Significance
The family with sequence similarity 20, member C (Fam20C) is a secretory pathway-specific kinase that phosphorylates secreted proteins on Ser-x-Glu/pSer motifs. Mutations in human FAM20C cause a devastating childhood disorder known as Raine syndrome. Some patients with FAM20C mutations as well as Fam20C KO mice develop hypophosphatemia due to elevated levels of the phosphate-regulating hormone FGF23. In this paper, we show that Fam20C phosphorylates FGF23 on a Ser-x-Glu motif that lies within a critical region of the hormone. The phosphorylation promotes FGF23 proteolysis by furin by blocking O-glycosylation by polypeptide N-acetylgalactosaminyltransferase 3. Our results have important implications for patients with abnormalities in phosphate homeostasis.
Keywords: phosphate homeostasis, rickets, Fam20, familial tumoral calcinosis, chronic kidney disease
Abstract
The family with sequence similarity 20, member C (Fam20C) has recently been identified as the Golgi casein kinase. Fam20C phosphorylates secreted proteins on Ser-x-Glu/pSer motifs and loss-of-function mutations in the kinase cause Raine syndrome, an often-fatal osteosclerotic bone dysplasia. Fam20C is potentially an upstream regulator of the phosphate-regulating hormone fibroblast growth factor 23 (FGF23), because humans with FAM20C mutations and Fam20C KO mice develop hypophosphatemia due to an increase in full-length, biologically active FGF23. However, the mechanism by which Fam20C regulates FGF23 is unknown. Here we show that Fam20C directly phosphorylates FGF23 on Ser180, within the FGF23 R176XXR179/S180AE subtilisin-like proprotein convertase motif. This phosphorylation event inhibits O-glycosylation of FGF23 by polypeptide N-acetylgalactosaminyltransferase 3 (GalNAc-T3), and promotes FGF23 cleavage and inactivation by the subtilisin-like proprotein convertase furin. Collectively, our results provide a molecular mechanism by which FGF23 is dynamically regulated by phosphorylation, glycosylation, and proteolysis. Furthermore, our findings suggest that cross-talk between phosphorylation and O-glycosylation of proteins in the secretory pathway may be an important mechanism by which secreted proteins are regulated.
Protein kinases are evolutionarily conserved enzymes that regulate numerous cellular processes by transferring a molecule of phosphate from ATP to target substrates (1, 2). The vast majority of theses enzymes function within the nucleus and cytosol. In contrast, there are several examples of phosphorylated proteins that are secreted from the cell, which raises the question: What are the kinases that phosphorylate these secreted phosphoproteins? We recently identified a small family of secretory pathway kinases that phosphorylate secreted proteins and proteoglycans (3). These enzymes have N-terminal signal sequences that direct them to the lumen of the endoplasmic reticulum, where they encounter the proteins or proteoglycans that they phosphorylate. One member of this atypical kinase family is the family with sequence similarity 20, member C (Fam20C), which phosphorylates secreted proteins on Ser(S)-x-Glu(E)/pSer(pS) (S-x-E/pS) motifs (3, 4). Because Fam20C localizes within the secretory pathway and the vast majority of secreted phosphoproteins are phosphorylated on S-x-E/pS motifs, Fam20C has been proposed to play a major role in the generation of the secreted phosphoproteome (5–7). For example, ∼75% of human serum and cerebrospinal fluid phosphoproteins are phosphorylated on S-x-E/pS motifs (8, 9). This includes proteins important for tooth and bone formation, as well as numerous hormones. In most cases, the functional importance of these phosphorylation events is unknown.
Loss-of-function mutations in the human FAM20C gene cause Raine syndrome, an often-fatal osteosclerotic bone dysplasia (10, 11). Most Raine patients die within the first few weeks of life. Nonlethal cases have also been reported, and these patients develop hypophosphatemia as a result of elevated levels of the phosphate-regulating hormone fibroblast growth factor 23 (FGF23) (12). Additionally, Fam20C knockout (KO) mice develop renal phosphate wasting due to an increase in circulating bioactive FGF23 as well as severe hypophosphatemic rickets (13, 14). Thus, Fam20C has been proposed to be a regulator of FGF23; however, the molecular mechanisms underlying the control of this hormone by Fam20C are unclear.
FGF23 is secreted from osteoblasts and osteocytes, and targets the kidney to regulate the reabsorption of phosphate and catabolism of 1,25-dihydroxyvitamin D3 (15, 16). FGF23 inhibits renal phosphate transport by activating FGF receptors (FGFRs) in a manner that requires binding the coreceptor α-klotho (α-KL) (17). Binding of FGF23 to FGFRs/α-KL induces urinary excretion of phosphate by decreasing the abundance of the type II sodium-dependent phosphate cotransporters NPT2a and NPT2c (15, 16). Several genetic disorders of renal phosphate wasting are associated with alterations in FGF23 (18). X-linked hypophosphatemia is the most prevalent form of hypophosphatemic rickets (1:20,000), and is caused by loss-of-function mutations in the gene encoding the phosphate-regulating endopeptidase homolog X-linked, which is associated with elevated FGF23 expression (19, 20). A similar situation exists in patients with an autosomal recessive form of hypophosphatemic rickets caused by mutations in the ectonucleotide pyrophosphatase ENPP1 (21) and the Fam20C substrate dentin matrix protein 1 (DMP1) (22). These disorders share the common denominator of elevated FGF23.
Other Mendelian disorders of renal phosphate handling have provided important insight into the regulation of FGF23 protein processing. FGF23 is inactivated in the Golgi by proteolysis within a highly conserved subtilisin-like proprotein convertase (SPC) site, 176RHTR179/S180AE182, generating inactive N- and C-terminal fragments (23). Gain-of-function missense mutations in FGF23 cause autosomal dominant hypophosphatemic rickets (ADHR) (24). These mutations substitute the Arg (R) residues within the SPC cleavage site and render the protein resistant to proteolysis (25). Conversely, loss-of-function mutations in FGF23 cause familial tumoral calcinosis (FTC), a hyperphosphatemic disorder characterized by often severe ectopic and vascular calcifications (26). FTC is also caused by loss-of-function mutations in polypeptide N-acetylgalactosaminyltransferase 3 (GalNAc-T3), a glycosyltransferase that O-glycosylates FGF23 at Thr178 within the SPC cleavage site (27, 28). These inactivating GalNAc-T3 mutations prevent O-glycosylation of FGF23, which produces a hormone more susceptible to SPC proteolysis (28, 29). Collectively, these studies suggest that FGF23 processing is a highly regulated and physiologically important process.
Here we demonstrate that Fam20C regulates FGF23 by phosphorylation of Ser180, a residue that neighbors the SPC site (R176H177T178R179/S180). We show that Ser180 phosphorylation inhibits GalNAc-T3 O-glycosylation of Thr178, thereby promoting furin (PCSK3)-dependent FGF23 proteolysis. Our results provide a plausible molecular mechanism by which loss of Fam20C leads to elevated levels of intact, biologically active FGF23 and subsequent hypophosphatemia. Furthermore, our results suggest that cross-talk between phosphorylation and O-glycosylation of proteins in the secretory pathway may be an important mechanism by which secreted proteins are dynamically regulated.
Results
FGF23 Is Phosphorylated by Fam20C in Vitro and in Cells.
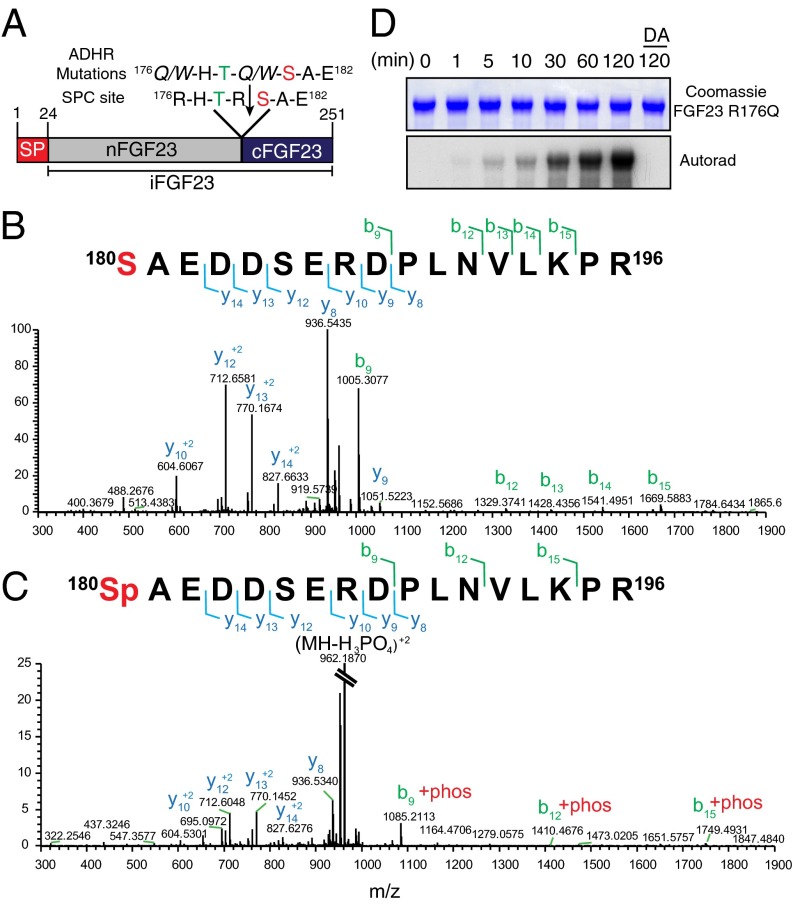
Contemporaneous with our finding that Fam20C is a protein kinase, Wang et al. (13) characterized Fam20C KO mice and demonstrated that these animals develop hypophosphatemic rickets as a result of elevated serum intact, bioactive FGF23. Furthermore, a recent report identified compound heterozygous mutations in FAM20C in two siblings referred for hypophosphatemia and severe dental demineralization disease (12). Biochemical analysis of the patients’ sera identified elevated intact FGF23. Because FGF23 is expressed in tissues that show high levels of Fam20C expression (30) and contains Fam20C consensus S-x-E/pS motifs (Fig. 1A), we hypothesized that Fam20C may directly regulate FGF23 by phosphorylation. To determine whether FGF23 is a phosphoprotein, we generated a HEK293T cell line stably expressing a protease-resistant mutant of FGF23 (FGF23 R176Q) (25). This construct contained a C-terminal Flag tag that allowed us to affinity-purify it from conditioned medium. We then digested FGF23 R176Q with trypsin or chymotrypsin, separated the peptides by liquid chromatography, and analyzed the peptides by mass spectrometry (MS). Tandem mass spectrometry (MS/MS) analysis identified 180pSAEDDSERDPLNVLKPR196 and 178TRpSAEDDSERDPL190 in the trypsin and chymotrypsin digests, respectively (Fig. 1 B and C and Fig. S1). These results suggest that FGF23 is phosphorylated in HEK293T cells on Ser180. Notably, Ser180 lies within a Fam20C S-x-E recognition motif and neighbors the SPC recognition site (Fig. 1A). To test whether Fam20C phosphorylates FGF23, we performed in vitro kinase reactions with recombinant Fam20C and FGF23 R176Q. Fam20C phosphorylated FGF23 R176Q in a time-dependent manner, whereas the catalytically inactive Fam20C D478A failed to incorporate phosphate (Fig. 1D). Furthermore, treatment of purified FGF23 R176Q with recombinant Fam20C increased the relative abundance of Ser180 phosphorylation (Fig. S2). By calculating the area under the selected ion species, we estimated that treatment with Fam20C increased the relative abundance of FGF23 R176Q Ser180 phosphorylation ∼10-fold.
Fig. 1.
Fam20C phosphorylates FGF23 on Ser180. (A) Schematic representation of human FGF23 indicating the signal peptide (SP), intact FGF23 (iFGF23), N-terminal fragment (nFGF23), and C-terminal fragment (cFGF23). The residues surrounding the SPC cleavage site (downward arrow) are shown. Mutations that replace the Arg in patients with autosomal dominant hypophosphatemic rickets (ADHR) are also shown. A potential Fam20C phosphorylation site is in red. (B and C) Representative MS/MS fragmentation spectra of a tryptic peptide (FGF23 180–196) depicting Ser180 phosphorylation of FGF23 R176Q purified from conditioned medium of HEK293T cells. (D) Time-dependent incorporation of 32P from [γ-32P]ATP into FGF23 R176Q by Fam20C or Fam20C D478A (DA). Reaction products were analyzed by SDS/PAGE and autoradiography.
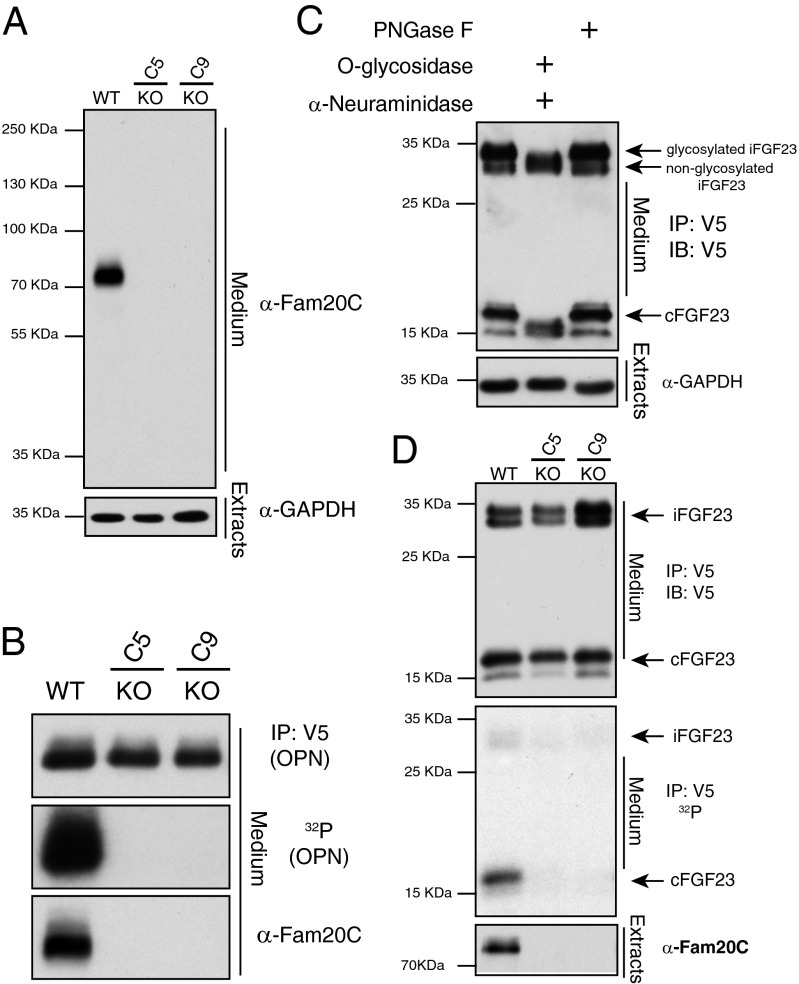
To determine whether Fam20C phosphorylates FGF23 in cells, we used clustered regularly interspaced short palindromic repeats (CRISPR/Cas9) genome-editing technology to delete the FAM20C gene from the osteoblast-like cell line U2OS (31, 32) (Fig. S3A). We generated U6-driven expression cassettes to express single-guide RNAs targeting exon 1 of the human FAM20C gene for expression in U2OS cells (Fig. S3 B and D). Two clones were detected to have insertions/deletions (indels) that resulted in frameshift mutations resulting in premature stop codons (Fig. S3 C and E). Protein immunoblotting with a polyclonal antibody raised against full-length Fam20C detected Fam20C protein in the conditioned medium of native U2OS cells but not the cells that were subjected to genome editing (Fig. 2A). To confirm loss of Fam20C activity, the phosphorylation status of the Fam20C substrate osteopontin (OPN) was analyzed in control and Fam20C KO cells that were metabolically labeled with [32P]orthophosphate. V5-tagged OPN was immunoprecipitated from conditioned medium, and OPN protein and phosphorylation levels were analyzed by autoradiography. OPN was phosphorylated in control cells, and no detectable phosphorylation was observed in Fam20C KO cells (Fig. 2B).
Fig. 2.
Fam20C phosphorylates FGF23 in mammalian cells (A) Protein immunoblotting of trichloroacetic acid precipitates from conditioned medium of control and Fam20C KO cells (C5, clone 5; C9, clone 9) using an affinity-purified rabbit anti-Fam20C polyclonal antibody. GAPDH from cell extracts is shown as loading control (Lower). (B) Control and Fam20C KO cells were metabolically labeled with 32PO43− and transfected with V5-tagged OPN. Protein immunoblotting of V5-immunoprecipitates from conditioned medium (Top) and autoradiography depicting 32P incorporation into OPN (Middle). Fam20C protein levels in conditioned medium are also shown (Bottom). (C) Protein immunoblotting of V5-immunoprecipitates from the conditioned medium of U2OS cells expressing V5-tagged FGF23. V5-immunoprecipitates were treated with O-glycosidase and α-(2→3,6,8,9)-neuraminidase to remove O-linked glycosylation or PNGase F to remove N-linked glycosylation. (D) Control and Fam20C KO cells were metabolically labeled with 32PO43− and transfected with V5-tagged FGF23. Protein immunoblotting of V5-immunoprecipitates from conditioned medium (Top) and autoradiography depicting 32P incorporation into FGF23 (Middle). Intact FGF23 and C-terminal fragments are shown. Fam20C protein levels in conditioned medium are also shown (Bottom). IB, immunoblotting; IP, immunoprecipitation.
In U2OS cells, ectopically expressed, C-terminal V5-tagged FGF23 is O-glycosylated, and we can detect the full-length hormone and the C-terminal fragments in the conditioned medium by protein immunoblotting of V5-immunoprecipitates (Fig. 2C). We transiently transfected V5-tagged FGF23 in control and Fam20C KO cells that we metabolically labeled with [32P]orthophosphate and analyzed V5-immunoprecipitates from conditioned medium. FGF23 was phosphorylated in control but not in Fam20C-deficient cells, and phosphorylation was more prevalent within the C-terminal fragment (Fig. 2D). Thus, Fam20C phosphorylates FGF23 in vitro and in cells.
Phosphorylation of FGF23 at Ser180 Prevents O-Glycosylation by GalNAc-T3.
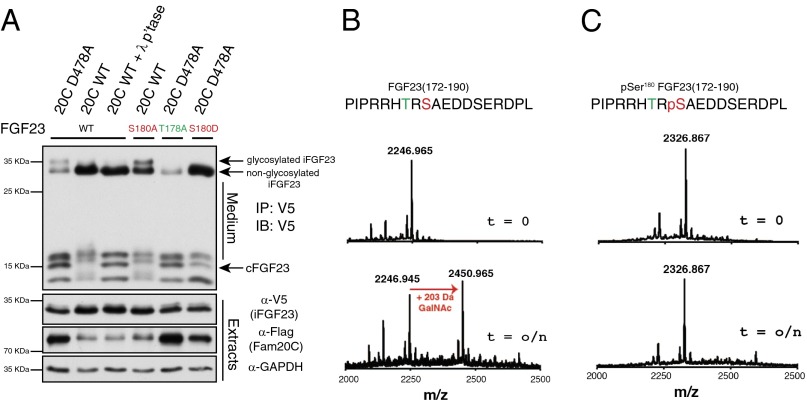
The physiological significance of FGF23 O-glycosylation was underscored when inactivating mutations in the gene encoding GalNAc-T3 (GALNT3) were found in patients with FTC (27). Patients with FTC develop hyperphosphatemia and severe ectopic calcifications, symptoms of which are the metabolic “mirror image” of the hypophosphatemic disorders (26). Subsequent studies identified inactivating mutations in FGF23 in FTC patients without mutations in GALNT3, suggesting that FGF23 and GalNAc-T3 operate in a common pathway to regulate phosphate homeostasis (26). The polypeptide N-acetylgalactosaminyltransferase (GalNAc-transferase) family consists of 20 enzymes that catalyze the initial step of mucin-type O-glycosylation by transferring GalNAc from UDP-GalNAc to Ser and Thr residues in secretory pathway proteins (33). GalNAc-T3 O-glycosylates FGF23 on Thr178 within the R176H177T178R179/S180 SPC site, and has been shown to protect FGF23 from proteolytic cleavage and thus inactivation (28). Because Fam20C phosphorylates FGF23 at Ser180, and this residue is located immediately adjacent to the SPC site (Fig. 1A), we hypothesized that phosphorylation may regulate FGF23 O-glycosylation and/or proteolytic processing. In Fam20C-deficient U2OS cells, we did not observe changes in O-glycosylation or proteolytic processing of ectopically expressed FGF23. However, it is likely that endogenous Fam20C levels and/or activity are much lower than the glycosyltransferases and/or proteases that modify FGF23 in these cells (i.e., Fam20C is limiting with respect to the glycosyltransferases and/or proteases). Deletion of Fam20C therefore would have no effect on O-glycosylation and/or processing. To explore the effect of phosphorylation of FGF23 on O-glycosylation and proteolytic processing, we coexpressed Flag-tagged Fam20C or the catalytically inactive Fam20C D478A mutant with V5-tagged FGF23 in U2OS cells and analyzed immunoprecipitates from conditioned medium. Wild-type Fam20C, but not the inactive D478A mutant, decreased the mobility of the C-terminal fragments of FGF23 that was reversed by λ-phosphatase treatment, suggesting a phosphorylation event (Fig. 3A, Lower, first, second, and third lanes). Interestingly, the doublet in full-length, intact FGF23 (iFGF23) was absent when active Fam20C, but not the inactive Fam20C D478A mutant, was expressed (Fig. 3A, Upper, first and second lanes). Given the close proximity of Ser180 to the SPC site, we reasoned that phosphorylation of FGF23 at Ser180 would prevent O-glycosylation at Thr178 and therefore explain the loss of the doublet observed in iFGF23 induced by Fam20C (Fig. 3A, second lane). Indeed, mutation of Ser180 to Ala prevented the loss of the doublet in iFGF23 when Fam20C was expressed (Fig. 3A, fourth lane). Consistently, mutation of Thr178 to Ala (a nonglycosylated mutant) or Ser180 to Asp (a phosphomimetic) resulted in a species that migrated similar to the phosphorylated WT iFGF23 (Fig. 3A, fifth and sixth lanes). These results support that Fam20C phosphorylation of FGF23 at Ser180 prevents O-glycosylation at Thr178.
Fig. 3.
Phosphorylation of FGF23 at Ser180 inhibits O-glycosylation by GalNAc-T3. (A) Protein immunoblotting of V5-immunoprecipitates from the conditioned medium of U2OS cells expressing V5-tagged FGF23 or mutants (S180A, T178A, S180D) with either WT (20C) or catalytically inactive D478A (20C D478A) FLAG-tagged Fam20C. V5-immunoprecipitates were treated with λ-phosphatase (λp’tase) (Upper). Extracts were analyzed for Fam20C, Fam20C D478A, FGF23, and GAPDH (Lower). (B and C) sGalNAc-T3 was incubated overnight (o/n) with FGF23(172–190) (B) or Ser180-phosphorylated FGF23(172–190) (C) and UDP-GalNAc. The products were analyzed by MALDI-TOF MS.
To determine whether phosphorylation of FGF23 Ser180 directly affects O-glycosylation of FGF23 at Thr178 by GalNAc-T3, we produced a HEK293T cell line stably expressing a secreted form of GalNAc-T3 lacking the first 37 residues encompassing the transmembrane domain and immunopurified the enzyme from conditioned medium (sGalNAc-T3; residues 38–633; Fig. S4). We then performed in vitro glycosylation experiments with sGalNAc-T3 and a peptide substrate surrounding the SPC cleavage site of FGF23 [residues 172–190 of human FGF23; hereafter referred to as FGF23(172–190)]. MALDI-TOF MS analysis of the reaction products demonstrated that sGalNAc-T3 catalyzed the transfer of GalNAc from UDP-GalNAc to Thr178 of FGF23(172–190) as expected (Fig. 3B and Figs. S5A and S6). However, when sGalNAc-T3 was assayed against Ser180-phosphorylated FGF23(172–190), no detectable glycosylation was observed upon extended incubation (Fig. 3C and Fig. S5B). Collectively, our data support that Fam20C phosphorylates FGF23 at Ser180 and inhibits O-glycosylation by GalNAc-T3 at Thr178. Importantly, these results provide a molecular mechanism that could account for the increase in serum full-length FGF23 in Fam20C KO mice and in humans with FAM20C mutations, namely reduced Fam20C phosphorylation allowing O-glycosylation and stabilization of FGF23.
Phosphorylated FGF23 Is Cleaved by the SPC Furin.
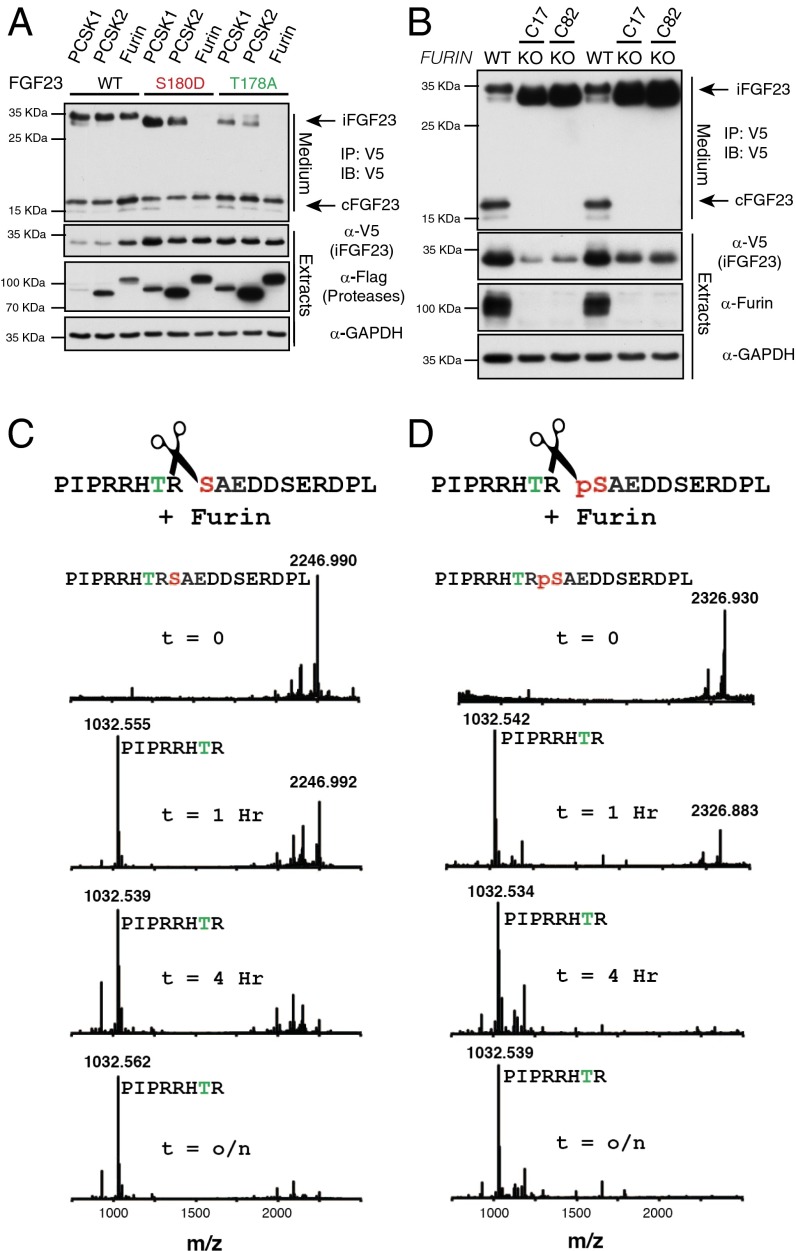
The importance of FGF23 cleavage is underscored in patients with ADHR who have mutations that substitute the Arg residues within the SPC cleavage site and render the protein resistant to proteolysis (Fig. 1A) (24, 25). As our above results support, phosphorylation of FGF23 at Ser180 inhibits O-glycosylation and would therefore promote hormone proteolysis and thus inactivation. The specific protease that inactivates FGF23 has yet to be conclusively identified, but is likely a member of the SPC family based upon the RXXR sequence comprising the cleavage site. With respect to our model, the protease must cleave FGF23 between Arg179 and a phosphorylated Ser180 (176RHTR↓pSAE182). To explore the proteolytic processing of phosphorylated FGF23, we coexpressed Flag-tagged PCSK1, PCSK2, and PCSK3 (furin) with V5-tagged FGF23 in U2OS cells. Coexpression of the proteases and FGF23 had no detectable effect on the relative levels of full-length secreted WT FGF23 (Fig. 4A). However, when FGF23 S180D (a phosphomimetic residue) or T178A (an O-glycosylation–defective mutant) was coexpressed with the proteases, the SPC furin completely abolished the iFGF23 but did not concurrently increase the levels of the C-terminal fragments (Fig. 4A). We reasoned that this could potentially be due to the presence of additional furin-specific RXXR recognition motifs within the C terminus of FGF23 that are cleaved by overexpressed furin. Therefore, to resolve this experimental possibility, we deleted furin in U2OS cells using CRISPR/Cas9 genome editing (Fig. S7). When WT FGF23 was expressed in furin KO cells, only intact FGF23 was detected, suggesting that furin cleaves FGF23 (Fig. 4B). Moreover, we analyzed the ability of recombinant furin to cleave the Ser180-phosphorylated FGF23(172–190) peptide by MALDI-TOF MS. Incubation of the nonphosphorylated or phosphorylated peptide with furin resulted in the time-dependent cleavage of both substrates (Fig. 4 C and D and Fig. S8). Thus, phosphorylation of FGF23 at Ser180 prevents Thr178 O-glycosylation by GalNAc-T3, which then allows furin-dependent proteolysis.
Fig. 4.
Furin cleaves phosphorylated FGF23. (A) Protein immunoblotting of V5-immunoprecipitates from the conditioned medium of U2OS cells expressing V5-tagged FGF23 or mutants (S180D and T178A) with FLAG-tagged PCSK1, PCSK2, or PCSK3 (furin) (Upper). Extracts were analyzed for PCSK1–3, FGF23, and GAPDH expression (Lower). (B) Protein immunoblotting of V5-immunoprecipitates from the conditioned medium of control or FURIN KO U2OS cells (C17, clone 17; C82, clone 82) (Upper). Extracts were analyzed for FGF23 and GAPDH expression (Lower). (C and D) Time-dependent cleavage of FGF23(172–190) (C) or Ser180-phosphorylated FGF23(172–190) (D). The products were analyzed by MALDI-TOF MS.
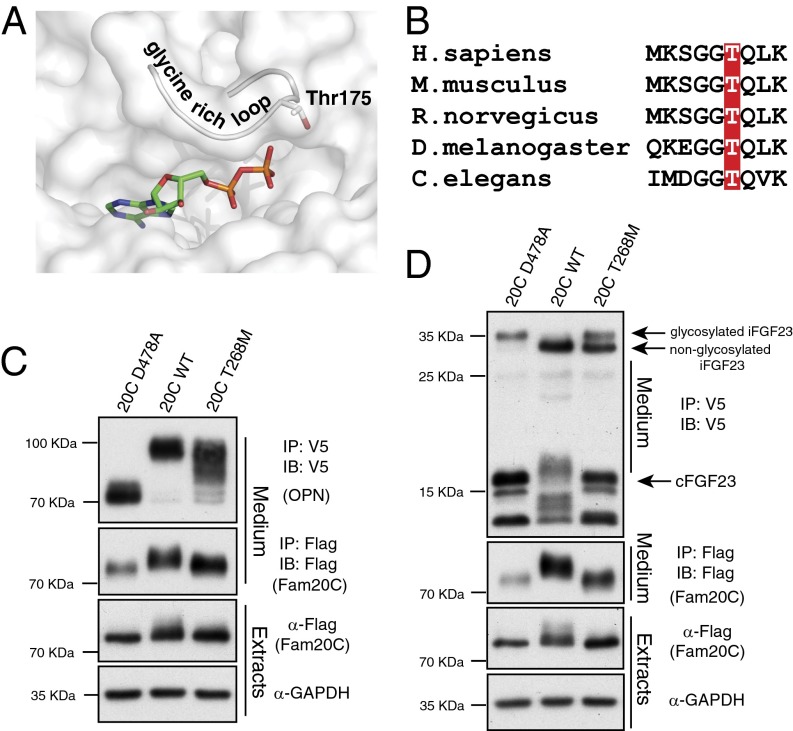
Incomplete Inhibition of FGF23 O-Glycosylation by Fam20C T268M.
Raine syndrome was originally described in 1989 as an aggressive, neonatal osteosclerotic bone dysplasia that results in death within the first few weeks of life (11). Subsequently, mutations in the FAM20C gene were found to be responsible for this disorder (10), and recent reports suggest a broader phenotypic spectrum for individuals with FAM20C mutations because some patients survive infancy (12, 34). Notably, Rafaelsen et al. (12) identified a novel missense mutation in Fam20C that substituted Thr268 with a Met in two compound heterozygous brothers who had elevated serum intact FGF23 and hypophosphatemia. Threonine 268 (Thr175 in Caenorhabditis elegans) is highly conserved within the Fam20C subfamily, and the C. elegans Fam20 crystal structure revealed that this Thr is located in the glycine-rich loop (β1-β2), a region important for nucleotide binding (35) (Fig. 5 A and B). To test the impact of this mutation on FGF23 phosphorylation and O-glycosylation, we generated Flag-tagged Fam20C with Thr268 mutated to Met (Fam20C T268M) and analyzed its activity as well as the ability to be secreted from U2OS cells. In contrast to other nonlethal Fam20C mutations (3), substitution of Thr268 with Met did not affect Fam20C secretion (Fig. 5 C and D). Fam20C T268M phosphorylated OPN and FGF23 much less efficiently than WT Fam20C, as judged by its ability to induce a mobility change in OPN (Fig. 5C) and the secreted, C-terminal fragment of FGF23 (Fig. 5D). Importantly, expression of Fam20C T268M did not completely prevent O-glycosylation of intact FGF23, whereas WT Fam20C achieved complete inhibition of FGF23 O-glycosylation (Fig. 5D). Taken together, these findings support that the elevated intact FGF23 and hypophosphatemia observed in patients with the Fam20C T268M mutation are a result of the inability of Fam20C to efficiently phosphorylate FGF23 to inhibit O-glycosylation. As a consequence, elevated biologically active, O-linked glycosylated FGF23 is secreted, ultimately resulting in hypophosphatemia.
Fig. 5.
Deficient inhibition of FGF23 O-glycosylation by Fam20C T268M. (A) Structural representation of the active site of Fam20 from C. elegans highlighting the position of Thr175 (human Thr268). The glycine-rich loop and nucleotide (ADP) are shown. (B) Sequence alignment of Fam20C from human (Homo sapiens), mouse (Mus musculus), rat (Rattus norvegicus), fly (Drosophila melanogaster), and worm (Caenorhabditis elegans) depicting the conservation of the Thr. (C and D) Protein immunoblotting of V5 and Flag-immunoprecipitates from conditioned medium of U2OS cells expressing V5-tagged OPN (C) or FGF23 (D) with Flag-tagged D478A (20C D478A), WT (20C WT), or mutant Fam20C found in patients with FGF23-related hypophosphatemia (20C T268M) (Upper). Extracts were analyzed for Fam20C and GAPDH expression (Lower).
Discussion
The results of this study expand our understanding of the complex control of mammalian phosphate homeostasis. Our model for the regulation of FGF23 envisions a highly dynamic interplay between O-glycosylation by GalNAc-T3 (or other members of the GalNAc-transferase family) and phosphorylation by Fam20C as a means by which to balance the processing of FGF23 as intact, biologically active protein (glycosylated > phosphorylated) or N- and C-terminal fragments (phosphorylated > glycosylated) (Fig. S9). This model is consistent with the marked elevation of intact, biologically active FGF23 observed in humans with the Fam20C T268M mutation (12) and in Fam20C KO mice (13, 14).
Sensing the need to adapt phosphate balance in vivo is a complex process, and our data support that the production of bioactive FGF23 will depend upon, in addition to the levels of FGF23 transcription, the relative expression and activities of osteoblast/osteocyte Fam20C, GalNAc-T3, and furin. The mechanisms by which Fam20C is functionally or expressionally regulated are unknown. Recent studies examining the production of intact and/or C-terminal fragments of FGF23 in fibrous dysplasia and during physiological situations of low iron, such as in ADHR (36, 37), support that GalNAc-T3 and furin are likely controlled by multiple factors including 3′,5′-cyclic adenosine monophosphate (38), iron or iron deficiency (39), and inorganic phosphate (40).
Site-specific O-glycosylation within, or neighboring, SPC sites is emerging as an important regulatory mechanism to control SPC-dependent processing of secreted proteins (41). Our results are in accord with the concept that phosphorylation of hormones may promote proteolysis by SPCs (or other proteases) by interfering with O-glycosylation by members of the GalNAc-transferase family. Our observation of competition between phosphorylation and O-glycosylation in the secretory pathway reflects prior observations on the cross-talk between phosphorylation and O-glycosylation by O-linked N-acetylglucosamine (O-GlcNAc) transferase, which transfers GlcNAc from UDP-GlcNAc to Ser and Thr residues in nuclear and cytosolic proteins (42). Interplay between O-glycosylation and phosphorylation of the tumor suppressor p53 has been shown to coordinately regulate p53 stability and activity (43). Analogous to what we observed in this work, phosphorylation of p53 promotes its degradation by the ubiquitin system by inhibiting O-GlcNAcylation. Currently, it is unknown whether the interplay between O-glycosylation by members of the GalNAc-transferase family and phosphorylation by secretory pathway kinases is a common mechanism within the secretory pathway or whether FGF23 is a unique example. However, it is worth noting that bone morphogenetic protein 15 (BMP15), a secreted maternal hormone essential for mammalian reproduction, is phosphorylated by Fam20C on an S-x-E motif (44). This phosphorylation site (Ser6) is located close to an O-glycosylated Thr (Thr10). When BMP15 is expressed in HEK293 cells, phosphorylation and O-glycosylation appear to be mutually exclusive (45).
The physiological scenario for the regulated control of circulating FGF23 is likely far more complicated than direct interactions between FGF23, Fam20C, GalNAc-T3, and furin. It is possible that Fam20C regulates FGF23 not only through direct mechanisms, as our results suggest, but also through emerging, indirect pathways. In this regard, we have shown that DMP1, a highly phosphorylated extracellular matrix protein critical for proper mineralization of bone (46), is a substrate for Fam20C (3, 4). Inactivating mutations in DMP1 result in autosomal recessive hypophosphatemic rickets, and Dmp1 KO mice share many phenotypic similarities with those of Fam20C-deficient animals, including elevated FGF23 (13, 22). DMP1 appears to regulate FGF23 expression indirectly, as loss of Dmp1 in vivo impairs the maturation of osteoblasts to osteocytes through unknown mechanisms and results in highly elevated FGF23 mRNA as well as circulating protein (22). Thus, loss of DMP1 phosphorylation in a state of Fam20C deficiency could also contribute to an increase in FGF23 in vivo. Certainly additional studies will be required to understand these new interactions.
In addition to Ser180, three Fam20C consensus S-x-E/pS sites are present in the C-terminal fragment of FGF23 (residues 180–250). We were unable to detect FGF23 peptides surrounding the predicted sites by MS, and therefore at this time we cannot rule out Fam20C-dependent phosphorylation of these residues. By quantitating the incorporated radioactivity in Fig. 1D, we calculated a stoichiometry of about 1.5–2 mol of phosphate per mol FGF23 R176Q, suggesting that other sites may be phosphorylated.
In conclusion, we have demonstrated that Fam20C regulates FGF23 by phosphorylation of Ser180. Phosphorylation of FGF23 inhibits GalNAc-T3 O-glycosylation, which then allows proteolysis by furin. Collectively, our results provide a mechanism by which loss of Fam20C leads to aberrations in phosphate homeostasis due to a cellular shift toward increased O-glycosylation and thus elevated levels of intact, biologically active FGF23 and, conversely, increased Fam20C activity shifts the FGF23 processing balance toward increased furin proteolysis and hormone inactivation. Furthermore, our data suggest that interplay between phosphorylation and O-glycosylation of proteins in the secretory pathway may be a critical posttranslational mechanism by which secreted proteins are regulated.
Methods
Protein Purification.
Flag-tagged Fam20C and FGF23 R176Q were immunopurified from conditioned medium of HEK293T cells as previously described (3). More details on protein purification are presented in SI Methods.
Generation of Anti-Fam20C Antibody.
Polyclonal antisera were raised in rabbits against recombinant Flag-tagged Fam20C produced in HEK293T cells (Cocalico Biologicals). More details on antibody generation are presented in SI Methods.
Supplementary Material
Acknowledgments
We thank Carolyn A. Worby, Gregory S. Taylor, Jenna L. Jewell, and members of the J.E.D. laboratory for insightful discussions and comments regarding the manuscript. We thank David King of the Howard Hughes Medical Institute’s Mass Spectrometry Laboratory (University of California, Berkeley) for peptide synthesis and Eric Durrant for technical assistance. This work was supported in part by National Institutes of Health Grants DK018849-36 and DK018024-37 (to J.E.D.), DK63934 (to K.E.W.), and K99DK099254 (to V.S.T.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1402218111/-/DCSupplemental.
References
- 1.Cohen P. The origins of protein phosphorylation. Nat Cell Biol. 2002;4(5):E127–E130. doi: 10.1038/ncb0502-e127. [DOI] [PubMed] [Google Scholar]
- 2.Fischer EH. Cellular regulation by protein phosphorylation. Biochem Biophys Res Commun. 2013;430(2):865–867. doi: 10.1016/j.bbrc.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 3.Tagliabracci VS, et al. Secreted kinase phosphorylates extracellular proteins that regulate biomineralization. Science. 2012;336(6085):1150–1153. doi: 10.1126/science.1217817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishikawa HO, Xu A, Ogura E, Manning G, Irvine KD. The Raine syndrome protein FAM20C is a Golgi kinase that phosphorylates bio-mineralization proteins. PLoS ONE. 2012;7(8):e42988. doi: 10.1371/journal.pone.0042988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tagliabracci VS, Pinna LA, Dixon JE. Secreted protein kinases. Trends Biochem Sci. 2013;38(3):121–130. doi: 10.1016/j.tibs.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tagliabracci VS, Xiao J, Dixon JE. Phosphorylation of substrates destined for secretion by the Fam20 kinases. Biochem Soc Trans. 2013;41(4):1061–1065. doi: 10.1042/BST20130059. [DOI] [PubMed] [Google Scholar]
- 7.Salvi M, Cesaro L, Tibaldi E, Pinna LA. Motif analysis of phosphosites discloses a potential prominent role of the Golgi casein kinase (GCK) in the generation of human plasma phospho-proteome. J Proteome Res. 2010;9(6):3335–3338. doi: 10.1021/pr100058r. [DOI] [PubMed] [Google Scholar]
- 8.Zhou W, et al. An initial characterization of the serum phosphoproteome. J Proteome Res. 2009;8(12):5523–5531. doi: 10.1021/pr900603n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bahl JM, Jensen SS, Larsen MR, Heegaard NH. Characterization of the human cerebrospinal fluid phosphoproteome by titanium dioxide affinity chromatography and mass spectrometry. Anal Chem. 2008;80(16):6308–6316. doi: 10.1021/ac800835y. [DOI] [PubMed] [Google Scholar]
- 10.Simpson MA, et al. Mutations in FAM20C are associated with lethal osteosclerotic bone dysplasia (Raine syndrome), highlighting a crucial molecule in bone development. Am J Hum Genet. 2007;81(5):906–912. doi: 10.1086/522240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raine J, Winter RM, Davey A, Tucker SM. Unknown syndrome: Microcephaly, hypoplastic nose, exophthalmos, gum hyperplasia, cleft palate, low set ears, and osteosclerosis. J Med Genet. 1989;26(12):786–788. doi: 10.1136/jmg.26.12.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rafaelsen SH, et al. Exome sequencing reveals FAM20c mutations associated with FGF23-related hypophosphatemia, dental anomalies and ectopic calcification. J Bone Miner Res. 2013;28(6):1378–1385. doi: 10.1002/jbmr.1850. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, et al. Inactivation of a novel FGF23 regulator, FAM20C, leads to hypophosphatemic rickets in mice. PLoS Genet. 2012;8(5):e1002708. doi: 10.1371/journal.pgen.1002708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogel P, et al. Amelogenesis imperfecta and other biomineralization defects in Fam20a and Fam20c null mice. Vet Pathol. 2012;49(6):998–1017. doi: 10.1177/0300985812453177. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharyya N, Chong WH, Gafni RI, Collins MT. Fibroblast growth factor 23: State of the field and future directions. Trends Endocrinol Metab. 2012;23(12):610–618. doi: 10.1016/j.tem.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrow EG, White KE. Recent advances in renal phosphate handling. Nat Rev Nephrol. 2010;6(4):207–217. doi: 10.1038/nrneph.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurosu H, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281(10):6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carpenter TO. The expanding family of hypophosphatemic syndromes. J Bone Miner Metab. 2012;30(1):1–9. doi: 10.1007/s00774-011-0340-2. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, et al. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab. 2006;291(1):E38–E49. doi: 10.1152/ajpendo.00008.2006. [DOI] [PubMed] [Google Scholar]
- 20.Consortium TH. The HYP Consortium A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet. 1995;11(2):130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- 21.Lorenz-Depiereux B, Schnabel D, Tiosano D, Häusler G, Strom TM. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am J Hum Genet. 2010;86(2):267–272. doi: 10.1016/j.ajhg.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng JQ, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38(11):1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergwitz C, Jüppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med. 2010;61:91–104. doi: 10.1146/annurev.med.051308.111339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White KE, et al. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26(3):345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 25.White KE, et al. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60(6):2079–2086. doi: 10.1046/j.1523-1755.2001.00064.x. [DOI] [PubMed] [Google Scholar]
- 26.Benet-Pagès A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet. 2005;14(3):385–390. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- 27.Topaz O, et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet. 2004;36(6):579–581. doi: 10.1038/ng1358. [DOI] [PubMed] [Google Scholar]
- 28.Kato K, et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem. 2006;281(27):18370–18377. doi: 10.1074/jbc.M602469200. [DOI] [PubMed] [Google Scholar]
- 29.Bergwitz C, et al. Defective O-glycosylation due to a novel homozygous S129P mutation is associated with lack of fibroblast growth factor 23 secretion and tumoral calcinosis. J Clin Endocrinol Metab. 2009;94(11):4267–4274. doi: 10.1210/jc.2009-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, et al. Expression of FAM20C in the osteogenesis and odontogenesis of mouse. J Histochem Cytochem. 2010;58(11):957–967. doi: 10.1369/jhc.2010.956565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cong L, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jinek M, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gill DJ, Clausen H, Bard F. Location, location, location: New insights into O-GalNAc protein glycosylation. Trends Cell Biol. 2011;21(3):149–158. doi: 10.1016/j.tcb.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Simpson MA, et al. Mutations in FAM20C also identified in non-lethal osteosclerotic bone dysplasia. Clin Genet. 2009;75(3):271–276. doi: 10.1111/j.1399-0004.2008.01118.x. [DOI] [PubMed] [Google Scholar]
- 35.Xiao J, Tagliabracci VS, Wen J, Kim SA, Dixon JE. Crystal structure of the Golgi casein kinase. Proc Natl Acad Sci USA. 2013;110(26):10574–10579. doi: 10.1073/pnas.1309211110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farrow EG, et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci USA. 2011;108(46):E1146–E1155. doi: 10.1073/pnas.1110905108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imel EA, et al. Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J Clin Endocrinol Metab. 2011;96(11):3541–3549. doi: 10.1210/jc.2011-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhattacharyya N, et al. Mechanism of FGF23 processing in fibrous dysplasia. J Bone Miner Res. 2012;27(5):1132–1141. doi: 10.1002/jbmr.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knutson MD. Iron-sensing proteins that regulate hepcidin and enteric iron absorption. Annu Rev Nutr. 2010;30:149–171. doi: 10.1146/annurev.nutr.012809.104801. [DOI] [PubMed] [Google Scholar]
- 40.Chefetz I, et al. GALNT3, a gene associated with hyperphosphatemic familial tumoral calcinosis, is transcriptionally regulated by extracellular phosphate and modulates matrix metalloproteinase activity. Biochim Biophys Acta. 2009;1792(1):61–67. doi: 10.1016/j.bbadis.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schjoldager KT, Clausen H. Site-specific protein O-glycosylation modulates proprotein processing—Deciphering specific functions of the large polypeptide GalNAc-transferase gene family. Biochim Biophys Acta. 2012;1820(12):2079–2094. doi: 10.1016/j.bbagen.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 42.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: Roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang WH, et al. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol. 2006;8(10):1074–1083. doi: 10.1038/ncb1470. [DOI] [PubMed] [Google Scholar]
- 44.Tibaldi E, et al. Golgi apparatus casein kinase phosphorylates bioactive Ser-6 of bone morphogenetic protein 15 and growth and differentiation factor 9. FEBS Lett. 2010;584(4):801–805. doi: 10.1016/j.febslet.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saito S, Yano K, Sharma S, McMahon HE, Shimasaki S. Characterization of the post-translational modification of recombinant human BMP-15 mature protein. Protein Sci. 2008;17(2):362–370. doi: 10.1110/ps.073232608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.George A, Veis A. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem Rev. 2008;108(11):4670–4693. doi: 10.1021/cr0782729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.