Significance
Centrioles form the core of centrosomes, which organize cilia and interphase and spindle microtubules in animal cells, but centrosome function has not been defined in mammals in vivo. We show that mouse embryos that lack centrioles and centrosomes survive to midgestation, when they lack primary cilia and cilia-dependent signaling. Despite the absence of centrosomes, bipolar spindle formation, chromosome segregation, cell-cycle profile, and DNA damage response are normal in the mutants. Unlike mutants that lack cilia, most cells in acentriolar embryos activate a p53-dependent apoptotic pathway. The data show that mammalian centrioles promote the efficient and rapid assembly of the mitotic spindle and that a short delay in prometaphase activates a checkpoint that leads to p53-dependent cell death in vivo.
Abstract
Centrosomes are the microtubule-organizing centers of animal cells that organize interphase microtubules and mitotic spindles. Centrioles are the microtubule-based structures that organize centrosomes, and a defined set of proteins, including spindle assembly defective-4 (SAS4) (CPAP/CENPJ), is required for centriole biogenesis. The biological functions of centrioles and centrosomes vary among animals, and the functions of mammalian centrosomes have not been genetically defined. Here we use a null mutation in mouse Sas4 to define the cellular and developmental functions of mammalian centrioles in vivo. Sas4-null embryos lack centrosomes but survive until midgestation. As expected, Sas4−/− mutants lack primary cilia and therefore cannot respond to Hedgehog signals, but other developmental signaling pathways are normal in the mutants. Unlike mutants that lack cilia, Sas4−/− embryos show widespread apoptosis associated with global elevated expression of p53. Cell death is rescued in Sas4−/− p53−/− double-mutant embryos, demonstrating that mammalian centrioles prevent activation of a p53-dependent apoptotic pathway. Expression of p53 is not activated by abnormalities in bipolar spindle organization, chromosome segregation, cell-cycle profile, or DNA damage response, which are normal in Sas4−/− mutants. Instead, live imaging shows that the duration of prometaphase is prolonged in the mutants while two acentriolar spindle poles are assembled. Independent experiments show that prolonging spindle assembly is sufficient to trigger p53-dependent apoptosis. We conclude that a short delay in the prometaphase caused by the absence of centrioles activates a previously undescribed p53-dependent cell death pathway in the rapidly dividing cells of the mouse embryo.
Centrioles are cylinders of triplet microtubules that provide the template for cilia and nucleate the centrosomes that act as microtubule organizing centers (MTOCs) at spindle poles and during interphase (1, 2). Genetic analysis has demonstrated that the biological roles of centrioles differ widely among organisms: Caenorhabditis elegans embryos without centrioles arrest at the two-cell stage, whereas zygotic removal of centrioles in Drosophila allows survival to adult stages (3–5). In humans, mutations in centriolar and centrosomal proteins are associated with microcephaly or microcephaly in the context of dwarfism (6–10). Abnormal numbers of centrioles are associated with cancer, although it is not clear whether abnormal centrosome number is a cause or an effect of tumorigenesis (1, 11–13). Studies in cultured cell lines have given conflicting results on the roles of vertebrate centrioles in mitosis, chromosome segregation, DNA damage response, and intercellular signaling (14–19), but the precise functions of mammalian centrioles have not been defined genetically.
A small number of core proteins have been shown to be required for centriole biogenesis in organisms ranging from Chlamydomonas reinhardtii to human cells. Spindle assembly defective-4 (SAS4), one of these core proteins, acts at an early step in the assembly pathway, when it is required for the addition of tubulin subunits to the forming procentrioles; it also is required for recruitment of the pericentriolar material (PCM) to form the centrosome (3, 20, 21). Mutations in Sas4 block centriole formation in Drosophila and C. elegans, and mutations in human SAS4 (CPAP/CENPJ) cause Seckel syndrome (dwarfism with microcephaly) (3–6). siRNA knockdown of SAS4 in cultured mammalian cells was reported to cause formation of multipolar spindles (14).
Here we use null mutations in Sas4 to define the cellular and developmental functions of centrioles in the mouse embryo. As expected, Sas4 is essential for formation of centrioles, centrosomes, and cilia and for cilia-dependent Hedgehog (Hh) signaling. Unexpectedly, Sas4−/− embryos arrest at an earlier stage than mutants that lack cilia and show widespread cell death associated with strong up-regulation of p53 in most cells in the embryo. Genetic removal of p53 rescues both the cell death and the early lethality of Sas4−/− mutants. Cell death in the mutants is not associated with defects in the cell-cycle profile, DNA damage response, or chromosome segregation. The data indicate that in Sas4−/− mouse embryos prolonged prometaphase, caused by a delay in spindle pole assembly, triggers a previously uncharacterized checkpoint that activates p53-dependent apoptosis in vivo.
Results
Sas4-Null Embryos Arrest at Midgestation.
Animals homozygous for a partial loss-of-function mutation in Sas4/Cenpj/Cpap generated by the International Knockout Mouse Consortium (IKMC) are viable but dwarfed and represent a model for human Seckel syndrome (22). To define the precise functions of mammalian centrioles, we generated a null allele from the IKMC partial loss-of-function allele (Materials and Methods). At embryonic day (E)8.5, Sas4−/− embryos had a normal anterior–posterior body axis and specified all three germ layers (Fig. 1 A, C, and D and Fig. S1 A and B). All Sas4−/− embryos arrested by E9.0, when they had made a heart and neural tube but failed to undergo embryonic turning (Fig. 1B). SAS4 protein was not detectable in E8.5 Sas4−/− embryos (Fig. S1C). To test whether a maternal contribution of SAS4 allowed early development in the mutants, we generated maternal/zygotic Sas4-null mutants (Materials and Methods), which were indistinguishable from the zygotic-null embryos (Fig. S1D). Thus, mouse embryos can survive to midgestation and lay down the basic body plan in the complete absence of SAS4.
Fig. 1.
Sas4−/− mouse embryos arrest at mid-gestation. (A) Dorsal view of wild-type and Sas4−/− embryos at E8.5. (B) Wild-type and Sas4−/− embryos at E9.5. (C and D) Cross-sections of wild-type and Sox2-Cre; Sas4fl/- embryos at E8.5 stained for E-cadherin (red). Arrows indicate the neural plate (ectoderm), arrowheads indicate the mesenchyme (mesoderm), and asterisks indicate the gut (endoderm). (Scale bars: 300 µm.)
Widespread p53-Dependent Cell Death in Sas4−/− Embryos.
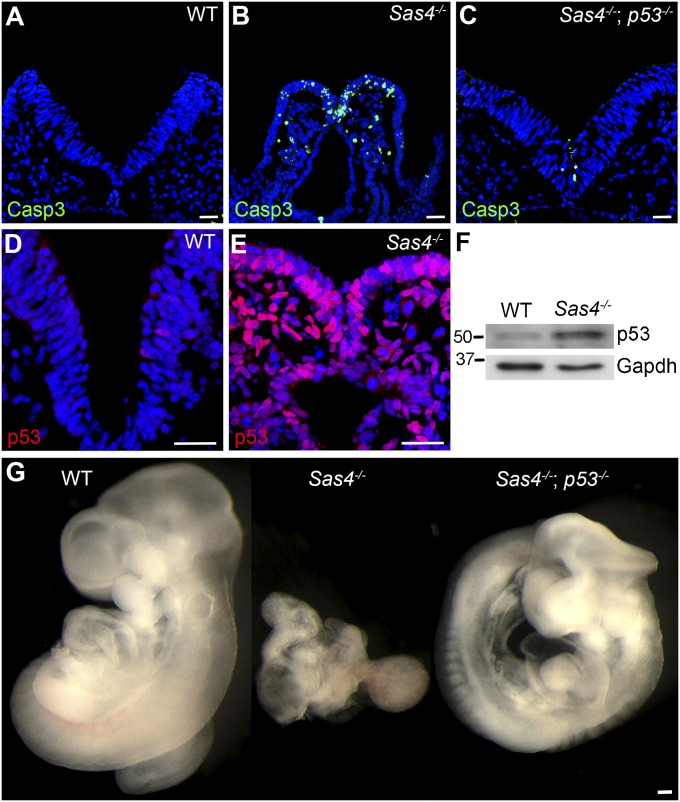
Sas4−/− embryos died more than a day earlier than mutants that lack cilia (23), indicating that centrioles in the developing embryo have functions in addition to acting as templates for cilia. DAPI-stained sections of Sas4−/− embryos showed many pyknotic and fragmented nuclei. Levels of cleaved caspase-3 (Casp3) were elevated in the mutants, revealing that there was widespread apoptosis in all cell types of the mutant at E8.5 (Fig. 2 A and B). Because up-regulation of p53 is a well-known mediator of apoptosis, we examined p53 expression in mutant embryos. In contrast to wild-type embryos, in which p53-positive (p53+) cells were rare, nuclear p53 was detected in nearly every healthy interphase cell in E8.5 mutant embryos (Fig. 2 D and E). By Western blot, the level of p53 was approximately fivefold higher in Sas4−/− embryos than in wild-type embryos (Fig. 2F).
Fig. 2.
Cell death and early lethality in Sas4−/− embryos are rescued by removal of p53. (A–C) Cleaved Casp3 (green) expression in cross-sections of wild-type embryos (0.6 ± 0.4%, n = 3,073 from two embryos) (A), Sas4−/− embryos (23 ± 4%, n = 3,150 from three embryos, P = 0.0048) (B), and Sas4−/− p53−/− embryos (2.7 ± 0.5%, n = 3,454 from two embryos, P = 0.0063) (C) at E8.5. (D and E) p53 (red) is rare in wild-type embryos (0.4 ± 0.1%, n = 3,534 from two embryos) but is strongly expressed in Sas4−/− mutant embryos (91 ± 1%, n = 2,629 from three embryos, P < 0.0001). (F) Western blot analysis of p53 in E8.5 embryo extracts. (G) Wild-type, Sas4−/−, and Sas4−/− p53−/− embryos at E9.5. P values are relative to wild type in each case. (Scale bars: 30 µm in A–E, 300 µm in G.)
To test whether the apoptosis in Sas4−/− mutants was caused by elevated p53, we generated Sas4−/− p53−/− double-mutant embryos. The double mutants survived until at least E9.5, and the number of Casp3+ apoptotic cells was greatly reduced as compared with Sas4−/− single mutants (Fig. 2 C and G). Sas4−/− p53−/− embryos at E9.5 had >20 somites, completed embryonic turning, and showed the randomized left–right situs and the abnormal brain morphology characteristic of mutants that lack cilia (Fig. 2G) (23). Ift88−/− mutants (24), which lack cilia but have normal centrosomes (Fig. S2 A and B), did not show elevated cell death or p53 expression (Fig. S2 D and E). Embryos homozygous for a mutation in another gene required for centriole biogenesis, centrosomal protein-152 (Cep152) (Cep152gt/gt) (Fig. S2C), also showed increased apoptosis and p53 expression (Fig. S2F).
Sas4−/− Embryos Lack Centrioles and Cilia.
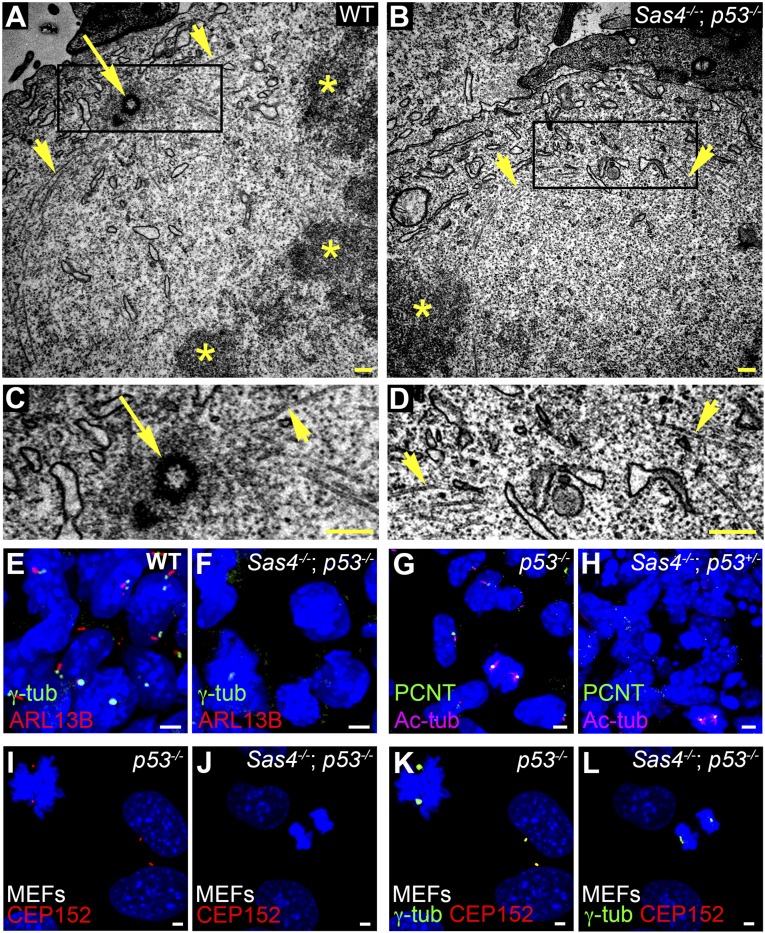
The longer survival of Sas4−/− embryos that lacked p53 facilitated the analysis of the cellular phenotypes caused by removal of SAS4. We used transmission electron microscopy (TEM) analysis of serial sections of E8.5 Sas4−/− p53−/− neural epithelium to assess whether centrioles were eliminated in the mutants. We observed 90 centrioles in 134 sections of control mitotic and nonmitotic cells (Fig. 3 A and C and Fig. S3 A and C). In contrast, no centrioles were seen in 174 sections of Sas4−/− p53−/− embryos (Fig. 3 B and D and Fig. S3 B and D). Even in mutant mitotic cells (n = 4) in which condensed chromosomes were in metaphase conformation, microtubules focused toward a pole that lacked centrioles in 23 serial sections (compare Fig. 3 B and D with Fig. 3 A and C).
Fig. 3.
Sas4−/− embryos lack centrioles, centrosomes, and cilia. (A–D) TEM of E8.5 neural folds. Microtubules (arrowheads) converge at metaphase spindle poles, where there are centrioles (arrow) in wild-type but not Sas4−/− p53−/− embryos. Asterisks mark the condensed chromosomes in A and B. C and D are magnifications of the boxed regions at the spindle poles in A and B, respectively. (E–H) Expression of the centrosomal and cilia markers in the mesenchymal cells in E8.5 wild-type and Sas4−/− embryos stained for γ-tubulin (γ-tub) or PCNT (green) and ARL13B (red) or Ac-tub (magenta). Cilia are absent, and focal staining of γ-tubulin and PCNT is detected only during mitosis in Sas4−/− mutants. (I–L) Expression of the PCM proteins CEP152 (red) and γ-tubulin (green) in p53−/− MEFs [45 ± 5 arbitrary units (A.U.)] and Sas4−/− p53−/− MEFs (25 ± 9 A.U., P < 0.0001, n = 14 poles each). CEP152 is not detected in either interphase or mitotic cells in Sas4−/− mutants. (Scale bars: 0.3 µm in A–D, 3 µm in E–L.)
Centrioles are required for the formation of cilia in all eukaryotes (25). The wild-type embryonic node has long cilia at E7.5 (Fig. S3E), but no cilia were detected by scanning electron microscopy in the Sas4−/− mutant node (Fig. S3F). Although cilia were present on most cells in wild-type embryos, no cilia were detected by immunostaining with the cilia markers ARL13B or acetylated α-tubulin (Ac-tub) in Sas4−/− embryos at E8.5 (Fig. 3 E–H).
No focal organization of the PCM proteins γ-tubulin or pericentrin (PCNT) was detected in interphase cells of E8.5 Sas4−/− embryos (Fig. 3 E–H), confirming that removal of SAS4 disrupted the organization of the centrosome. Western blot analysis showed that total γ-tubulin levels were comparable in E8.5 wild-type and mutant embryos, indicating that γ- tubulin was still present in the absence of a centriole (Fig. S4). Unlike Drosophila Sas4−/− mutants (5), both γ-tubulin and PCNT were enriched at the spindle poles of dividing cells in Sas4−/− mutants at E8.5, albeit at lower levels than in wild-type embryos (Fig. 3 E–H; see also Fig. S8 A–F). Similarly, in mouse embryonic fibroblasts (MEFs) derived from Sas4−/− p53−/− embryos, γ-tubulin staining was not detected during interphase but was present at lower levels at the mitotic poles than in p53−/− control cells (Fig. 3 K and L). In contrast, focal staining of CEP152, a PCM protein that is required for centriole formation, was not detected in either interphase or mitotic cells of Sas4−/− p53−/− MEFs (Fig. 3 I–L), indicating that CEP152 recruitment to the centrosome depends on SAS4.
Hh Signaling Is Disrupted in Sas4−/− Embryos, but Wnt Signaling Is Normal.
Primary cilia are required for responses to Hh family ligands in the mouse (23, 26). As expected, two direct targets of Hh signaling, Gli1 and Ptch1, were not expressed in Sas4−/− mutants at E8.0–8.5 (Fig. S5 A and B) because of the absence of cilia. Dorsal–ventral patterning of the neural tube provides an assay for different levels of Sonic hedgehog (Shh) signaling. The survival of Sas4−/− p53−/− mutants to E9.5 made it possible to analyze neural patterning in the absence of centrioles. The ventral neural cell types that depend on high or intermediate levels of Shh signaling (FOXA2 and NKX2.2) were absent in the double mutants (Fig. 4 A and B), and markers that normally are repressed by Shh (e.g., PAX6) spread into the ventral neural tube (Fig. 4 C and D). Thus, Shh-dependent neural patterning was disrupted in Sas4−/− p53−/− mutants in the same way as in mutants that lack cilia (23).
Fig. 4.
Hh, but not Wnt, signaling is disrupted in Sas4−/− mutants. (A–D) Hh-dependent ventral cell types in the neural tube of E9.5 wild-type and Sas4−/− p53−/− embryos. In Sas4−/− p53−/− embryos floor plate cells (FOXA2, red) and V3 interneurons (NKX2.2, green) are absent (compare A and B), and PAX6+ interneurons (green) occupy the ventral neural tube (compare C and D). (E and F) The activity of the canonical Wnt reporter TOPGAL (blue) is normal in E8.5 Sas4−/− mutants. (G–L) The PCP noncanonical Wnt signaling protein VANGL2 (green) localizes to the anterior/posterior (horizontal) faces of the cells in the embryonic node of both wild-type (G–I) and Sas4−/− p53−/− embryos (J–L) at E7.75. Rhodamine-conjugated phalloidin (red) marks F-actin at cell borders. (Scale bars: 300 µm in A–F, 3 µm in G–L.)
A number of components of Wnt signaling pathways are enriched at centrosomes, including β-catenin (19), AXIN1 (27), AXIN2 (28), and Dishevelled (29), and studies have suggested that both canonical and noncanonical Wnt signaling depend on centrosomes or cilia (30–32). However, the phenotypes of Sas4−/− embryos do not resemble those of Wnt pathway mutants. For example, Wnt3 and Axin1 mutants arrest at gastrulation, and Wnt3a mutants are posteriorly truncated (33–35); neither phenotype was seen in Sas4−/− p53−/− mutants (Fig. 2G), suggesting that canonical Wnt signaling was not strongly disrupted. Expression of TOPGAL, a transgenic reporter of canonical Wnt signaling, was indistinguishable in Sas4−/− mutants and wild-type embryos at E7.5 and E8.5 (Fig. 4 E and F and Fig. S5C). Expression of Axin2, a direct target of canonical Wnt signaling, also was normal in Sas4−/− embryos at E7.5 (Fig. S5D).
Mutations in the noncanonical Wnt pathway cause characteristic morphological abnormalities; the most prominent defect is the failure to close the entire neural tube posterior to the midbrain (craniorachischisis) (36). In contrast, the spinal neural tube in Sas4−/− p53−/− mutants was closed (Figs. 2G and 4 A–D). An objective assay for the activity of the noncanonical Wnt pathway is the polarized expression of planar cell polarity (PCP) proteins on the anterior and posterior faces of cells of the embryonic node (37–39), where noncanonical Wnt signaling is required to polarize node cilia and initiate left–right asymmetry. Although Sas4−/− mutants lack cilia and basal bodies, the PCP protein VANGL2 localized correctly to the anterior/posterior faces of Sas4−/− p53−/− node cells (Fig. 4 G–L and Fig. S5 E and F), indicating that the activity of the noncanonical Wnt pathway is normal in the absence of centrioles.
The Cell-Cycle Profile, Chromosome Segregation, and DNA Damage Response Are Normal in Sas4−/− Embryos.
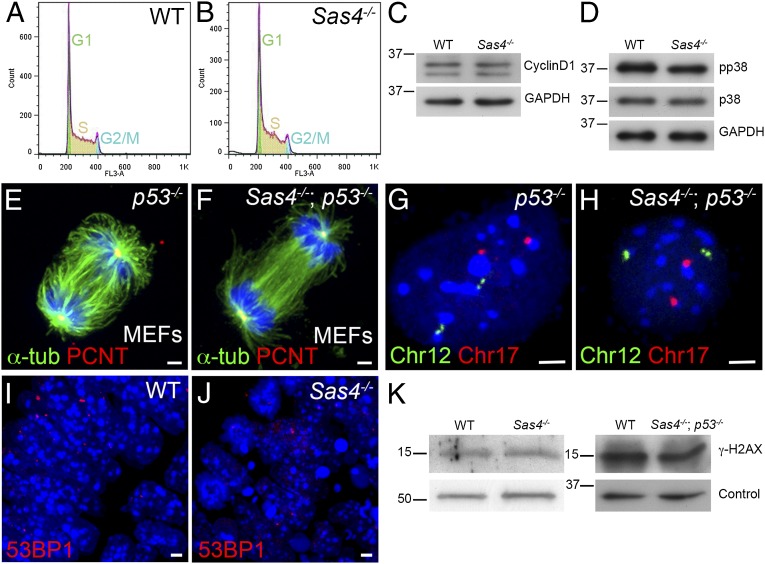
To account for the up-regulation of p53 in the absence of centrioles, we evaluated functions that have been ascribed to centrioles and centrosomes that could regulate p53. Studies that used laser ablation, microsurgery, or siRNA knockdown indicated that disruption of centrosomes in cultured mammalian cells causes a p38-, p53-, and p21-dependent G1 cell-cycle arrest (15, 16). Therefore we analyzed the cell cycle by FACS of dissociated cells from Sas4−/− embryos at E8.5. There were no obvious differences in cell-cycle profiles between Sas4−/− and wild-type embryos (Fig. 5 A and B; G1: 27%, S: 60–63%, and G2/M: 8–12%); in particular, there was no G1/S arrest in the mutants. Consistent with the FACS results, Western blot analysis showed that the level of cyclin D1 (a G1 cyclin) was unchanged in Sas4−/− embryos at E8.5 (Fig. 5C). Unlike the results in cell culture, active phospho-p38 (T180/Y182) was not up-regulated in Sas4−/− embryos at E8.5 (Fig. 5D).
Fig. 5.
Cell-cycle profile, chromosome segregation, and the DNA damage response are normal in Sas4−/− mutants. (A and B) Cell-cycle profiles of cells isolated from E8.5 wild-type (A) and Sas4−/− (B) embryos. (C) Western blots show similar levels of cyclin D1 in wild-type and mutant E8.5 embryo extracts. (D) Phospho-p38 (pp38, T180/Y182) is not elevated in E8.5 Sas4−/− embryo extracts. (E and F) Mitotic spindles from p53−/− (control) and Sas4−/− p53−/− MEFs stained with α-tubulin (green) and PCNT (red). (G and H) Sample FISH signals from dissociated cells of p53−/− control and Sas4−/− p53−/− embryos at E8.5 with two copies of chromosome 12 (green) and two copies of chromosome 17 (red). (I and J) Wild-type (I) and Sas4−/− (J) embryo sections at E8.5 (neural plate) stained with the DNA damage marker 53BP1. (K) Western blots of embryo extracts show that γ-H2AX is not elevated in Sas4−/− or Sas4−/− p53−/− cells at E8.5 relative to the respective wild types. For loading controls, α-tubulin was used for Sas4−/− and GAPDH for Sas4−/− p53−/−. (Scale bars: 3 µm.)
Many studies have correlated centrosomal aberrations with abnormal spindles and chromosomal instability (17, 40–42). Staining for α-tubulin in Sas4−/− p53−/− MEFs showed that bipolar spindles formed in the absence of SAS4 (Fig. 5 E and F). Astral microtubules were present, although they were decreased in number in the double mutant MEFs (Fig. 5 E and F).
To test whether the absence of centrioles caused aneuploidy, we examined chromosome number in cells from Sas4−/− p53−/− double-mutant embryos, reasoning that any aneuploid cells generated by removal of SAS4 would survive and accumulate in the absence of p53. We used chromosome 12- and 17-specific probes for FISH on dissociated cells from E8.5 Sas4−/− p53−/− double-mutant, wild-type, and p53−/− embryos. Indistinguishable levels of apparent aneuploidy were seen by this assay in wild-type (6.0%), p53−/− (5.3%), and Sas4−/− p53−/− (6.7%) embryos (n = 150 cells total from two embryos of each genotype) (Fig. 5 G and H). Supporting the conclusion that removal of SAS4 does not cause detectable aneuploidy, metaphase chromosome counts were indistinguishable in cells from E8.5 Sas4−/− and wild-type embryos (Fig. S6 A and B).
A number of proteins in the DNA damage response pathway, including CHK1 and ATM, appear to be enriched at centrosomes (43), and it has been suggested that mutations in specific centrosomal proteins disrupt the DNA damage response pathway (18). We did not detect any difference between E8.5 wild-type and Sas4−/− embryos in the staining pattern of 53BP1, which marks the sites of DNA damage (Fig. 5 I and J). Western blot analysis showed that the level of another marker of DNA damage, γ-H2AX, was not increased in Sas4−/− or Sas4−/− p53−/− embryos (Fig. 5K). To test whether Sas4−/− cells are able to activate the normal response to DNA damage, we treated Sas4−/− p53−/− and p53−/− MEFs with ionizing radiation for 1 h, which increased the levels of γ-H2AX and pCHK1 (S345) to the same extent in mutants and controls (Fig. S6C). The cell cycle in both Sas4−/− p53−/− and p53−/− MEFs arrested in response to irradiation, as assayed by decreased levels of phospho-histone H3 (pHH3) (Fig. S6C). Treatment with UV radiation or hydroxyurea for 2 h also induced a robust DNA damage response in Sas4−/− p53−/− MEFs (Fig. S6D).
Prometaphase Is Prolonged in Sas4−/− Embryonic Cells.
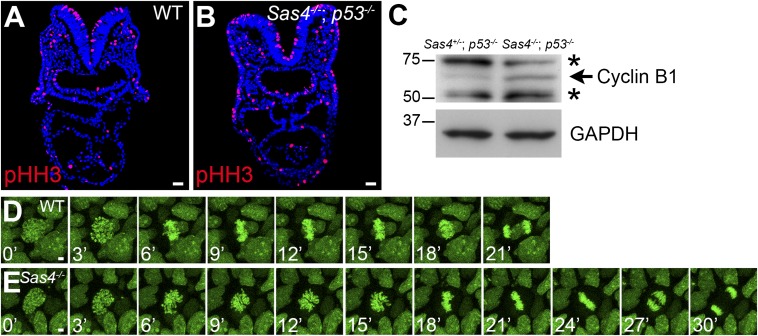
Because of the localization of centrioles at mitotic spindle poles, we examined mitosis in the absence of centrioles in Sas4−/− embryos. Embryo sections stained for the mitotic marker pHH3 (Fig. 6 A and B) showed a 70% increase in the fraction of mitotic cells in Sas4−/− embryos at E8.5 (9.1 ± 0.8%, n = 19,212 cells from nine embryos) compared with wild type (5.4 ± 0.5%, n = 16,277 cells from five embryos; P = 0.0014). Consistent with the increase in mitotic cell number, the level of cyclin B1 (a mitotic cyclin) increased ∼1.6-fold in Sas4−/− embryos (Fig. 6C).
Fig. 6.
Prolonged mitosis in Sas4−/− embryos. (A and B) Cross-sections of E8.5 embryos show an increase in the number of mitotic cells (pHH3+; red) in Sas4−/− p53−/− embryos. (C) Western blots show an increase in cyclin B1 (arrow) in E8.5 Sas4−/− p53−/− embryos. Asterisks mark nonspecific bands. (D and E) Snapshots from time-lapse imaging of dividing cells in cultured E7.5 wild-type (D) and Sas4−/− (E) H2B-GFP+ embryos. (Scale bars: 30 µm in A and B, 3 µm in D and E.)
To follow the dynamics of mitosis in the embryo, we generated Sas4−/− mutants that carried an H2B-GFP transgene (44) and imaged the chromosomes of dividing cells in intact cultured embryos beginning at E7.5, a stage when mutant embryos showed increased p53 and apoptosis (Fig. S7). In every case (n = 124 cells from five embryos), the condensed chromosomes successfully segregated to two poles. We observed no examples of multipolar spindles, and lagging chromosomes were observed in only two cases in the mutant and in two cases in wild-type embryos (Fig. 6 D and E and Movies S1 and S2).
Despite the successful completion of mitosis, live imaging showed that the time from prophase to anaphase was significantly longer in Sas4−/− than in wild-type embryos: Mitosis lasted 30.1 ± 4.8 min in Sas4−/− embryos (n = 52 cells from three embryos) compared with 21.0 ± 2.9 min in wild type (P < 0.0001, n = 112 cells from three embryos) (Fig. 6 D and E). The delay occurred during prometaphase, when chromosomes organized in rosette-like arrangements for ∼15 min in the mutant, compared with ∼6 min in wild type. In every mitosis, however, the mutant chromosome rosette resolved into two equal groups of chromosomes that segregated to two poles.
Spindle Pole Assembly in Sas4−/− Cells.
The delay in anaphase onset and chromosome segregation in Sas4−/− embryos was paralleled by delayed formation of two defined spindle poles, as assessed by staining for the PCM proteins γ-tubulin, PCNT, and aurora kinase A (AURKA) (Fig. S8 A–H). Wild-type mitotic cells in the embryo invariably had two well-defined and brightly stained γ-tubulin+ spindle poles throughout mitosis (Fig. S8A). In contrast, multiple small γ-tubulin+ foci or a faint cloud of γ-tubulin were observed at prometaphase in Sas4−/− embryos (Fig. 3F and Fig. S8B). Despite the abnormal γ-tubulin foci during prometaphase, γ-tubulin always was focused at the two opposing poles at anaphase (Fig. S8 C and D). PCNT and AURKA showed the same gradual coalescence at spindle poles as γ-tubulin during mitosis in Sas4−/− embryos (Fig. S8 E–H). The longer duration of prometaphase was confirmed by assay of the spindle assembly checkpoint (SAC). The SAC proteins MAD2 and BUBR1 were both enriched at kinetochores in mutant cells during prometaphase (Fig. S8 I–L). We conclude that during acentriolar mitosis in mouse embryos prometaphase is extended because of a slower alternative mechanism for spindle pole assembly.
Prolonged Mitosis Activates p53 and Apoptosis in the Wild-Type Embryo.
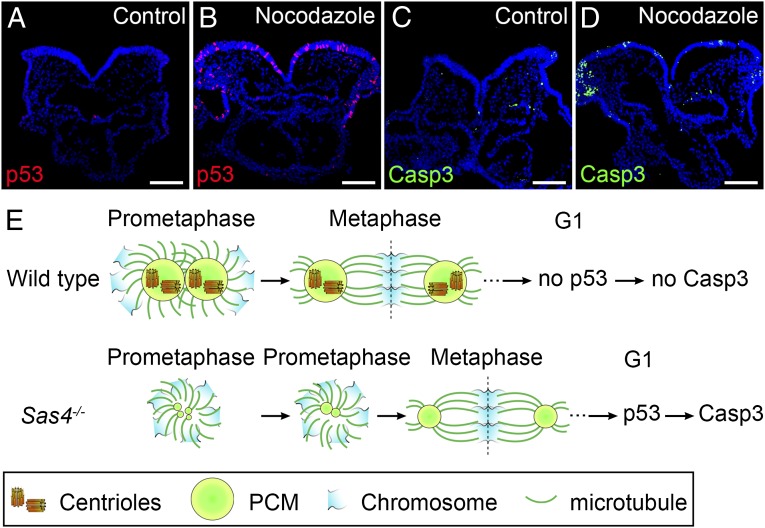
It is remarkable that a short (∼10 min) increase in the duration of prometaphase appeared to cause induction of p53 in Sas4−/− embryos. In cultured cells, treatment with the microtubule-depolymerizing agent nocodazole prolongs mitosis and activates p53 without affecting interphase progression (45, 46). Therefore, to test whether a short delay in mitosis could activate p53 in the mouse embryo, we cultured E8.5 wild-type embryos for 30 or 60 min in the presence of a low concentration (1 µM) of nocodazole followed by a washout and then 2 or 4 h of culture (Table S1). After a 60-min treatment, 10.7% of the cells in treated embryos were p53+ at 2 or 4 h after nocodazole removal, but p53+ cells were rare (1.5%) in control cultured embryos (Fig. 7 A and B and Table S1). The number of apoptotic Casp3+ cells was elevated at 4 h but not at 2 h after nocodazole removal (Fig. 7 C and D), suggesting that the cells delayed in mitosis up-regulated p53 rapidly during the subsequent G1 phase and initiated apoptosis shortly thereafter. A similar increase in nuclear p53 was obtained after 60-min treatment with 0.5 mM monastrol (Table S1), which inhibits the Eg5 kinesin and spindle assembly (46, 47). Incubation of wild-type embryos in 1 µM nocodazole for only 30 min, which is the length of time that acentriolar cells spend in mitosis, led to elevated levels p53 in 6.1% of the cells at 4 h after drug removal (Table S1). These data suggest that every cell delayed in mitosis in this experiment activated a pathway that led to elevated levels of p53. We conclude that interference with mitotic spindle assembly in the mouse embryo by preventing microtubule polymerization, by inhibiting Eg5, or by removing centrioles causes a prolonged mitosis that leads to p53-dependent apoptosis.
Fig. 7.
Prolonged prometaphase activates p53 and apoptosis in the wild-type mouse embryo. (A–D) Cross-sections of cultured wild-type E8.5 embryos treated with vehicle (DMSO) (A and C) or 1 µM nocodazole for 1 h (B and D) and harvested 4 h after nocodazole removal. Approximately 11% of the cells in nocodazole-treated embryos are p53+ (red) (B). The number of Casp3+ (green) cells also was elevated in nocodazole-treated embryos (D), indicating increased apoptotic cell death. (E) Model for the activation of p53 and apoptosis by acentriolar mitosis. (Upper) Mitotic cells in wild-type embryos use centrioles to recruit more PCM and form mature centrosomes that organize microtubules and establish the bipolar mitotic spindle during prometaphase. The chromosomes are aligned quickly and efficiently at the metaphase plate of a well-structured bipolar spindle. Mitosis proceeds normally, and p53 and Casp3 are not up-regulated in the daughter cells. (Lower) Mitotic cells in Sas4−/− embryos lack centrioles and rely primarily on a chromatin-based pathway to organize the mitotic spindle. The establishment of bipolar spindle poles is slower in the absence of centrioles, but acentriolar cells eventually assemble a bipolar spindle and align the chromosomes at the metaphase plate, followed by proper chromosome segregation. However, the daughter cells of the acentriolar division retain the memory of the prolonged prometaphase and up-regulate p53, which increases the probability of cell death (marked by Casp3 expression). (Scale bars: 100 µm.)
Discussion
Sas4-null mutant mouse embryos lack centrioles and die at midgestation with disrupted Hh signaling, elevated levels of p53, and increased apoptotic cell death. Mutations in three other mouse genes that now are known to be required for centriole duplication, Plk4/Sak, Sil/Stil, and Rotatin/Ana-3, were shown previously to cause arrest at midgestation (48–52). Defects in Hh-dependent patterning were described in Sil/Stil mutant embryos (53), and increased cell death was observed in Plk4 and Rotatin mutants (48, 50). We also observed midgestation lethality associated with increased cell death and up-regulation of p53 in mutant embryos homozygous for a gene trap allele of Cep152, which encodes another core protein of the centriole duplication pathway. Thus, the absence of centrioles, rather than the absence of SAS4 per se, triggers up-regulation of p53, which causes widespread cell death and leads to midgestation lethality in the mouse embryo.
The centrosome has been thought of as a signaling platform organized by the centriole (54). Many developmental pathways are required for survival to midgestation, and some of these have been inferred to be dependent on cilia or centrosomes. Nevertheless, we observe that specification of the anterior–posterior body axis, which requires Wnt, Nodal, BMP, and FGF signaling, is normal in the absence of centrioles and centrosomes. It has been suggested that centrosomes are important in directed cell migration (55), but the polarized collective migration of the anterior visceral endoderm (56) is normal in the absence of centrioles, as shown by the formation of a single anterior–posterior body axis in Sas4−/− embryos. Similarly, the apical–basal oscillations of nuclei in pseudostratified epithelia (interkinetic nuclear migration) are thought to depend on an apically anchored centrosome (57), but restriction of mitotic cells to the apical surface of the neural epithelium is normal in Sas4−/− mutants (Fig. 6 A and B).
The majority of cells in E8.5 Sas4−/− embryos express high levels of p53. Increased p53 can be caused by DNA damage, but there is no detectable increase in DNA damage in Sas4−/− embryos, and Sas4−/− mutant cells can activate the DNA damage response pathways normally. Aneuploidy also can activate p53, but live imaging of E7.5–8.5 Sas4−/− embryos showed that bipolar spindles formed and that chromosomes segregated successfully in every case observed. FISH analysis confirmed that the rate of aneuploidy is not elevated in Sas4 mutants. Our results contrast with experiments that indicated that centrosome amplification in the mouse central nervous system leads to the formation of multipolar spindles, aneuploidy, cell death, and microcephaly (42). Removal of p53 in the background of multiple centrosomes led to only a partial rescue (∼12%) of brain size (42). Thus, the absence of centrosomes and centrosome amplification appear to have very different consequences.
Because p53 is expressed at high levels in the majority of cells in Sas4-null mutants, but DNA damage and aneuploidy were not detected, we conclude that some other defect caused by the absence of centrioles is responsible for the up-regulation of p53 and subsequent cell death. Our data do not rule out the possibility of a low frequency of DNA damage or chromosome loss, which have been observed in the absence of centrosomes in other species (58) and in Sas4 partial loss-of-function mutants (22). Indeed, we assume that the function of p53 pathway activation is to prevent the survival of cells in the embryo that have the potential to experience genomic aberrations.
Although the prominent location of centrosomes at the spindle poles led to the belief that they are required for accurate chromosome segregation, experiments have shown that either centrosomes or chromosomes can act as the prime organizers of the spindle pole (59). The early mitoses in the preimplantation mouse embryo are acentriolar, demonstrating that the mouse has robust machinery to support acentriolar mitosis (60–62). The onset of gastrulation correlates with more rapid proliferation, with average cell-cycle times of 4–8 h and cell cycles as fast as 2 h in regions of the epiblast (63, 64). In our hands, the highest mitotic index in Sas4−/− E7.5 embryo is in the epiblast, whereas the mitotic index is lowest in the endoderm (Table S2). The difference in proliferation rate between the germ layers parallels the higher expression of p53 and Casp3 in the epiblast than in the endoderm (Fig. S7B). These data suggest that acentriolar mitosis is most likely to cause p53-dependent cell death in rapidly proliferating cell types.
Live imaging showed that Sas4−/− cells take about 50% longer to complete mitosis than wild-type cells because of an increase in the length of prometaphase from ∼6 to ∼15 min. During the prolonged prometaphase, the chromosomes are arranged in a rosette that could easily be mistaken for a monopolar spindle. However, the live imaging shows this phase is transient and resolves to give two distinct spindle poles, paralleling the gradual assembly of two distinct PCM foci at the poles. The coalescence of PCM foci appears to be similar to the gradual fusion of MTOCs during the acentriolar cell cycles in the preimplantation mouse embryo (61). A delay in mitosis also was reported in Drosophila Sas4 mutants: Mitosis takes 30–40% longer in Drosophila Sas4 mutant neuroblasts than in wild-type cells because of a prolonged phase of spindle assembly (5), although cell death was not reported in Drosophila Sas4 mutants.
The role of centrioles in microtubule organization appears to be remarkably conserved in evolution. Centrioles are required for the formation of cilia in every organism studied (25). Unlike results obtained in cell-culture studies using vertebrate cells, mouse centrioles do not appear to be essential for bipolar spindle formation or proper chromosome segregation (14, 17). Similar to Drosophila zygotic mutants (5), the mouse embryo can proceed through many cell divisions without centrioles, but in the mouse the delay in mitosis leads to an efficient up-regulation of p53 that does not occur in Drosophila.
It is remarkable that a delay in prometaphase of only about 10 min in acentriolar cells in the mouse embryo was sufficient to increase the level of p53 by approximately fivefold (Figs. 2F and 6E). A short treatment of wild-type embryos with nocodazole also caused rapid up-regulation of p53 followed by cell death. Thus, the data indicate that a short delay in mitosis, caused by either absence of centrioles or disruption of mitotic microtubules, initiates a p53-dependent apoptotic pathway in the embryo (Fig. 7E). Interference with microtubule dynamics during mitosis of cultured human cell lines also causes increased levels of p53 in the absence of DNA damage but leads to a p38/p21-dependent G1 cell-cycle arrest rather than apoptosis; this cell-cycle arrest is seen only after treatments that caused arrest in prometaphase for >1.5 h (46). We suggest that a prolonged mitosis initiates a common pathway that up-regulates p53 after exit from mitosis; the outcome of p53 up-regulation in cultured cells is cell-cycle arrest, whereas the outcome in the embryo is apoptosis. The precise molecular mechanism that activates p53 could be the prolonged activation of the SAC, mislocalization of a PCM component, or inappropriate activity of a cell-cycle regulator; elucidation of this mechanism will be important for understanding this previously unidentified role of the p53 tumor suppressor in maintenance of genomic integrity.
Materials and Methods
Animals and Genotyping.
The Sas4 knockout-first ES cell line (EPD0028_7_G05) was obtained from the IKMC [Cenpjtm1a(EUCOMM)Wtsi] (www.mousephenotype.org/martsearch_ikmc_project/martsearch/ikmc_project/34777). The null allele was generated by crossing Cenpjtm1a(EUCOMM)Wtsi mice to CAGG-Cre mice (65). Actin-FLP transgenic mice [B6.Cg-Tg(ACTFLPe)9205Dym/J; Jax stock no. 005703] were used to excise the gene trap and obtain the Sas4+/fl (fl, floxed allele) conditional allele. Sox2-Cre transgenic mice were used to delete Sas4 from all embryonic tissues excluding the extraembryonic lineages (66). To generate maternal/zygotic Sas4 mutants, ZP3-Cre/+ (67) Sas4+/− males were crossed to Sas4fl/fl females to generate Sas4fl/- ZP3-Cre/+ females, which were crossed to Sas4+/− males to give 50% maternal/zygotic Sas4−/− mutant embryos. The Cep152 knockout-first ES cell line [EPD0061_1_E05, Cep152tm1a(EUCOMM)Wtsi] was obtained from IKMC (www.mousephenotype.org/martsearch_ikmc_project/martsearch/ikmc_project/30116). H2B-GFP transgenic mice were a gift from Anna-Katerina Hadjantonakis (Sloan–Kettering Institute, New York) (44). Mice carrying the Trp53tm1Tyj null allele (Jax stock no. 002080) were a gift from Andrea Ventura (Sloan–Kettering Institute, New York). Trp53ftm1Brn mice (Jax stock no. 008462) were a gift from David Solit (Sloan–Kettering Institute, New York). The TOPGAL Wnt-reporter and the Ptch-LacZ knockin mice were previously described (68, 69). Conditional Ift88fl/fl mice were a gift of Bradley Yoder (University of Alabama at Birmingham, Birmingham, AL) (24). Phenotypes were analyzed in the FVB/NJ background. Genotyping was carried out using standard PCR protocols. Animal experiments were approved by the institutional animal care and use committee at the Sloan–Kettering Institute.
Immunofluorescence and Embryo Culture and Imaging.
Cryosections of embryos were prepared using standard conditions (70). Cryosections (8–12 μm) were postfixed in methanol at −20 °C for 20 min for centrosomal staining or were used directly for immunofluorescence. MEFs were fixed in methanol for centrosomal staining or in 4% (wt/vol) paraformaldehyde (PFA) for 10 min for nuclear staining followed by methanol. For α-tubulin staining, the cells were fixed in 4% PFA in 1× PBS at 37 °C for 5 min. Images were obtained using a Zeiss Axioplan2 wide-field microscope equipped with an MRC Axiocam camera (Zeiss) or a Leica SP2 or SP5 confocal microscope (Leica Microsystems). Confocal images were analyzed using the Volocity software package (Improvision). Nuclei were counted and fluorescence intensity was measured using ImageJ (National Institutes of Health).
For embryo cultures, embryos were dissected at E7.5–8.5, with their yolk sac and ectoplacental cone intact, at 37 °C with DMEM:F12 supplemented with 10% (vol/vol) FBS and 1% penicillin-streptomycin. For the nocodazole experiments, embryos at E8.5 were cultured in 1 µM nocodazole or 0.5 mM monastrol (Sigma-Aldrich) in rat serum for 30 or 60 min, washed, and then incubated in rat serum for an additional 2 or 4 h.
For live imaging, H2B-GFP+ embryos at E7.75 were inserted into a hole created in a freshly prepared collagen matrix (BD Biosciences) on a glass-bottomed, 35-mm dish and were covered with rat serum. The embryos were imaged on an inverted Leica SP5 confocal microscope equipped with an incubation chamber at 37 °C and CO2 for 6–12 h with frames taken every 3 or 5 min. The resulting time-lapse movies were analyzed using the Volocity (Improvision) or Imaris software (Bitplane).
MEFs were prepared from E9.5 embryos by removing the heart and triturating before seeding onto cell culture-treated 12-well plates. MEFs were cultured in DMEM medium (Invitrogen) containing 10% FBS and 1% penicillin-streptomycin.
LacZ Staining, in Situ Hybridization, and FISH.
β-Galactosidase activity was detected using standard described protocols (71). The Brachyury, Gli1, and Axin2 in situ probes were previously described (72–74). Whole-mount in situ hybridization was performed on embryos following standard methods (70). The embryos were photographed using an HRC Axiocam (Zeiss) fitted onto a Leica stereomicroscope (Leica). FISH was performed on dissociated embryos at E8.5 (see Cell-Cycle Analysis below for details) using probes corresponding to BAC clones mapping to chromosomes 12 and 17 according to published methods (75).
Western Blot Analysis.
Embryos were dissected in cold 1× PBS with 0.1% Tween-20 and were frozen at −80 °C to be lysed later in Laemmli buffer (BioRad). Western blots were performed according to standard protocols. The luminescent detection reaction was performed using ECL Plus or Prime reagent (Amersham) according to the manufacturer’s recommendations. Relative band intensities was quantified using ImageJ (National Institutes of Health).
Antibodies.
The following antibodies were used in this study, The rabbit anti-SAS4 antibody was a gift from Pierre Gönczy (Institut Suisse de Recherches Experimentales sur le Cancer, Lausanne, Switzerland) (1/1,000 for Western blots). The rabbit anti-ARL13B (1/2,000) was described previously (76). The rabbit anti-VANGL2 antibody was a gift from Mireille Montcouquiol (University of Bordeaux, Bordeaux, France) (1/1,000) (77). The following antibodies were from Sigma-Aldrich: mouse anti–γ-tubulin (clone GTU-88, 1/1,000 for immunofluorescence, 1/5,000 for Western blots), mouse anti–α-tubulin (1/1,000 for immunofluorescence, 1/10,000 for Western blots), mouse anti–acetylated α-tubulin (1/1,000), and rabbit anti-CEP152 (1/200). The following antibodies were from Developmental Studies Hybridoma Bank: mouse anti-PAX6 (1/10) and mouse anti-NKX2.2 (1/10). The following antibodies were from Santa Cruz Biotechnology: mouse anti-cyclin D1 (A12, 1/200), mouse anti-cyclin B1 (1/500), and rabbit anti–N-cadherin (1/200). The following antibodies were from Cell Signaling: mouse anti-p53 (1/200 for immunofluorescence), rabbit anti–γ-H2AX (1/1,000), rabbit anti-pCHK1 (S345; 1/1,000), rabbit anti-pCHK1 (S317; 1/1,000), mouse anti-AURKA (1/200), and rabbit anti-pp38 (T180/Y182) and anti-p38 (1/1,000). The following antibodies were from Abcam: rabbit anti–γ-H2AX (1/1,000) and rabbit anti-FOXA2 (1/1,000). The following antibodies were from BD Biosciences: mouse anti-MAD2 (1/200) and mouse anti-BUBR1 (1/200). The following antibodies were from Millipore: rabbit anti–phospho-histone H3 (1/500 for immunofluorescence, 1/2,000 for Western blot). Rabbit anti-pericentrin (1/5,000) was from Covance. Other antibodies used were rabbit anti–cleaved caspase-3 (1/200, Promega), rabbit anti-p53 (CM5; 1/500 for Western blot and 1/200 for immunofluorescence; Leica Microsystems), and rabbit anti-53BP1 (1/1,000, Novus Biologicals). Secondary antibodies for immunofluorescence were Alexa Fluor 488-, 568-, 594-, 633-, or 647-conjugated (Life Technologies) and used at 1/1,000. Secondary antibodies for Western blot were from GE Healthcare and used at 1/10,000. Rhodamine-conjugated phalloidin (1/400) was obtained from Invitrogen.
Scanning Electron Microscopy and TEM.
Embryos for scanning electron microscopy and TEM were dissected in 1× PBS at room temperature and directly fixed in half-strength Karnovsky’s fixative (2% PFA, 2.5% glutaraldehyde, 0.1 M cacodylate buffer) (Electron Microscopy Sciences) overnight or longer at 4 °C and processed as previously described (23, 78). For TEM, embryos were postfixed in tannic acid to enhance microtubule visibility (79). TEM sections were observed using a JEOL 1200EX transmission electron microscope at 80 kV. For scanning electron microscopy, embryos were observed using Zeiss SUPRA 25 FESEM.
Cell-Cycle Analysis and DNA Damage Studies.
Embryos were dissected in cold 1× PBS and dissociated in 0.25% trypsin/EDTA (Invitrogen) for 20–30 min at 37 °C with occasional trituration with a fine pipette. The dissociated cells were washed in 1× PBS and fixed in 70% ethanol at −20 °C until the time of analysis. Propidium iodide staining was used for DNA content analysis on a FACSCalibur machine (BD Biosciences). FlowJo software was used to analyze the cell-cycle data. Both the Watson Pragmatic model and Dean–Jett–Fox models were used and showed similar distributions of cell-cycle phases. The experiment was performed twice on pooled embryos. In the experiment shown, n = 9 wild-type embryos and n = 16 Sas4−/− mutant embryos.
Control p53−/− MEFs and Sas4−/− p53−/− MEFs (passage 4–5) were irradiated with a dose of 10 Gy using an X-Rad 225 machine (Precision X-Ray) and were harvested 1 h after irradiation. Higher-passage MEFs (passage 20) were subjected to UV irradiation using a StrataLinker 2400 machine (Agilent Technologies) at 20 j/m2 or hydroxyurea (Sigma-Aldrich) treatment at 5 mM and were harvested 2 h after irradiation or treatment.
Chromosome Spreads.
Embryos were dissected for culture at E8.5 as described above and were incubated in embryo culture medium supplemented with 10 μM colcemid (Roche Applied Science) for 4 h to enrich for mitotic metaphases. Six to seven mutant or three wild-type embryos were genotyped, pooled, and incubated in 0.25% trypsin/EDTA (Invitrogen) for ∼20–30 min at 37 °C for dissociation. After the trypsin was inactivated in culture medium and the cells were washed, the dissociated cells were incubated at 37 °C in 0.1 M KCl hypotonic solution for ∼25 min. The cells then were fixed in 3:1 methanol:acetic acid mixture on ice and spread on slides for metaphase preparations according to described protocols (80). The slides were dried overnight and stained with DAPI, and the chromosomes were imaged using a 63× oil objective on a Leica SP5 confocal microscope (Leica).
Statistical Analysis.
Two groups or more of data were compared using a two-tailed Student t test or ANOVA with multiple comparisons (Dunnett’s test) with a cutoff for significance of <0.05 (Prism software, GraphPad). The data are presented as the mean ± SD in all cases except in Fig. S5 E and F where the error bars represent SEM. The detailed analyses of the intensity of the signals of VANGL2 and F-actin using ImageJ was described previously (39).
Supplementary Material
Acknowledgments
We thank the Mouse Genetics, Cytogenetics, Molecular Cytology, and Electron Microscopy core facilities at Memorial Sloan–Kettering Cancer Center. We thank Kunihiro Uryu of the electron microscopy core facility at Rockefeller University for help with TEM; Pierre Gönczy for the SAS4 antibody; James Mahaffey for analysis of planar polarity images; Prasad Jallepali, Andrew Koff, Bryan Tsou, John Petrini, and their laboratories for reagents and advice; Anna-Katerina Hadjantonakis for the H2B-GFP mice; Elizabeth Lacy for Actin-FLP mice; Andrea Ventura for p53−/− mice; David Solit for p53fl/fl mice; and Bradley Yoder for Ift88 mice. We thank Bryan Tsou, Prasad Jallepalli, Tim Bestor and members of the K.V.A. laboratory for comments on the manuscript. This work was supported by National Institutes of Health Grant NS044385 (to K.V.A.) and Memorial Sloan–Kettering Cancer Center Support Grant P30 CA008748. H.B. was supported by National Kidney Foundation and National Research Service Award postdoctoral fellowships.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1400568111/-/DCSupplemental.
References
- 1.Boveri T. Concerning the origin of malignant tumours by Theodor Boveri. Translated and annotated by Henry Harris. J Cell Sci. 2008;121(Suppl 1):1–84. doi: 10.1242/jcs.025742. [DOI] [PubMed] [Google Scholar]
- 2.Bettencourt-Dias M, Glover DM. Centrosome biogenesis and function: Centrosomics brings new understanding. Nat Rev Mol Cell Biol. 2007;8(6):451–463. doi: 10.1038/nrm2180. [DOI] [PubMed] [Google Scholar]
- 3.Kirkham M, Müller-Reichert T, Oegema K, Grill S, Hyman AA. SAS-4 is a C. elegans centriolar protein that controls centrosome size. Cell. 2003;112(4):575–587. doi: 10.1016/s0092-8674(03)00117-x. [DOI] [PubMed] [Google Scholar]
- 4.Leidel S, Gönczy P. SAS-4 is essential for centrosome duplication in C elegans and is recruited to daughter centrioles once per cell cycle. Dev Cell. 2003;4(3):431–439. doi: 10.1016/s1534-5807(03)00062-5. [DOI] [PubMed] [Google Scholar]
- 5.Basto R, et al. Flies without centrioles. Cell. 2006;125(7):1375–1386. doi: 10.1016/j.cell.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 6.Bond J, et al. A centrosomal mechanism involving CDK5RAP2 and CENPJ controls brain size. Nat Genet. 2005;37(4):353–355. doi: 10.1038/ng1539. [DOI] [PubMed] [Google Scholar]
- 7.Rauch A, et al. Mutations in the pericentrin (PCNT) gene cause primordial dwarfism. Science. 2008;319(5864):816–819. doi: 10.1126/science.1151174. [DOI] [PubMed] [Google Scholar]
- 8.Griffith E, et al. Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling. Nat Genet. 2008;40(2):232–236. doi: 10.1038/ng.2007.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar A, Girimaji SC, Duvvari MR, Blanton SH. Mutations in STIL, encoding a pericentriolar and centrosomal protein, cause primary microcephaly. Am J Hum Genet. 2009;84(2):286–290. doi: 10.1016/j.ajhg.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalay E, et al. CEP152 is a genome maintenance protein disrupted in Seckel syndrome. Nat Genet. 2011;43(1):23–26. doi: 10.1038/ng.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nigg EA. Origins and consequences of centrosome aberrations in human cancers. Int J Cancer. 2006;119(12):2717–2723. doi: 10.1002/ijc.22245. [DOI] [PubMed] [Google Scholar]
- 12.Basto R, et al. Centrosome amplification can initiate tumorigenesis in flies. Cell. 2008;133(6):1032–1042. doi: 10.1016/j.cell.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nigg EA, Raff JW. Centrioles, centrosomes, and cilia in health and disease. Cell. 2009;139(4):663–678. doi: 10.1016/j.cell.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 14.Cho J-H, Chang C-J, Chen C-Y, Tang TK. Depletion of CPAP by RNAi disrupts centrosome integrity and induces multipolar spindles. Biochem Biophys Res Commun. 2006;339(3):742–747. doi: 10.1016/j.bbrc.2005.11.074. [DOI] [PubMed] [Google Scholar]
- 15.Mikule K, et al. Loss of centrosome integrity induces p38-p53-p21-dependent G1-S arrest. Nat Cell Biol. 2007;9(2):160–170. doi: 10.1038/ncb1529. [DOI] [PubMed] [Google Scholar]
- 16.Uetake Y, et al. Cell cycle progression and de novo centriole assembly after centrosomal removal in untransformed human cells. J Cell Biol. 2007;176(2):173–182. doi: 10.1083/jcb.200607073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sir J-H, et al. Loss of centrioles causes chromosomal instability in vertebrate somatic cells. J Cell Biol. 2013;203(5):747–756. doi: 10.1083/jcb.201309038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaki M, et al. Exome capture reveals ZNF423 and CEP164 mutations, linking renal ciliopathies to DNA damage response signaling. Cell. 2012;150(3):533–548. doi: 10.1016/j.cell.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mbom BC, Nelson WJ, Barth A. β-catenin at the centrosome: Discrete pools of β-catenin communicate during mitosis and may co-ordinate centrosome functions and cell cycle progression. Bioessays. 2013;35(9):804–809. doi: 10.1002/bies.201300045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dammermann A, Maddox PS, Desai A, Oegema K. SAS-4 is recruited to a dynamic structure in newly forming centrioles that is stabilized by the gamma-tubulin-mediated addition of centriolar microtubules. J Cell Biol. 2008;180(4):771–785. doi: 10.1083/jcb.200709102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gopalakrishnan J, et al. Sas-4 provides a scaffold for cytoplasmic complexes and tethers them in a centrosome. Nat Commun. 2011;2:359. doi: 10.1038/ncomms1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McIntyre RE, et al. Sanger Mouse Genetics Project Disruption of mouse Cenpj, a regulator of centriole biogenesis, phenocopies Seckel syndrome. PLoS Genet. 2012;8(11):e1003022. doi: 10.1371/journal.pgen.1003022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huangfu D, et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426(6962):83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 24.Haycraft CJ, et al. Intraflagellar transport is essential for endochondral bone formation. Development. 2007;134(2):307–316. doi: 10.1242/dev.02732. [DOI] [PubMed] [Google Scholar]
- 25.Carvalho-Santos Z, et al. Stepwise evolution of the centriole-assembly pathway. J Cell Sci. 2010;123(Pt 9):1414–1426. doi: 10.1242/jcs.064931. [DOI] [PubMed] [Google Scholar]
- 26.Goetz SC, Anderson KV. The primary cilium: A signalling centre during vertebrate development. Nat Rev Genet. 2010;11(5):331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fumoto K, Kadono M, Izumi N, Kikuchi A. Axin localizes to the centrosome and is involved in microtubule nucleation. EMBO Rep. 2009;10(6):606–613. doi: 10.1038/embor.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadjihannas MV, Brückner M, Behrens J. Conductin/axin2 and Wnt signalling regulates centrosome cohesion. EMBO Rep. 2010;11(4):317–324. doi: 10.1038/embor.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Itoh K, Jenny A, Mlodzik M, Sokol SY. Centrosomal localization of Diversin and its relevance to Wnt signaling. J Cell Sci. 2009;122(Pt 20):3791–3798. doi: 10.1242/jcs.057067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simons M, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37(5):537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corbit KC, et al. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10(1):70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- 32.Lancaster MA, Schroth J, Gleeson JG. Subcellular spatial regulation of canonical Wnt signalling at the primary cilium. Nat Cell Biol. 2011;13(6):700–707. doi: 10.1038/ncb2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu P, et al. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22(4):361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- 34.Zeng L, et al. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90(1):181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 35.Takada S, et al. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994;8(2):174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- 36.Torban E, et al. Genetic interaction between members of the Vangl family causes neural tube defects in mice. Proc Natl Acad Sci USA. 2008;105(9):3449–3454. doi: 10.1073/pnas.0712126105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hashimoto M, et al. Planar polarization of node cells determines the rotational axis of node cilia. Nat Cell Biol. 2010;12(2):170–176. doi: 10.1038/ncb2020. [DOI] [PubMed] [Google Scholar]
- 38.Song H, et al. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature. 2010;466(7304):378–382. doi: 10.1038/nature09129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahaffey JP, Grego-Bessa J, Liem KF, Jr, Anderson KV. Cofilin and Vangl2 cooperate in the initiation of planar cell polarity in the mouse embryo. Development. 2013;140(6):1262–1271. doi: 10.1242/dev.085316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ganem NJ, Godinho SA, Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460(7252):278–282. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holland AJ, Cleveland DW. Boveri revisited: Chromosomal instability, aneuploidy and tumorigenesis. Nat Rev Mol Cell Biol. 2009;10(7):478–487. doi: 10.1038/nrm2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marthiens V, et al. Centrosome amplification causes microcephaly. Nat Cell Biol. 2013;15(7):731–740. doi: 10.1038/ncb2746. [DOI] [PubMed] [Google Scholar]
- 43.Löffler H, Lukas J, Bartek J, Krämer A. Structure meets function—centrosomes, genome maintenance and the DNA damage response. Exp Cell Res. 2006;312(14):2633–2640. doi: 10.1016/j.yexcr.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Hadjantonakis A-K, Papaioannou VE. Dynamic in vivo imaging and cell tracking using a histone fluorescent protein fusion in mice. BMC Biotechnol. 2004;4:33. doi: 10.1186/1472-6750-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uetake Y, Sluder G. Cell-cycle progression without an intact microtuble cytoskeleton. Curr Biol. 2007;17(23):2081–2086. doi: 10.1016/j.cub.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uetake Y, Sluder G. Prolonged prometaphase blocks daughter cell proliferation despite normal completion of mitosis. Curr Biol. 2010;20(18):1666–1671. doi: 10.1016/j.cub.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayer TU, et al. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science. 1999;286(5441):971–974. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- 48.Hudson JW, et al. Late mitotic failure in mice lacking Sak, a polo-like kinase. Curr Biol. 2001;11(6):441–446. doi: 10.1016/s0960-9822(01)00117-8. [DOI] [PubMed] [Google Scholar]
- 49.Izraeli S, et al. The SIL gene is required for mouse embryonic axial development and left-right specification. Nature. 1999;399(6737):691–694. doi: 10.1038/21429. [DOI] [PubMed] [Google Scholar]
- 50.Faisst AM, Alvarez-Bolado G, Treichel D, Gruss P. Rotatin is a novel gene required for axial rotation and left-right specification in mouse embryos. Mech Dev. 2002;113(1):15–28. doi: 10.1016/s0925-4773(02)00003-5. [DOI] [PubMed] [Google Scholar]
- 51.Stevens NR, Dobbelaere J, Wainman A, Gergely F, Raff JW. Ana3 is a conserved protein required for the structural integrity of centrioles and basal bodies. J Cell Biol. 2009;187(3):355–363. doi: 10.1083/jcb.200905031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang C-JC, et al. The human microcephaly protein STIL interacts with CPAP and is required for procentriole formation. EMBO J. 2011;30(23):4790–4804. doi: 10.1038/emboj.2011.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Izraeli S, et al. Genetic evidence that Sil is required for the Sonic Hedgehog response pathway. Genesis. 2001;31(2):72–77. doi: 10.1002/gene.10004. [DOI] [PubMed] [Google Scholar]
- 54.Bettencourt-Dias M, Hildebrandt F, Pellman D, Woods G, Godinho SA. Centrosomes and cilia in human disease. Trends Genet. 2011;27(8):307–315. doi: 10.1016/j.tig.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang N, Marshall WF. Centrosome positioning in vertebrate development. J Cell Sci. 2012;125(Pt 21):4951–4961. doi: 10.1242/jcs.038083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Migeotte I, Omelchenko T, Hall A, Anderson KV. Rac1-dependent collective cell migration is required for specification of the anterior-posterior body axis of the mouse. PLoS Biol. 2010;8(8):e1000442. doi: 10.1371/journal.pbio.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spear PC, Erickson CA. Interkinetic nuclear migration: A mysterious process in search of a function. Dev Growth Differ. 2012;54(3):306–316. doi: 10.1111/j.1440-169X.2012.01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zamora I, Marshall WF. A mutation in the centriole-associated protein centrin causes genomic instability via increased chromosome loss in Chlamydomonas reinhardtii. BMC Biol. 2005;3:15. doi: 10.1186/1741-7007-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Connell CB, Khodjakov AL. Cooperative mechanisms of mitotic spindle formation. J Cell Sci. 2007;120(Pt 10):1717–1722. doi: 10.1242/jcs.03442. [DOI] [PubMed] [Google Scholar]
- 60.Gueth-Hallonet C, et al. gamma-Tubulin is present in acentriolar MTOCs during early mouse development. J Cell Sci. 1993;105(Pt 1):157–166. doi: 10.1242/jcs.105.1.157. [DOI] [PubMed] [Google Scholar]
- 61.Courtois A, Schuh M, Ellenberg J, Hiiragi T. The transition from meiotic to mitotic spindle assembly is gradual during early mammalian development. J Cell Biol. 2012;198(3):357–370. doi: 10.1083/jcb.201202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Howe K, FitzHarris G. A non-canonical mode of microtubule organization operates throughout pre-implantation development in mouse. Cell Cycle. 2013;12(10):1616–1624. doi: 10.4161/cc.24755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mukherjee AB. Cell cycle analysis and X-chromosome inactivation in the developing mouse. Proc Natl Acad Sci USA. 1976;73(5):1608–1611. doi: 10.1073/pnas.73.5.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Snow MH. Gastrulation in the mouse: Growth and regionalization of the epiblast. J Embryol Exp Morphol. 1977;42:293–303. [Google Scholar]
- 65.Sakai K, Miyazaki Ji. A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochem Biophys Res Commun. 1997;237(2):318–324. doi: 10.1006/bbrc.1997.7111. [DOI] [PubMed] [Google Scholar]
- 66.Hayashi S, Lewis P, Pevny L, McMahon AP. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech Dev. 2002;119(Suppl 1):S97–S101. doi: 10.1016/s0925-4773(03)00099-6. [DOI] [PubMed] [Google Scholar]
- 67.de Vries WN, et al. Expression of Cre recombinase in mouse oocytes: A means to study maternal effect genes. Genesis. 2000;26(2):110–112. [PubMed] [Google Scholar]
- 68.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126(20):4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 69.Goodrich LV, Milenković L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277(5329):1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 70.Eggenschwiler JT, Anderson KV. Dorsal and lateral fates in the mouse neural tube require the cell-autonomous activity of the open brain gene. Dev Biol. 2000;227(2):648–660. doi: 10.1006/dbio.2000.9918. [DOI] [PubMed] [Google Scholar]
- 71.Hogan BL, Blessing M, Winnier GE, Suzuki N, Jones CM. Growth factors in development: The role of TGF-beta related polypeptide signalling molecules in embryogenesis. Dev Suppl. 1994;1994(Suppl):53–60. [PubMed] [Google Scholar]
- 72.Wilkinson DG, Bhatt S, Herrmann BG. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature. 1990;343(6259):657–659. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
- 73.Hui CC, Slusarski D, Platt KA, Holmgren R, Joyner AL. Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm- and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev Biol. 1994;162(2):402–413. doi: 10.1006/dbio.1994.1097. [DOI] [PubMed] [Google Scholar]
- 74.Jho E-H, et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22(4):1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leversha MA. Mapping of genomic clones by fluorescence in situ hybridization. Methods Mol Biol. 2001;175:109–127. doi: 10.1385/1-59259-235-X:109. [DOI] [PubMed] [Google Scholar]
- 76.Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12(5):767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 77.Montcouquiol M, et al. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci. 2006;26(19):5265–5275. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liem KF, Jr, et al. The IFT-A complex regulates Shh signaling through cilia structure and membrane protein trafficking. J Cell Biol. 2012;197(6):789–800. doi: 10.1083/jcb.201110049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roholl PJ, Leene W, Kapsenberg ML, Vos JG. The use of tannic acid fixation for the electron microscope visualization of fluorochrome-labelled antibodies attached to cell surface antigens. J Immunol Methods. 1981;42(3):285–289. doi: 10.1016/0022-1759(81)90157-5. [DOI] [PubMed] [Google Scholar]
- 80.Evans EP, Burtenshaw MD, Ford CE. Chromosomes of mouse embryos and newborn young: Preparations from membranes and tail tips. Stain Technol. 1972;47(5):229–234. doi: 10.3109/10520297209116541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.