Significance
The 2010 Deepwater Horizon (MC252) disaster in the northern Gulf of Mexico released more than 4 million barrels of crude oil. Oil rose from the ocean floor to the surface where many large pelagic fish spawn. Here we describe the impacts of field-collected oil samples on the rapidly developing embryos of warm-water predators, including bluefin and yellowfin tunas and an amberjack. For each species, environmentally relevant MC252 oil exposures caused serious defects in heart development. Moreover, abnormalities in cardiac function were highly consistent, indicating a broadly conserved developmental crude oil cardiotoxicity. Losses of early life stages were therefore likely for Gulf populations of tunas, amberjack, swordfish, billfish, and other large predators that spawned in oiled surface habitats.
Keywords: oil spill, damage assessment, heart development, embryology
Abstract
The Deepwater Horizon disaster released more than 636 million L of crude oil into the northern Gulf of Mexico. The spill oiled upper surface water spawning habitats for many commercially and ecologically important pelagic fish species. Consequently, the developing spawn (embryos and larvae) of tunas, swordfish, and other large predators were potentially exposed to crude oil-derived polycyclic aromatic hydrocarbons (PAHs). Fish embryos are generally very sensitive to PAH-induced cardiotoxicity, and adverse changes in heart physiology and morphology can cause both acute and delayed mortality. Cardiac function is particularly important for fast-swimming pelagic predators with high aerobic demand. Offspring for these species develop rapidly at relatively high temperatures, and their vulnerability to crude oil toxicity is unknown. We assessed the impacts of field-collected Deepwater Horizon (MC252) oil samples on embryos of three pelagic fish: bluefin tuna, yellowfin tuna, and an amberjack. We show that environmentally realistic exposures (1–15 µg/L total PAH) cause specific dose-dependent defects in cardiac function in all three species, with circulatory disruption culminating in pericardial edema and other secondary malformations. Each species displayed an irregular atrial arrhythmia following oil exposure, indicating a highly conserved response to oil toxicity. A considerable portion of Gulf water samples collected during the spill had PAH concentrations exceeding toxicity thresholds observed here, indicating the potential for losses of pelagic fish larvae. Vulnerability assessments in other ocean habitats, including the Arctic, should focus on the developing heart of resident fish species as an exceptionally sensitive and consistent indicator of crude oil impacts.
The Deepwater Horizon disaster resulted in the release of more than 4 million barrels (636 million L) of oil into the offshore waters of the northern Gulf of Mexico between April 10 and July 14, 2010 (1). Although subsurface application of dispersant near the wellhead resulted in retention of a considerable portion of oil in the bathypelagic zone (2), oil also traveled to the upper surface waters where it formed a large and dynamic patchwork of slicks (e.g., covering an estimated 17,725 km2 during May 2010) (3). In the decades following the last major US oil spill (the 1989 Exxon Valdez spill in Alaska), developing fish embryos have been shown to be especially vulnerable to the toxicity of crude oil (4). The northern Gulf provides critical spawning and rearing habitats for a range of commercially and ecologically important pelagic fish species, and the timing of oil release into the ecosystem from the damaged Deepwater Horizon/MC252 well coincided with the temporal spawning window for bluefin and yellowfin tunas, mahi mahi, king and Spanish mackerels, greater and lesser amberjack, sailfish, blue marlin, and cobia (5–13). Yellowfin tuna (Thunnus albacares) and greater amberjack (Seriola dumerili) contribute to important commercial fisheries (48,960,000 pounds in 2010 and 4,348,000 pounds in 2004, respectively) (14, 15). The Atlantic bluefin tuna (Thunnus thynnus) population from the Gulf of Mexico is currently at a historically low level (16), and was recently petitioned for listing under the US Endangered Species Act. For these and other pelagics, the extent of early-life stage loss from oiled spawning habitats is an important outstanding question for fisheries management and conservation.
The developing fish heart is known as a sensitive target organ for the toxic effects of crude oil-derived polycyclic aromatic hydrocarbons (PAHs) (4). Of the multiple two- to six-ringed PAH families contained in crude oil, the most abundant three-ringed compounds are sufficient to drive the cardiotoxicity of petroleum-derived PAH mixtures. These compounds (fluorenes, dibenzothiophenes, and phenanthrenes) directly disrupt fish cardiac function (17, 18), thereby interfering with the interdependent processes of circulation and heart chamber formation. Exposure of fish embryos to PAH mixtures derived from crude oil slows the heartbeat (bradycardia) and reduces contractility (17, 19–21). The underlying mechanism was recently shown to be blockade of key potassium and calcium ion channels involved in cardiac excitation-contraction coupling (22). These collective effects of PAHs during embryonic and larval stages can influence the structure and function of the adult fish heart in ways that permanently reduce cardiac performance (23), potentially leading to delayed mortality. Consistent with this, mark-recapture studies on pink salmon following the Exxon Valdez spill found that transient and sublethal exposures to crude oil at very low levels during embryogenesis reduced subsequent marine survival to adulthood by 40% (24, 25). Exposures to relatively higher PAH concentrations cause embryonic heart failure and death soon after fish hatch into free-swimming larvae (19, 20, 23). These effects occur at a total PAH concentration range as low as 1–10 µg/L for more sensitive species (26, 27), levels as much as an order-of-magnitude lower than those measured in some samples collected both at depth and at the surface during the Deepwater Horizon active spill phase (28, 29).
The above crude oil cardiotoxicity syndrome has been extensively characterized in zebrafish embryos exposed to several geologically distinct oils (17, 21, 23, 30, 31), including the Mississippi Canyon 252 (MC252) crude oil released from the blown out Deepwater Horizon wellhead (20, 32). Similar effects have been reported for temperate marine and anadromous species, such as Pacific herring (19, 26, 27, 33) and pink salmon (34, 35), following exposure to Alaska North Slope crude oil. Although zebrafish are a tropical freshwater model species, the embryos of herring and salmon assessed in the aftermath of the Exxon Valdez spill develop at cold temperatures (4–12 °C) over relatively long intervals (weeks to months). In contrast, pelagic species spawning in the warm surface waters of the northern Gulf of Mexico (e.g., 24–29 °C) develop rapidly (24–48 h to hatch) (36, 37). The influence of development duration on PAH uptake and toxicity, if any, is not well understood. The higher temperatures characteristic of waters in the Gulf of Mexico may also influence how the chemical composition of crude oil in surface habitat(s) changes over time (i.e., weathers). Processes that determine weathering are generally accelerated at higher temperatures, potentially influencing the fraction of cardiotoxic PAHs that is bioavailable for uptake by floating fish embryos in the mixed layer and thermocline regions. To address these information gaps, controlled laboratory exposures are necessary to determine the sensitivity of Gulf species to Deepwater Horizon crude oil.
To assess potential early life-stage losses from large pelagic predator populations that were actively spawning in habitats affected by the Deepwater Horizon spill, we determined the effects of field-collected MC252 oil samples on the development of embryos from representative warm water open-ocean fish species. Our approach extended earlier work in zebrafish, a laboratory model species and Pacific herring, a marine nearshore spawner (19, 27, 38). Zebrafish and herring both produce large demersal embryos that are relatively easy to manipulate (i.e., collect, dechorionate, and image at consistent ontogenetic intervals). In contrast, Gulf pelagic species produce small, fragile, buoyant embryos that develop relatively rapidly (on a timescale of hours relative to days or weeks for zebrafish and herring, respectively) and are not amenable to dechorionation. Moreover, the embryos hatch into buoyant larvae. Normally present in infinite-volume pelagic habitats, they are very sensitive to any form of physical contact, thereby complicating conventional embryology in small-volume laboratory cultures. Finally, access to embryos is difficult, with only a few land-based facilities capable of maintaining spawning broodstocks throughout the world.
In the present study we overcome the aforementioned challenges for focal pelagic species that included yellowfin tuna, Southern bluefin tuna (Thunnus maccoyii), and yellowtail amberjack (or kingfish, Seriola lalandi). The yellowfin tuna are the same species that spawn in the Gulf of Mexico, and the other two species are closely related congenerics to T. thynnus and S. dumerili, respectively. Controlled bluefin tuna spawning is exceptionally difficult to achieve in a husbandry facility, and we used the only land-based captive broodstock available in the world for experiments. Similarly, we relied on a commercial broodstock of yellowtail amberjack and a research broodstock of yellowfin tuna, the latter the only worldwide source of fertilized embryos for this species. Embryos were exposed to high-energy water-accommodated fractions (HEWAFs) (20) that generated PAH concentrations and compositional profiles closely matching water samples collected during active MC252 crude oil release phase.
Results
PAH Concentrations and Composition in HEWAF Preparations.
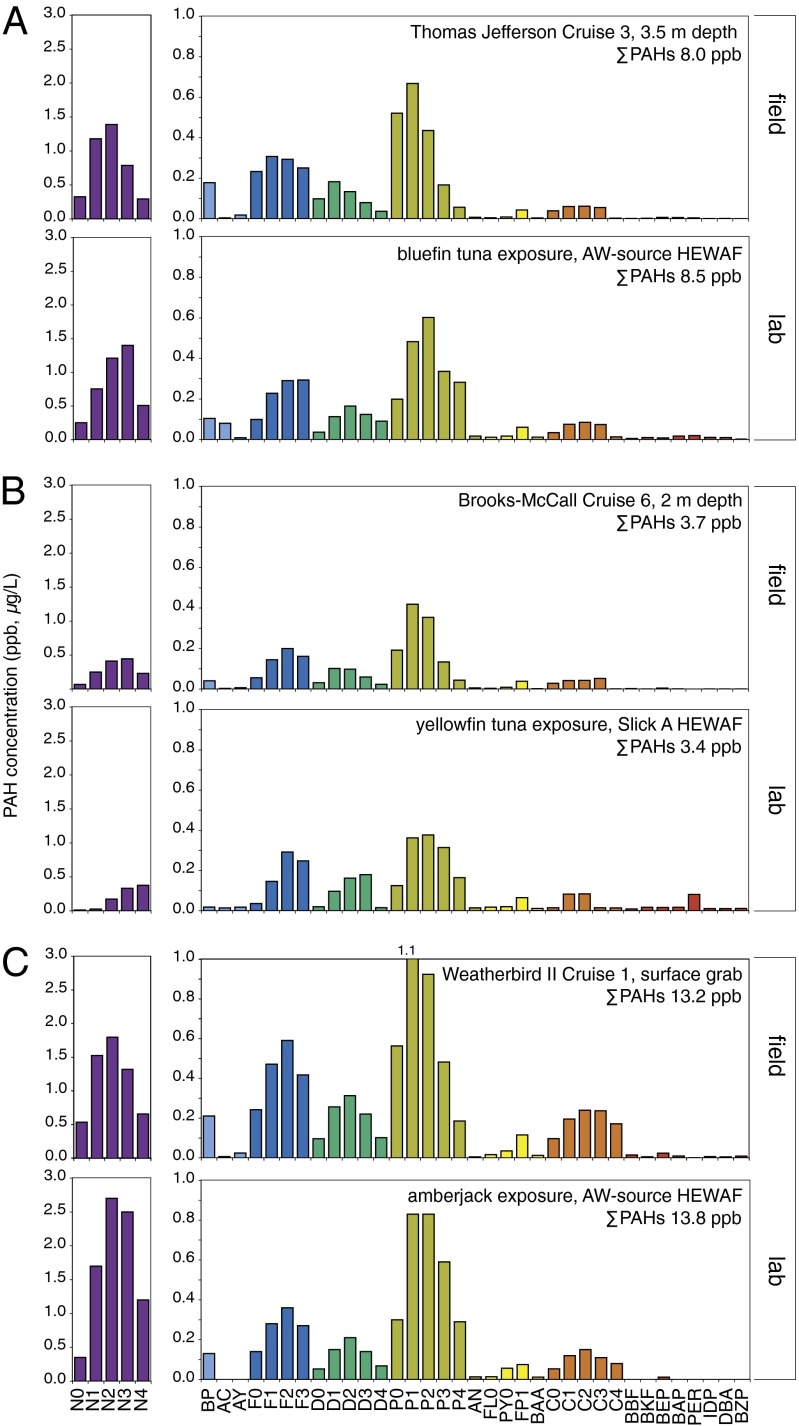
Embryos were exposed to two different oil samples, one collected from surface-skimming operations in the open Gulf of Mexico (slick A) and one taken from the source pipe attached to the damaged well head that was subsequently weathered artificially (Methods) to remove most of the volatile monoaromatic compounds (artificially weathered source oil; AW source). The two samples were weathered to different degrees, as reflected primarily in the relative ratios of naphthalenes to tricyclic PAHs and four-ringed chrysenes (Fig. S1). Relative to unweathered fresh-source oil (Fig. S1A), the AW-source oil showed an increase in C2–C4 naphthalenes and C1–C4 phenanthrenes (Fig. S1B). The slick A sample collected from the Gulf surface waters was considerably more weathered, with ∼90% depletion of all naphthalenes per unit mass and ∼50% depletion of tricyclic PAHs (Fig. S1C). However, the tricyclic PAHs represented 65% of the total ΣPAHs of slick A but only 34% of ΣPAHs in fresh source oil.
Each of the three species tested was exposed to independently generated HEWAFs. PAHs in HEWAFs were measured at the onset of exposure only for tuna embryos, and at both the beginning and end of exposure for amberjack embryos (Fig. 1 and Fig. S2). HEWAFs were unfiltered, and measurements therefore included PAHs that were both dissolved and droplet-associated. HEWAFs used in laboratory exposures for each species closely matched the PAH composition and concentrations measured in representative water samples collected from the upper pelagic zone during the active spill phase, with ΣPAH concentrations in the range of 3–14 µg/L at the highest tested oil load (Fig. 1; measured PAHs are provided in Table S1). HEWAFs used for bluefin (Fig. 1A) and yellowfin tuna (Fig. 1B) exposures were similar to water samples collected in the Gulf of Mexico in late and early June 2010, respectively, and the amberjack exposure closely matched a representative sample from late May 2010 (Fig. 1C). Notably, C1-phenanthrenes are one of the most abundant three-ringed PAH classes in MC252 crude oil samples (Fig. S1). Across all tests, the slick A HEWAF exposure to yellowfin tuna had the lowest concentration of C1-phenanthrenes (0.36 µg/L at the highest tested HEWAF dilution) (Fig. 1B). Among all of the water samples collected within and external to the spill zone (between April 28 and July 30, 2010), 2.3% (61 of 2,647) contained C1-phenanthrene concentrations above 0.36 µg/L. Levels in many samples were considerably higher, with mean (± SEM) and median C1-phenanthrene concentrations of 1.76 ± 0.64 (n = 61) and 0.55 µg/L, respectively. For samples collected in the pelagic zone of the northern Gulf of Mexico (above 200-m depth, an area bounded by latitude 29.6–27.25°, longitude –90.6 to –87.09; a rectangular area of roughly 96,000 km2 centered over the wellhead, extending offshore from 73 to 333 km), a larger fraction (4.6%, 25 of 548) had concentrations of C1-phenanthrenes ≥ 0.36 µg/L.
Fig. 1.
HEWAF preparations produced environmentally relevant PAH exposures. PAH profiles in HEWAFs used in pelagic embryo exposures are compared with water samples collected during the active spill phase of the Deepwater Horizon/MC252 incident. Water samples shown are representative of 78 samples collected during May–July 2010 that had comparable PAH compositions to HEWAFs, 44 of which had ΣPAH levels that exceeded the highest concentrations tested in embryo exposures. These data were generated as part of the NRDA sampling program carried out by the Deepwater Horizon oil spill trustee team, and are publicly available (Methods). (A) Field sample from late June 2010 (Upper) and the artificially weathered source oil HEWAF used in a bluefin tuna test (Lower). (B) Field sample from early June 2010 (Upper) and the slick A HEWAF used in a yellowfin tuna test (Lower). (C) Field sample from late May 2010 (Upper) and the artificially weathered source oil HEWAF used in the amberjack test (Lower). Abbreviations for PAHs are listed in Table S1 and all values for individual PAHs are provided in Dataset S1. Focal compounds are naphthalenes (N0–N4, purple), fluorenes (F0–F3, blue), dibenzothiophenes (D0–D4, green), phenanthrenes (P0–P4, olive), and chrysenes (C0–C4, orange).
PAH concentrations declined during each exposure. For example, in the amberjack tests the PAH profile at the start of the exposure generally matched the profile for the whole AW-source oil, albeit with a slightly higher level of the more water-soluble naphthalenes (Fig. S2A). By the end of incubation (Fig. S2B), the naphthalenes, fluorenes, and dibenzothiophenes were mostly lost from the HEWAF, most likely because of volatilization, whereas ∼25% of the phenanthrenes remained.
Normal Cardiac Development in Tunas and Amberjack.
We characterized the timing and general morphological sequence of heart development in unexposed control tuna and amberjack embryos. Heart development was very similar among bluefin and yellowfin tunas and amberjack, both in terms of morphogenetic sequence and timing in relation to developmental stage. There were parallels and contrasts in comparison with the well-described development of the zebrafish heart (39). In all three species, the heart was first visible microscopically in late segmentation-stage embryos (e.g., 24 somites) (Fig. S3B) as a cone that was symmetrical along the anterior-posterior axis with a ventrally located base (Fig. S3D). At the time the first cardiomyocyte contractions were observed, some left-right asymmetry was already apparent as the cardiac cone began to rotate to position the atrial opening on the left side (Fig. S3E). By the free-tail stage (several hours later), the heart showed regular, rapid contractions of both the atrium and ventricle, and was rotated nearly 90° so that the lumen of the atrium was visible in left lateral views (amberjack, Fig. S3 F and G; yellowfin tuna, Fig. S3 J and K). After the hatching stage, the heart elongated along the anterior-posterior axis as the head extended and yolk absorbed, bringing the atrium posterior to the ventricle, but still slightly on the left side (amberjack, Fig. S3 H and I; yellowfin tuna, Fig. S3 L–O). Cardiac development in bluefin tuna was virtually indistinguishable from yellowfin tuna, and was not photographically documented because of a limited availability of embryos and time constraints for sample processing.
Gross Morphological Defects in Response to MC252 Oil Exposure.
Exposures were carried out at temperatures appropriate for broodstock maintenance and routine hatchery rearing for each species (bluefin tuna and amberjack, 25 °C; yellowfin tuna, 27 °C), which generally resulted in high survival rates for unexposed (control) embryos. Bluefin tuna embryos had the lowest control survival at 60 ± 5% (n = 4). The control survival rates for yellowfin tuna and amberjack were 72 ± 9% (n = 6) and 93 ± 3% (n = 4), respectively.
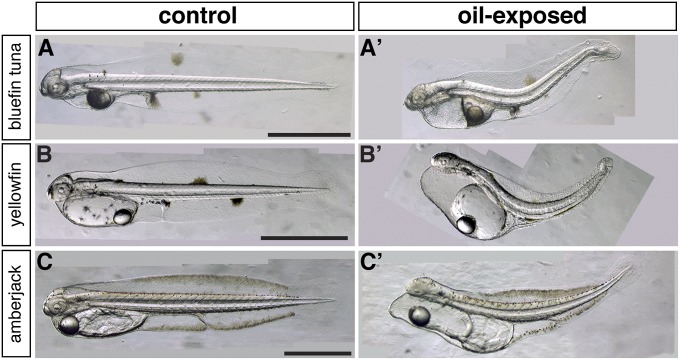
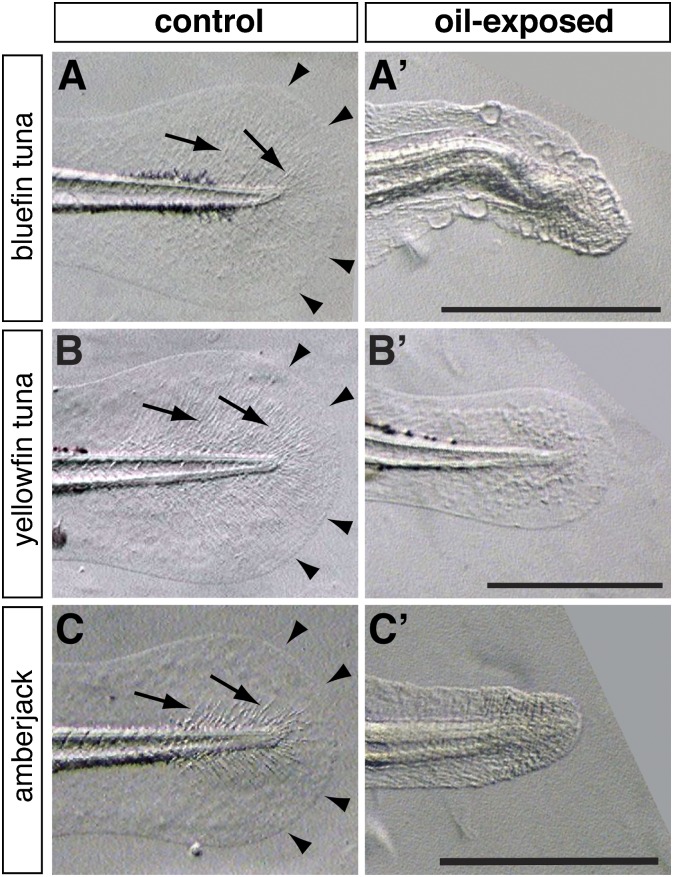
Exposure to each MC252 sample type (source- or surface-collected) produced a virtually identical suite of defects in the pelagic fish embryos (Figs. 2–4) that were consistent with the previously described effects of other crude oils in other fishes, including neritic spawning marine species such as herring. Each pelagic species showed comparable morphological responses, marked by accumulation of pericardial edema, expanding to yolk-sac edema in more severely affected larvae (representative examples shown in Fig. 3). Notably, all three species also showed similar types of extracardiac defects at the highest exposure concentration (8.5, 3.4, and 13.8 µg/L for bluefin, yellowfin, and amberjack, respectively) (Fig. 2). These morphological abnormalities included a lack of actinotrichia (fin ray precursors), reduction in the outgrowth of the finfolds or finfold blisters (Fig. 4), a dorsal or upward curvature of the body axis, and marked reduction in the growth of the eye (Fig. 3). Bluefin tuna showed the highest percentage of larvae with the entire suite of defects at a ΣPAH level of 8.5 µg/L (73%) (Table 1). In the pair of assays conducted with yellowfin tuna embryos, one assay included the addition of the broad-spectrum antibiotic oxytetracycline (10 mg/L) to inhibit potential bacterial growth. The presence or absence of oxytetracycline in the exposure water (see, for example, Fig. S5) did not influence the morphological effects of MC252 crude oil (see below). Similar effects on finfold outgrowth and a dorsal flexion of the body axis were also observed in yellowfin tuna, although the lower exposure level (ΣPAH 3.4 µg/L) resulted in a lower frequency of this suite of defects (15.6%) (Table 1).
Fig. 2.
Gross morphology of hatching stage larvae exposed to MC252 HEWAFs oil during embryonic development. Embryos were exposed from shortly after fertilization to 12–16 h after hatching. Unexposed controls incubated in clean water are shown in A–C, and oil-exposed (highest-dose tested) in A′–C′. Representative examples are shown for (A, A′) bluefin tuna exposed to artificially weathered source oil (1 mg/L oil, 8.5 µg/L ΣPAH), (B, B′) yellowfin tuna exposed to slick A (2 mg/L oil, 3.4 µg/L ΣPAH), (C, C′) amberjack exposed to artificially weathered source oil (1 mg/L oil, 13.8 µg/L ΣPAH). (Scale bars, 1 mm.)
Fig. 4.
Caudal finfold defects in oil-exposed larvae. Higher magnification views are shown for bluefin tuna (A, A′), yellowfin tuna (B, B′), and amberjack (C, C′) from the same exposure shown in Fig. 1. A–C show the tails of controls and A′–C′ show the corresponding caudal fins of oil-exposed larvae. Arrowheads indicate the margins of the caudal finfolds in controls, and arrows indicate actinotrichia. The specific oil exposures (source and concentration) correspond to those in Figs. 2 and 3. (Scale bars, 0.5 mm.)
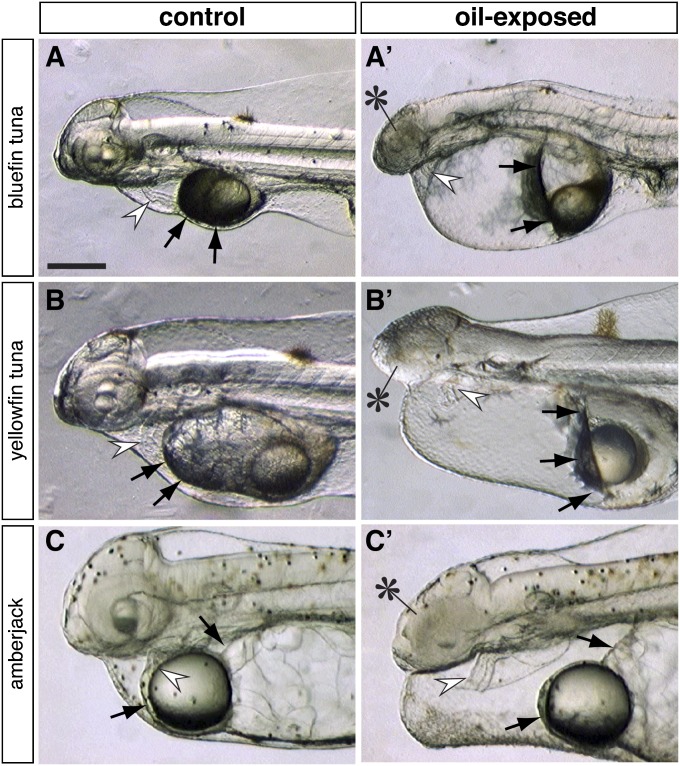
Fig. 3.
Oil-induced circulatory failure and corresponding fluid accumulation (edema) in pelagic fish embryos. Higher magnification views are shown for (A, A′) bluefin tuna exposed to artificially weathered source oil (1 mg/L oil, 8.5 µg/L ΣPAH); (B, B′) yellowfin tuna exposed to slick A (2 mg/L oil, 3.4 µg/L ΣPAH), (C, C′) amberjack exposed to artificially weathered source oil (1 mg/L oil, 13.8 µg/L ΣPAH). Controls are shown in A–C and oil-exposed larvae in A′–C′. Arrows indicate the anterior edges of the yolk masses, which are distorted and forced posteriorly in the oil-exposed larvae; white arrowheads indicate the location of the heart. Reduction of the eyes is indicated in the oil-exposed embryos with asterisks. (Scale bar, 0.2 mm.)
Table 1.
Occurrence of extracardiac morphological defects in relation to egg size and ΣPAH
| Species | Egg diameter (mm) | Percent with tail and axial defects (%) | ΣPAH (µg/L)* |
| Bluefin tuna | 1 | 73.4 ± 8.6 | 8.5 (9.4) |
| Yellowfin tuna | 1 | 15.6 ± 6.0 | 3.4 (3.9) |
| Amberjack | 1.2 | 29 ± 4.6 | 13.8 (ND) |
Values in parentheses are sum of 50 PAHs listed in Table S1.
MC252 Oil Caused Pericardial Edema at ΣPAH Concentrations in the Range of 1–15 µg/L, Irrespective of Weathering.
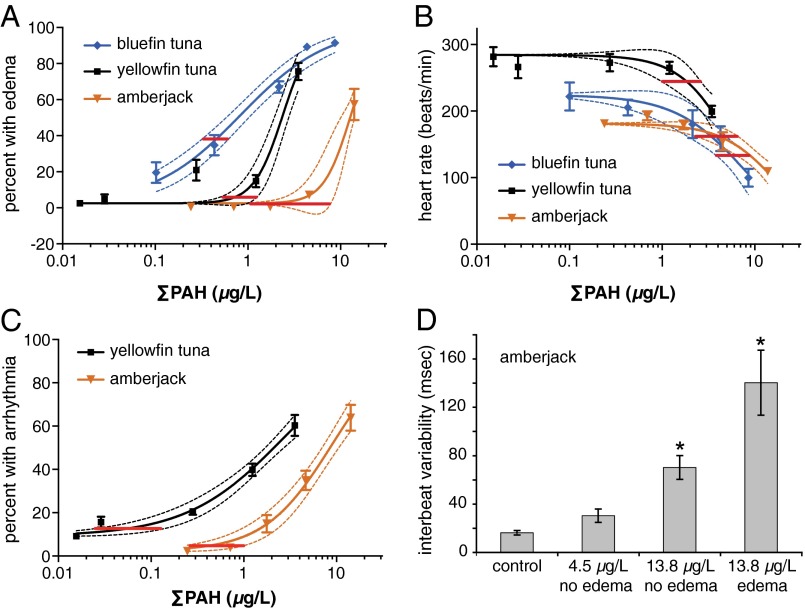
Pericardial fluid accumulation (edema) caused by a failing circulatory system is a consistent and sensitive indicator of the petrogenic PAH cardiotoxicity syndrome. Both tuna species and amberjack showed edema and cardiac-specific abnormalities in response to both naturally and artificially weathered MC252 oil. In fish early life stages, accumulating fluid distorts the rounded yolk mass into a shape that is either angular or flattened anteriorly. Distortion of the anterior end of the yolk mass was therefore used to quantify edema in tunas and amberjack. For all three species, the percentage of hatched larvae showing pericardial and yolk-sac edema in response to oil exposure was dose-dependent (Fig. 5A). The EC50 values (Table 2) were lower for the smaller (≤1 mm) bluefin tuna (0.8 µg/L ΣPAHs) and yellowfin tuna (2.3 µg/L) embryos relative to the larger (1.2 mm) amberjack embryos (12.4 µg/L). Based on the intersection between the upper 95% confidence limit of controls and the 95% confidence band for individual concentration-response nonlinear regressions (Fig. 5A), the thresholds for edema were in the range of 0.3–0.6 µg/L, 0.5–1.3 µg/L, and 1.0–6.0 µg/L for bluefin tuna, yellowfin tuna, and amberjack, respectively (Table 2). Based on C1-phenanthrenes, ∼20% (112 of 548) of water samples collected in the northern Gulf pelagic zone had concentrations above these lower thresholds. The larger volume amberjack embryo exposures yielded similar results, with ΣPAH 13.5 µg/L causing significant edema (Fig. S4A). However, the single-replicate design for larger volumes (Methods) precluded corresponding EC50 determinations.
Fig. 5.
Dose-dependent heart failure and defects in cardiac function following MC252 crude oil exposure. Dose–response curves are shown for (A) occurrence of heart failure leading to edema and (B) bradycardia in southern bluefin tuna, yellowfin tuna, and amberjack. (C) Occurrence of arrhythmia defined as prolongation of atrial systole in yellowfin tuna and amberjack. (D) Quantification of the regularity of the heart beat in amberjack by beat-to-beat variability. Data are mean ± SEM. Red horizontal lines (A–C) indicate estimations for PAH exposure thresholds based on the overlap of the 95% confidence limits of controls and the 95% confidence band of the nonlinear regression. Asterisks in D indicate groups significantly different from control (α < 0.05). Because only control and highest concentrations were measured for southern bluefin tuna, intermediate PAH concentrations shown in A and B are predictions based on dilution.
Table 2.
Calculated EC50 and IC50 values for cardiotoxic endpoints
| Species | Edema | Heart rate | Arrhythmia | |||||||||
| EC50 | 95% CI | R2 | Threshold | IC50 | 95% CI | R2 | Threshold | EC50 | 95% CI | R2 | Threshold | |
| Bluefin tuna | 0.8 (0.9) | 0.6–1.1 | 0.9 | 0.3–0.6 | 7.7 (8.5) | 5.0–11.9 | 0.7 | 4.0–8.5 | NC | NC | ||
| Yellowfin tuna | 2.3 (2.5) | 2.0–2.8 | 0.8 | 0.5–1.3 | 6.1 (7.5) | 3.3–11.2 | 0.5 | 1.0–2.6 | 2.6 (2.9) | 2.1–3.4 | 0.9 | 0.026–0.13 |
| Amberjack | 12.4 (ND) | 10.9–14.1 | 0.9 | 1.0–6.0 | 18.2 | 12.7–26.0 | 0.8 | 2.2–6.5 | 8.6 | 7.0–10.6 | 0.9 | 0.27–1.0 |
Exposure to MC252 Oil Caused Defects in Cardiac Function.
The effects of MC252 crude oil on heart rate and rhythm were assessed using digital video analyses. Heart rate was measured in a single assay each for bluefin tuna and amberjack, and two independent assays for yellowfin tuna. Each species showed a dose-dependent slowing of heart rate (Fig. 5B), or bradycardia, following exposure to either artificially weathered source oil (bluefin tuna and amberjack) or slick A (yellowfin tuna). The IC50 values for oil-induced bradycardia were somewhat higher than the EC50 values for edema (Table 2), and showed a similar relationship to egg size. The IC50s for reduction in heart rate were ΣPAH 7.7 and 6.1 µg/L for the smaller bluefin and yellowfin tuna embryos, respectively, and 18.2 µg/L for the larger amberjack embryos. Based on the intersection between the lower 95% confidence limit of controls and the 95% confidence band for individual concentration-response nonlinear regressions (Fig. 5B), the exposure thresholds for bradycardia were in the range of 4.0–8.5 µg/L and 1.0–2.6 µg/L for bluefin and yellowfin tunas, respectively, and 2.2–6.5 µg/L for amberjack (Table 2). The lower thresholds in yellowfin tuna and amberjack are most likely because of higher power associated with a larger sample size and lower variability in control heart rates. Bradycardia was also observed in amberjack embryos exposed in larger volumes, at a ΣPAH concentration as low as 1.2 µg/L (Fig. S4B). Addition of oxytetracycline to the exposure solution did not influence functional cardiotoxicity in yellowfin tuna embryos (i.e., the inhibitory effects of increasing ΣPAH concentrations on yellowfin heart rates were comparable in the presence or absence of the antibiotic) (Fig. S5C).
In addition to a slowing heartbeat, oil exposure resulted in an irregular arrhythmia observable in all three species (Fig. 5 C and D and Movie S1). These dose-dependent effects were quantified for yellowfin tuna and amberjack embryos. For both species, the durations of atrial systole (contraction) and atrial diastole (relaxation) were assessed by digital video analysis. In hatched yellowfin tuna larvae, systole was shorter than diastole in 91.4 ± 3.1% of control animals, but oil exposure from fertilization through the hatching stage lengthened the systolic phase and increased the frequency of larvae with systole longer than diastole in a concentration-dependent manner (Fig. 5C).
Similarly, in hatching-stage amberjack larvae, 96.7 ± 3.3% of unexposed controls had heartbeats with a systolic phase that was shorter on average (115 ± 8 ms) than the corresponding diastolic phase (200 ± 0 ms). Amberjack larvae exposed to the highest MC252 treatment (ΣPAH 13.8 µg/L) had systolic and diastolic phases lasting 350 ± 69 and 158 ± 16 ms, respectively, and the frequency of larvae with prolonged systole also increased in a concentration-dependent manner (Fig. 5C). The calculated EC50s for prolongation of systole in yellowfin tuna and amberjack were ΣPAH 2.6 and 8.6 µg/L, respectively. Based on the upper 95% confidence limits of controls, exposure thresholds for arrhythmia were 0.026–0.13 µg/L and 0.27–1.0 µg/L for yellowfin tuna and amberjack, respectively (Fig. 5C). Based on C1-phenanthrenes, 30% (165 of 548) of water samples collected in the northern Gulf pelagic zone had concentrations above these lower thresholds.
The slower basal heart rate in amberjack larvae also allowed an assessment of beat-to-beat variability, or heart rate regularity (19). The average heart rate of unexposed amberjack controls was ∼180 beats per minute, with a beat-to-beat variability (Methods) of only 16 ± 2 ms (Fig. 5D). Heart rhythm irregularities increased with both crude oil-exposure concentration (beginning at 4.5 µg/L ΣPAH), as well as the severity of the cardiotoxicity phenotype. Heartbeat irregularity was significant in larvae exposed to 13.8 µg/L ΣPAH, and larvae with visible edema were more severely affected (Fig. 5D).
Discussion
The Deepwater Horizon disaster is the largest oil spill yet to occur in the pelagic zone of an oceanic ecosystem. Crude oil released at depth eventually rose to warm mixed layer and surface waters of the northern Gulf of Mexico during the spawning windows for bluefin tuna and many other large predator species (e.g., mackerel, amberjack, sailfish, marlin, mahi mahi, and other tunas). These pelagic fish all produce fertilized eggs that float in the upper layers of the water column. It is therefore highly likely that the early life stages of many northern Gulf pelagics were exposed to crude oil. Despite differences in temperature and species-specific developmental rate, we show here that warm-water pelagic embryos are similarly sensitive to crude oil cardiotoxicity, with an injury phenotype that aligns closely with previous reports for temperate and boreal species. Additionally, the higher exposure concentrations produced extracardiac defects very similar to those observed in other species (zebrafish, herring) with several crude oils, including MC252 (17, 20, 21). In particular, finfold defects and reduced fin growth appear to be specific effects of crude oil exposure, and not necessarily because of developmental delay in embryos with severe edema. For example, reduced finfold outgrowth was observed in MC252-exposed zebrafish embryos that only had mild to moderate edema (20).
For all three species assessed here, exposures to ΣPAHs below 15 µg/L caused cardiotoxicity in the form of heart failure and corresponding edema. Our exposure protocols produced PAH concentrations and mixture compositions that overlapped with measured PAHs in northern Gulf surface waters during the active spill phase, with field detections in many cases at levels that were considerably higher than those used here in experiments [e.g., up to 84 µg/L ΣPAH (28, 29)]. Despite relative differences in weathering, each of the MC252 crude oil samples produced concentration-dependent, stereotypical crude oil cardiotoxicity to the embryos of tunas and amberjack. The spatial extent of injury to fish early life stages may therefore have been large, in response to both fresher oil proximal to the wellhead and more weathered oil further afield.
As anticipated from previous studies, pericardial edema was a sensitive indicator of reduced cardiac function or heart failure in the tunas and amberjack. Oil exposures yielding ΣPAH concentrations in the range of 2–3 µg/L caused gross edema in ≥50% of exposed tuna embryos. Yolk-sac larvae with severe edema would not be expected to survive into the feeding stage (20, 23), indicating that the early life stages of pelagic fish that spawned in or near Gulf of Mexico surface waters containing ΣPAHs at levels above a few micrograms per liter may have been lost from wild populations. Importantly, the most sensitive physiological measurement indicative of cardiotoxicity was atrial arrhythmia, with thresholds near or below 1-µg/L ΣPAH for both yellowfin tuna and the larger amberjack embryos. Larvae with arrhythmia did not have gross pericardial edema. However, at lower exposure concentrations, the pathophysiological processes leading to arrhythmia are likely to also disrupt cardiac morphogenesis [e.g., calcium ion homeostasis (40)], potentially leading to permanent impacts on cardiac form and function (23) and delayed mortality (25). Our analysis shows that a considerable portion of upper pelagic water samples (nearly one-third) collected during the active spill phase had PAH concentrations above thresholds for arrhythmias in the developing hearts of tunas and amberjack. However, this approximation may be low given the limited sampling in areas with high surface oiling because of worker health and safety concerns and other vessel access restrictions.
In conjunction with previous studies, the findings here demonstrate that the response of teleost embryos to petroleum-derived PAHs in both the laboratory and the field is highly conserved among species tested thus far. Tunas and amberjacks develop at higher water temperatures, and yet they display heart failure and other abnormalities that are remarkably similar to those previously reported for species, such as herring and salmon, which develop at very cold temperatures. Pacific herring were a focal species for natural resource injury assessments following the Exxon Valdez (41) (Alaskan waters) and Cosco Busan oil spills (38) (California Current). Herring early life stages exposed to both oil types, in both the field and the laboratory, developed cardiotoxic defects in the form of bradycardia (38) and pericardial edema (38, 41), with strikingly similar results to those reported here for subtropical spawning pelagic fish. The remarkably consistent morphological and physiological responses to oil across diverse fish species indicate that the core mechanisms of PAH-induced cardiotoxicity are conserved. Namely, the cardiotoxic injury stemming from embryonic exposure to crude oil observed in the scombrid and carangid species in this study is essentially identical to the response of a boreal clupeid (herring) (19), representing families that are separated by roughly 100 million y of evolution (42). Our findings thus have implications beyond the upper pelagic zone of the Gulf of Mexico, and are likely to be indicative of sensitivity to oil over a wider range of fish species spawning in other habitats contaminated by MC252 crude oil.
Previous studies have commonly used flow-through columns packed with oiled gravel to generate PAH exposures (19, 26, 27). In zebrafish embryos, the effects of two geologically distinct oils (Alaskan and Iranian) were indistinguishable irrespective of whether PAHs originated from oiled gravel columns or HEWAFs (17, 21). The similarity of the injury phenotype observed here in pelagic embryos and larvae, in the presence or absence of a broad-spectrum antibiotic, provides further evidence that crude oil cardiotoxicity to fish embryos is both independent of exposure methodology and consistent with direct actions of individual PAH compounds on the developing fish heart (17, 18, 43).
Subtle variations in heart failure and edema among pelagic species are likely the result of a combination of factors. For example, PAHs would be expected to accumulate more rapidly in smaller eggs with higher surface-to-volume ratios, yielding higher effective tissue concentrations and hence more severe toxicity at the same exposure concentration. Although tissue PAH concentrations could not be measured in these pelagic species, this has been shown for other species with different egg sizes (e.g., herring and salmon) (26, 34). Species may also differ in the degree of metabolic capacity at a given time point within prehatching development. Relative to tunas, zebrafish have a larger embryo (>1 mm) and hatch at a more advanced stage of organogenesis. Zebrafish exposed to Deepwater Horizon oil primarily show reduced ventricular contractility rather than bradycardia and atrial arrhythmia (20). Also important is the interaction of individual components of complex PAH mixtures with multiple cellular targets in the fish heart (17–19, 21, 43), each of which may vary across species with different cardiovascular physiology. Crude oil HEWAFs similar to those used here were shown to block key voltage-gated potassium and calcium ion channels controlling excitation-contraction coupling in fish cardiomyocytes (22), and the precise impacts on cardiac function may depend on the relative levels of these targets in different species. In addition, results from studies on alkylated phenanthrenes and more weathered crude oil mixtures enriched with higher levels of alkylated tricyclic PAHs suggest mechanisms of cardiotoxicity that are downstream of aryl hydrocarbon receptor (AHR) activation in cardiac muscle cells (21, 43). The AHR is the ligand-activated transcription factor that induces the up-regulation of protective PAH-metabolizing enzymes (e.g., cytochrome P4501A, CYP1A). Inappropriate AHR activation in heart muscle cells also underlies the cardiotoxicity of some higher molecular weight PAHs, as well as dioxins and polychlorinated biphenyls (43–47). Species-specific developmental variation in ion channel expression and other targets for PAHs in cardiac tissues may underlie the observed subtle differences in MC252 oil-induced functional defects in the developing hearts of tunas, amberjack, and zebrafish.
In closing, determining early life-stage cardiotoxic injury for open-ocean spawners during a large spill is practically impossible given the logistical constraints of sampling live but fragile yolk-sac larvae and identifying individual species with uncertain developmental staging in mixed populations of zooplankton. Moreover, land-based facilities capable of maintaining captive broodstocks of large pelagic predators are rare, making experiments challenging. For example, the capacity to spawn bluefin tunas in captivity in controlled land-based facilities has only been accomplished recently, at a single location. At this time, therefore, the studies described here constitute the only feasible means for providing an environmentally realistic assessment of the potential impacts to the offspring of bluefin and yellowfin tunas that spawned in or near the Deepwater Horizon spill zone. Finally, these studies demonstrate the feasibility of collecting quantitative information for species that are logistically very difficult to handle and study. A similar approach can be used for ecologically and commercially important fishes in other ocean habitats where oil exploration and extraction is ongoing or increasing, such as the Arctic.
Methods
Oil Samples and WAF Preparation.
All oil samples were collected under chain of custody during Deepwater Horizon response efforts. Samples used in toxicity assays included MC252 source oil that was artificially weathered by heating with gentle mixing to 90–105 °C until the mass was reduced by 33–38% (sample 072610-W-A), and slick A (sample CTC02404-02), a spill-zone sample collected July 29, 2010 from a barge holding mixed oil offloaded from a number of different skimmers. HEWAFs were prepared using a commercial stainless steel blender as described in detail elsewhere (20). HEWAF stocks (1:1,000 dilutions of oil) and further dilutions for exposure incubations were prepared in high-quality seawater used for culture purposes at each facility supplying fertilized eggs. All HEWAF preparations were allowed to separate for 1 h, but were not filtered, and thus contained whole-particulate oil in addition to dissolved PAHs.
Embryo Exposures.
Experiments with each species were approved by animal care and use committees at Stanford University (protocol no. 14706) and the University of Miami (protocol no. 12–011 RENEWAL 09–001). Fertilized eggs were obtained from yellowfin tuna (Achotines Laboratory, Peninsula Azuero, Panamá), Southern bluefin tuna (Cleanseas Tuna, Arno Bay, South Australia), and yellowtail amberjack (Cleanseas Tuna, Arno Bay, South Australia). For yellowfin tuna, eggs were removed from an egg collector attached to the broodstock tank and placed in a 20-L bucket. Water was saturated with dissolved oxygen (100% D.O.) using an airstone and nonviable eggs were allowed to settle for 15 min, after which floating eggs were scooped off the surface using a soft mesh net and placed into another 20-L bucket of 1-μm filtered/UV-sterilized seawater at a density of ∼300–500 eggs per liter. After gentle flushing with clean 1-μm filtered/UV sterilized seawater for 10–15 min, formalin [37% (vol/vol) formaldehyde solution] was applied to the eggs at a final concentration of 100 ppm for 1 h as a prophylactic treatment. Aeration was supplied at a low rate to maintain dissolved oxygen levels at or above saturation levels (6.1–6.5 mg/L at 27–29 °C) throughout formalin treatment. Eggs were then rinsed using 1-μm filtered/UV-sterilized seawater for at least five full volume changes before use in exposure assays. Temperature was maintained within 1 °C of the spawning tank temperature throughout the collection and formalin treatment periods. For bluefin tuna and amberjack, fertilized eggs were collected from the spawning captive broodstock by hatchery operators and treated to reduce microbes using nonformaldehyde proprietary methods per standard practice at the facility for routine larval production. For yellowfin tuna exposures, oil exposures and embryological analyses were carried out at the Achotines Laboratory adjacent to the captive broodstock operation. For bluefin tuna and amberjack exposures, bagged oxygenated eggs were transported in seawater in a cooler ∼1 h to a laboratory at the Lincoln Marine Science Center (Port Lincoln, South Australia). All embryos were staged and sorted under Olympus and Nikon stereomicroscopes. For yellowfin tuna, exposures began during the cleavage stage. For bluefin tuna and amberjack, embryos undergoing normal gastrulation were selected and exposure was started at 50% epiboly. Two exposures were carried out sequentially with yellowfin tuna, one with an addition of 10 ppm oxytetracycline to the water used for processing eggs and HEWAF dilutions for exposure.
Exposures were carried out in 1-L beakers with densities of 20 embryos per liter (bluefin and yellowfin tuna) or 80 embryos per liter (amberjack). Amberjack embryos were also exposed in 10-L plastic buckets with an exposure volume of 7 L and the same embryo density. Because of a limited availability of embryos, bluefin tuna was restricted to a single assay. Assays with beakers used either four replicates (bluefin tuna, amberjack) or six replicates (yellowfin tuna) per treatment (unexposed control and four concentrations of HEWAF). Amberjack exposure in buckets used a single replicate per treatment (control and four exposure concentrations) with an embryo density of 79 ± 4 per liter (mean ± SEM; n = 5). At source hatcheries, each pelagic species was typically incubated at densities of ∼100 embryos per liter in large tanks with constant upwelling agitation, allowing very high hatch rates of morphologically normal larvae. Initial studies were carried out with bluefin tuna embryos during a narrow window of availability, and used a static incubation protocol. Bluefin tuna embryos could only be incubated at low density (20 per liter), with hatch rates lower than that achieved in large volume culture. In addition, it was determined that newly hatched larvae could not be viably mounted and imaged without anesthesia, and a protocol was developed that relied on imaging of anesthetized larvae 6–10 h posthatch. Subsequent experiments with amberjack and yellowfin tuna embryos determined that hatch rates were markedly improved with gentle agitation of beakers that kept hatching-stage embryos away from the air–water interface and beaker bottoms. In amberjack assays, beakers were agitated on a modified horizontal shaking water bath and buckets were agitated with magnetic stir bars. Hatching rates could not be precisely quantified for buckets because of the large numbers of embryos (> 500 per bucket), but generally appeared similar to beakers. Despite agitation on an orbital shaker, yellowfin tuna hatch rates still declined at densities above 20 per liter.
Dilutions of a 1:1,000 stock WAF were distributed to the replicate beakers for each concentration tested, and embryos added using glass transfer pipets. Depending on the timing of incubation to hatch for each species, exposures were carried out without WAF renewal up to several hours after hatch. All exposures for the data shown were carried out in temperature-controlled rooms (bluefin tuna and amberjack, 25 °C; yellowfin tuna, 27 °C). Water quality was monitored daily, and included measurements of pH, dissolved oxygen, salinity, ammonia, and temperature.
Image Collection.
For all data collection, room temperatures were held at the same temperature as exposures. Larvae were anesthetized with clove oil before capture and imaging. The dilution of clove oil required to immobilize larvae was titrated for each species. Clove oil was diluted 1:10,000 into exposure beakers, with 10 min allowed for anesthetic effect before initiation of imaging. Only one beaker at a time was imaged, so that all replicates had equal exposure time in clove oil. For all imaging (with or without anesthetic), two to three larvae were captured at a time and transferred to a Petri dish, where they were individually mounted on top of 2% methylcellulose in seawater. This process ensured rapid imaging of specimens while avoiding potential temperature elevation on the microscope stage. Two stereoscope stations allowed sequential processing of two replicates at a time, and larvae were imaged continuously until all replicates were completed. Replicates were processed in random order, except control replicates that were evenly spaced throughout the entire time of processing, which ranged from 5 to 8 h. For amberjack embryos exposed in buckets, 10 hatched larvae were randomly captured from each treatment and imaged within 1 h. Images (640 × 480 pixels) were collected using FireI-400 industrial digital video cameras (Unibrain) mounted on Nikon SMZ800 stereomicroscopes, using MacBook laptops and BTV Carbon Pro software. Images were calibrated using a stage micrometer.
Image Analysis and Statistics.
For scoring the presence or absence of edema, still frames and videos were assessed for the shape of the yolk mass. Larvae were scored as normal if the anterior portion of the yolk sac was smooth and rounded with a bullet-shaped tip and if there were no obvious indentations on the yolk sac because of pressure from fluid buildup in the pericardial area. Edema was scored positive if the anterior portion of the yolk sac was concave or pushed to a sharp point, or if indentations indicated by dark, angular lines were seen pushing on the yolk sac because of pressure from fluid buildup in the pericardial area. For all species, there was a range of normal yolk-sac shapes in control fish. Yolk sacs that did not have a perfect rounded, bullet shape (e.g., slightly blunted or semipointed) were still considered within the range of normal. Because amberjack embryos exposed in buckets were all imaged within a narrow time window (1 h) and were thus at the same developmental stage, pericardial areas were measured in lateral images as a quantitative assessment of edema as described elsewhere (20).
Cardiac function was assessed in ≥10-s digital video clips of all viable embryos collected from each replicate exposure. Heart rate was determined by simply counting the total number of heart beats in a given video clip played back at half speed. Arrhythmia was assessed by relating the onset of contraction and relaxation for cardiac chambers to individual video frames, and counting the total number of frames for each phase. The actual mean duration of systole and diastole was determined for amberjack from four randomly selected videos for control and high-dose treatment groups. Duration of systole and diastole was not determined for yellowfin tuna because of higher heart rates. For amberjack, heart rate (beat-to-beat) variability was determined by the number of video frames between the initiation of contractions for an entire video clip, and calculated as described elsewhere (19), measured in 10 randomly selected videos from each replicate (e.g., Movie S1).
Statistical Analysis.
Parametric statistical analyses were performed using JMP (versions 6.0.2 and 8.0.1 for Macintosh; SAS Institute), and dose–response relationships analyzed by nonlinear models using Prism 6.0b for Macintosh (GraphPad Software). For nonlinear regressions, ΣPAH data were log-transformed and a normalized-response model was used for occurrence data (percent with edema or arrhythmia); heart rate data were not normalized. For parametric analyses, occurrence data (arrhythmia) (Fig. 5D) were analyzed by one-way ANOVA. If ANOVA was significant for effect of treatment (P < 0.05), means were compared between controls and treatment groups (exposure concentrations) using Dunnett’s post hoc test (α = 0.05). Heart-rate data (Fig. S5) were analyzed by one-way ANOVA with replicate nested in treatment group to assess “tank” effect and to avoid pseudoreplication. If ANOVA was significant for effect of treatment (P < 0.05), means were compared between controls and treatment groups (exposure concentrations) using Dunnett’s test (α = 0.05).
Analytical Chemistry.
PAH concentrations were determined by gas chromatography/mass spectrometry (GC/MS) by either Columbia Analytical Services (CAS; bluefin and yellowfin tuna) or the Northwest Fisheries Science Center (NWFSC; amberjack). The 250-mL water samples were stabilized with either hydrochloric acid (CAS samples) or dichloromethane (NWFSC samples), stored at 4 °C until shipment, and shipped to each analytical laboratory by expedited courier. Samples were then extracted with dichloromethane and processed for GC/MS. Details of sample extraction, cleanup and GC/MS analysis, including limits of detection, are provided elsewhere (38). PAH concentrations were determined for each dilution of the dose–response series. For the bluefin tuna exposure, PAHs were measured in only the highest concentration and control samples because of sample loss during shipping between Australia and the United States. Measured PAHs are provided in Table S1. For consistency with previously published work, a conventional list of 40 analytes was used for PAH composition figures and calculations of ΣPAH. A larger list of 50 analytes was used to provide supplemental EC50/IC50 values comparable to other ongoing Deepwater Horizon Natural Resource Damage Assessment (NRDA) studies. Individual PAH measurements for all tests are provided in Dataset S1. HEWAF PAH data were compared with field sample PAH data generated within the larger NRDA field sampling program. The NRDA sampling plans, protocols, and raw data are publicly available through links at www.gulfspillrestoration.noaa.gov/oil-spill/gulf-spill-data. For comparison with PAH levels in field-collected water samples, the source file for all validated Gulf water sample PAH values was obtained at http://54.243.205.138/gulfspillrestoration/qmmatrix/Water_chem_export.zip. The file Water_chem_export.csv was filtered in Microsoft Excel using columns “matrix” (water), “chemname” (C1-phenanthrenes/anthracenes), “sampdate” (20100428–20100730), “upperdepth” (greater than or equal to 0 m), “lower depth” (less than or equal to 200 m), “latitude” (≥27.25, ≤29.6), and “longitude” (≥−90.6, ≤−87.09). These filtering criteria resulted in a list of 548 pelagic water samples from a total of 2,647. The map locations of water samples can be found at http://gomex.erma.noaa.gov/erma.html#x=-88.25810&y=27.03211&z=6&layers=19129. For comparison of overall PAH composition between HEWAFs and field samples (Fig. 1), a subset of 77 water samples was selected that showed weathering as a relative loss of naphthalenes. The ΣPAH concentrations for these samples ranged from 2.6–354.4 µg/L, with a mean (±SD) of 22.3 ± 42.7 µg/L and a median value of 13.2 µg/L. Water samples from the field that showed similar PAH concentration and composition (i.e., weathering state) to HEWAF preps (represented in Fig. 1) were thus in the low end of the range. The data subset used for this comparison is publically available as a package at http://54.243.205.138/gulfspillrestoration/PNAS/NOAA_WAF_PNAS_2014.zip.
Supplementary Material
Acknowledgments
The authors thank Adam Miller, Morten Deichmann, and Craig Foster of Cleanseas Tuna for providing bluefin tuna and amberjack embryos, and advice on culture conditions; Erin Bubner and Bob Delaine for facilities access and logistical support at the Lincoln Marine Science Center; Tor Linbo and Robbie Schallert for assistance with bluefin tuna and amberjack embryo exposures; the Inter-American Tropical Tuna Commission members Guillermo Campeán, Richard Deriso, Daniel Margulies, Vernon Scholey, and the staff at the Achotines Laboratory for providing access to the yellowfin tuna broodstock and laboratory facilities; Bernadita Anulacion, Daryle Boyd, and Ron Pearce for assistance with polycyclic aromatic hydrocarbon analyses; and National Oceanic and Atmospheric Administration National Ocean Service staff and contractors for reviewing the experimental design and a draft of the manuscript. M.G. is a Maytag professor of ichthyology. This work was funded as a contributing study to the Deepwater Horizon/MC252 Incident Natural Resource Damage Assessment.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.A.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1320950111/-/DCSupplemental.
References
- 1.Crone TJ, Tolstoy M. Magnitude of the 2010 Gulf of Mexico oil leak. Science. 2010;330(6004):634. doi: 10.1126/science.1195840. [DOI] [PubMed] [Google Scholar]
- 2.Kujawinski EB, et al. Fate of dispersants associated with the Deepwater Horizon oil spill. Environ Sci Technol. 2011;45(4):1298–1306. doi: 10.1021/es103838p. [DOI] [PubMed] [Google Scholar]
- 3.Labson VF, et al. 2010. Estimated Lower Bound for Leak Rates from the Deepwater Horizon Spill—Interim Report to the Flow Rate Technical Group from the Mass Balance Team, U.S. Geological Survey Open–File Report (US Geological Survey, Reston, VA), p 4. [Google Scholar]
- 4.Incardona JP, Collier TK, Scholz NL. Oil spills and fish health: Exposing the heart of the matter. J Expo Sci Environ Epidemiol. 2011;21(1):3–4. doi: 10.1038/jes.2010.51. [DOI] [PubMed] [Google Scholar]
- 5.Lang KL, Grimes CB, Shaw RF. Variations in the age and growth of yellowfin tuna larvae, Thunnus albacares, collected about the Mississippi River plume. Environ Biol Fishes. 1994;39(3):259–270. [Google Scholar]
- 6.Arocha F, Lee DW, Marcano LA, Marcano JS. Update information on the spawning of yellowfin tuna, Thunnus albacares, in the western central Atlantic. Col Vol Sci Pap ICCAT. 2001;52(1):167–176. [Google Scholar]
- 7.Grimes CB, Finucane JH, Collins AL, DeVries DA. Young king mackerel, Scomberomorus cavalla, in the Gulf of Mexico, a summary of the distribution and occurrence of larvae and juveniles, and spawning dates for Mexican juveniles. Bull Mar Sci. 1990;46(3):640–654. [Google Scholar]
- 8.McEachran JD, Finucane JH, Hall LS. Distribution, seasonality and abundance of king and Spanish mackerel larvae in the northwestern Gulf of Mexico (Pisces: Scombridae) Northeast Gulf Science. 1980;4:1–16. [Google Scholar]
- 9.Block BA, et al. Electronic tagging and population structure of Atlantic bluefin tuna. Nature. 2005;434(7037):1121–1127. doi: 10.1038/nature03463. [DOI] [PubMed] [Google Scholar]
- 10.Teo S, et al. Annual migrations, diving behavior, and thermal biology of Atlantic bluefin tuna, Thunnus thynnus, on their Gulf of Mexico breeding grounds. Mar Biol. 2007;151:1–18. [Google Scholar]
- 11.Rooker JR, et al. Distribution and habitat associations of billfish and swordfish larvae across mesoscale features in the Gulf of Mexico. PLoS ONE. 2012;7(4):e34180. doi: 10.1371/journal.pone.0034180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murie DJ, Parkyn DC. Marine Fisheries Research Initiative (MARFIN) Program, Final Report. 2008. Age, growth and sex maturity of greater amberjack (Seriola dumerili) in the Gulf of Mexico. NA05NMF4331071 (National Marine Fisheries Service, St. Petersburg, FL) [Google Scholar]
- 13.Thompson BA, Wilson CA, Render JH, Beasley M, Cauthron C. Marine Fisheries Research Initiative (MARFIN) Program, Final Report. 1991. Age, growth, and reproductive biology of greater amberjack and cobia from Louisiana waters. NA90AAH-MF722 (National Marine Fisheries Service, St. Petersburg, FL) [Google Scholar]
- 14. National Marine Fisheries Service (2010) Available at www.st.nmfs.noaa.gov/sisPortal/sisTimeSeriesPdf.jsp?tsPdfIndex=1&entityName=Greater%20amberjack%20%20Gulf%20of%20Mexico&asmtId=2758&asmtYear=2010&asmtMonth=December. Accessed March 10, 2014.
- 15.National Marine Fisheries Service 2011. Available at www.st.nmfs.noaa.gov/sisPortal/sisTimeSeriesPdf.jsp?tsPdfIndex=1&entityName=Yellowfin%20tuna%20%20Western%20Atlantic&asmtId=3143&asmtYear=2011&asmtMonth=October. Accessed March 10, 2014.
- 16.Taylor NG, McAllister MK, Lawson GL, Carruthers T, Block BA. Atlantic bluefin tuna: A novel multistock spatial model for assessing population biomass. PLoS ONE. 2011;6(12):e27693. doi: 10.1371/journal.pone.0027693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Incardona JP, et al. Aryl hydrocarbon receptor-independent toxicity of weathered crude oil during fish development. Environ Health Perspect. 2005;113(12):1755–1762. doi: 10.1289/ehp.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Incardona JP, Collier TK, Scholz NL. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol. 2004;196(2):191–205. doi: 10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Incardona JP, et al. Cardiac arrhythmia is the primary response of embryonic Pacific herring (Clupea pallasi) exposed to crude oil during weathering. Environ Sci Technol. 2009;43(1):201–207. doi: 10.1021/es802270t. [DOI] [PubMed] [Google Scholar]
- 20.Incardona JP, et al. Exxon Valdez to Deepwater Horizon: Comparable toxicity of both crude oils to fish early life stages. Aquat Toxicol. 2013;142–143:303–316. doi: 10.1016/j.aquatox.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Jung J-H, et al. Geologically distinct crude oils cause a common cardiotoxicity syndrome in developing zebrafish. Chemosphere. 2013;91(8):1146–1155. doi: 10.1016/j.chemosphere.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Brette F, et al. Crude oil impairs cardiac excitation-contraction coupling in fish. Science. 2014;343(6172):772–776. doi: 10.1126/science.1242747. [DOI] [PubMed] [Google Scholar]
- 23.Hicken CE, et al. Sublethal exposure to crude oil during embryonic development alters cardiac morphology and reduces aerobic capacity in adult fish. Proc Natl Acad Sci USA. 2011;108(17):7086–7090. doi: 10.1073/pnas.1019031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heintz RA. Chronic exposure to polynuclear aromatic hydrocarbons in natal habitats leads to decreased equilibrium size, growth, and stability of pink salmon populations. Integr Environ Assess Manag. 2007;3(3):351–363. [PubMed] [Google Scholar]
- 25.Heintz RA, et al. Delayed effects on growth and marine survival of pink salmon Oncorhynchus gorbuscha after exposure to crude oil during embryonic development. Mar Ecol Prog Ser. 2000;208:205–216. [Google Scholar]
- 26.Carls MG, Rice SD, Hose JE. Sensitivity of fish embryos to weathered crude oil: Part I. Low-level exposure during incubation causes malformations, genetic damage, and mortality in larval Pacific herring (Clupea pallasi) Environ Toxicol Chem. 1999;18:481–493. [Google Scholar]
- 27.Incardona JP, et al. Potent phototoxicity of marine bunker oil to translucent herring embryos after prolonged weathering. PLoS ONE. 2012;7(2):e30116. doi: 10.1371/journal.pone.0030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diercks A-R, et al. Characterization of subsurface polycyclic aromatic hydrocarbons at the Deepwater Horizon site. Geophys Res Lett. 2010;37:L20602. [Google Scholar]
- 29. Wade TL, et al. (2011) Analyses of water samples from the Deepwater Horizon oil spill: Documentation of the subsurface plume. Monitoring and Modeling the Deepwater Horizon Oil Spill: A Record-Breaking Enterprise, eds Liu Y, MacFadyen A, Ji Z-G, Weisberg RH, Geophys Monogr Ser (AGU, Washington, DC), 195:77–82. [Google Scholar]
- 30.Carls MG, et al. Fish embryos are damaged by dissolved PAHs, not oil particles. Aquat Toxicol. 2008;88(2):121–127. doi: 10.1016/j.aquatox.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Hatlen K, et al. Natural sunlight and residual fuel oils are an acutely lethal combination for fish embryos. Aquat Toxicol. 2010;99(1):56–64. doi: 10.1016/j.aquatox.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 32.de Soysa TY, et al. Macondo crude oil from the Deepwater Horizon oil spill disrupts specific developmental processes during zebrafish embryogenesis. BMC Biol. 2012;10:40. doi: 10.1186/1741-7007-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barron MG, Carls MG, Short JW, Rice SD. Photoenhanced toxicity of aqueous phase and chemically dispersed weathered Alaska North Slope crude oil to Pacific herring eggs and larvae. Environ Toxicol Chem. 2003;22(3):650–660. [PubMed] [Google Scholar]
- 34.Heintz RA, Short JW, Rice SD. Sensitivity of fish embryos to weathered crude oil: Part II. Increased mortality of pink salmon (Oncorhynchus gorbuscha) embryos incubating downstream from weathered Exxon Valdez crude oil. Environ Toxicol Chem. 1999;18(3):494–503. [Google Scholar]
- 35.Marty GD, et al. Ascites, premature emergence, increased gonadal cell apoptosis, and cytochrome P4501A induction in pink salmon larvae continuously exposed to oil-contaminated gravel during development. Can J Zool. 1997;75(6):989–1007. [Google Scholar]
- 36.Margulies D, et al. Spawning and early development of captive yellowfin tuna (Thunnus albacares) Fish Bull. 2007;105:249–265. [Google Scholar]
- 37.Ditty JG, Shaw RF, Grimes CB, Cope JS. Larval development, distribution, and abundance of common dolphin, Coryphaena hippurus, and pompano dolphin, C equiselis (family, coryphaenidae), in the northern Gulf of Mexico. Fish Bull. 1994;92(2):275–291. [Google Scholar]
- 38.Incardona JP, et al. Unexpectedly high mortality in Pacific herring embryos exposed to the 2007 Cosco Busan oil spill in San Francisco Bay. Proc Natl Acad Sci USA. 2012;109(2):E51–E58. doi: 10.1073/pnas.1108884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Staudt D, Stainier D. Uncovering the molecular and cellular mechanisms of heart development using the zebrafish. Annu Rev Genet. 2012;46:397–418. doi: 10.1146/annurev-genet-110711-155646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rottbauer W, et al. Growth and function of the embryonic heart depend upon the cardiac-specific L-type calcium channel alpha1 subunit. Dev Cell. 2001;1(2):265–275. doi: 10.1016/s1534-5807(01)00023-5. [DOI] [PubMed] [Google Scholar]
- 41.Marty GD, Hose JE, McGurk MD, Brown ED, Hinton DE. Histopathology and cytogenetic evaluation of Pacific herring larvae exposed to petroleum hydrocarbons in the laboratory or in Prince William Sound, Alaska, after the Exxon Valdez oil spill. Can J Fish Aquat Sci. 1997;54(8):1846–1857. [Google Scholar]
- 42.Betancur-R R, et al. The tree of life and a new classification of bony fishes. PLOS Curr. 2013 doi: 10.1371/currents.tol.53ba26640df0ccaee75bb165c8c26288. 10.1371/currents.tol.53ba26640df0ccaee75bb165c8c26288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott JA, Incardona JP, Pelkki K, Shepardson S, Hodson PV. AhR2-mediated; CYP1A-independent cardiovascular toxicity in zebrafish (Danio rerio) embryos exposed to retene. Aquat Toxicol. 2011;101(1):165–174. doi: 10.1016/j.aquatox.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 44.Carney SA, Peterson RE, Heideman W. 2,3,7,8-Tetrachlorodibenzo-p-dioxin activation of the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator pathway causes developmental toxicity through a CYP1A-independent mechanism in zebrafish. Mol Pharmacol. 2004;66(3):512–521. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 45.Clark BW, Matson CW, Jung D, Di Giulio RT. AHR2 mediates cardiac teratogenesis of polycyclic aromatic hydrocarbons and PCB-126 in Atlantic killifish (Fundulus heteroclitus) Aquat Toxicol. 2010;99(2):232–240. doi: 10.1016/j.aquatox.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Incardona JP, Day HL, Collier TK, Scholz NL. Developmental toxicity of 4-ring polycyclic aromatic hydrocarbons in zebrafish is differentially dependent on AH receptor isoforms and hepatic cytochrome P4501A metabolism. Toxicol Appl Pharmacol. 2006;217(3):308–321. doi: 10.1016/j.taap.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 47.Incardona JP, Linbo TL, Scholz NL. Cardiac toxicity of 5-ring polycyclic aromatic hydrocarbons is differentially dependent on the aryl hydrocarbon receptor 2 isoform during zebrafish development. Toxicol Appl Pharmacol. 2011;257(2):242–249. doi: 10.1016/j.taap.2011.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.