Significance
Expression of a productively rearranged T-cell receptor (TCR)-β chain induces a program of αβ T-lineage differentiation, whereas thymocytes that productively rearranged TCR-γ and TCR-δ typically give rise to γδ-lineage T cells. However, given that all three TCR gene loci simultaneously undergo gene rearrangements, the possibility exists that a developing thymocyte may express a γδ-TCR together with a TCR-β or pre-TCR complex, and it is not clear to what this outcome would give rise in terms of T-lineage differentiation. Our findings point to a striking conclusion, in that rather than transmitting signals that exclusively promote αβ-lineage commitment/differentiation, the pre-TCR can function in concert with the γδ-TCR to promote γδ commitment/differentiation, a result that supports a signal strength model of αβ/γδ-lineage choice.
Keywords: T-cell development, β-selection, Notch, γδ T-lineage
Abstract
Developing thymocytes bifurcate from a bipotent precursor into αβ- or γδ-lineage T cells. Considering this common origin and the fact that the T-cell receptor (TCR) β-, γ-, and δ-chains simultaneously rearrange at the double negative (DN) stage of development, the possibility exists that a given DN cell can express and transmit signals through both the pre-TCR and γδ-TCR. Here, we tested this scenario by defining the differentiation outcomes and criteria for lineage choice when both TCR-β and γδ-TCR are simultaneously expressed in Rag2−/− DN cells via retroviral transduction. Our results showed that Rag2−/− DN cells expressing both TCRs developed along the γδ-lineage, down-regulated CD24 expression, and up-regulated CD73 expression, showed a γδ-biased gene-expression profile, and produced IFN-γ in response to stimulation. However, in the absence of Inhibitor of DNA-binding 3 expression and strong γδ-TCR ligand, γδ-expressing cells showed a lower propensity to differentiate along the γδ-lineage. Importantly, differentiation along the γδ-lineage was restored by pre-TCR coexpression, which induced greater down-regulation of CD24, higher levels of CD73, Nr4a2, and Rgs1, and recovery of functional competence to produce IFN-γ. These results confirm a requirement for a strong γδ-TCR ligand engagement to promote maturation along the γδ T-cell lineage, whereas additional signals from the pre-TCR can serve to enforce a γδ-lineage choice in the case of weaker γδ-TCR signals. Taken together, these findings further cement the view that the cumulative signal strength sensed by developing DN cells serves to dictate its lineage choice.
T cells can differentiate along distinct αβ- or γδ-cell lineages, but bifurcate from a common bipotent precursor (1, 2). In mice, the earliest subset of T cells contains CD4− CD8− or double-negative (DN) thymocytes, and this can further be divided into four subgroups (DN1–4) based on the expression of CD25 and CD44 (3, 4). Single-cell progenitor analyses have identified the DN3 stage as the point of T-lineage commitment, and also the final stage at which a DN cell specifies its lineage fate as αβ or γδ (1, 5). The αβ- or γδ-lineage choice decision is governed by several factors. Two competing models have been proposed for this process: the stochastic and instructional models (2). Although evidence exists to support either model, a version of the instructional model posits that the strength of signal transduced by the T-cell receptor (TCR) expressed by the DN3 cell dictates its lineage specification (6, 7).
The apparent connection between lineage choice and the TCR expressed by the cell can be severed by manipulations of TCR signal strength. We previously noted that stimulating stronger signals via expression of the ERK/MAPK-induced Inhibitor of DNA-binding 3 (Id3) appears to promote the γδ-lineage fate in developing DN3 cells in the absence of TCR expression (8), suggesting a critical role for Id3 in mediating αβ- versus γδ-lineage decisions at this developmental checkpoint. Nevertheless, absence of Id3 also appears to favor the emergence of innate-like Vγ1.1/Vδ6.3 γδ-TCR–bearing T cells from the thymus over other γδ-TCR subsets (9, 10).
Several studies have shown that ligand engagement highly influences the αβ- versus γδ-lineage decision because of its effects on γδ-TCR signal strength (6, 7, 9, 11, 12). γδ-TCR–expressing DN3 cells develop along the αβ-lineage and become CD4+ CD8+ (double-positive, DP) cells in the absence of ligand engagement (7), whereas provision of the ligand, or the use of antibodies to mimic ligand engagement (11), allows these cells to adopt the γδ-lineage fate, remain DN, and down-regulate expression of CD24. Additional signals, such as those mediated by Notch, can also influence αβ- versus γδ-lineage fate outcomes (1, 13–16). We showed that γδ-TCR–bearing thymocytes adopting the γδ-lineage do not require concurrent signals from Notch to mature past the DN3 stage, whereas their pre-TCR–expressing counterparts are completely dependent upon Notch signaling to facilitate their pre-TCR–dependent differentiation to the DP stage (1, 17).
Considering the common origin of αβ- and γδ-lineage cells, it is possible for a bipotent DN3 cell to simultaneously express and transmit signals through a functional pre-TCR and a functional γδ-TCR, especially considering that TCR-β, -γ, and -δ genes complete their rearrangements at the DN3 stage. Additionally, γδ-T cells have been shown to contain TCR-β rearrangements (18) and αβ-lineage cells show evidence of both TCR-γ and -δ rearrangements (19–21). In a previous study looking to address the consequences of simultaneously expressing a TCR-β and γδ-TCR in vivo using transgenic (Tg) mice, the numbers of αβ- and γδ-lineage cells in TCR-β/γδ–expressing cells were both high, and comparable to TCR-β- and γδ-TCR-Tg mice, respectively (22). In this case, however, the TCR chains were expressed earlier than physiological for T-cell development, and premature expression of αβ-TCR transgene can lead to aberrant developmental progression (23, 24).
Here, we attempt to definitively answer the question of lineage choice by simultaneously expressing TCR-β and γδ-TCR in Rag2−/− DN3 cells via retroviral transduction followed by in vitro coculture, including limiting dilution and clonal analyses. We now find that Rag2−/− DN3 cells expressing both pre-TCR and γδ-TCR mature along the γδ-lineage into functionally competent cells that produce IFN-γ in response to stimulation. However, in the absence of Id3 expression and strong γδ-TCR ligand, γδ-expressing cells show a lower propensity to differentiate along the γδ-lineage, but when expressing both pre- and γδ-TCRs, these cells showed increased γδ-lineage differentiation and recover functional competence to produce IFN-γ, indicating that the pre-TCR can serve to enforce to a γδ-lineage choice in the case of weaker γδ-TCR signals. Taken together, these findings further cement the view that the cumulative signal strength sensed by developing DN cells dictates its lineage choice.
Results
DN3 Cells Simultaneously Expressing Productive TCR-β and γδ-TCR Chains Develop and Mature Along the γδ-Lineage.
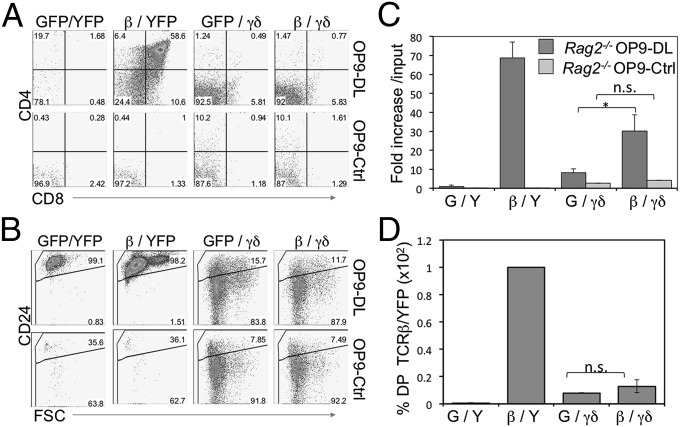
Previously, we found that TCR-β– and γδ-TCR–expressing DN3 cells have a high propensity to develop along their respective lineages (1). Furthermore, the pre-TCR and γδ-TCR produced quantitatively different signals, which was responsible for directing lineage-fate outcomes (7, 8). Considering these results, we sought to determine the lineage choice of a DN3 cell that simultaneously expresses a pre-TCR (TCR-β) and a γδ-TCR (KN6-TCR) (1). Specifically, we wanted to address whether strong signals transmitted from a γδ-TCR would be sufficient to override developmental signals emitted from a pre-TCR. To test this possibility, we ectopically expressed TCR-β, γδ-TCR, or both in Rag2−/− DN3 cells and assessed the role of each TCR in determining lineage fate in the presence of Notch signals by culturing them on OP9-DL cells (25). As reported previously (1), pre-TCR- (TCR-β–transduced) expressing Rag2-deficient DN3 cells proliferated robustly, developed along the αβ-lineage to the DP stage, and maintained high expression of CD24 (Fig. 1). In contrast, γδ-TCR–expressing DN3 cells showed lower cellular expansion, developed along the γδ-lineage, and matured into DN CD24lo γδ cells, with only a very small proportion of cells differentiating along the αβ-lineage to become DP cells (Fig. 1D). Of note, Rag2−/− DN3 cells that did not bear any TCRs (MigR1/MIY-transduced), did not proliferate, and remained DN and CD24hi, indicating their inability to progress along either lineage (Fig. 1 A–C).
Fig. 1.
γδ-TCR–expressing DN3 cells predominantly differentiate along the γδ-lineage, irrespective of TCR-β coexpression and the availability of Notch signals. (A and B) Developmental progression of in vitro-derived Rag2−/− DN3 cells retrovirally transduced to express TCR-β, γδ-TCR, neither, or both, and cultured for 6 d with OP9-DL or OP9-Ctrl cells. Flow cytometric analysis of cell surface expression for CD4 and CD8 (A), and CD24 along with forward size scatter (FSC) (B) are shown for GFP+ YFP+-gated cells; C shows the corresponding fold-expansion in cellularity. Fold-expansion was obtained from the total cellularity divided by the number of cells used at the start of the culture (input). (D) Bar graph showing percentage of DP from the indicated cocultures in relation to TCR-β/YFP–transduced cells set at 100. Data are derived from at least three independent experiments. GFP(G), MigR1-transduced; YFP(Y), MIY-transduced.
Strikingly, DN3 cells simultaneously expressing both TCR-β and γδ-TCR followed a developmental path much like γδ-TCR–only cells, in that they progressed to become DN CD24lo γδ cells, with only a very small proportion of cells differentiating to become DP cells (Fig. 1 A and B). Interestingly, TCR-β expression with γδ-TCR slightly increased the level of CD24 down-regulation compared with γδ-TCR alone (Fig. 1B). Of note, TCR-β/γδ–expressing DN3/OP9-DL cocultures showed higher cellularity compared with their γδ-TCR-only–expressing counterparts, but still much lower than that of TCR-β–only/OP9-DL cocultures (Fig. 1C). Additionally, to rule out any effects as a result of a potential limiting availability of shared CD3 complex subunits, we found that TCR-β/γδ–expressing cells had similar levels of γδ-TCR cell-surface expression and TCR-β expression as γδ-TCR–expressing cells and TCR-β–expressing cells, respectively, suggesting that expression of both pre-TCR and γδ-TCR does not result in lower γδ-TCR or pre-TCR expression (Fig. S1 A–C).
We previously showed a differential requirement for Notch signaling between DN3 cells expressing the pre-TCR and committing to the αβ-lineage, and those expressing a γδ-TCR and differentiating along the γδ-lineage (1). Considering this finding, we addressed the role of Notch signals in the differentiation of DN3 cells simultaneously expressing both TCR-β and γδ-TCR by culturing them without Notch signals on OP9-Ctrl cells. As expected, Rag2-deficient cells that did not express a TCR, or those expressing a pre-TCR, were unable to develop (17). These cells remained DN and apoptosed in the absence of the trophic signals provided by Notch signaling (26), as indicated by the complete loss of cellularity in these cultures (Fig. 1 A and C). Conversely, both γδ-TCR– and TCR-β/γδ–expressing Rag2−/− DN3 cells were able to differentiate along the γδ-lineage, remained DN, and matured to become CD24lo, albeit with reduced cell numbers compared with the same cells cultured with Notch signals (Fig. 1C), in agreement with previous findings (1).
γδ-TCR and Id3 Signal to Induce Maturation Along the γδ-Lineage.
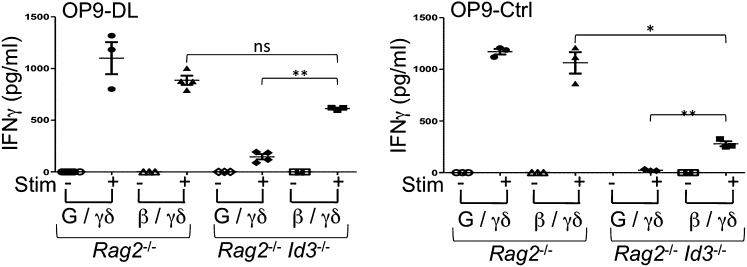
Id3 is an important molecular effector of the strong signals that dictate γδ-fate choice and maturation, but it is dispensable for adoption of the αβ-fate (8). Considering this finding, we sought to determine whether the loss of Id3 in TCR-β/γδ–expressing DN3 cells would affect lineage choice. As reported previously (8), differentiation of pre-TCR–expressing DN3 cells was not impaired by Id3-deficiency, and these cells were able to proliferate and develop to the DP stage (Fig. S2A). Notably, the maturation potential of γδ-TCR–only DN3 cells into IFN-γ–producing γδ-T cells was severely affected in the absence of Id3, but a significant rescue in IFN-γ production was seen in TCR-β/γδ–expressing cells (Fig. 2). Furthermore, in the absence of Notch signals, γδ-TCR–expressing Rag2−/−Id3−/− DN3 cells showed no functionality in terms of IFN-γ production. However, and as seen in the presence of Notch signals, TCR-β/γδ–expressing Rag2−/−Id3−/− DN3 cells showed a rescued in their ability to produce IFN-γ (Fig. 2).
Fig. 2.
Loss of Id3 inhibits the maturation of γδ-TCR–expressing T cells as IFN-γ–producers. Sorted CD45+ GFP+ cells were obtained from in vitro-derived Rag2−/− DN3 or Rag2−/−Id3−/− DN3 cells retrovirally transduced to express TCR-β, γδ-TCR, neither, or both, and cultured for 6 d with OP9-DL or OP9-Ctrl cells (as indicated) and were stimulated for 36 h (P+I, PMA+Iono) or placed in culture without stimulation (Unstim). Levels of IFN-γ in culture supernatants were quantified by an antibody-capture ELISA. Data are derived from at least three independent experiments. *P ≤ 0.05; **P ≤ 0.01.
The CD24hi DN phenotype is shared between preselection DN3 thymocytes and cells that have committed but not yet matured along the IFN-γ–producing γδ T-cell lineage (1, 8). Considering this ambiguity associated with using the CD24 marker, we sought to verify the identity of CD24hi and CD24lo DN cells present in the cocultures (Fig. 1B) by determining their gene-expression profiles. Interestingly, γδ-TCR– and TCR-β/γδ–expressing Rag2−/−Id3−/− DN3 cells expressed similar or greater transcript levels of γδ-biased genes Crem, Nurr1, and Rgs1 compared with their Rag2−/− DN3 counterparts (Fig. S2B). This finding did not seem to be a consequence of increased Id2 expression in Id3-deficient cells. However, despite retention of γδ-identity in the absence of Id3, Rag2−/−Id3−/− DN3 cells showed a greater induction of germ-line Tcra transcripts in the presence of Notch signals (Fig. S2).
γδ-TCR Ligand Strength Affects the Maturation Status of γδ-TCR–Bearing Rag2−/− DN3 Cells.
Results from our previous work (8) and Fig. 2 highlight the necessity for Id3 in inducing strong signals downstream of γδ-TCR and mediating maturation along the γδ-lineage. However, the role of ligand involvement in mediating strong signals remains controversial, particularly in light of recent evidence supporting a ligand-independent development of IL-17–producing γδ-T cells (27). In light of this, we sought to further examine the mechanistic basis behind how weak and strong signaling is sensed, by addressing whether ligand engagement plays a necessary role for the induction of a signal strength differential between pre-TCR and γδ-TCR. In H2b mice (or in H2k OP9 cells), the KN6 γδ-TCR recognizes the nonclassic MHC class I molecule T22, in association with β2-microglobulin. In H2d mice, the T22d gene is defective (28), thus another β2M-associated molecule, T10 (also known as T9), which is closely related to the T22 gene, interacts weakly with KN6 TCR in these mice. T10d and T22b proteins differ by four residues in the a1I and a2 domains (28), and whereas T10 is able to mediate positive selection of KN6 cells, it is characterized as a “weak” ligand that fails to induce negative selection and fails to activate mature, peripheral KN6 TCR-Tg cells (28–31). Considering this finding, we generated BALB/c (H2d) stromal cell lines expressing the weak KN6 γδ-TCR ligand to determine whether it could affect αβ- versus γδ-lineage fate decisions.
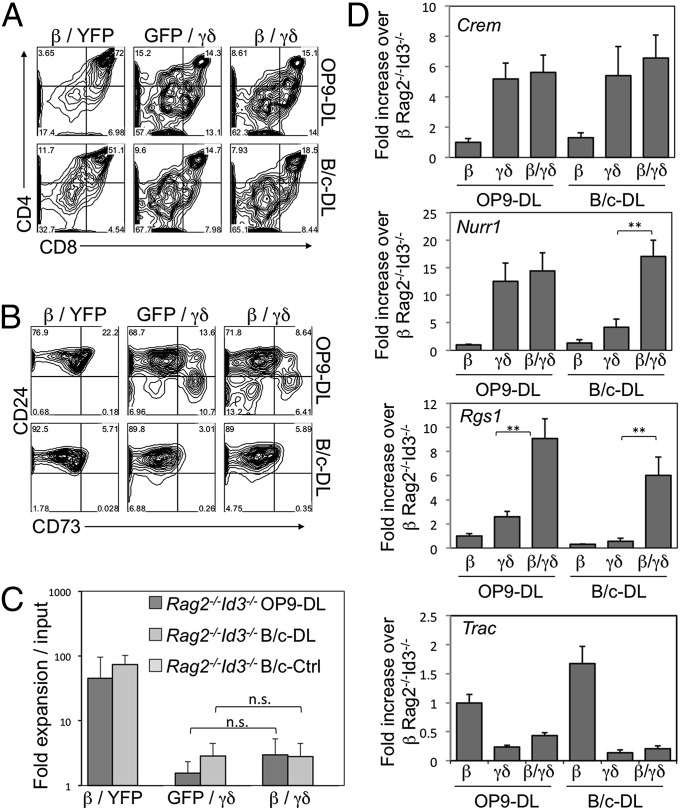
H2d stromal cells from BALB/c (B/c) mice, with or without ectopic Dll4 expression (32), B/c-DL, or B/c-Ctrl, respectively, were used for cocultures with Rag2−/− DN3 cells transduced to express a pre-TCR, γδ-TCR, or both. As expected, the differentiation of pre-TCR– (TCR-β–transduced) expressing DN3 cells was not impaired on B/c-DL cells, as cells proliferated robustly and developed to the DP stage in a Notch ligand-dependent fashion (Fig. 3A and Fig. S3 A and B). Interestingly, absence of T22b on B/c-DL cells reduced the ability of γδ-TCR–only DN3 cells to develop along the γδ-lineage, as a greater percentage of γδ-TCR–only DN3 cells developed into αβ-lineage DP cells, compared with cocultures with B/c-DL cells ectopically expressing T22b (B/c-DL+T22) (Fig. S3C) in which a lower frequency of DP cells was observed (Fig. 3A). Additionally, provision of both TCRs led to a lower percentage of DP cells in B/c-DL4 cocultures compared with γδ-TCR–only DN3 cells, suggesting that in the presence of the weak γδ-TCR ligand, T10d, additional signals derived from the pre-TCR appear to enforce a γδ-lineage outcome. Moreover, the generation of DP cells was further decreased by TCR-β/γδ–expressing cells in the presence of T22b (Fig. 3A). Thus, by increasing the TCR-ligand affinity from the endogenous T10d to T22b, the propensity to yield DP cells is reduced by γδ-transduced cells, and this is further reduced when coexpressing a pre-TCR.
Fig. 3.
Provision of T22b expression and TCRβ enhances the development of γδ-TCR–expressing DN3 cells toward the γδ-lineage. (A) Development of in vitro-derived Rag2−/− DN3 cells retrovirally transduced to express TCR-β, γδ-TCR, or both, and cultured for 5.5 d with B/c-DL cells expressing T22b or MICherry alone. Flow cytometric analysis of cell surface expression for CD4 and CD8 (A) and CD73 and FSC (B). Data are derived from at least two independent experiments.
Not only did we observe a reduction toward the αβ-lineage outcome, but equally important, we noted an increase in CD73+ (γδ-lineage) cells (33, 34) when B/c-DL+T22 cells were used for the cocultures, and this was further increased with the addition of TCR-β (Fig. 3B). Of note, TCR-β/γδ–expressing DN3 cells differentiated along the γδ-lineage with similar frequency and cellularity, regardless of whether they were cultured on B/c-DL or OP9-DL cells (Fig. S4). Nevertheless, TCR-β/γδ–expressing cells, in comparison with γδ-TCR–only cells, showed higher levels of CD73 expression, and a trend to lower levels of CD24 expression, in B/c-DL cocultures (Fig. S4B). However, on B/c-Ctrl cells, γδ-TCR–expressing cells behaved like their vector-transduced counterparts in that they remained as DNs and failed to thrive in the absence of Notch signals (Fig. 3 and Figs. S3 and S4).
To further elucidate whether the CD24hi CD73− CD4−CD8− population, which make up the majority of cells within γδ-TCR–expressing Rag2−/− DN3 B/c-DL cocultures, are progressing toward the γδ-lineage, we assessed for the expression of γδ-lineage–biased genes. Surprisingly, provision of weak ligand (T10d) did not prevent γδ-TCR–transduced DN3 cells from inducing expression of Crem, Nurr1, and Rgs1, nor did it lead to increased TCR-α germ-line transcription (Fig. S4D).
To directly determine lineage outcomes in TCR-β/γδ–expressing cells, we performed single-cell and limiting dilution analyses of Rag2−/− DN3 cells transduced to express TCR-β or γδ-TCR, cultured on OP9-DL or B/c-DL stromal cells, and measured their αβ- or γδ-lineage precursor frequency (1). Table 1 shows that both stromal cells supported a near-perfect capacity to yield DP cells from TCR-β–expressing cells, with frequencies approaching unity. Importantly, the KN6 γδ-TCR–expressing cells showed a low DP precursor frequency as expected (1), but when cultured on the weaker TCR-ligand expressing cells (B/c-DL), this frequency was more than doubled (from 1/116–1/48). A more striking change was seen with TCR-β/γδ–expressing cells, which showed a dramatically lower frequency in generating DP cells, with a fivefold reduction seen on B/c-DL cells (1/230; P < 0.05). A similar effect, but in reverse, was seen in γδ-lineage outcomes, with a twofold increase in TCR-β/γδ–expressing cells compared with γδ-only cells cultured on B/c-DL cells (Table 1).
Table 1.
Limiting dilution analysis of precursor potential of TCR-transduced DN3 cells
| αβ-Lineage* |
γδ-Lineage* |
|||
| TCR/stromal cell | 1/freq | (95% C.L.) | 1/freq. | (95% C.L.) |
| TCR-β/OP9-DL4 | 1.05 | (0.73–1.52) | Nil | N/A |
| TCR-β/B/c-DL4 | 1.02 | (0.72–1.46) | Nil | N/A |
| TCR-γδ/OP9-DL4 | 116.23 | (49.97–270.39) | 1.35 | (0.95–1.91) |
| TCR-γδ/B/c-DL4 | 47.56 | (26.81–84.35) | 3.38 | (2.40–4.77) |
| TCR(β+γδ)/OP9-DL4 | 353.00 | (84.36–1,477.09) | 1.39 | (0.98–1.97) |
| TCR(β+γδ)/B/c-DL4 | 233.99 | (72.38–756.47) | 1.67 | (1.18–2.36) |
αβ-Lineage outcome was determined by the detection of CD4 and CD8 expressing cells, with >50 cells per well analyzed. γδ-Lineage outcome was determined by the presence of CD4 and CD8 DN cells that expressed high levels of CD73, with >30 cells per well analyzed. C.L., confidence limits for P ≤ 0.05; N/A, not applicable. Limiting dilution analyses coculture conditions and TCR used for transductions of Rag2−/− DN3 cells are indicated in SI Methods. Numbers in bold represent frequencies of respective lineage outcomes for KN6-transduced DN3s exposed to weak ligand in the absence and presence of TCR-β, respectively.
Taken together, these data suggest that a weak ligand leads to an increase in differentiation of γδ-TCR–only DN3 cells into DP cells, but the diversion to the αβ fate is incomplete, because these cells continue to express γδ-lineage–biased genes. Furthermore, coexpression of TCR-β with γδ-TCR in Rag2−/− DN3 cells cultured on B/c-DL reduces their ability to adopt the αβ-lineage fate, and increases the propensity of these cells to develop along the γδ-lineage, rather than hindering it.
γδ-TCR Ligand Strength Affects the Maturation Status of γδ-TCR–Bearing Rag2−/−Id3−/− DN3 Cells.
We have already observed that, in the presence of Notch signaling, Id3-deficiency decreased the percentage of γδ-TCR–only DN3 cells that matured along the γδ-lineage (8), and this effect was reduced in TCR-β/γδ–expressing cells (Fig. 2). As expected, in the absence of Id3 and strong γδ-TCR ligand, differentiation of pre-TCR–expressing DN3 cells progressed to the DP stage (Fig. 4 A and C). Interestingly, in the presence of weak ligand (B/c-DL), γδ-TCR–expressing Id3-deficient DN3 cells did not further divert their lineage choice to become αβ-lineage DP cells (Fig. 4A). However, maturation was more severely impaired by the combined loss of both Id3 and strong ligand, as the already decreased CD24 down-regulation seen with Id3-deficiency was even more pronounced when combined with the weak γδ-TCR ligand expressed on B/c-DL cells and CD73 induction was completely abrogated (Fig. 4B). γδ-TCR–expressing Rag2−/−Id3−/− DN3 cells behaved much like their vector-transduced counterparts in that they remained DN, did not down-regulate CD24, up-regulate CD73, or survive in the absence of Notch signaling (Fig. 4C). This finding supports the notion that Notch is required for the differentiation of γδ-TCR–expressing DN3 cells in the absence of Id3 and a strong γδ-TCR ligand.
Fig. 4.
Provision of a weak γδ-TCR ligand with loss of Id3 does not promote αβ-lineage choice in γδ-TCR–expressing DN3 cells, both in the presence and absence of Notch signals. (A and B) Development of culture-derived Rag2−/−Id3−/− DN3 cells retrovirally transduced to express TCR-β, γδ-TCR, neither, or both, and cultured for 6 d with OP9-DL4 and B/c-DL4. Flow cytometric analysis of cell-surface expression for CD4 and CD8 (A), and CD24 and CD73 (B) are shown for GFP+ YFP+-gated cells; C shows the corresponding fold expansion in cellularity. (D) Quantitative RT-PCR analysis of γδ-biased genes: Crem, Nurr1, and Rgs1, and Trac, in transduced Rag2−/−Id3−/− DN3 cells cultured for 6 d on the indicated cells, with mRNA levels normalized to β-actin. β, γδ, and β/γδ represent TCR-β/MIY–, MigR1/TCR-γδ–, and TCR-β/TCR-γδ–transduced DN3 cells, respectively. Data are derived from at least three independent experiments. **P ≤ 0.01.
To further characterize the identity of CD24hi CD73− CD4−CD8− cells within the γδ-TCR–expressing Rag2−/−Id3−/− DN3 B/c-DL cocultures, we assessed cells for the expression of γδ-lineage–biased genes. Notably, we found that the provision of weak signal in combination with Id3-deficiency failed to induce the expression of Nurr1, and Rgs1 in γδ-TCR–only transduced DN3 cells, but did so in TCR-β/γδ–transduced cells, suggesting that they had received sufficient signals to induce the expression of these γδ-lineage–biased genes. Nevertheless, Crem expression was similarly induced in cultures containing a strong or weak ligand, and Tcra expression remained low under both conditions.
Taken together, these data suggest that decreasing TCR signal strength by combining low-affinity ligand with Id3-deficiency does partially divert γδ-TCR–expressing DN3 cells away from a γδ-lineage fate; however, coexpression of the γδ-TCR with the pre-TCR signals restores adoption of the γδ-lineage fate.
Discussion
We have addressed the question of how TCR signals influence DN3 thymocytes to adopt the αβ- or γδ-lineage fate. Specifically, we investigated whether weak γδ-TCR signals can be transformed into effective inducers of γδ-fate specification when combined with pre-TCR signals, which are normally associated with adoption of the αβ fate. Here, we find that DN3 cells that simultaneously express a TCR-β and γδ-TCR choose a γδ T-cell fate, in agreement with the principles of the signal strength model. We also confirm the importance of Id3 for the maturation along the IFN-γ–producing γδ-lineage (8).
Previously, Pereira and colleagues found that TCR-β/γδ transgenic mice generated similar numbers of αβ cells as those transgenic for TCR-β only, and similar numbers of γδ cells as those transgenic for γδ-TCR only (22). However, the timing of TCR expression in the thymocytes of these transgenic mice was premature, and the Vγ transgene included the flanking DNA sequences encoding a putative silencer element, both of which could affect development along the γδ-lineage (35). To overcome these issues, we used an in vitro T-cell differentiation system that allows us to temporally regulate TCR expression precisely, as well as a TCR-γ gene used that was cloned without flanking sequences. Finally, we used Rag2−/− DN3 cells to prevent the expression of TCR variants before, with, or instead of, the rearranged TCRs provided by retroviral transduction.
Here, we find that αβ- versus γδ-lineage choice in γδ-TCR–expressing DN3 cells is independent of TCR-β coexpression, in agreement with the findings published by Pereira’s group (22). However, unlike this previous study, we find that the majority of γδ-TCR–expressing DN3 cells, in the presence of a strong ligand, develop along the γδ T-cell lineage and remain DN, instead of along the αβ-lineage to gain expression of CD4 and CD8. This difference is an important distinction, as it affects how the results are interpreted. The finding that the majority of γδ-TCR–expressing DN3 cells differentiate along the γδ-lineage is in agreement with previously published results (1, 11), and reflects the idea that strong signals, such as those transmitted from the γδ-TCR in the presence of ligand, promote the γδ-lineage fate and oppose an αβ choice.
In the context of the signal-strength model for αβ- versus γδ-lineage bifurcation, and in contrast to the instructional model, one would predict that coexpression of a TCR-β in γδ-TCR–expressing DN3 cells would not impact lineage choice. In principle, the addition of TCR-β could only increase the total strength of TCR signal received by the DN3 cell at the bifurcation point, and drive lineage choice to the γδ-fate. In support of this notion, we find that TCR-β coexpression with γδ-TCR increases CD24 down-regulation and CD73 up-regulation.
Importantly, differentiation is not the only consequence of TCR-selection at the DN3 developmental checkpoint. Another critical outcome is proliferation, and this could, in theory, be differentially regulated in TCR-β, γδ-TCR, and TCR-β/γδ–expressing DN3 cells. Here, we find that in certain situations, such as in the presence of a weak ligand, TCR-β/γδ–expressing Rag2−/− DN3 cells proliferate more extensively than their γδ-TCR-only–expressing counterparts. This increase in proliferation may indicate that, although the TCR-β chain cannot drive differentiation along the αβ-lineage when coexpressed with a γδ-TCR, it can promote some αβ-like proliferation, suggesting that survival, differentiation, and proliferation can be separated and distinctly driven by different TCRs (36).
Recent insights into the molecular basis for αβ- versus γδ-lineage choice have culminated in support of a signal strength model to dictate lineage fate. However, it remains unclear whether differential signal initiation mechanisms lead to changes in quantitative strength. It is now widely accepted that the pre-TCR is capable of cell-autonomous signaling (37–39), which combined with its low expression levels suggests that it emits either weak or transient signals. Although ligands have been characterized for some γδ-TCR subsets (40, 41), the role of ligand engagement for γδ T-cell development is much less clear. Removal of γδ-TCR ligand reduces transcription of Egr1 and Egr3, impairs γδ-lineage development and maturation, and simultaneously promotes αβ-lineage commitment to the DP stage (7). However, recent evidence suggests that IL-17–producing γδ-T cells may develop independently of ligand availability, but still induce of ERK1 and ERK2 activation through their TCR (27). Nevertheless, ligand-independent γδ-TCR–derived signals would appear to be stronger than those from the pre-TCR to drive γδ-lineage commitment.
Here, we attempt to clarify the role of γδ-TCR ligand at the αβ- versus γδ-bifurcation point by providing KN6 γδ-TCR–expressing T cells with a weak or strong ligand, via culture with BALB/c stromal cells expressing endogenous T10d or ectopic T22b, respectively. Our results show that provision of weak γδ-TCR ligand does not lead γδ-TCR–expressing DN3 cells to fully abandon a γδ-fate and develop as αβ-lineage DP cells, but appears to affect the ability of γδ-TCR–bearing cells to mature into the CD24lo CD73+ stage. Nevertheless, provision of a strong ligand led to enhanced CD73 expression and a decreased appearance of DP cells. Weak ligand may be sufficient to drive differentiation into the γδ-lineage as retroviral transduction of γδ-TCR leads to high levels of γδ-TCR expression on DN3 cells, and weak signaling through each individual γδ-TCR may additively equate to a sufficiently strong signal received by the cell that is capable of inducing γδ-lineage commitment and expression of γδ-biased genes. Because a fair proportion of γδ cells express the pre-TCR, it might be of interest to speculate as to the character of their receptors; perhaps these are TCRs transducing weak signals that need the pre-TCR to enforce the γδ fate. Thus, low affinity γδ-TCR or those that do not encounter ligand may develop into γδ-T cells because of the presence of a pre-TCR, which would add to the overall signal strength.
It remains to be determined whether differences in CD24 and CD73 expression can accurately reflect the maturation status of a γδ-T cell, or mark the bifurcation between cells committing to the αβ- versus γδ-lineage (33). Expression of γδ-biased genes in CD24hi DN γδ-TCR+ Rag2−/−Id3−/− DN3 cells, or DN3 cells from the B/c-DL cocultures, suggests that these cells have indeed chosen the γδ-lineage. Thus, CD24 expression may not be a reliable marker of lineage commitment, unlike CD4 and CD8. CD24hi CD4+ CD8+ reliably marks αβ-lineage DP cells, but to clearly define the lineage of CD24hi CD4− CD8− and CD24lo CD4− CD8− cells requires further analysis of their gene-expression profile.
TCR-selection at the DN3 stage can be Notch-dependent or -independent, and this decision is based on the final lineage choice, which can be based on the strength of TCR signals, as measured by Id3 induction (8). In this case, strong TCR signals that induce high levels of Id3 are able to suppress E protein activity beyond the threshold required for passage across γδ-selection, independently of Notch signaling. Conversely, the weak signals that emanate from a pre-TCR require concurrent Notch signals to achieve down-regulation of E2A to levels that allow for successful traversal of the β-selection checkpoint. No DP cells appear in γδ-TCR–expressing Rag2−/− DN3 cocultures without Notch signaling, even in the presence of pre-TCR expression. Interestingly, we also find that provision of a weak γδ-TCR ligand alters the requirement for Notch signaling in γδ-TCR–expressing cells. Here, γδ-TCR–expressing DN3 cells can no longer survive, differentiate, and proliferate in the absence of Notch signaling (B/c-Ctrl cells), in support of the notion that Notch-independence at this stage of development requires strong signals from the TCR.
Taken together, these data confirm previous reports that the strength of TCR signal dictates lineage choice. More importantly, these data provide novel insights into the lineage decisions of a DN3 cell that expresses both a TCR-β and γδ-TCR. In this case, TCR-β coexpression with γδ-TCR appears to provide additive TCR signals, and further promote γδ-lineage selection, maturation, and function. Further work in this area may be needed to address the differential ligand, differential maturation (as determined by CD24 and CD73), and differential TCR signal strength requirements for the functional maturation of γδ T cells.
Methods
Mice, retroviral transductions of Rag2−/− DN3 cells, cocultures of hematopoietic stem cells on OP9-DL1 cells, quantitative real-time PCR for mRNA analysis, generation of BALB/c stromal cell lines, T-cell stimulation assays, and statistical analysis are described in SI Methods. For flow cytometry, all single-cell suspensions were stained with commercially available antibodies (BD Biosciences and e-Biosciences) and analyzed with a BD-LSRII flow cytometer, using Flowjo software (Treestar). Dead cells were excluded from the analyses using DAPI gating.
Supplementary Material
Acknowledgments
We thank Courtney MacIntosh and Gisele Knowles for their expert flow cytometry support, and Y. H. Chien (Stanford University) for generously providing the anti-T22 (7H9-PE) antibody. This work was supported by funds from the Canadian Institutes of Health Research (CIHR-MOP-42387) and a Canadian Institutes of Health Research Studentship Award (to G.W.W.). J.C.Z.-P. is a recipient of a Canada Research Chair in Developmental Immunology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. H.v.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312872111/-/DCSupplemental.
References
- 1.Ciofani M, Knowles GC, Wiest DL, von Boehmer H, Zúñiga-Pflücker JC. Stage-specific and differential notch dependency at the alphabeta and gammadelta T lineage bifurcation. Immunity. 2006;25(1):105–116. doi: 10.1016/j.immuni.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Ciofani M, Zúñiga-Pflücker JC. Determining γδ versus αß T cell development. Nat Rev Immunol. 2010;10(9):657–663. doi: 10.1038/nri2820. [DOI] [PubMed] [Google Scholar]
- 3.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8- triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150(10):4244–4252. [PubMed] [Google Scholar]
- 4.Zúñiga-Pflücker JC. When three negatives made a positive influence in defining four early steps in T cell development. J Immunol. 2012;189(9):4201–4202. doi: 10.4049/jimmunol.1202553. [DOI] [PubMed] [Google Scholar]
- 5.Pereira P, Boucontet L, Cumano A. Temporal predisposition to αβ and γδ T cell fates in the thymus. J Immunol. 2012;188(4):1600–1608. doi: 10.4049/jimmunol.1102531. [DOI] [PubMed] [Google Scholar]
- 6.Hayes SM, Li L, Love PE. TCR signal strength influences alphabeta/gammadelta lineage fate. Immunity. 2005;22(5):583–593. doi: 10.1016/j.immuni.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Haks MC, et al. Attenuation of gammadeltaTCR signaling efficiently diverts thymocytes to the alphabeta lineage. Immunity. 2005;22(5):595–606. doi: 10.1016/j.immuni.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Lauritsen JP, et al. Marked induction of the helix-loop-helix protein Id3 promotes the gammadelta T cell fate and renders their functional maturation Notch independent. Immunity. 2009;31(4):565–575. doi: 10.1016/j.immuni.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alonzo ES, et al. Development of promyelocytic zinc finger and ThPOK-expressing innate gamma delta T cells is controlled by strength of TCR signaling and Id3. J Immunol. 2010;184(3):1268–1279. doi: 10.4049/jimmunol.0903218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ueda-Hayakawa I, Mahlios J, Zhuang Y. Id3 restricts the developmental potential of gamma delta lineage during thymopoiesis. J Immunol. 2009;182(9):5306–5316. doi: 10.4049/jimmunol.0804249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreslavsky T, Garbe AI, Krueger A, von Boehmer H. T cell receptor-instructed alphabeta versus gammadelta lineage commitment revealed by single-cell analysis. J Exp Med. 2008;205(5):1173–1186. doi: 10.1084/jem.20072425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauritsen JP, Haks MC, Lefebvre JM, Kappes DJ, Wiest DL. Recent insights into the signals that control alphabeta/gammadelta-lineage fate. Immunol Rev. 2006;209:176–190. doi: 10.1111/j.0105-2896.2006.00349.x. [DOI] [PubMed] [Google Scholar]
- 13.Maillard I, et al. The requirement for Notch signaling at the beta-selection checkpoint in vivo is absolute and independent of the pre-T cell receptor. J Exp Med. 2006;203(10):2239–2245. doi: 10.1084/jem.20061020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Washburn T, et al. Notch activity influences the alphabeta versus gammadelta T cell lineage decision. Cell. 1997;88(6):833–843. doi: 10.1016/s0092-8674(00)81929-7. [DOI] [PubMed] [Google Scholar]
- 15.Doerfler P, Shearman MS, Perlmutter RM. Presenilin-dependent gamma-secretase activity modulates thymocyte development. Proc Natl Acad Sci USA. 2001;98(16):9312–9317. doi: 10.1073/pnas.161102498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanigaki K, et al. Regulation of alphabeta/gammadelta T cell lineage commitment and peripheral T cell responses by Notch/RBP-J signaling. Immunity. 2004;20(5):611–622. doi: 10.1016/s1074-7613(04)00109-8. [DOI] [PubMed] [Google Scholar]
- 17.Ciofani M, et al. Obligatory role for cooperative signaling by pre-TCR and Notch during thymocyte differentiation. J Immunol. 2004;172(9):5230–5239. doi: 10.4049/jimmunol.172.9.5230. [DOI] [PubMed] [Google Scholar]
- 18.Burtrum DB, Kim S, Dudley EC, Hayday AC, Petrie HT. TCR gene recombination and alpha beta-gamma delta lineage divergence: productive TCR-beta rearrangement is neither exclusive nor preclusive of gamma delta cell development. J Immunol. 1996;157(10):4293–4296. [PubMed] [Google Scholar]
- 19.Heilig JS, Tonegawa S. T-cell gamma gene is allelically but not isotypically excluded and is not required in known functional T-cell subsets. Proc Natl Acad Sci USA. 1987;84(22):8070–8074. doi: 10.1073/pnas.84.22.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson A, de Villartay JP, MacDonald HR. T cell receptor delta gene rearrangement and T early alpha (TEA) expression in immature alpha beta lineage thymocytes: implications for alpha beta/gamma delta lineage commitment. Immunity. 1996;4(1):37–45. doi: 10.1016/s1074-7613(00)80296-4. [DOI] [PubMed] [Google Scholar]
- 21.Winoto A, Baltimore D. Separate lineages of T cells expressing the alpha beta and gamma delta receptors. Nature. 1989;338(6214):430–432. doi: 10.1038/338430a0. [DOI] [PubMed] [Google Scholar]
- 22.Gerber D, Boucontet L, Pereira P. Early expression of a functional TCRbeta chain inhibits TCRgamma gene rearrangements without altering the frequency of TCRgammadelta lineage cells. J Immunol. 2004;173(4):2516–2523. doi: 10.4049/jimmunol.173.4.2516. [DOI] [PubMed] [Google Scholar]
- 23.Erman B, Feigenbaum L, Coligan JE, Singer A. Early TCRalpha expression generates TCRalphagamma complexes that signal the DN-to-DP transition and impair development. Nat Immunol. 2002;3(6):564–569. doi: 10.1038/ni800. [DOI] [PubMed] [Google Scholar]
- 24.Serwold T, Hochedlinger K, Inlay MA, Jaenisch R, Weissman IL. Early TCR expression and aberrant T cell development in mice with endogenous prerearranged T cell receptor genes. J Immunol. 2007;179(2):928–938. doi: 10.4049/jimmunol.179.2.928. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt TM, Zúñiga-Pflücker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17(6):749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 26.Ciofani M, Zúñiga-Pflücker JC. Notch promotes survival of pre-T cells at the beta-selection checkpoint by regulating cellular metabolism. Nat Immunol. 2005;6(9):881–888. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- 27.Jensen KD, et al. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29(1):90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito K, et al. Recognition of the product of a novel MHC TL region gene (27b) by a mouse gamma delta T cell receptor. Cell. 1990;62(3):549–561. doi: 10.1016/0092-8674(90)90019-b. [DOI] [PubMed] [Google Scholar]
- 29.Schild H, et al. The nature of major histocompatibility complex recognition by gamma delta T cells. Cell. 1994;76(1):29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 30.Dent AL, et al. Self-reactive gamma delta T cells are eliminated in the thymus. Nature. 1990;343(6260):714–719. doi: 10.1038/343714a0. [DOI] [PubMed] [Google Scholar]
- 31.Bonneville M, et al. Blockage of alpha beta T-cell development by TCR gamma delta transgenes. Nature. 1989;342(6252):931–934. doi: 10.1038/342931a0. [DOI] [PubMed] [Google Scholar]
- 32.Mohtashami M, Shah DK, Kianizad K, Awong G, Zúñiga-Pflücker JC. Induction of T-cell development by Delta-like 4-expressing fibroblasts. Int Immunol. 2013;25(10):601–611. doi: 10.1093/intimm/dxt027. [DOI] [PubMed] [Google Scholar]
- 33.Coffey F, et al. The TCR ligand-inducible expression of CD73 marks γδ lineage commitment and a metastable intermediate in effector specification. J Exp Med. 2014;211(2):329–343. doi: 10.1084/jem.20131540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narayan K, et al. Immunological Genome Project Consortium Intrathymic programming of effector fates in three molecularly distinct γδ T cell subtypes. Nat Immunol. 2012;13(5):511–518. doi: 10.1038/ni.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishida I, et al. T-cell receptor gamma delta and gamma transgenic mice suggest a role of a gamma gene silencer in the generation of alpha beta T cells. Proc Natl Acad Sci USA. 1990;87(8):3067–3071. doi: 10.1073/pnas.87.8.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tourigny MR, Mazel S, Burtrum DB, Petrie HT. T cell receptor (TCR)-beta gene recombination: dissociation from cell cycle regulation and developmental progression during T cell ontogeny. J Exp Med. 1997;185(9):1549–1556. doi: 10.1084/jem.185.9.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saint-Ruf C, et al. Different initiation of pre-TCR and gammadeltaTCR signalling. Nature. 2000;406(6795):524–527. doi: 10.1038/35020093. [DOI] [PubMed] [Google Scholar]
- 38.Panigada M, et al. Constitutive endocytosis and degradation of the pre-T cell receptor. J Exp Med. 2002;195(12):1585–1597. doi: 10.1084/jem.20020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamasaki S, et al. Mechanistic basis of pre-T cell receptor-mediated autonomous signaling critical for thymocyte development. Nat Immunol. 2006;7(1):67–75. doi: 10.1038/ni1290. [DOI] [PubMed] [Google Scholar]
- 40.Lewis JM, et al. Selection of the cutaneous intraepithelial gammadelta+ T cell repertoire by a thymic stromal determinant. Nat Immunol. 2006;7(8):843–850. doi: 10.1038/ni1363. [DOI] [PubMed] [Google Scholar]
- 41.Chien YH, Konigshofer Y. Antigen recognition by gammadelta T cells. Immunol Rev. 2007;215:46–58. doi: 10.1111/j.1600-065X.2006.00470.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.