector reported mold issues and those without. There were no differences in concentrations of the individual or the total summed culturable fungi, (1–3, 1–6)-β-D-glucan, and ergosterol between the controls and the multiple allergic case children, or individual diagnosis of asthma, rhinitis or eczema.
Conclusion
Culturable fungi, (1–3, 1–6)-β-D-glucan, and ergosterol in dust were not associated with qualitative markers of indoor dampness or mold or indoor humidity. Furthermore, these agents in dust samples were not associated with any health outcomes in the children.
Keywords: indoor, asthma, allergies, children, dampness, mold
INTRODUCTION
Detectable moisture and moisture-related problems in the indoor environment are associated with respiratory diseases and disorders such as asthma and wheezing in children and adults (Bornehag et al., 2001; Bornehag et al., 2004; Institute of Medicine, 2004; WHO, 2006; WHO, 2009). Yet, in most studies that identified an elevated risk due to such problems, most predictive markers of indoor dampness or mold (IDOM) remain qualitative in nature (Belanger et al., 2003; Iossifova et al., 2009; Pekkanen et al., 2007). Such indicators include qualitatively assessed dampness, visible mold, visible moisture damage of housing structure, or ‘moldy’ smell (Belanger et al., 2003; Iossifova et al., 2009; Pekkanen et al., 2007). While the evidence is strong enough for an association between IDOM and a wide range of respiratory and allergic diseases, the evidence does not yet support the causal role. In addition, the specific dampness-related agents underlying these diseases, and the mechanisms of their action remain unknown (Institute of Medicine, 2004; Mendell et al., 2011).
On the other hand, quantitatively determined concentrations of microbial agents do not show a consistent association with respiratory health outcomes; in some cases, exposure to microbial factors is protective againt asthma-related symptoms and wheezing, particularly for those who are exposed very early in life (Mendell et al., 2011). In particular, culturable mold spore concentrations in air samples from the children’s rooms are not related to doctor diagnosed asthma, asthmoid-spastic or obstructive bronchitis, hay fever, atopic eczema or sensitization (Jovanovic et al., 2004). Taken together, the strength of evidence on quantitatively assessed home indoor fungal exposure is inconsistent in regards to the development asthma and allergic disease (Bush et al., 2006; Hardin et al., 2003). As a result, while the qualitative markers of IDOM (e.g., signs of mold, presence of moldy or musty odor, visible water damage) have been associated with health outcomes, the critical gap in our knowledge lies in identifying the specific causative agent(s). In order to address these questions, we measured the culturable fungi, (1–3, 1–6)-β-D-glucan, and ergosterol concentrations from the household dust samples of those taking part in the Dampness in Buildings and Health (DBH) study in Sweden. Both ergosterol and (1–3, 1–6)-β-D-glucan are fungal specific cellular markers used for indirect quantification of fungal biomass (Szponar et al., 2000; Chew et al., 2001). Dust is a time-integrated carrier for environmental contaminants, including fungal agents (Munir et al., 1995; Rudel et al., 2003). Quantification of such agents in dust is therefore of assessment importance in indoor exposure, and could indicate a sustained exposure. It is therefore necessary to assess dust as a potential inhalation and/or ingestion purveyor of exposure to a risk factor asthma and allergy.
In our earlier analysis, we found no association between airborne culturable fungi and allergic diseases in children (Holme et al., 2010). Here, we further investigate a related line of inquiry by examining the culturable fungi, ergosterol, and (1–3, 1–6)-β-D-glucan in dust and the risks of doctor-diagnosed asthma, and multiple allergy symptoms in the same group of pre-school age case-control children. We also examined the relationship between these fungal agents in dust and parental reporting of home moisture issues, as well as the professional inspections of the home environment.
METHODS
Detailed descriptions of the study methods are provided elsewhere (Bornehag et al., 2005c; Holme et al., 2010). Briefly, this study is part of the on-going Dampness in Buildings and Health (DBH) study focusing on the impact of indoorenvironmental factors on asthma and allergy among children in Sweden. The first Phase of the DBH study was a cross-sectional questionnaire investigation of the parents of all children aged 1– 6 years (n = 14,077) in Värmland County, Sweden (Bornehag et al., 2003, 2005). The current study is part of the Phase II investigation, which is a nested case control investigation on 198 symptomatic children and 202 non-symptomatic controls representing 390 households (including 10 sibling pairs).
Case and Control Definition
In order to meet the definition for a case, the following conditions were required for the child; (i) in the baseline questionnaire (DBH Phase I), the parent had to report ≥ two symptoms of wheezing, rhinitis, and/or eczema within the last 12 months. Eighteen months later, at DBH phase II the child’s parent had to report ≥ two symptoms of wheezing, rhinitis, and/or eczema without a cold within the prior 12-months period. From the pool of DBH phase I, we sought and recruited clinically diagnosed cases of asthma, rhinitis, and eczema, respectively. Diagnostic criteria for asthma include a) at least three wheezing episodes prior to age 2; b) onset of wheezing since age 2; c) an onset of wheezing in addition to other atopic diseases; d) current asthma medication use; and e) clinical diagnosis of asthma at any age. Diagnostic criteria for rhinitis required: (a) ever having allergic rhinitis symptoms; (b) symptoms presentation in the nose and/or eyes following the contact with furred animals or pollen; (c) present use of rhinitis medicine. Eczema case definition required that the child have at least six months of remitting itching and redness in typical body locations. For the control children, only those whose parent reported an absence of symptoms during both Phase I and II were invited.
In order to preclude misclassification of exposure history, the cases and controls were further excluded if: (i) the home was renovated or remodeled due to moisture problems; and (ii) family relocated to a new residence since the phase I. The medical examinations of the 400 children as well as the air, dust sample collection, and home inspection were completed during October 2001 and April 2002 (i.e. 18–24 months after phase I). A team of four medical doctors examined all children following a structured anamnesis (Hederos et al., 2007). Blood samples (n = 387) from all available children were screened for IgE antibodies to 10 airborne allergens (Phadiatop®, Pharmacia & Upjohn Diagnostics, Uppsala, Sweden), including timothy, mugwort, birch, cat, horse, dog, house dust mites (D. pteronyssinus and D. farinae), and two mold genera (Penicillium and Cladosporium). Cut-off value (1.2 kUA/l) was used to define a dichotomous category for IgE-positive status. Institutional ethical committee in Örebro, Sweden, approved the study.
Here we present culturable fungi as colony-forming unit (CFU) per gram dust of the five most prevalent fungal genera as well as yeasts, (1–3,1–6)-β-d-glucan (μg/g dust), and ergosterol (μg/g dust), and total culturable fungi (CFU/g) from the dust sample collected in the child’s bedroom. Consistent with our earlier analysis (Holme et al., 2010), we considered three separate definitions of the moisture and fungal problems as below:
Parental-reporting of moisture problems (in DBH Phase I questionnaire)
Home building (walk-through) assessment by professional inspectors in Phase II
Semi-quantitative mold index by the microbiology lab
-
Parental-Reporting of Moisture Problems: Parents qualitatively reported in a questionnaire (DBH Phase I) regarding the signs of chronic moisture issues and mold in the child’s, the parent’s, or other rooms. We chose the following questions:
Visible dampness: mold or obvious water stains on the ceiling, walls, or floor in the child’s bedroom or the parents’ bedroom.
Floor moisture: Discolored/blackened parquet or cork-flooring; bubbly, loosening, or discolored vinyl or linoleum floor covering in the child’s, parents’, or other room.
Moldy odor: Moldy, earthy, or “cellar-odor” sometimes or often within the last 3 months.
Condensation on windows: visible condensation (≥5cm diameter) during winter in the child and/or parents’ bedroom.
-
Home Building Assessment: Six inspectors conducted a visual and olfactory exam of mold and water damage, and collected air and dust samples in the homes. The inspectors were blind to the case–control status of the children. The inspector graded each home on the scale of 0 to 3 regarding four aspects of the mold presence:
Mold y odor: First impression upon entering the home or in at least one room
Moldy odor along the skirting board in at least one room of the dwelling
Discolored damp stains on walls or ceiling in at least one room
-
Blackened, bubbly, or loosening floor-covering material, or any other sign of floor dampness in at least one room
Grade of zero indicated no mold problem; grade 1–2 indicated mild issue for possible smell or a small visible indication of moisture; and grade 3 indicated severe issue for clear and strong odor or obvious moisture damage.
Semi-Quantitative Mold Index: The genera and quantity of culturable fungi from each house was validated by four microbiologists. The fungi concentration from an air sample (Holme et al., 2010) was compared to the corresponding outdoor concentration and categorized into one of the four groups (range, 0–3) as developed by the Norwegian standard for building survey (Tilstandsanalyse for byggverk, NS 3424.) The score of 0 denote no signs of fungal spores compared to the reference sample taken outdoors; 1 denotes a limited signs of fungal spores; 2 denotes moderate signs of fungal spores; and 3 denotes an obvious signs of fungal spores. Consistent with our earlier analysis, we further reduced the categories into two, in which score of 0–1 denote houses with no fungal contamination, and score of 2–3 denote houses with fungal contamination (Holme et al., 2010).
Ventilation rates of the entire home and of the bedroom of the index-child were measured during one week with a passive tracer gas method (i.e. the homogeneous emission technique) (Bornehag et al., 2005a). This PFT (perfluorocarbon tracer) technique, described in NT VVS 118, has been validated (Nordtest, 1997; Stymne et al., 1994). Temperature (°C) and relative humidity (%) were measured instantaneously during the home visit (VL2000 Temperature & Humidity Sensor, Vaisala, Helsinki, Finland) and continuously at every hour for a week (Mitec Satelite-TH, Mitec Instrument AB, Säffle, Sweden).
Quantification of Microbial Agents in Dust
A single dust samples was collected with a vacuum from the floor in the child’s bedroom by six licensed building inspectors. A random subset of the building inspectors visited each home during the heating season (October 2001–April 2002). Regardless of the type of the floor, the inspectors vacuumed at a sampling a rate of 2 minutes/m2 until a sample of approximately 300 – 500 mg was attained. The dust samples were analyzed for 388 of the 390 families/houses that are participating in this study; two homes did not have dust samples. A 90-mm membrane filter made of pure cellulose was used to collect the dust. The filters were placed in holders made of tyrene-acrylonitrile polymer mounted on a sampler made of polypropylene (Petersen Bach, Bjerringbro, Denmark) connected to a vacuum cleaner. The filters were wrapped in aluminum foil and placed in a polyethylene bag and stored in the refrigerator for 2–3 days following sample collection. The filter was weighed before and immediately after vacuuming under controlled conditions. Before weighing, the filters were conditioned at 23° C and 50% relative humidity. Once the samples arrived in the lab, 30 mg of unsieved dust from each sample was spread directly onto V8 agar (Vegetable juice, Campbell Soup. Ltd.) plates. The plates were incubated for a week at 26 °C (Gravesen, 1978). To reduce bacterial growth, penicillin and streptomycin were added. Fungal growth was quantified microscopically as CFU/30 mg of dust (Wickman et al., 1992). Fungi were identified at the level of genera. Molds were considered fungal species which have a predominately multi-cellular growth habit characterized by hyphae, while yeasts are species which can adopt a unicellular growth habit (Madigan et al., 2005). Whenever possible, fungi were identified to species using direct microscopy using established methods (Andersen et al., 2011). After counting fungi/microorganisms, the values were converted to CFUs per gram of dust (CFU/g).
Ergosterol
Upon arrival at the lab, the dust samples were stored at −20 °C prior to analysis. Two to four milligram aliquots of unsieved dust samples were analyzed for ergosterol using gas chromatography-tandem mass spectrometry. In brief, samples were subjected to alkaline hydrolysis following clean-up and derivatization prior to analysis (Sebastian et al., 2003).
(1–3, 1–6)-β-D-glucan
Approximately 30 mg of the unsieved dust sample from each home was used for glucan analysis. Samples were extracted in phosphate-buffered saline plus 0.05% Tween 20 with 1 hr shaking, 1 hr autoclaving (120 °C), followed by centrifugation at 600 xG for 20 min at 4 °C. The concentration of fungal glucan in the supernatants was quantified by enzyme immunoassay (EIA) using mouse anti-(1–3, 1–6)-β-D-glucan monoclonal antibody as the capture antibody, rabbit anti-scleroglucan polyclonal antibody as the second antibody and goat anti-rabbit IgG (Biosource, Inc. Camarillo, CA) as the labeling antibody as previously described (Blanc et al., 2005). The reaction was monitored at 650 nm and was read at 405 nm in a microplate reader (SpectraMax Plus 384, Molecular Devices, Sunnyvale, CA).
Statistical Analysis
Here we present the observed CFUs, for each genera of fungi, and a group of non-specific yeasts separately, and the sum of these groups as total fungi (CFU/g) in the child’s bedroom. We identified in total 31 genera. We restricted our analyses to those fungal genera or fungal groups (yeasts) detected in ≥ 30% of 390 homes — Penicillium spp., Alternaria spp., Aspergillus spp., Yeasts, Rhodotorula spp., Trichoderma spp., and total fungi CFUs. Yeasts include all yeasts other than Rhodotorula spp., which were prevalent enough to warrant separation. In the remaining 25 genera, the prevalence among 390 homes was too low to statistically compare between the outcome groups. The Mann–Whitney U-test was used to assess the associations, when the independent variable was coded as a dichotomous variable. If the independent variable had more than two categories, a Kruskal-Wallis test was used. Specifically, we used Kruskal-Wallis test to compare the distribution patterns of culturable fungi among the categories of parent reported mold problems, building inspector ratings, semi-quantitative home mold index. Similar distributions between the respective outcomes (i.e. asthma, rhinitis, or eczema) vs. the controls were compared using the Mann-Whitney’s U-test. Both tests non-parametrically compare the distributions underlying the samples without assuming a normal distribution of the main exposure variable. We did not develop any logistic regression model for the exposure-outcome association, because the results of the descriptive and non-parametric tests did not warrant the development of multivariate models. We examined the modification of mold effect by humidity by stratifying the dataset according to quartiles of absolute (g/m3) or relative humidity (%).
RESULTS
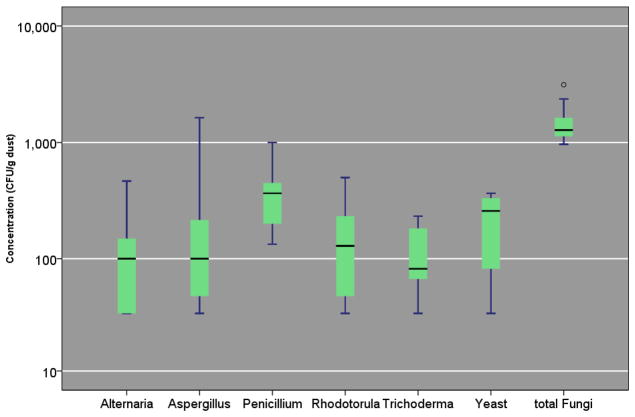
Overall, the CFU concentrations of the six most prevalent mold genera were low in 388 homes (Figure 1). Median concentration of the six genera ranged between 67 CFU/g for Trichoderma spp. to 400 CFU/g for Penicillium spp. The interquartile ranges for the six genera were: 200—733 CFU/g for Penicillium spp.; 100—400 CFU/g for yeasts; 67—200 CFU/g for Alternaria spp.; 33—250 CFU/g for Aspergillus spp.; 67—233 CFU/g for Rhodotorula spp.; and 33—133 CFU/g for Trichoderma spp.. In all, except Penicillium spp., the 90th percentile value was below 1,000 CFU/g. Accordingly, the distributions of all six genera were highly skewed to the right (Figure 1). While (1–3, 1–6)-β-D-glucan and ergosterol were overall prevalent (n = 367 and 383 homes, respectively), their arithmetic mean and standard deviation were 29 ±97 μg/g and 3.47 ±8.36 μg/g. The 25th, 50th, and 75th percentile of (1–3, 1–6)-β-D-glucan were 3, 6, and 19 μg/g dust. Similar values for ergosterol were 0.63, 1.69, and 3.72 μg/g dust, respectively.
FIGURE 1. Culturable Fungi Concentration (CFU/g) in Home Dust (n=390).
Box and the bar within it show 25th, 50th, and 75th percentile of the concentration; the whiskers show the 5th and the 95th percentiles, respectively. The symbols, ● and *, represent concentrations that are >1.5– and >3–fold of the 75th percentile value.
Parental questionnaire reports of mold and moisture-related damages (taken 18–24 months prior to the presentfungal measurements) were not positively associated with any concentration trends for any of the five genera of fungi, but were significantly positively associated with concentration of non-specific yeasts. Yeasts and Aspergillus spp. were significantly negatively associated with parental report of dampness and floor moisture respectively (Table 1).
Table 1.
Distribution of Fungal Genera and Yeasts in Dust Sample and Parent-Reported Dampness Problems.
| Parental Report | Unanswered | NO | Yes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| N | Percentiles | N | Percentiles | N | Percentiles | ||||||||
| 5th | 50th | 95th | 5th | 50th | 95th | 5th | 50th | 95th | P-value | ||||
| Visible Dampness | |||||||||||||
| Penicillium | 4 | 100 | 417 | -- | 316 | 67 | 400 | 1938 | 8 | 100 | 433 | 0.980 | |
| Alternaria | 2 | 33 | 117 | -- | 226 | 33 | 100 | 500 | 6 | 67 | 167 | 0.246 | |
| Aspergillus | 2 | 33 | 500 | -- | 212 | 33 | 100 | 1135 | 3 | 33 | 67 | 0.240 | |
| Yeasts | 2 | 67 | 683 | -- | 194 | 33 | 200 | 925 | 7 | 133 | 300 | 0.055 | |
| Rhodotorula | 2 | 133 | 333 | -- | 198 | 33 | 100 | 1142 | 5 | 100 | 233 | 0.130 | |
| Trichoderma | 0 | -- | -- | -- | 129 | 33 | 67 | 233 | 5 | 33 | 67 | 0.780 | |
| Total Fungi | 5 | 467 | 1067 | -- | 374 | 392 | 1267 | 3808 | 9 | 533 | 2200 | 0.137 | |
| (1–3, 1–6)-β-D-glucan | 4 | 2 | 6 | -- | 355 | 1 | 6 | 95 | 8 | 0 | 6 | 0.365 | |
| Ergosterol | 5 | 0.626 | 2.058 | -- | 369 | 0.079 | 1.658 | 9.826 | 9 | 96 | 1.722 | 0.423 | |
| Floor Moisture | |||||||||||||
| Penicillium | 69 | 50 | 400 | 2650 | 229 | 67 | 400 | 1783 | 30 | 85 | 383 | 3040 | 0.768 |
| Alternaria | 50 | 33 | 117 | 530 | 166 | 33 | 100 | 467 | 18 | 33 | 83 | 0.962 | |
| Aspergillus | 53 | 33 | 100 | 1360 | 142 | 33 | 100 | 1412 | 22 | 33 | 67 | 428 | 0.043 |
| Yeasts | 41 | 37 | 233 | 1480 | 146 | 33 | 200 | 1143 | 16 | 33 | 233 | 0.414 | |
| Rhodotorula | 42 | 33 | 167 | 1667 | 142 | 33 | 100 | 962 | 21 | 33 | 133 | 1570 | 0.202 |
| Trichoderma | 26 | 33 | 67 | 308 | 95 | 33 | 67 | 233 | 13 | 33 | 67 | 0.792 | |
| Total Fungi | 85 | 420 | 1467 | 3713 | 269 | 367 | 1267 | 3933 | 34 | 258 | 1150 | 4233 | 0.557 |
| (1–3, 1–6)-β-D-glucan | 84 | 1 | 6 | 84 | 252 | 1 | 6 | 107 | 31 | 1 | 6 | 85 | 0.727 |
| Ergosterol | 84 | 0.069 | 1.756 | 10.168 | 265 | 81 | 1.680 | 10.209 | 34 | 0.105 | 1.589 | 7.264 | 0.492 |
| Moldy Odor | |||||||||||||
| Penicillium | 94 | 67 | 450 | 3333 | 212 | 67 | 367 | 1760 | 22 | 72 | 267 | 2080 | 0.201 |
| Alternaria | 73 | 33 | 100 | 477 | 145 | 33 | 100 | 500 | 16 | 33 | 67 | 0.886 | |
| Aspergillus | 63 | 33 | 133 | 1547 | 140 | 33 | 67 | 1100 | 14 | 33 | 67 | 0.646 | |
| Yeasts | 55 | 60 | 300 | 1953 | 140 | 33 | 200 | 667 | 8 | 100 | 200 | 0.421 | |
| Rhodotorula | 63 | 33 | 133 | 1233 | 129 | 33 | 100 | 1667 | 13 | 33 | 133 | 0.442 | |
| Trichoderma | 40 | 33 | 67 | 198 | 86 | 33 | 67 | 298 | 8 | 33 | 67 | 0.645 | |
| Total Fungi | 114 | 425 | 1383 | 4383 | 251 | 353 | 1233 | 3767 | 23 | 333 | 1400 | 4540 | 0.717 |
| (1–3, 1–6)-β-D-glucan | 109 | 1 | 6 | 210 | 237 | 1 | 6 | 75 | 21 | 0 | 5 | 67 | 0.523 |
| Ergosterol | 113 | 0.114 | 1.716 | 13.524 | 247 | 0.078 | 1.783 | 9.966 | 23 | 0.019 | 1.174 | 6.093 | 0.099 |
| Condensation | |||||||||||||
| Penicillium | 16 | 100 | 383 | -- | 252 | 67 | 400 | 1667 | 60 | 67 | 333 | 6625 | 0.793 |
| Alternaria | 13 | 33 | 167 | -- | 184 | 33 | 100 | 500 | 37 | 33 | 100 | 350 | 0.590 |
| Aspergillus | 12 | 33 | 100 | -- | 164 | 33 | 100 | 1100 | 41 | 33 | 67 | 1543 | 0.242 |
| Yeasts | 7 | 67 | 300 | -- | 160 | 33 | 233 | 1293 | 36 | 33 | 150 | 1692 | 0.034 |
| Rhodotorula | 13 | 33 | 267 | -- | 156 | 33 | 100 | 1158 | 36 | 33 | 100 | 1802 | 0.647 |
| Trichoderma | 4 | 33 | 67 | -- | 105 | 33 | 67 | 233 | 25 | 33 | 67 | 427 | 0.646 |
| Total Fungi | 19 | 467 | 1500 | -- | 300 | 368 | 1267 | 3732 | 69 | 383 | 1400 | 6417 | 0.362 |
| (1–3, 1–6)-β-D-glucan | 18 | 1 | 6 | -- | 284 | 1 | 6 | 96 | 65 | 1 | 7 | 94 | 0.902 |
| Ergosterol | 19 | 0.007 | 1.547 | -- | 296 | 0.084 | 1.742 | 10.003 | 68 | 0.083 | 1.588 | 9.036 | 0.445 |
Concentration units are CFU/g for the fungal and yeasts, μg/g dust for (1–3, 1–6)-β-D-glucan and ergosterol. There were no reported values at the 95th percentile for those with answers for the mold index. These are denoted as --. Kruskal-Wallis test was used to obtain the P-values.
In addition, no positive correlation was observed between the inspectors’ rating of mold and moisture-related damages, IDOM issues, and the concentrations of the five fungal genera or yeasts. However, the concentration of Rhodotorula spp. was negatively associated with moldy odor (Table 2).
Table 2.
Distribution of the Fungal Genera and Yeasts and Other Markers According to Home Inspector Grading of Dampness Problem in Dwelling.
| Inspector Rating | No Evidence | Weak Indication | Strong Indication | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| N | Percentiles | N | Percentiles | N | Percentiles | ||||||||
| 5th | 50th | 95th | 5th | 50th | 95th | 5th | 50th | 95th | |||||
| Moldy Odor | |||||||||||||
| Penicillium | 195 | 93 | 400 | 1987 | 83 | 67 | 400 | 1907 | 50 | 52 | 333 | 3333 | 0.895 |
| Alternaria | 139 | 33 | 100 | 567 | 58 | 33 | 83 | 503 | 37 | 33 | 100 | 477 | 0.612 |
| Aspergillus | 130 | 33 | 100 | 1633 | 49 | 33 | 67 | 1333 | 38 | 33 | 100 | 937 | 0.592 |
| Yeasts | 115 | 33 | 200 | 1667 | 54 | 33 | 250 | 1275 | 34 | 33 | 167 | 1250 | 0.179 |
| Rhodotorula | 126 | 33 | 133 | 1632 | 55 | 33 | 133 | 1080 | 24 | 33 | 67 | 608 | 0.023 |
| Trichoderma | 81 | 33 | 67 | 323 | 34 | 33 | 67 | 208 | 19 | 33 | 67 | 0.567 | |
| Total Fungi | 235 | 400 | 1300 | 3813 | 89 | 367 | 1300 | 3867 | 64 | 375 | 1133 | 4483 | 0.203 |
| (1–3, 1–6)-β-D-glucan | 227 | 1 | 7 | 100 | 79 | 1 | 6 | 70 | 61 | 1 | 5 | 131 | 0.474 |
| Ergosterol | 232 | 0.079 | 1.940 | 9.245 | 87 | 0.043 | 0.1355 | 7.149 | 64 | 0.121 | 1.799 | 16.063 | 0.190 |
| Moldy Odor Along Skirting Board | |||||||||||||
| Penicillium | 174 | 67 | 367 | 2233 | 99 | 67 | 400 | 1667 | 55 | 60 | 400 | 3333 | 0.501 |
| Alternaria | 115 | 33 | 100 | 667 | 84 | 33 | 83 | 425 | 35 | 33 | 133 | 473 | 0.073 |
| Aspergillus | 110 | 33 | 67 | 1355 | 70 | 33 | 67 | 1082 | 37 | 33 | 133 | 1633 | 0.086 |
| Yeasts | 101 | 33 | 200 | 1280 | 65 | 67 | 233 | 1310 | 37 | 33 | 167 | 3457 | 0.108 |
| Rhodotorula | 110 | 33 | 133 | 1772 | 67 | 33 | 100 | 720 | 28 | 33 | 100 | 340 | 0.527 |
| Trichoderma | 66 | 33 | 67 | 298 | 44 | 33 | 67 | 233 | 24 | 33 | 67 | 192 | 0.760 |
| Total Fungi | 203 | 407 | 1333 | 3827 | 122 | 372 | 1233 | 3823 | 63 | 340 | 1200 | 4740 | 0.931 |
| (1–3, 1–6)-β-D-glucan | 194 | 1 | 6 | 71 | 116 | 1 | 6 | 179 | 57 | 1 | 6 | 149 | 0.637 |
| Ergosterol | 202 | 0.083 | 1.874 | 7.988 | 118 | 0.065 | 1.563 | 10.326 | 63 | 0.079 | 1.358 | 15.829 | 0.209 |
| Damp Stain | |||||||||||||
| Penicillium | 243 | 67 | 367 | 1667 | 72 | 100 | 433 | 3333 | 13 | 33 | 367 | 0.812 | |
| Alternaria | 173 | 33 | 100 | 567 | 50 | 33 | 100 | 463 | 11 | 33 | 67 | 0.352 | |
| Aspergillus | 169 | 33 | 100 | 1333 | 40 | 33 | 100 | 995 | 8 | 33 | 83 | 0.430 | |
| Yeasts | 157 | 33 | 233 | 1180 | 37 | 33 | 233 | 1667 | 9 | 67 | 200 | 0.781 | |
| Rhodotorula | 156 | 33 | 100 | 1355 | 42 | 33 | 117 | 898 | 7 | 33 | 100 | 0.828 | |
| Trichoderma | 101 | 33 | 67 | 233 | 30 | 33 | 67 | 233 | 3 | 100 | 133 | 0.089 | |
| Total Fungi | 292 | 367 | 1267 | 3790 | 81 | 510 | 1267 | 4043 | 15 | 400 | 1267 | 0.548 | |
| (1–3, 1–6)-β-D-glucan | 273 | 1 | 6 | 69 | 79 | 1 | 7 | 139 | 15 | 1 | 6 | 0.467 | |
| Ergosterol | 288 | 0.079 | 1.563 | 8.176 | 80 | 0.085 | 2.115 | 15.742 | 15 | 0.032 | 2.135 | 0.617 | |
| Floor Moisture | |||||||||||||
| Penicillium | 300 | 67 | 400 | 1798 | 24 | 75 | 300 | 5833 | 4 | 167 | 1367 | 0.175 | |
| Alternaria | 221 | 33 | 100 | 500 | 12 | 33 | 150 | 1 | 33 | 33 | 33 | 0.271 | |
| Aspergillus | 200 | 33 | 100 | 1195 | 15 | 33 | 67 | 2 | 167 | 267 | 0.094 | ||
| Yeasts | 192 | 33 | 233 | 948 | 11 | 67 | 133 | 0 | 0.194 | ||||
| Rhodotorula | 194 | 33 | 100 | 1175 | 10 | 33 | 83 | 1 | 733 | 733 | 733 | 0.393 | |
| Trichoderma | 128 | 33 | 67 | 233 | 6 | 33 | 33 | 0 | |||||
| Total Fungi | 359 | 400 | 1267 | 3800 | 25 | 220 | 1400 | 5927 | 4 | 600 | 2150 | ||
| (1–3, 1–6)-β-D-glucan | 340 | 1 | 6 | 90 | 24 | 1 | 5 | 523 | 3 | 5 | 5 | 0.758 | |
| Ergosterol | 354 | 0.093 | 1.719 | 9.757 | 25 | 0.065 | 1.200 | 51.304 | 4 | 0.558 | 1.721 | 0.752 | |
Concentration units are CFU/g for the Fungal genera and yeasts, μg/g dust for (1–3, 1–6)-β-D-glucan and ergosterol. Kruskal-Wallis test was used to obtain the P-values.
Based on semi-qualitative mold index, the medians of three mold genera (Penicillium, Aspergillus, Rhodotorula) as well as yeasts were higher in the homes with mold issues compared to the homes with no mold issues. The concentration of Alternaria spp. was lower and Trichoderma spp. was approximately equal for the same comparison. Total fungi was significantly higher in houses with mold issues based on semi-quantitative mold index (p = 0.047, Table 3).
Table 3.
Distribution of Fungal Genera, Yeasts, and Other Markers in Dust Sample and Semi-Quantitative Mold Index.
| Mold Index | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Missing | No Mold | Houses with Mold | P | ||||||||||
| N | Percentiles | N | Percentiles | N | Percentiles | ||||||||
| 5th | 50th | 95th | 5th | 50th | 95th | 5th | 50th | 95th | |||||
| Penicillium | 8 | 200 | 383 | -- | 254 | 67 | 367 | 1942 | 66 | 45 | 417 | 3147 | 0.259 |
| Alternaria | 6 | 33 | 133 | -- | 177 | 33 | 100 | 567 | 51 | 33 | 67 | 327 | 0.297 |
| Aspergillus | 7 | 33 | 67 | -- | 169 | 33 | 100 | 1550 | 41 | 33 | 133 | 1030 | 0.160 |
| Yeasts | 5 | 100 | 567 | -- | 155 | 33 | 200 | 1233 | 43 | 40 | 233 | 1173 | 0.720 |
| Rhodotorula | 3 | 100 | 167 | -- | 156 | 33 | 100 | 992 | 46 | 33 | 133 | 1632 | 0.255 |
| Trichoderma | 3 | 67 | 67 | -- | 105 | 33 | 67 | 233 | 26 | 33 | 67 | 222 | 0.373 |
| Total Fungi | 10 | 733 | 1800 | -- | 301 | 367 | 1233 | 3893 | 77 | 587 | 1500 | 3803 | 0.047 |
| (1–3, 1–6)-β-D-glucan | 8 | 1 | 4 | -- | 288 | 1 | 6 | 93 | 71 | 1 | 7 | 97 | 0.404 |
| Ergosterol | 10 | 0.079 | 1.402 | -- | 296 | 0.083 | 1.686 | 10.248 | 77 | 0.078 | 1.734 | 8.980 | 0.751 |
Concentration units are CFU/g for the fungal genera and yeasts, μg/g dust for (1–3, 1–6)-β-D-glucan and ergosterol. There were no reported values at the 95th percentile for those with answers for the mold index. These are denoted as --. Kruskal-Wallis test was used to obtain the P-values.
Neither (1–3,1–6)-β-d-glucan nor ergosterol was associated with a clear trend in concentration difference, according to the parental reports of IDOM, inspectors’ rating of IDOM, and semi-quantitative mold index, respectively (Tables 1, 2, and 3).
When we stratified the homes according to quartile of indoor absolute humidity, the concentrations of the six mold genera were overall non-significantly lower for those within the highest quartile compared to those within the lowest quartile (data not shown). Similar trend was observed when we compared the concentrations of the mold genera in terms of the child’s bedroom relative humidity (data not shown).
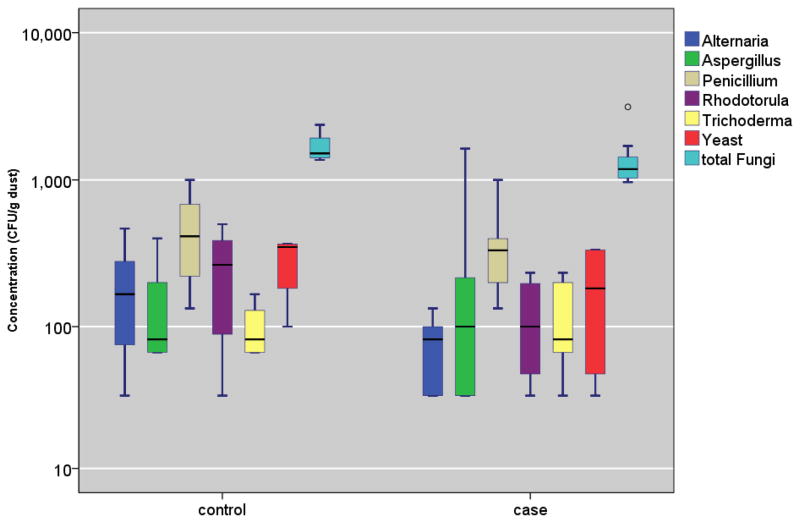
The concentrations of the mold genera, (1–3,1–6)-β-d-glucan, and ergosterol were not associated with any notable differences, comparing the controls versus the cases, asthma-diagnosed, rhinitis-diagnosed, and eczema-diagnosed, respectively (all P-values > 0.05, Table 4 and Figure 2). Furthermore, when we restrict the analysis to the cases, culturable fungi, (1–3,1–6)-β-d-glucan, and ergosterol were not significantly different between the IgE- sensitized versus the non-sensitized children (data not shown).
Table 4.
Association between Fungal Concentration, Yeasts and Other Fungal Markers in Dust and Multiple Allergic Symptom Presentation (i.e. case status) or Clinical Diagnosis of Asthma, Rhinitis, or Eczema.
| N | Percentiles | P | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 5th | 50th | 95th | ||||
| Penicillium | control | 162 | 67 | 367 | 1695 | |
| case | 166 | 100 | 400 | 3147 | 0.122 | |
| asthma | 102 | 67 | 400 | 1922 | 0.358 | |
| rhinitis | 83 | 73 | 400 | 3333 | 0.263 | |
| eczema | 107 | 80 | 400 | 3333 | 0.270 | |
| Alternaria | control | 119 | 33 | 100 | 667 | |
| case | 115 | 33 | 100 | 500 | 0.774 | |
| asthma | 68 | 33 | 100 | 470 | 0.810 | |
| rhinitis | 62 | 33 | 100 | 495 | 0.839 | |
| eczema | 77 | 33 | 100 | 470 | 0.793 | |
| Aspergillus | control | 119 | 33 | 100 | 1033 | |
| case | 98 | 33 | 100 | 1635 | 0.701 | |
| asthma | 64 | 33 | 67 | 1550 | 0.292 | |
| rhinitis | 56 | 33 | 67 | 2473 | 0.419 | |
| eczema | 64 | 33 | 100 | 1658 | 0.889 | |
| Yeasts | control | 97 | 33 | 200 | 717 | |
| case | 106 | 33 | 233 | 1667 | 0.928 | |
| asthma | 64 | 33 | 233 | 1667 | 0.665 | |
| rhinitis | 46 | 33 | 250 | 1003 | 0.919 | |
| eczema | 69 | 33 | 200 | 1483 | 0.559 | |
| Rhodotorula | control | 98 | 33 | 100 | 1930 | |
| case | 107 | 33 | 100 | 1013 | 0.460 | |
| asthma | 65 | 33 | 100 | 1367 | 0.617 | |
| rhinitis | 57 | 33 | 100 | 670 | 0.475 | |
| eczema | 77 | 33 | 100 | 1337 | 0.272 | |
| Trichoderma | control | 69 | 33 | 67 | 233 | |
| case | 65 | 33 | 67 | 233 | 0.560 | |
| asthma | 33 | 33 | 67 | 233 | 0.546 | |
| rhinitis | 38 | 33 | 67 | 233 | 0.484 | |
| eczema | 39 | 33 | 67 | 233 | 0.582 | |
| Total Fungi | control | 198 | 367 | 1283 | 3767 | |
| case | 190 | 418 | 1250 | 5113 | 0.973 | |
| asthma | 117 | 330 | 1200 | 3973 | 0.400 | |
| rhinitis | 96 | 385 | 1200 | 6272 | 0.897 | |
| eczema | 125 | 353 | 1233 | 4673 | 0.721 | |
| (1–3, 1–6)-β-D-glucan | control | 187 | 1 | 6 | 66 | |
| case | 180 | 1 | 6 | 137 | 0.674 | |
| asthma | 112 | 1 | 6 | 108 | 0.999 | |
| rhinitis | 89 | 1 | 6 | 204 | 0.521 | |
| eczema | 119 | 1 | 6 | 69 | 0.747 | |
| Ergosterol | control | 195 | 0.138 | 1.750 | 10.367 | |
| case | 188 | 0.078 | 1.629 | 7.572 | 0.182 | |
| asthma | 115 | 0.079 | 1.631 | 7.077 | 0.346 | |
| rhinitis | 94 | 0.076 | 1.482 | 7.083 | 0.138 | |
| eczema | 124 | 0.083 | 1.561 | 7.145 | 0.101 | |
Concentration units are CFU/g for the mold genera, μg/g dust for (1–3, 1–6)-β-D-glucan, pg/mg for ergosterol. Mann–Whitney U-test was used to obtain the P-values.
FIGURE 2. Culturable Fungi Concentration Distributions (CFU/g) in Homes of Case and Control Children.
Box and the bar within it show 25th, 50th, and 75th percentile of the concentration; the whiskers show the 5th and the 95th percentiles, respectively. The symbols, ● and * represent concentrations that are >1.5– and >3–fold of the 75th percentile value.
DISCUSSION
In the present investigation, we investigated whether culturable fungi, ergosterol, and (1–3,1–6)-β-d-glucan in indoor dust are associated with IDOM rating by parents, professional inspectors, or mold index. We subsequently examined whether the five most common fungal genera and yeasts pose independent risks on the respective diagnosis of asthma, rhinitis, eczema, or multiple allergic symptom presentation. We additionally examined risk of ergosterol and (1–3,1–6)-β-d-glucan on these allergic outcomes.
Overall, CFU counts/g dust for the five most common fungal genera and yeasts in all homes within our investigation were low. The mean ± S.D. and median for total culturable fungi in house dust was 1680±1443 CFU/g and 1267 CFU/g dust, respectively. While directly plating the dust on the agar medium may increase the diversity of observed fungi, this method might have yielded low detected concentration of culturable fungi due to crowding compared to serial dilution method (Verhoeff et al., 1994). Therefore, we interpreted the CFU counts from directly plated dust as semi-quantitative measures of growth intensity rather than quantitative counts of culturable fungi (Yang et al., 2007). As such, our references to concentrations of culturable fungi indicate observed CFUs on the plate.
Ergosterol concentrations from this study are comparable to those found in other studies. In a study of two nursing homes, one with and one without fungal contamination, the range of ergosterol concentrations in dust was between 1.6 and 3.3 ng/mg and 0.5 and 5.7 ng/mg, respectively (Pitkäranta et al., 2008). Similarly, ergosterol ranged from 2—16.5 ng/mg dust in homes not obviously contaminated with fungi; ergosterol concentrations were positively correlated with total CFU in dust (Saraf et al., 1997). Measured (1–3, 1–6)-β-D- glucan concentrations in the current study are low compared to concentrations in dust from the homes of children from five European countries, where median concentrations were over 1000 μg/g (Schram et al., 2005).
Fungal Agents and Health Outcomes
As shown in Table 4, neither the total CFU nor specific fungal genera or yeasts group were associated with an elevated likelihood of being a case, or any specific diagnosis of asthma, rhinitis, or eczema. As stated above, direct plating of dust may have underestimated the true culturable fungi concentrations. At the same time, this method has been validated for its ability to capture diverse mold genera (Wanner et al., 1993).
Several other studies have reported similar absence of association between quantified mold or mold related factors in dust and asthma or allergic disease. Wickman et al., (1992) found that sum of Alternaria spp., Penicillium spp. and Cladosporium spp. spores in house dust was lower among atopic households as opposed to asymptomatic controls. The authors posited that lower concentrations might reflect sanitary measures put in place by families with atopic children to reduce total allergen levels (Wickman et al., 1992). In a case control study of German children, fungal CFU counts in dust and air were not different between cases and controls. However, the IgE sensitization rate to fungi was higher among cases (9.2%) as compared to controls (4.4%) (Jovanovic et al., 2004). In a prospective study, visually observed mold in the homes of 8 month old children was associated with a positive asthma predictive index (API) at three years of age, while (1–3, 1–6)-β-D-glucan concentration in dust was associated with a negative API at the same age; the protective effect was not significant (Iossifova et al., 2009). In a study of 226 adults with asthma and rhinitis residing in California, using the same methods as in this paper, Blanc et al. (2005) reported (1–3,1–6)-β-d-glucan median concentration of 211 μg/g in house dust (25th – 75th percentile, 124—426 μg/g). The authors noted no association between glucan levels and forced expiratory volume (FEV1) (Blanc et al., 2005). There was also no significant relationship between visible mold, wall dampness or air humidity with FEV1 (Blanc et al., 2005). At the same time, laboratory studies have shown that (1–3, 1–6)-β-D-glucans could cause or exacerbate allergic symptoms (Douwes, 2005).
Other studies have found an association between fungal concentrations in house dust and adverse health outcomes. In a birth cohort study, dust-borne fungal concentrations were positively associated with the development of allergic rhinitis in the first five years of life after controlling for dampness and several other covariates (Stark et al., 2005). In this investigation, median mold concentrations in dust are not reported, however the 90th percentiles are approximately one to two orders of magnitude above those reported in our investigation (Stark et al., 2005). Reponen et al., (2012) found that both environmental moldiness index, a measure of prevalence of dampness related vs. non-dampness related molds, and the sum of three mold species Aspergillus ochraceus, Apergillus unguis and Penicillium variable measured in home at age 1 year significantly predict asthma at age 7. Respective geometric mean concentrations for these species, in cell equivalents/mg dust, were 6.8, 2.6 and 12.6 for cases and 2.0, 1.0 and 4.0 among controls (Reponen et al., 2012). These findings suggest a lack of species-specific fungal identification may contribute to misclassification of exposure and bias the results toward the null.
Our present observation regarding ergosterol is contrary to other investigations, which observed significantly elevated risks of asthma. For example, in a group of adult employees at a building with a history of water-damage, ergosterol concentration in floor and chair dust samples was significantly correlated with asthma, independent of culturable fungi concentration (Park et al., 2008). In our present investigation, we observed overall similar ranges of ergosterol exposure as those in Park et al. (2008). However, the distributions of ergosterol were consistently lower in all four outcome groups in our investigation compared to that in controls. Inconsistent associations between ergosterol and health outcomes may be partially explained by the non-specificity of this fungal marker (i.e. it is present in innocuous fungi) and the variable concentrations of ergosterol among different fungal species (Pasanen et al., 1999).
The inconsistent association between exposure to household fungus and health outcomes in different studies could in part be due to lack of standard methodology for assessing mold exposure (Mendell et al., 2011). Stark et al., (2005) noted that total fungal concentrations would pool diverse genera into a single exposure variable that may not accurately predict risk (Stark et al., 2005). In addition, Muller et al., (2002) found significant associations between Penicillium and Aspergillus exposure with respiratory infections and IgE sensitization to grass respectively. However total CFU in air were not associated with health outcomes (Müller et al., 2002). Species level identification and molecular quantification methods may alleviate some of the inconsistencies.
Our present observation adds to the body of literature and suggests a lack of association between exposure to fungal agents and a range of respiratory outcomes. In a comprehensive review and meta-analysis, Mendell et al., (2011) found that while there is ample evidence that dampness related factors adversely affects health, dust-borne fungal and bacterial burdens had little predictive value for asthma and allergy outcomes, noting that both positive and negative associations were present (Mendell et al. 2011). Other reviews have come to similar conclusions about the role of indoor molds in the development of asthma and allergic disease (Bornehag et al., 2001; Institute of Medicine, 2004; Portnoy et al., 2008).
Culturable Fungi, Ergosterol, (1–3,1–6)-β-d-glucan and Dampness and Building Characteristics
In the present investigation, Penicillium was the most common genus, yielding the highest mean concentration. Alternaria spp. and Yeasts were also frequently detected and tended to be present at elevated concentrations compared to other genera. The low concentrations of culturable fungi and fungal markers found in the current investigation suggest that exposure to fungal material from dust is probably low. Furthermore, poor associations between IDOM and culturable fungi suggest that IDOM do not necessarily indicate fungal exposure. This is supported by two separate observations. First, there is low agreement between parental report of mold and the mold index, as well as the inspector ratings of mold and the mold index (Holme et al., 2010). Second, there is a general lack of association between qualitative IDOM assessments and culturable fungi in dust samples, with the exception of yeasts.
There is poor and inconsistent correlation between IDOM and quantified concentrations of fungal agents in the literature. While some studies found an association, other studies could not replicate such findings. Lignell et al. (2008) found that history of moisture damage is significantly associated with concentrations of several bacterial and fungal genera identified from the house dust (Lignell et al., 2008). Reponen et al. (2010) assessed the correlation between perception of mold and quantitative measures of mold in dust. The authors reported that visual perception of mold was not associated with concentrations of mold as measure by (1–3,1–6)-β-D-glucan in dust and air and airborne fungal spores. However, mold odor and environmental relative moldiness index (ERMI) were associated with mold burden (Reponen et al., 2010). Hyvärinen et al., (2006) measured fungal spores in vacuum cleaner dust and found culturable spore counts to be associated with presence of visible mold in the home (Hyvärinen et al., 2006).
Another study reported that airborne spore concentrations (CFU/m3) are associated with indicators of dampness in homes such as water intrusion, indoor humidity, musty odor, and low ventilation (Garrett et al., 1998). Dekoster and Thorne (1995) demonstrated that airborne mold spore concentrations in complaint homes were two-fold higher than non-complaint homes and four-fold higher than intervention homes (DeKoster et al., 1995). Genera-level determinations were performed for Penicillium, Aspergillus, Cladosporium, Alernaria, Fusarium and yeasts and showed marked differences between specific organisms. Penicillium and Aspergillus species were over 20-fold higher in the basements of complaint homes than non-complaint homes while other molds were not markedly different. Concentrations of airborne culturable molds were significantly associated with basement humidity levels and a history of a leaky roof (DeKoster et al., 1995). In contrast, in a birth cohort study, no association was observed between home characteristics (e.g., visible mold or water damage) and concentrations of Alternaria spp. antigen (Cho et al., 2006). However, use of a dehumidifier and indoor dryer venting were associated with Alternaria spp. concentrations (Cho et al., 2006). In German homes, neither the individual mold genera, nor the summed total of all mold genera were associated with relative humidity, temperature, visible mold, carpet, dampness, or ventilation (Jovanovic et al., 2004). Based on short-term air sampling, no association was found between visible mold or moisture damage and CFU concentrations in air (Müller et al., 2002). Such evidence highlights the inconsistent relationship between IDOM and quantifiable fungal agents.
It is also important to consider that indoor mold may be correlated with other multiple proximal correlates of IDOM, including, but not limited to, dust mites, and select synthetic chemicals. For example, indoor humidity increases concentrations of phthalate degradation by-products from PVC (Norback et al., 2000). Previous research within this cohort suggests that propylene glycol and glycol ethers are correlated with indoor dampness (Choi et al., 2010). Thus, the use of indoor dampness as an indirect measure of mold exposure, or vice versa, is problematic. While high levels of excess moisture in the home may precipitate mold growth they are not shown to be consistently correlated enough to suggest equivalency. In our repeated examination of dampness as a risk factor multiple allergic symptoms, we observed an inconsistent role of indoor moisture. While the parental reporting of home dampness was a strong risk factor of multiple allergic symptoms on a cross-sectional examination (Bornehag et al., 2005b), most of such associations disappeared when the same questions were repeated 5 years after the initial examination and the analyses were conducted with a longitudinal design (Larsson et al., 2011). Thus, the causal agent underlying the home dampness and health outcomes remains an open question.
Strengths
Here, we conducted a direct measurement of culturable fungi in dust samples taken from the floor in the children’s bedroom. Dust is a major route of exposure for numerous indoor environmental agents, both biogenic and abiogenic (Munir et al., 1995; Rudel et al., 2003). Measurement of the culturable fungi in dust enabled us to examine possibly integrated human exposure in an indoor setting, independent of dampness. Fungi in dust samples are less likely to be influenced by outdoor sources as compared to air samples (Chew et al., 2003). Furthermore, indoor fungal concentrations in air and dust are not always highly correlated (Reponen et al. 2010). Analysis of ergosterol and (1–3,1–6)-β-d-glucan provide non-culture dependent markers of fungal contamination and exposure. Inclusion of these agents provides independent markers of fungal exposure, and thereby, overcomes the limitations of methods for culturable fungi measurement.
Limitations
Potential for selection biases for cases and controls has already been described and discussed (Bornehag et al., 2006). Briefly, participating families were more likely to have health problems, and also more likely to have health promoting factors such as non-smoking parents and higher socio-economic status. However, our earlier investigation revealed that there were no apparent selection biases regarding home dampness measures or other building characteristics (Bornehag et al., 2006). It is also possible that the children in the present study were exposed to allergenic factors in other non-home environment (e.g., school, nursery). The cross sectional nature of the exposure and outcome assessment make temporal relationships impossible to determine, thus, reverse causality is possible. The methods for determining cases and controls selected the most and least symptomatic children respectively for inclusion in the study. This sampling strategy makes identifying associations more likely; however, results are less generalizable. Our case-control selection strategy examines the risk of fungal exposure in a vulnerable segment of the population, compared to very healthy controls. Our present sample selection strategy does not threaten the validity of our conclusion. This is because the expected direction of the bias would be away from the null association. Our present observation of the null association between home fungal exposure and risk in the vulnerable segment of children is not likely to be different from that in the general population.
One limitation of our culture dependent method is that it fails to capture non-culturable fungal spores or other fungal material, which may still contribute allergic response. For example, Douwes et al., found that Penicillium and Aspergillus specific extracellular polysaccharides isolated from house dust were associated with doctor diagnosed asthma (Douwes et al., 1999). Additionally culture dependent methods select for easily culturable mold spores, and those that grow rapidly (Pasanen, 2001). Direct plating of dust may cause a reduction in apparent CFU compared to plating of serial dilutions, due to inhibitory effects of high fungal density on the plate (Verhoeff et al., 1994). Culture dependent methods underestimate concentrations and diversity of fungus present in the home as compared to molecular quantification methods such as quantitative PCR (Lignell et al., 2008; Pitkäranta et al., 2008). Additionally, qualitative assessments of visual mold may have underestimated its presence due to mold hidden inside walls or building materials. In addition, our reliance on reservoir dust samples as a proxy for airborne bioaerosol exposures might have introduced an exposure misclassification. This is because some of the materials in reservoir dust are never airborne.
This study reports the absence of an apparent association between exposure to culturable fungi, and fungal agents in dust and asthma and allergic disease symptoms. However, geographic variation in this association due to climatic conditions and endemic mold genera are possible. There is evidence that the association between mold and health outcomes is different in warmer and more humid areas (Hamilos, 2010). A review focusing on fungal rhinitis and rhinosinusitis suggested that geographic areas with higher natural fungal spore concentrations have higher rates of fungal allergy (Hamilos, 2010). In contrast, the occurrence of visible mold on building surfaces is less common in Scandinavian countries as compared to warmer climates (Bornehag et al., 2001). It is possible the low levels of mold present in the homes of this study were not sufficient to elicit asthma or allergic symptoms. Thus, these results may not be generalizable to areas outside of Scandinavian countries.
CONCLUSION
This study demonstrates that culturable fungi, ergosterol, and (1–3, 1–6)-β-D-glucan in dust are not significantly associated with qualitatively determined degree of mold presence. Culturable fungi, ergosterol, and (1–3, 1–6)-β-D-glucan in dust do not pose an independent risk on individual diagnosis of asthma, rhinitis, eczema, as well as the presence of multiple symptoms of allergies.
Practical Implications.
There is no consistent agreement between qualitative indicators of indoor dampness and mold and the level of culturable fungi in dust. Culturable fungi and fungal factors in house dust do not predict asthma or allergic disease outcomes among children.
Acknowledgments
The study has been supported by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (Formas), Swedish Asthma and Allergy Association’s Research Foundation, the Swedish Foundation for Health Care Sciences and Allergy Research, Norwegian Research Council, the U.S. National Research Service Award (T32 ES 07069), The American Scandinavian Foundation (Research Grant for Young Investigators), and County Council of Värmland, Sweden. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This article was partially made possible by EPA fellowship number 91743401. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the EPA. Further, the EPA does not endorse the purchase of any commercial products or services mentioned in the publication. The authors also thank Dr. Linda Hägerhed Engman for helpful comments on the manuscript.
Contributor Information
Hyunok Choi, Email: hchoi@albany.edu.
Sam Byrne, Email: sbyrne@albany.edu.
Lisbeth Suldrup Larsen, Email: lsl@teknologisk.dk.
Torben Sigsgaard, Email: TS@MIL.AU.DK.
Peter S. Thorne, Email: peter-thorne@uiowa.edu.
Lennart Larsson, Email: lennart.larsson@med.lu.se.
Aleksandra Sebastian, Email: aleksandra.sebastian@windowslive.com.
Carl-Gustaf Bornehag, Email: Carl-Gustaf.Bornehag@kau.se.
References
- Andersen B, Frisvad JC, Søndergaard I, Rasmussen IS, Larsen LS. Associations between Fungal Species and Water-Damaged Building Materials. Applied and Environmental Microbiology. 2011;77:4180–4188. doi: 10.1128/AEM.02513-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger K, Beckett W, Triche E, Bracken MB, Holford T, Ren P, Mcsharry J-E, Gold DR, Platts-Mills TaE, Leaderer BP. Symptoms of Wheeze and Persistent Cough in the First Year of Life: Associations with Indoor Allergens, Air Contaminants, and Maternal History of Asthma. American Journal of Epidemiology. 2003;158:195–202. doi: 10.1093/aje/kwg148. [DOI] [PubMed] [Google Scholar]
- Blanc PD, Eisner MD, Katz PP, Yen IH, Archea C, Earnest G, Janson S, Masharani UB, Quinlan PJ, Hammond SK, Thorne PS, Balmes JR, Trupin L, Yelin EH. Impact of the Home Indoor Environment on Adult Asthma and Rhinitis. J Occup Environ Med. 2005;47:362–372. doi: 10.1097/01.jom.0000158708.32491.9d. [DOI] [PubMed] [Google Scholar]
- Bornehag C-G, Sundell J, Sigsgaard T, Janson S. Potential self-selection bias in a nested case-control study on indoor environmental factors and their association with asthma and allergic symptoms among pre-school children. Scand J Public Health. 2006;34:534–543. doi: 10.1080/14034940600607467. [DOI] [PubMed] [Google Scholar]
- Bornehag CG, Blomquist G, Gyntelberg F, Jarvholm B, Malmberg P, Nordvall L, Nielsen A, Pershagen G, Sundell J. Dampness in buildings and health. Nordic interdisciplinary review of the scientific evidence on associations between exposure to “”. dampness” in buildings and health effects (NORDDAMP) Indoor Air. 2001;11:72–86. doi: 10.1034/j.1600-0668.2001.110202.x. [DOI] [PubMed] [Google Scholar]
- Bornehag CG, Sundell J, Bonini S, Custovic A, Malmberg P, Skerfving S, Sigsgaard T, Verhoeff V. Dampness in buildings as a risk factor for health effects, (EUROEXPO). A multidisciplinary review of the literature (1998–2000) on dampness and mite exposure in buildings and health effects. Indoor Air. 2004;14:243–257. doi: 10.1111/j.1600-0668.2004.00240.x. [DOI] [PubMed] [Google Scholar]
- Bornehag CG, Sundell J, Hägerhed-Engman L, Sigsgaard T. Association between ventilation rates in 390 Swedish homes and allergic symptoms in children. 2005a;15:275–280. doi: 10.1111/j.1600-0668.2005.00372.x. [DOI] [PubMed] [Google Scholar]
- Bornehag CG, Sundell J, Hagerhed-Engman L, Sigsggard T, Janson S, Aberg N. “Dampness” at home and its association with airway, nose and skin symptoms among 10 851 preschool children in Sweden: a cross sectional study. Indoor Air. 2005b;15:48–55. doi: 10.1111/j.1600-0668.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- Bornehag CG, Sundell J, Lundgren B, Weschler CJ, Sigsgaard T, Hagerhed-Engman L. Phthalates in indoor dust and their association with building characteristics. Environmental health perspectives. 2005c;113:1399–1404. doi: 10.1289/ehp.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush RK, Portnoy JM, Saxon A, Terr AI, Wood RA. The medical effects of mold exposure. Journal of Allergy and Clinical Immunology. 2006;117:326–333. doi: 10.1016/j.jaci.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Chew GL, Douwes J, Doekes G, Higgins KM, Van Strien R, Spithoven J, Brunekreef B. Fungal extracellular polysaccharides, beta (1-->3)-glucans and culturable fungi in repeated sampling of house dust. Indoor Air. 2001;11:171–178. doi: 10.1034/j.1600-0668.2001.011003171.x. [DOI] [PubMed] [Google Scholar]
- Chew GL, Rogers C, Burge HA, Muilenberg ML, Gold DR. Dustborne and airborne fungal propagules represent a different spectrum of fungi with differing relations to home characteristics. Allergy. 2003;58:13–20. doi: 10.1034/j.1398-9995.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- Cho SH, Reponen T, Bernstein DI, Olds R, Levin L, Liu X, Wilson K, Lemasters G. The effect of home characteristics on dust antigen concentrations and loads in homes. Sci Total Environ. 2006;371:31–43. doi: 10.1016/j.scitotenv.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Schmidbauer N, Sundell J, Hasselgren M, Spengler J, Bornehag CG. Common household chemicals and the allergy risks in pre-school age children. PLoS One. 2010;5:e13423. doi: 10.1371/journal.pone.0013423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekoster JA, Thorne PS. Bioaerosol Concentrations in Noncomplaint, Complaint, and Intervention Homes in the Midwest. Am Ind Hyg Assoc J. 1995;56:573–580. [Google Scholar]
- Douwes J. (1→3)-β-D-glucans and respiratory health: a review of the scientific evidence. Indoor Air. 2005;15:160–169. doi: 10.1111/j.1600-0668.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- Douwes J, Van Der Sluis B, Doekes G, Van Leusden F, Wijnands L, Van Strien R, Verhoeff A, Brunekreef B. Fungal extracellular polysaccharides in house dust as a marker for exposure to fungi: Relations with culturable fungi, reported home dampness, and respiratory symptoms. Journal of Allergy and Clinical Immunology. 1999;103:494–500. doi: 10.1016/s0091-6749(99)70476-8. [DOI] [PubMed] [Google Scholar]
- Garrett MH, Rayment PR, Hooper MA, Abramson MJ, Hooper BM. Indoor airborne fungal spores, house dampness and associations with environmental factors and respiratory health in children. Clin Exp Allergy. 1998;28:459–467. doi: 10.1046/j.1365-2222.1998.00255.x. [DOI] [PubMed] [Google Scholar]
- Gravesen S. Identification and prevalence of culturable mesophilic micro fungi in house dust from 100 Danish homes. Allergy. 1978;33:269–272. doi: 10.1111/j.1398-9995.1978.tb01547.x. [DOI] [PubMed] [Google Scholar]
- Hamilos DL. Allergic Fungal Rhinitis and Rhinosinusitis. Proceedings of the American Thoracic Society. 2010;7:245–252. doi: 10.1513/pats.200909-098AL. [DOI] [PubMed] [Google Scholar]
- Hardin B, Kelman B, Saxon A. Adverse Human Health Effects Associated with Molds in the Indoor Environment. Journal of Occupational and Environmental Medicine. 2003;45:8. doi: 10.1097/00043764-200305000-00006. [DOI] [PubMed] [Google Scholar]
- Hederos C-A, Hasselgren M, Hedlin G, Bornehag C-G. Comparison of clinically diagnosed asthma with parental assessment of children’s asthma in a questionnaire. Pediatric Allergy and Immunology. 2007;18:135–141. doi: 10.1111/j.1399-3038.2006.00474.x. [DOI] [PubMed] [Google Scholar]
- Holme J, Hägerhed-Engman L, Mattsson J, Sundell J, Bornehag CG. Culturable mold in indoor air and its association with moisture-related problems and asthma and allergy among Swedish children. Indoor Air. 2010;20:329–340. doi: 10.1111/j.1600-0668.2010.00658.x. [DOI] [PubMed] [Google Scholar]
- Hyvärinen A, Sebastian A, Pekkanen J, Larsson L, Korppi M, Putus T, Nevalainen A. Characterizing Microbial Exposure With Ergosterol, 3-Hydroxy Fatty Acids, and Viable Microbes in House Dust: Determinants and Association With Childhood Asthma. Archives of Environmental & Occupational Health. 2006;61:149–157. doi: 10.3200/AEOH.61.4.149-157. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Damp Indoor Spaces and Health. New York: The National Academies; 2004. [PubMed] [Google Scholar]
- Iossifova YY, Reponen T, Ryan PH, Levin L, Bernstein DI, Lockey JE, Hershey GKK, Villareal M, Lemasters G. Mold exposure during infancy as a predictor of potential asthma development. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology. 2009;102:131–137. doi: 10.1016/S1081-1206(10)60243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic S, Felder-Kennel A, Gabrio T, Kouros B, Link B, Maisner V, Piechotowski I, Schick K-H, Schrimpf M, Weidner U, Zöllner I, Schwenk M. Indoor fungi levels in homes of children with and without allergy history. International Journal of Hygiene and Environmental Health. 2004;207:369–378. doi: 10.1078/1438-4639-00302. [DOI] [PubMed] [Google Scholar]
- Larsson M, Hagerhed-Engman L, Moniruzzaman S, Janson S, Sundell J, Bornehag CG. Can we trust cross-sectional studies when studying the risk of moisture-related problems indoor for asthma in children? International journal of environmental health research. 2011;21:237–247. doi: 10.1080/09603123.2010.533368. [DOI] [PubMed] [Google Scholar]
- Lignell U, Meklin T, Rintala H, Hyvärinen A, Vepsäläinen A, Pekkanen J, Nevalainen A. Evaluation of quantitative PCR and culture methods for detection of house dust fungi and streptomycetes in relation to moisture damage of the house. Letters in Applied Microbiology. 2008;47:303–308. doi: 10.1111/j.1472-765x.2008.02431.x. [DOI] [PubMed] [Google Scholar]
- Madigan M, Martinko J. Brock Biology of Microorganisms. Prentice Hall; 2005. [Google Scholar]
- Mendell MJ, Mirer AG, Cheung K, Tong M, Douwes J. Respiratory and Allergic Health Effects of Dampness, Mold, and Dampness-Related Agents: A Review of the Epidemiologic Evidence. Environmental health perspectives. 2011:119. doi: 10.1289/ehp.1002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A, Lehmann I, Seiffart A, Diez U, Wetzig H, Borte M, Herbarth O. Increased incidence of allergic sensitisation and respiratory diseases due to mould exposure: Results of the Leipzig Allergy Risk children Study (LARS) International Journal of Hygiene and Environmental Health. 2002;204:363–365. doi: 10.1078/1438-4639-00110. [DOI] [PubMed] [Google Scholar]
- Munir AKM, Einarsson R, Dreborg SKG. Mite (Der p I, Der f I), cat (Fel d I) and dog (Can f I) allergens in dust from Swedish day-care centres. Clinical & Experimental Allergy. 1995;25:119–126. doi: 10.1111/j.1365-2222.1995.tb01016.x. [DOI] [PubMed] [Google Scholar]
- Norback D, Wieslander G, Nordstr K, Ouml RW, Aring, Linder Asthma symptoms in relation to measured building dampness in upper concrete floor construction, and 2-ethyl-1-hexanol in indoor air. The International Journal of Tuberculosis and Lung Disease. 2000;4:1016–1025. [PubMed] [Google Scholar]
- Nordtest. Nordtest method NT VVS 118. Nordtest, Finland: 1997. Ventilation: Local mean age of air - homogenous emission techniques. [Google Scholar]
- Park JH, Cox-Ganser JM, Kreiss K, White SK, Rao CY. Hydrophilic fungi and ergosterol associated with respiratory illness in a water-damaged building. Environmental health perspectives. 2008;116:45–50. doi: 10.1289/ehp.10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasanen AL. A Review: Fungal Exposure Assessment in Indoor Environments. Indoor Air. 2001;11:87–98. doi: 10.1034/j.1600-0668.2001.110203.x. [DOI] [PubMed] [Google Scholar]
- Pasanen AL, Yli-Pietila K, Pasanen P, Kalliokoski P, Tarhanen J. Ergosterol content in various fungal species and biocontaminated building materials. Appl Environ Microbiol. 1999;65:138–142. doi: 10.1128/aem.65.1.138-142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekkanen J, Hyvärinen A, Haverinen-Shaughnessy U, Korppi M, Putus T, Nevalainen A. Moisture damage and childhood asthma: a population-based incident case–control study. European Respiratory Journal. 2007;29:509–515. doi: 10.1183/09031936.00040806. [DOI] [PubMed] [Google Scholar]
- Pitkäranta M, Meklin T, Hyvärinen A, Paulin L, Auvinen P, Nevalainen A, Rintala H. Analysis of Fungal Flora in Indoor Dust by Ribosomal DNA Sequence Analysis, Quantitative PCR, and Culture. Applied and Environmental Microbiology. 2008;74:233–244. doi: 10.1128/AEM.00692-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy J, Barnes C, Kennedy K. Importance of mold allergy in asthma. Current Allergy and Asthma Reports. 2008;8:71–78. doi: 10.1007/s11882-008-0013-y. [DOI] [PubMed] [Google Scholar]
- Reponen T, Lockey J, Bernstein DI, Vesper SJ, Levin L, Khurana Hershey GK, Zheng S, Ryan P, Grinshpun SA, Villareal M, Lemasters G. Infant origins of childhood asthma associated with specific molds. Journal of Allergy and Clinical Immunology. 2012;130:639–644. doi: 10.1016/j.jaci.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reponen T, Singh U, Schaffer C, Vesper S, Johansson E, Adhikari A, Grinshpun SA, Indugula R, Ryan P, Levin L, Lemasters G. Visually observed mold and moldy odor versus quantitatively measured microbial exposure in homes. Sci Total Environ. 2010;408:5565–5574. doi: 10.1016/j.scitotenv.2010.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environmental Science & Technology. 2003;37:4543–4553. doi: 10.1021/es0264596. [DOI] [PubMed] [Google Scholar]
- Saraf A, Larsson L, Burge H, Milton D. Quantification of ergosterol and 3-hydroxy fatty acids in settled house dust by gas chromatography-mass spectrometry: comparison with fungal culture and determination of endotoxin by a Limulus amebocyte lysate assay. Applied and Environmental Microbiology. 1997;63:2554–2559. doi: 10.1128/aem.63.7.2554-2559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram D, Doekes G, Boeve M, Douwes J, Riedler J, Ublagger E, Von Mutius E, Budde J, Pershagen G, Nyberg F, Alm J, Braun-Fahrländer C, Waser M, Brunekreef B. Bacterial and fungal components in house dust of farm children, Rudolf Steiner school children and reference children--the PARSIFAL Study. Allergy. 2005;60:611–618. doi: 10.1111/j.1398-9995.2005.00748.x. [DOI] [PubMed] [Google Scholar]
- Sebastian A, Larsson L. Characterization of the Microbial Community in Indoor Environments: a Chemical-Analytical Approach. Applied and Environmental Microbiology. 2003;69:3103–3109. doi: 10.1128/AEM.69.6.3103-3109.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark PC, Celedón JC, Chew GL, Ryan LM, Burge HA, Muilenberg ML, Gold DR. Fungal Levels in the Home and Allergic Rhinitis by 5 Years of Age. Environmental health perspectives. 2005:113. doi: 10.1289/ehp.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stymne H, Boman C, Kronvall J. Measuring ventilation rates in the Swedish building stock. Building and Environment. 1994;29:373–379. [Google Scholar]
- Szponar B, Larsson L. Determination of microbial colonisation in water-damaged buildings using chemical marker analysis by gas chromatography-mass spectrometry. Indoor Air. 2000;10:13–18. doi: 10.1034/j.1600-0668.2000.010001013.x. [DOI] [PubMed] [Google Scholar]
- Verhoeff AP, Van Reenen-Hoekstra ES, Samson RA, Brunekreef PJ, Van Wijnen JH. Fungal propagules in house dust. I. Allergy. 1994;49:533–539. doi: 10.1111/j.1398-9995.1994.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Wanner H, Verhoeff A, Colombi A, Flannigan B, Gravesen S, Mouilleseaux A, Nevalainen A, Papadakis J, Seidel K. Directorate General for Science, Research and Development. Vol. 12. Luxembourg: Joint Research Centre - Environment Institute; 1993. European Collaborative Action: Indoor Air Quality and Its Impact on Man, Environment and Quality of Life. [Google Scholar]
- Who. Second technical meeting on quantifying disease from inadequate housing; Bonn: WHO Regional Office for Europe; 2006. [Google Scholar]
- Heseltine E, Rosen J, editors. Who. WHO guidelines for indoor air quality: dampness and mould. WHO Regional Office for Europe; 2009. [PubMed] [Google Scholar]
- Wickman M, Gravesen S, Nordvall SL, Pershagen G, Sundell J. Indoor viable dust-bound microfungi in relation to residential characteristics, living habits, and symptoms in atopic and control children. Journal of Allergy and Clinical Immunology. 1992;89:752–759. doi: 10.1016/0091-6749(92)90384-e. [DOI] [PubMed] [Google Scholar]
- Yang C, Heinsohn P. Sampling and Analysis of Indoor Microorganisms. Hoboken, NJ: John Wiley and Sons; 2007. [Google Scholar]