Significance
This work addresses the molecular basis for interspecies signaling effects of antibiotics, which have been a controversial but potentially significant emerging topic over the last few years. The “pseudo” gamma-butyrolactone (GBL) receptor (i.e., those GBL receptor homologues often found in Streptomyces genomes, but apparently not binding or responding to GBLs), ScbR2, was identified as the receptor of JdB. It has an extraordinary ability to bind and respond to exogenous angucyclines, as well as to be able to directly regulate the biosynthesis of different endogenous antibiotics and the morphological development of Streptomyces. Our findings significantly extend understanding of antibiotic-mediated signaling mechanisms and the ecological impact of antibiotics.
Abstract
The angucycline antibiotic jadomycin B (JdB) produced by Streptomyces venezuelae has been found here to induce complex survival responses in Streptomyces coelicolor at subinhibitory concentration. The receptor for JdB was identified as a “pseudo” gamma-butyrolactone receptor, ScbR2, which was shown to bind two previously unidentified target promoters, those of redD (redDp) and adpA (adpAp), thus directly regulating undecylprodigiosin (Red) production and morphological differentiation, respectively. Because AdpA also directly regulates the expression of redD, ScbR2, AdpA, and RedD together form a feed-forward loop controlling both differentiation and Red production phenotypes. Different signal strengths (i.e., JdB concentrations) were shown to induce the two different phenotypes by modulating the relative transcription levels of adpA vs. redD. The induction of morphological differentiation and endogenous antibiotic production by exogenous antibiotic exemplifies an important survival strategy more sophisticated than the induction of antibiotic resistance.
Antibiotics are well known and important for their ability to inhibit microbial growth by highly specific interactions with such key targets as ribosomes, cell-wall biosynthetic machinery, etc. However, even at concentrations too low to affect the growth of target organisms, exposure to antibiotics can bring about changes in global gene expression, as first visually displayed by a promoter–reporter fusion method (1). Such effects gave rise to the concept of antibiotics as signaling molecules (2–4). However, only in a few cases could the receptors for antibiotic signals be identified (5–8), and in most cases, the signaling mechanisms remain poorly understood. Many known antibiotics are produced by streptomycetes, soil bacteria that engage in active reciprocal interactions in natural communities (9). There is evidence to suggest that many of these interactions are mediated by antibiotics (4, 10, 11). Thus, antibiotics offer great tools to probe the responses of cellular and ecological networks at the systems level; antibiotic–cell interactions could be used as models to dissect the molecular mechanisms underlying cellular responses.
In our previous works, an important type of antibiotic receptors was defined and designated “pseudo” gamma-butyrolactone (GBL) receptors, due to the fact that they are homologous to GBL receptors, but bind antibiotics instead of GBL molecules (12). The pseudo GBL receptor, ScbR2—situated in the coelimycin gene cluster in Streptomyces coelicolor—was shown to bind two structurally distinct endogenous antibiotics, actinorhodin (Act) and undecylprodigiosin (Red). Thus, it was implicated in cross-talk between different antibiotic biosynthetic pathways (12, 13). In this study, we describe a previously unidentified interaction between an exogenous antibiotic, jadomycin B (JdB) and S. coelicolor. At subinhibitory concentrations, the Streptomyces venezuelae angucycline antibiotic JdB induces S. coelicolor to undergo premature differentiation (formation of sporulating aerial mycelium) and early production of the red-pigmented antibiotic Red. This effect involves the binding of JdB by ScbR2, thereby relieving ScbR2-mediated repression of important activators of differentiation and Red production. Other angucyclines also elicited similar phenotypes, suggesting that they also triggered this signal transduction system as signals.
Results
Discovery of JdB as an Antibiotic Signal, Inducing Complex Survival Responses in S. coelicolor.
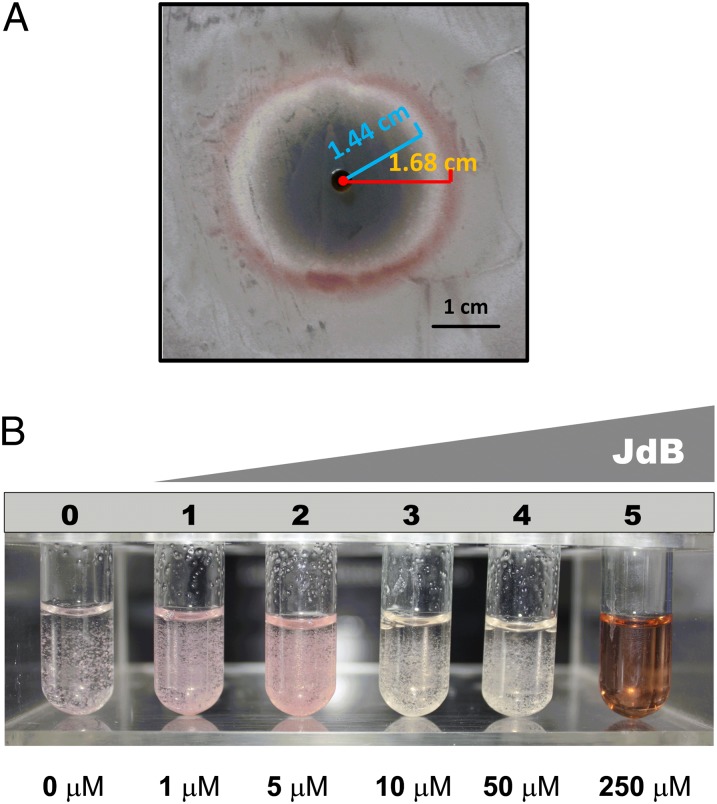
GBLs are growth cycle-related signaling molecules produced by streptomycetes (13, 14). Previously, a bioassay method based on the pigment production by S. coelicolor was developed as a convenient assay for some GBL molecules (15). When we applied S. venezuelae culture extracts harvested at different times to S. coelicolor indicator plates, several fractions (12, 24, 30, 36, and 42 h) were observed to induce pigment production (Fig. S1). The early (12-h) fraction could have contained a GBL molecule, but the later fractions were deduced to contain a substance different from GBL molecules, because GBL production should have stopped at this stage (13). Upon further fractionation on HPLC, the active molecule was identified as JdB, an atypical angucycline antibiotic produced by S. venezuelae (16). JdB is active against Gram-positive bacteria and human cancer cell lines (17, 18), but its cellular targets in bacteria and human cells are not known. To demonstrate the responses of S. coelicolor to JdB, a lawn of S. coelicolor mycelium grown on supplemented minimal medium (SMM) agar was spotted with JdB, and a pink zone surrounding the spot of antibiotic addition was observed (Fig. 1A). Interestingly, early aerial growth was observed internal to the pigmented zone (Fig. 1A). Pigment production is a specific response to JdB, because three other antibiotics—ampicillin, erythromycin, and kanamycin—showed growth inhibition but did not induce obvious phenotypes. The ability of subinhibitory concentrations of JdB (1–5 μM) to induce pink pigment production was also observed in liquid cultures (Fig. 1B), and when analyzed by HPLC, the pigment showed a retention time matching that of a Red standard (Fig. S2A). Mass spectrometry (MS) of the collected peaks confirmed that the pigment was indeed Red (Fig. S2B).
Fig. 1.
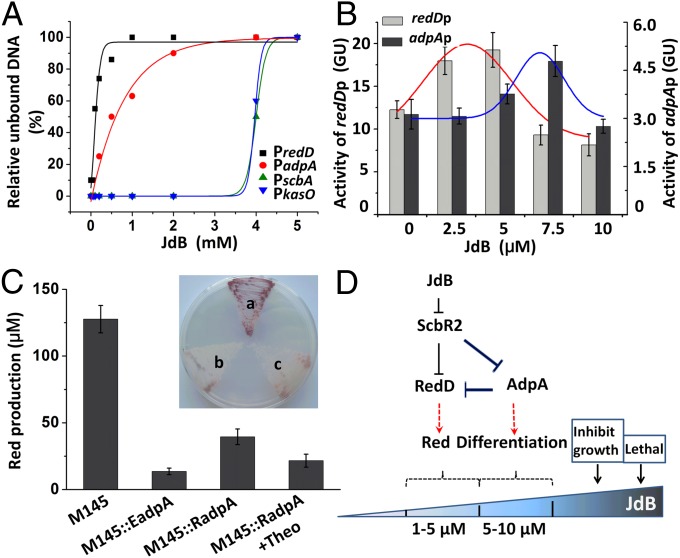
The phenotypic responses of S. coelicolor M145 to JdB. (A) A plate assay of the response to JdB on SMM agar. JdB was dissolved in 100% DMSO, and 10 μL (10 mM) was placed in a well cut in SMM agar covering a lawn of S. coelicolor. The photograph was taken from the top of the plate. A red line shows the radius of the Red production zone; a blue line shows the radius of the aerial hyphal zone. (B) The responses of S. coelicolor M145 to increasing concentrations of JdB in liquid SMM, highlighting the production of a pink pigment at 1–5 μM JdB.
Identification of ScbR2 as the Receptor of JdB in S. coelicolor.
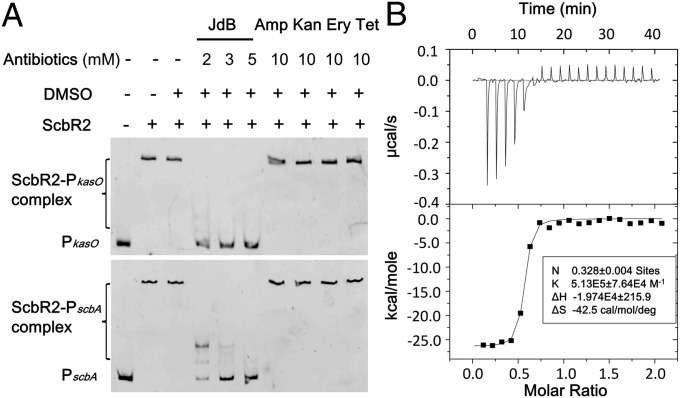
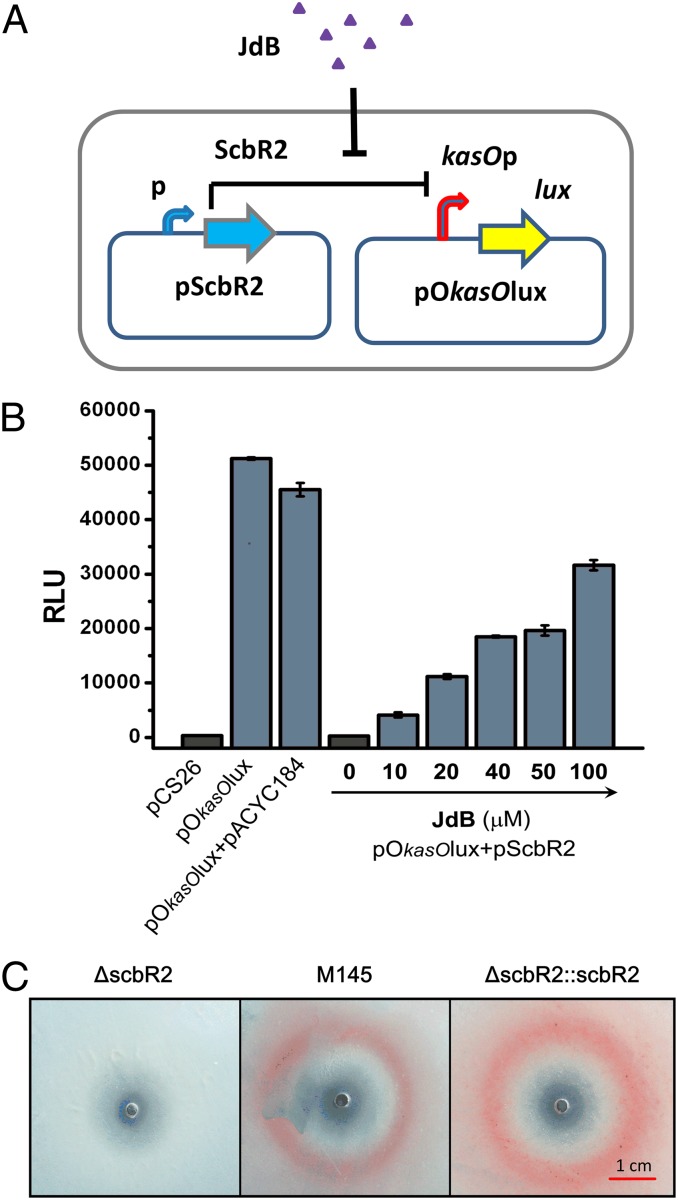
To identify the receptor of JdB in S. coelicolor, we focused our attention on the pseudo GBL receptor, ScbR2. ScbR2 is a close homolog of JadR2 in S. venezuelae, which is known to recognize JdB as a ligand (12). We have previously reported that ScbR2 mediates cross-talk between different antibiotic biosynthetic pathways within S. coelicolor, because its interaction with target promoters is relieved by promiscuously binding to diverse pathway end products (12). The responses of ScbR2 to JdB and other antibiotics were analyzed by electrophoretic mobility shift assays (EMSAs) using two previously identified DNA targets of ScbR2 (PkasO and PscbA) that contained the promoter regions of kasO and scbA, respectively (12, 13). The ScbR2–DNA complexes were dissociated by JdB, but not by ampicillin, kanamycin, erythromycin, or tetracycline (Fig. 2A). To characterize the pattern of interaction between ScbR2 and JdB, the two molecules were purified and subjected to isothermal titration calorimetry (ITC) analysis, which measures the heat released or absorbed in the process of molecular interactions (19). The binding between ScbR2 and JdB was further verified, and the titration curve suggested that ScbR2 and JdB interact at a ratio near 1:1 (Fig. 2B). We then designed a reporter system in Escherichia coli to investigate whether JdB could relieve the repression of the kasO promoter (kasOp) by ScbR2 in vivo (Fig. 3A). The reporter system involved two plasmids: pScbR2, expressing ScbR2; and pOkasOlux, expressing the lux reporter genes under the control of kasOp. In the absence of JdB, ScbR2 repressed kasOp, thus turning off the expression of lux genes and bioluminescence (Fig. 3B). When JdB was added, bioluminescence was turned on in a dose-dependent manner (Fig. 3B). These results suggested that ScbR2 could sense and respond to JdB. The conclusion was supported by in vivo experiments in S. coelicolor, showing that the scbR2 mutant (ΔscbR2) lost the JdB-dependent induction of pink pigment, whereas the parental strain (M145) and the complemented scbR2 mutant (ΔscbR2::scbR2) both responded to JdB by producing pink zones (Fig. 3C).
Fig. 2.
Specific binding between the receptor ScbR2 and JdB in vitro. (A) Gel mobility shift assays of the DNA-binding activity of ScbR2 in the presence of various antibiotics. The assays were performed in 0.3 μM ScbR2, with 6.6 ng (0.13 nM) of PscbA or 6.6 ng (0.08 nM) of PkasO probes. Amp, ampicillin; Ery, erythromycin; Kan, kanamycin; Tet, tetracycline. (B) ITC analysis of the binding of ScbR2 to JdB. ScbR2 (100 μM) was injected into a solution of 20 μM JdB. (Upper) Heat changes measured as a function of time at 25 °C are shown. (Lower) Normalized heat changes (black square) and the best-fit curve (solid line) and calculated parameters are shown. (Inset) The estimated binding stoichiometry constant and enthalpy are shown.
Fig. 3.
Demonstration of the response of ScbR2 to JdB. (A) A schematic representation of the reporter system used to investigate the response of ScbR2 to JdB. (B) Investigation of the interactions of JdB with ScbR2 using a Lux reporter system in vivo. pCS26 contains a promoterless lux operon, and pACYC184 was used to express scbR2. They were used as controls. Relative light units (RLU) are represented as the average of at least three independent readings; error bars indicate ±SDs. (C) Plate assay of the responses of ΔscbR2 (Left), M145 (Center), and ΔscbR2::scbR2 (Right) to JdB on SMM agar. JdB was dissolved in DMSO, and 10 μL (10 mM) was spotted onto a lawn of S. coelicolor. The photographs were from the bottom of the plates.
Identification of Two Targets of ScbR2 in S. coelicolor.
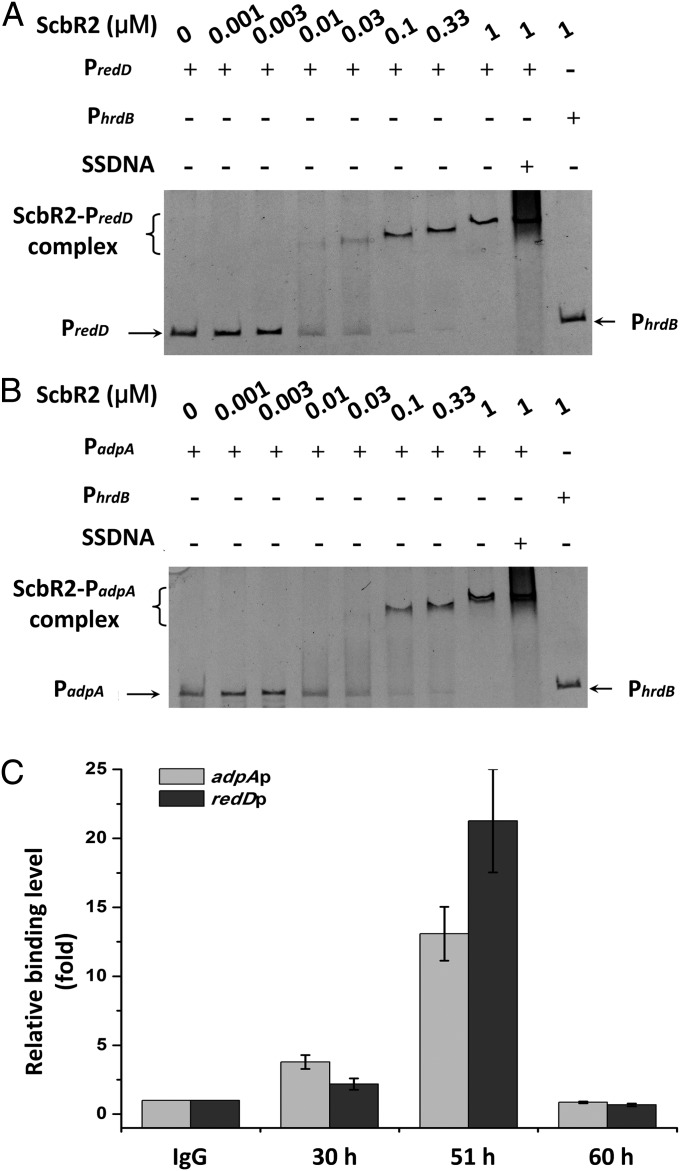
To identify the JdB signal-transduction pathways, we used phenotypes on plates as a guide. Because the most readily observed phenotype observed on plates is the production of Red (Fig. 1A), we first focused on the red gene cluster (20). By scanning the promoter regions in front of redD, redQ, redV, and redZ with ScbR2 using EMSAs, we identified the promoter of redD (redDp) as the only target of ScbR2 in the red cluster (Fig. 4A). The result is logical because RedD is known to be the transcriptional activator of the red biosynthetic genes (21).
Fig. 4.
Binding of ScbR2 with PredD and PadpA in vitro and in vivo. (A and B) Gel mobility shift assays of the binding of ScbR2 to PredD (A) and PadpA (B). The three probes (PredD, PadpA, and PhrdB) were used at a concentration of 0.12 nM in the assays. SSDNA, random fragments of salmon sperm DNA. PhrdB was used as negative control. (C) ChIP-qPCR assays in vivo. Anti-ScbR2 antibodies were used to immunoprecipitate ScbR2–DNA complexes from 30-, 51-, and 60-h cultures treated with formaldehyde. IgG-immunoprecipitated complexes were used as the negative control. The y axis represents the relative enrichment of PredD and PadpA compared with the control. The relative values are means ± SD from three independent experiments.
In addition, based on the observed early aerial hyphal growth internal to Red production zones (Fig. 1A) and the fact that ScbR2 shows ∼31% identity to ArpA of Streptomyces griseus—which was known to bind the promoter region of adpA to repress its transcription (22)—we also tested the binding of ScbR2 to the promoter of adpA (adpAp), the product of which is the master regulator of aerial hyphal production in S. coelicolor (23–25). Remarkably, binding between ScbR2 and adpAp was observed (Fig. 4B).
To confirm these findings, we performed chromosome immunoprecipitation-quantitative PCR (ChIP-qPCR) experiments using a monoclonal antibody against ScbR2 to measure its binding with redDp and adpAp at different times in vivo. Samples were taken from 30-, 51-, and 60-h liquid cultures (in SMM). Compared with the control, higher relative amounts of adpAp and redDp were observed in samples immunoprecipitated at 30 and 51 h (Fig. 4C), confirming direct binding between ScbR2 and these target promoters at those time points in vivo. However, the binding disappeared at 60 h, suggesting a dynamic temporal binding of ScbR2 with these targets.
Characterization of the Interactions Between ScbR2 and its Targets in Response to JdB.
Our results showed that ScbR2 could directly bind adpAp and redDp, in addition to two previously identified targets, the scbA/R intergenic promoter (scbA/Rp) and kasOp (12, 26). To characterize the interactions between ScbR2 and these four targets in response to JdB, we examined the dissociation of ScbR2 from these promoters by EMSAs and observed a growing degree of dissociation from all four target promoters in response to increasing concentrations of JdB (Fig. S3). As summarized in Fig. 5A, when the concentrations of ScbR2 and probes were kept constant, the sequence of dissociation is redDP > adpAP > kasOP > scbA/Rp with increasing concentrations of JdB. From these results, we deduced that ScbR2 functions as a repressor of redDp and adpAp, with low concentrations of JdB being able to induce dissociation from redDp, leading to the production of Red; conversely, at higher JdB concentration, expression of the adpA regulon should be induced. These are exactly what we observed during earlier phenotype experiments on plates—i.e., the aerial hyphal zone showed a shorter radius than the Red pigment zone (Fig. 1A). To accurately determine the concentration range of JdB capable of inducing Red production, we repeated the JdB induction experiment in liquid SMM and carefully quantified Red production levels at different JdB concentrations. We again found that Red is only produced in the 1- to 5-μM JdB concentration range but is not produced at higher JdB concentrations (Fig. S4). To rule out the possibility that Red production was due to a lower growth rate, the growth of M145 at different JdB concentrations was also measured simultaneously: In the 1- to 10-μM range, JdB did not cause apparent growth inhibition (Fig. S5). Although the in vitro and in vivo results were basically consistent with the phenotypes of M145 in response to JdB, they did not explain the phenotype of ΔscbR2 and the lack of Red production by M145 at high concentrations (>5 μM) of JdB. If ScbR2 indeed functions as a repressor of both redDp and adpAp, its mutant ΔscbR2 would be expected to overproduce Red and to differentiate earlier. The ΔscbR2 mutant did show early aerial hyphae production and sporulation compared with M145 (Fig. S6A), but it did not overproduce Red; in fact, it completely lost the ability to produce Red. In contrast, the scbR2-overexpressing strain (M145::scbR2) lost the ability to differentiate on SMM plates (Fig. S6A).
Fig. 5.
The feed-forward loop controlling different behaviors of S. coelicolor M145 in respond to different concentrations of JdB. (A) Dissociation curves of ScbR2–DNA complexes in response to JdB. The concentrations of JdB used were 0, 0.05, 0.1, 0.2, 0.5, 1, 2, 4, and 5 mM. Black squares, red closed circles, green triangles, and blue triangles represent the estimated relative unbound DNA of the PredD, PadpA, PscbA, and PkasO probes, respectively. (B) The activities of redDp and adpAp at different concentrations of JdB reported by the gusA reporter gene. Values are means and SDs from triplicate cultures. The trends of expression are fitted by Gaussian function above the columns of expression levels at different concentrations of JdB. (C) Red production of different adpA-overexpression strains. A concentration of 4 mM theophylline (Theo) was used to induce M145::RadpA. The values represent the average of at least three independent cultures plus SDs. (Inset) The Red production phenotypes of the adpA-overexpression strains on SMM plate: a, M145; b, M145::EadpA; c, M145::RadpA. (D) The signal transduction circuit controlling survival responses to JdB in S. coelicolor M145.
To understand why Red production is turned off at high JdB concentration, we designed an in vivo experiment to monitor the expression levels of redD and adpA in M145, ΔscbR2, and M145::scbR2 using the gusA reporter gene. Two reporter plasmids capable of monitoring redDp or adpAp were introduced into M145, ΔscbR2, and M145::scbR2, respectively, and the expression levels of redDp and adpAp in response to different JdB concentrations in liquid SMM were measured. As shown in Fig. 5B, the expression levels of redDp and adpAp (measured as GusA activities) displayed two different trends: Higher levels of redD expression were observed at a lower range of JdB concentrations (2.5–5 μM), but, in contrast, higher levels of adpA expression were detected at 7.5 μM JdB in the M145 transformants. Thus, interestingly, when the expression of adpA rose, the expression of redDp quickly declined (Fig. 5B). In contrast, in ΔscbR2, the expression of redD and adpA showed no response to JdB (Fig. S7), again indicating that the dynamic pattern of the expression of redD and adpA was controlled by ScbR2. Importantly, in ΔscbR2, the expression of redD constantly remained at a low level, whereas adpA was continuously expressed at a high level (Figs. S6B and S7), which are similar to the adpA and redD expression profiles of M145 transformants to 7.5 μM JdB. The low expression level of redD under these conditions provides a molecular explanation for the lack of Red production in ΔscbR2.
A Feed-Forward Loop Controlling Two Distinct Phenotypes.
Based on previous reports, the pleiotropic regulatory protein AdpA could directly bind to and negatively regulate redDp expression (24, 27, 28). Overexpression of adpA reduced Red production, whereas disruption of adpA increased Red production (24, 27). The direct binding of AdpA with redD promoter was demonstrated by EMSA (28). To prove that the decline of redD expression level at 7.5 μM JdB is due to the induced overexpression of AdpA, strains in which adpA overexpressed at different levels were constructed. M145::EadpA contains an additional copy of adpA expressed from the constitutive promoter ermEp*, and M145::RadpA contains an additional copy of adpA expressed from a riboswitch-controlled ermEp1-E* promoter (29). As expected, Red production was severely reduced in the adpA-overexpressing strains both in liquid medium and on plate (Fig. 5C): The riboswitch-controlled adpA could be induced to overexpress by the corresponding signal (theophylline), and a reduction of Red production in response to this signal could be observed (Fig. 5C). These results from in vivo experiments, in which adpA levels were quantitatively adjusted, were fully consistent with redD expression being negatively modulated by AdpA. Together, our results reveal a clear mechanism by which different JdB concentrations could induce different phenotypes by controlling the relative levels of AdpA and RedD (Fig. 5D). This dynamic control is made possible by the differential dissociation of ScbR2 from the two targets: adpAp and redDp. When the concentration of external JdB is low (1–5 μM), JdB could only relieve the repression of ScbR2 on redDp so to trigger Red production; when JdB levels increase further (to ∼7.5 μM), the transcription of adpA is de-repressed from ScbR2, thus turning on aerial hyphae differentiation, and at the same time turning off Red production due to the repression of redDp by AdpA.
Prevalence of Angucycline Signals.
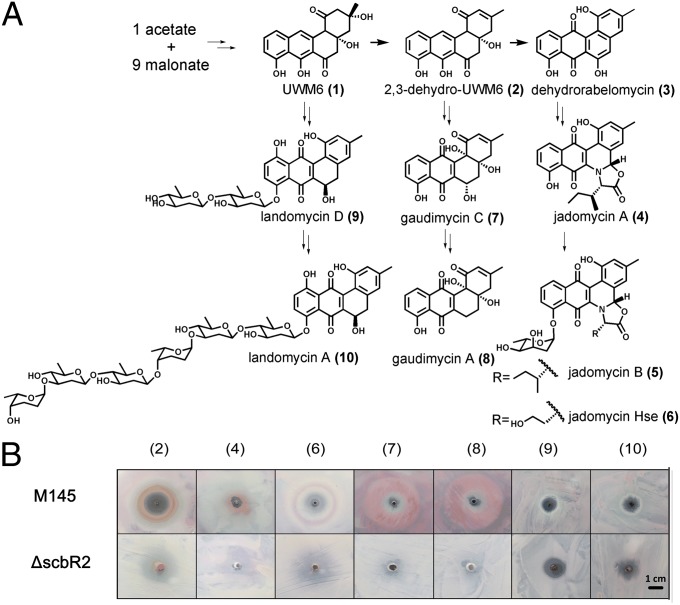
To find out whether angucyclines from different streptomycetes could function as signals and be sensed by ScbR2, the dissociation of ScbR2 from scbA/Rp was evaluated. The experiment was first carried out in the presence of 2,3-dehydro-UWM6 (DHU; 2) (Fig. 6A), a common intermediate in angucycline biosynthesis (30). DHU indeed inhibited the binding of ScbR2 to the target DNA (Fig. S8). We then tested the activity of a series of angucycline compounds to induce Red production in the plate assay: DHU; Jadomycin A (4) and Hse (6) from the jadomycin pathway; gaudimycin A (8) and C (7) from Streptomyces sp. PGA64 (31); and landomycin A (10) and D (9) found in Streptomyces cyanogenus S136 (32) (Fig. 6A). As shown in Fig. 6B, most compounds induced Red production, except 10 and 9. The lack of signalling effects of 10 and 9 may be due to the long sugar chain attached to the landomycin core structure. ΔscbR2 lost such response, suggesting that these angucyclines were sensed by ScbR2.
Fig. 6.
A simplified biosynthetic route to representative angucyclines and the phenotypic responses of S. coelicolor to various angucyclines. (A) A simplified biosynthetic route to representative angucyclines; the key structures are numbered in brackets. (B) The phenotypic responses of S. coelicolor to various angucyclines on SMM plates. The angucyclines were dissolved in 100% DMSO, and 10 μL (10 mM) was spotted on a lawn of S. coelicolor grown on SMM.
Discussion
Angucyclines are the largest group of polycyclic aromatic polyketides identified in actinomycetes (30). Previously, they were mainly recognized for their diverse chemical scaffolds and biological activities. In this work, these molecules are discovered as agents capable of inducing complex survival phenotypes in a streptomycete. This finding implies they may act as signaling molecules in interspecies interactions.
The receptor of exogenous angucycline signals was firmly identified as ScbR2, which represents a recently characterized class of antibiotic signal receptors, widely distributed among streptomycetes (13). The fact that ScbR2 could recognize multiple structurally distinct antibiotics—including the endogenous antibiotics, Act and Red (12), and exogenous angucyclines—endows to it the property to integrate information from multiple signals; thus, ScbR2 could play important roles not only in intracellular communication but also in interspecies interaction and cellular adaptation to environmental signals. Hitherto, four targets (adpA, redD, kasO, and scbA/R) directly controlled by ScbR2 have been identified (Fig. S9). AdpA is the master regulator of differentiation and secondary metabolism that coordinates the expression of hundreds of genes involved in aerial hyphae production and sporulation (23–25). scbA and scbR (encoding GBL receptor and synthase, respectively) form a divergent gene pair controlling the temporal production of GBL signal in S. coelicolor (33). Both redD and kasO are cluster-situated regulatory genes, controlling production of Red and the yellow-colored coelimycin, respectively (21, 26). All these genes governed by ScbR2 are key regulatory genes in the growth cycle of S. coelicolor, so ScbR2 is a pleiotropic regulator of many downstream genes. It is important to point out that the binding between ScbR2 and adpAp and redDp disappeared at 61 h during the stationary phase (Fig. 4C), probably because ScbR2 is released from these targets by the endogenous antibiotics, Act and Red, which are programmed to appear at the transition phase before the onset of differentiation (12). Therefore, these endogenous antibiotics may function as physiological signals to fine-tune the timing of cellular behaviors via ScbR2. These predictions underpin ScbR2 as one of the most important regulators of the growth cycle and as a key regulator of survival responses to antibiotic signals in S. coelicolor.
Understandably, the regulation of adpA is complex in S. coelicolor (34). It is repressed by its own product (27), as well as by ScbR2 and another pleiotropic regulator, BldD (35). In addition, adpA mRNA is a target of RNase III processing and contains a bldA-dependent UUA codon, so the level of AdpA is controlled by the abundance of bldA tRNA and mRNA stability (25, 36). Recent results showed that the expression of AdpA and bldA is highly mutually dependent. AdpA directly activates bldA expression, and the latter is needed for the functional translation of AdpA. Therefore, AdpA and bldA form a strong positive-feedback regulatory relationship that is biologically important in that it could trigger the irreversible onset of morphological differentiation and sporulation (37). AdpA also plays an important role in the regulation of secondary metabolism (34): There is evidence suggesting that it directly regulates both Red and Act production in S. coelicolor (24, 28). In this work, the finding that AdpA negatively regulates Red production when it is highly expressed is biologically relevant because it is important to shut down antibiotic production once the cells have made the commitment to differentiate, which will allow the allocation of limited resources to aerial growth. Because there is also ample evidence to suggest that AdpA could trigger antibiotic production (34), it is possible that AdpA plays dual roles in the regulation of endogenous antibiotic production—i.e., acting both as activators and repressors on the same target genes at different expression levels (38). A high level of AdpA kept redD at a low level, despite the fact that AdpA directly activates bldA expression, which is required for the translation of redZ, and RedZ is the activator of redD expression (39). Thus, in theory, a high level of AdpA should indirectly up-regulate redD expression via bldA and redZ; however, due to the fact that AdpA also directly regulates redD, the combined outcome is that a high level of AdpA will override the activation by RedZ and thus shut down redD expression.
ScbR2, AdpA, and RedD form an incoherent type II feed-forward loop (FFL) (40) controlling two distinct physiological behaviors (morphological differentiation and Red production) of M145 in response to an exogenous JdB signal (Fig. 5D). It is important to point out that, within this FFL, RedD is the bottom-level regulator regulated by both ScbR2 and AdpA, so the expression of redD is controlled by the dynamic levels of ScbR2 and AdpA. By such a design, the regulatory loop has gained the property of responding to signal concentrations. Thus, two distinct physiological behaviors (aerial hyphae differentiation and Red production) of M145 in response to exogenous JdB signal have been explained mechanistically at molecular levels. It is important to note that this FFL is part of a network consisting of two top-level regulators—ScbR and ScbR2—and three lower-level regulators—AdpA, KasO, and RedD—which also control their own regulons (Fig. S9). Based on this network, we could predict the behavior of S. coelicolor at higher (inhibitory) concentrations of JdB. At these concentrations, the scbR/A and kasO should be induced to express, so that GBL and coelimycin should be produced. Therefore, a concentration gradient of antibiotic could induce a series of different responses; such complex responses are highly relevant to the survival and effective competition of streptomycetes. These findings represent a significant advance in the understanding of the ecological and physiological consequences of antibiotic production.
Materials and Methods
Bacterial Strains and Growth Conditions.
Bacterial strains used in this study are listed in Table S1. S. coelicolor strains M145, ΔscbR2 (scbR2DM), M145::scbR2 (scbR2OE), and ΔscbR2::scbR2 (scbR2COM) were handled as described (12). S. venezuelae ISP5230 was grown in glucose–Mops (GM) medium (13). SMM agar was used to observe the phenotypic responses of S. coelicolor M145, ΔscbR2, M145::scbR2, and ΔscbR2::scbR2 to angucyclines. The effect of JdB to S. coelicolor was also tested in liquid SMM (41). The growth rates of S. coelicolor M145 were measured by the diphenylamine method (42). E. coli strains were grown in Luria–Bertani containing ampicillin (100 μg/mL), kanamycin (50 μg/mL), or chloramphenicol (50 μg/mL) when necessary.
Preparations of S. venezuelae ISP5230 Culture Extracts and Bioassay.
Cultures of S. venezuelae ISP5230 grown in GM medium for different times were harvested and extracted by ethyl acetate. The extracts were dried, dissolved in DMSO, and then used in bioassay on a lawn of S. coelicolor M145. The plate indicator bioassay for GBL molecules was carried out as described by Takano et al. (15). JdB was isolated and purified from S. venezuelae ISP5230 cultures as described (43).
Liquid Culture Analysis of the Effects of JdB on S. coelicolor M145 and Measurement of Red Production.
S. coelicolor M145 was grown in liquid SMM. Replicate cultures were inoculated with increasing amounts of JdB, and the cultures were incubated at 30 °C at 220 rpm for 48 h before observation.
The mycelia were harvested, treated, and used to measure Red production by HPLC; the peak corresponding to Red was analyzed by LC-MS. JdB and other angucyclines samples were purified to show a single peak on HPLC. Details of sample preparation, conditions to perform HPLC, and HPLC-MS are presented in SI Materials and Methods.
Gel Mobility Shift Assays (EMSAs).
His6-tagged ScbR2 was purified from E. coli BL21 (DE3) harboring pET23b::scbR2, as described (12). To identify target binding sites of ScbR2, the promoter regions of adpA, kasO, scbA, redD, redQ, redV, redZ, and hrdB were obtained by PCR from the genomic DNA of S. coelicolor M145 using the primers listed in Table S2. The subsequent binding experiments were performed by using a modified gel mobility shift assay as described (12) (SI Materials and Methods).
Bioluminescence Detection in E. coli and β-Glucuronidase Activity Assay in S. coelicolor.
The plasmids used are listed in Table S1, and the primers are listed in Table S2. The plasmid pOkasOplux containing kasOp controlled lux reporter genes, and ScbR2 expression vector pScbR2 were previously constructed in our laboratory (12). The bioluminescence of E. coli reporter cultures was measured after 12-h incubation by using a single-tube luminometer (Turner Biosystems 20/20n). The construction of gusA reporter plasmids and strains and β-glucuronidase activity assay in S. coelicolor are described in SI Materials and Methods.
ITC.
Protein concentration was determined spectrophotometrically at 280 nm by using a molar extinction coefficient (ε280). Both ScbR2 and JdB were dissolved in the same buffer [5 mM NaCl, 2 mM Tris⋅HCl, pH 7.0, 1 mM DTT, 5% (vol/vol) glycerol]. The titration was initiated with a first injection of 2 μL of ScbR2, followed by 19 injections of 4 μL at 25 °C. The instrument software was used to calculate the normalized heats released from each injection.
ChIP-qPCR.
The ChIP protocol was modified from Johnson et al. (44). Details are presented in SI Materials and Methods.
Promoter Dissociation Assays and Calculation of Dissociation Curves.
By using the above-described EMSA conditions, small molecules were added at various concentrations. The relative abundance of bound probes was measured by grayscale. The dissociation curves of ScbR2–DNA complexes in respond to JdB were fitted by the software Origin (Version 8.0).
Construction of AdpA Overexpression Strains.
The construction of different AdpA overexpression plasmids and strains is presented in SI Materials and Methods. The effects on Red production were observed in liquid SMM, and the levels of Red production were measured by HPLC.
Preparations of Jadomycin Pathway Intermediates and Other Angucyclines.
Details are presented in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Profs. Keith Chater, Julian Davies, and Chunbo Lou for their critical comments; Dr. Xiaoyu Yang and Prof. Biao Yu (Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences) for the samples of landomycins; Dr. M. Metsä-Ketelä (University of Turku) for the samples of gaudimycins; Prof. Beatrix Suess (Technische Universität Darmstadt) for the riboswitch plasmid pGusT-ermE-E*; and Prof. Xiaoming Ding (Fudan University) for the plasmid pLC803. This work was supported by National Natural Science Foundation of China Grant 31130001 and Ministry of Science and Technology of China Grant 2013CB734001.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1324253111/-/DCSupplemental.
References
- 1.Goh EB, et al. Transcriptional modulation of bacterial gene expression by subinhibitory concentrations of antibiotics. Proc Natl Acad Sci USA. 2002;99(26):17025–17030. doi: 10.1073/pnas.252607699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yim G, Wang HH, Davies J. Antibiotics as signalling molecules. Philos Trans R Soc Lond B Biol Sci. 2007;362(1483):1195–1200. doi: 10.1098/rstb.2007.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fajardo A, Martínez JL. Antibiotics as signals that trigger specific bacterial responses. Curr Opin Microbiol. 2008;11(2):161–167. doi: 10.1016/j.mib.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Romero D, Traxler MF, López D, Kolter R. Antibiotics as signal molecules. Chem Rev. 2011;111(9):5492–5505. doi: 10.1021/cr2000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman LR, et al. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature. 2005;436(7054):1171–1175. doi: 10.1038/nature03912. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich LE, Teal TK, Price-Whelan A, Newman DK. Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science. 2008;321(5893):1203–1206. doi: 10.1126/science.1160619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koteva K, et al. A vancomycin photoprobe identifies the histidine kinase VanSsc as a vancomycin receptor. Nat Chem Biol. 2010;6(5):327–329. doi: 10.1038/nchembio.350. [DOI] [PubMed] [Google Scholar]
- 8.Chiu ML, et al. Broad spectrum thiopeptide recognition specificity of the Streptomyces lividans TipAL protein and its role in regulating gene expression. J Biol Chem. 1999;274(29):20578–20586. doi: 10.1074/jbc.274.29.20578. [DOI] [PubMed] [Google Scholar]
- 9.Vetsigian K, Jajoo R, Kishony R. Structure and evolution of Streptomyces interaction networks in soil and in silico. PLoS Biol. 2011;9(10):e1001184. doi: 10.1371/journal.pbio.1001184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amano S, et al. Promomycin, a polyether promoting antibiotic production in Streptomyces spp. J Antibiot (Tokyo) 2010;63(8):486–491. doi: 10.1038/ja.2010.68. [DOI] [PubMed] [Google Scholar]
- 11.Amano SI, et al. A cryptic antibiotic triggered by monensin. J Antibiot (Tokyo) 2011;64(10):703. doi: 10.1038/ja.2011.69. [DOI] [PubMed] [Google Scholar]
- 12.Xu G, et al. “Pseudo” gamma-butyrolactone receptors respond to antibiotic signals to coordinate antibiotic biosynthesis. J Biol Chem. 2010;285(35):27440–27448. doi: 10.1074/jbc.M110.143081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, et al. A novel role of ‘pseudo’γ-butyrolactone receptors in controlling γ-butyrolactone biosynthesis in Streptomyces. Mol Microbiol. 2011;82(1):236–250. doi: 10.1111/j.1365-2958.2011.07811.x. [DOI] [PubMed] [Google Scholar]
- 14.Takano E. Gamma-butyrolactones: Streptomyces signalling molecules regulating antibiotic production and differentiation. Curr Opin Microbiol. 2006;9(3):287–294. doi: 10.1016/j.mib.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Takano E, et al. Purification and structural determination of SCB1, a gamma-butyrolactone that elicits antibiotic production in Streptomyces coelicolor A3(2) J Biol Chem. 2000;275(15):11010–11016. doi: 10.1074/jbc.275.15.11010. [DOI] [PubMed] [Google Scholar]
- 16.Doull JL, Singh AK, Hoare M, Ayer SW. Conditions for the production of jadomycin B by Streptomyces venezuelae ISP5230: Effects of heat shock, ethanol treatment and phage infection. J Ind Microbiol. 1994;13(2):120–125. doi: 10.1007/BF01584109. [DOI] [PubMed] [Google Scholar]
- 17.Jakeman DL, et al. Antimicrobial activities of jadomycin B and structurally related analogues. Antimicrob Agents Chemother. 2009;53(3):1245–1247. doi: 10.1128/AAC.00801-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng JT, et al. Cytotoxic activities of new jadomycin derivatives. J Antibiot (Tokyo) 2005;58(6):405–408. doi: 10.1038/ja.2005.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Draczkowski P, Matosiuk D, Jozwiak K. Isothermal titration calorimetry in membrane protein research. J Pharm Biomed Anal. 2014;87:313–325. doi: 10.1016/j.jpba.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Malpartida F, Niemi J, Navarrete R, Hopwood DA. Cloning and expression in a heterologous host of the complete set of genes for biosynthesis of the Streptomyces coelicolor antibiotic undecylprodigiosin. Gene. 1990;93(1):91–99. doi: 10.1016/0378-1119(90)90141-d. [DOI] [PubMed] [Google Scholar]
- 21.Takano E, et al. Transcriptional regulation of the redD transcriptional activator gene accounts for growth-phase-dependent production of the antibiotic undecylprodigiosin in Streptomyces coelicolor A3(2) Mol Microbiol. 1992;6(19):2797–2804. doi: 10.1111/j.1365-2958.1992.tb01459.x. [DOI] [PubMed] [Google Scholar]
- 22.Ohnishi Y, Yamazaki H, Kato JY, Tomono A, Horinouchi S. AdpA, a central transcriptional regulator in the A-factor regulatory cascade that leads to morphological development and secondary metabolism in Streptomyces griseus. Biosci Biotechnol Biochem. 2005;69(3):431–439. doi: 10.1271/bbb.69.431. [DOI] [PubMed] [Google Scholar]
- 23.Wolański M, Jakimowicz D, Zakrzewska-Czerwińska J. AdpA, key regulator for morphological differentiation regulates bacterial chromosome replication. Open Biol. 2012;2(7):120097. doi: 10.1098/rsob.120097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen KT, et al. Colonial differentiation in Streptomyces coelicolor depends on translation of a specific codon within the adpA gene. J Bacteriol. 2003;185(24):7291–7296. doi: 10.1128/JB.185.24.7291-7296.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takano E, et al. A rare leucine codon in adpA is implicated in the morphological defect of bldA mutants of Streptomyces coelicolor. Mol Microbiol. 2003;50(2):475–486. doi: 10.1046/j.1365-2958.2003.03728.x. [DOI] [PubMed] [Google Scholar]
- 26.Takano E, et al. A bacterial hormone (the SCB1) directly controls the expression of a pathway-specific regulatory gene in the cryptic type I polyketide biosynthetic gene cluster of Streptomyces coelicolor. Mol Microbiol. 2005;56(2):465–479. doi: 10.1111/j.1365-2958.2005.04543.x. [DOI] [PubMed] [Google Scholar]
- 27.Wolanski M, et al. The level of AdpA directly affects expression of developmental genes in Streptomyces coelicolor. J Bacteriol. 2011;193(22):6358–6365. doi: 10.1128/JB.05734-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park SS, et al. Mass spectrometric screening of transcriptional regulators involved in antibiotic biosynthesis in Streptomyces coelicolor A3(2) J Ind Microbiol Biotechnol. 2009;36(8):1073–1083. doi: 10.1007/s10295-009-0591-2. [DOI] [PubMed] [Google Scholar]
- 29.Rudolph MM, Vockenhuber MP, Suess B. Synthetic riboswitches for the conditional control of gene expression in Streptomyces coelicolor. Microbiology. 2013;159(Pt 7):1416–1422. doi: 10.1099/mic.0.067322-0. [DOI] [PubMed] [Google Scholar]
- 30.Kharel MK, et al. Angucyclines: Biosynthesis, mode-of-action, new natural products, and synthesis. Nat Prod Rep. 2012;29(2):264–325. doi: 10.1039/c1np00068c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kallio P, Liu Z, Mäntsälä P, Niemi J, Metsä-Ketelä M. Sequential action of two flavoenzymes, PgaE and PgaM, in angucycline biosynthesis: Chemoenzymatic synthesis of gaudimycin C. Chem Biol. 2008;15(2):157–166. doi: 10.1016/j.chembiol.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Henkel T, Rohr J, Beale JM, Schwenen L. Landomycins, new angucycline antibiotics from Streptomyces sp. I. Structural studies on landomycins A-D. J Antibiot (Tokyo) 1990;43(5):492–503. doi: 10.7164/antibiotics.43.492. [DOI] [PubMed] [Google Scholar]
- 33.Takano E, Chakraburtty R, Nihira T, Yamada Y, Bibb MJ. A complex role for the gamma-butyrolactone SCB1 in regulating antibiotic production in Streptomyces coelicolor A3(2) Mol Microbiol. 2001;41(5):1015–1028. doi: 10.1046/j.1365-2958.2001.02562.x. [DOI] [PubMed] [Google Scholar]
- 34.Liu G, Chater KF, Chandra G, Niu G, Tan H. Molecular regulation of antibiotic biosynthesis in streptomyces. Microbiol Mol Biol Rev. 2013;77(1):112–143. doi: 10.1128/MMBR.00054-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.den Hengst CD, et al. Genes essential for morphological development and antibiotic production in Streptomyces coelicolor are targets of BldD during vegetative growth. Mol Microbiol. 2010;78(2):361–379. doi: 10.1111/j.1365-2958.2010.07338.x. [DOI] [PubMed] [Google Scholar]
- 36.Xu W, Huang J, Lin R, Shi J, Cohen SN. Regulation of morphological differentiation in S. coelicolor by RNase III (AbsB) cleavage of mRNA encoding the AdpA transcription factor. Mol Microbiol. 2010;75(3):781–791. doi: 10.1111/j.1365-2958.2009.07023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higo A, Horinouchi S, Ohnishi Y. Strict regulation of morphological differentiation and secondary metabolism by a positive feedback loop between two global regulators AdpA and BldA in Streptomyces griseus. Mol Microbiol. 2011;81(6):1607–1622. doi: 10.1111/j.1365-2958.2011.07795.x. [DOI] [PubMed] [Google Scholar]
- 38.Pan Y, Liu G, Yang H, Tian Y, Tan H. The pleiotropic regulator AdpA-L directly controls the pathway-specific activator of nikkomycin biosynthesis in Streptomyces ansochromogenes. Mol Microbiol. 2009;72(3):710–723. doi: 10.1111/j.1365-2958.2009.06681.x. [DOI] [PubMed] [Google Scholar]
- 39.White J, Bibb M. bldA dependence of undecylprodigiosin production in Streptomyces coelicolor A3(2) involves a pathway-specific regulatory cascade. J Bacteriol. 1997;179(3):627–633. doi: 10.1128/jb.179.3.627-633.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mangan S, Alon U. Structure and function of the feed-forward loop network motif. Proc Natl Acad Sci USA. 2003;100(21):11980–11985. doi: 10.1073/pnas.2133841100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA. 2000. in Practical Streptomyces Genetics (John Innes Foundation, Norwich, U.K.), pp 410–413.
- 42.Zhao Y, Xiang S, Dai X, Yang K. A simplified diphenylamine colorimetric method for growth quantification. Appl Microbiol Biotechnol. 2013;97(11):5069–5077. doi: 10.1007/s00253-013-4893-y. [DOI] [PubMed] [Google Scholar]
- 43.Fan K, et al. Evaluation of the cytotoxic activity of new jadomycin derivatives reveals the potential to improve its selectivity against tumor cells. J Antibiot (Tokyo) 2012;65(9):449–452. doi: 10.1038/ja.2012.48. [DOI] [PubMed] [Google Scholar]
- 44.Johnson DS, Mortazavi A, Myers RM, Wold B. Genome-wide mapping of in vivo protein-DNA interactions. Science. 2007;316(5830):1497–1502. doi: 10.1126/science.1141319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.