Significance
Antiretroviral therapy cannot eradicate HIV-1 because the virus can become transcriptionally inactive in resting memory CD4+ T cells (and other cell types), which are long-lived, thus generating a reservoir undetectable by the immune system. When therapy is stopped, the latent viral reservoir is activated and HIV-1 rebounds. Our understanding of HIV-1 latency and reactivation is incomplete. Here we report that the heat shock protein 90 (Hsp90) regulates HIV-1 reactivation from latency by controlling the NF-kB pathway. Therefore Hsp90 is a key molecule linking HIV-1 reactivation from latency to CD4+ T-cell activation. Selective Hsp90 inhibitors combined with PKC-ϑ inhibitors, all in phase II clinical trials, potently suppressed HIV-1 reactivation, thus Hsp90 may be a novel target to control HIV-1 latency.
Abstract
Latency allows HIV-1 to persist in long-lived cellular reservoirs, preventing virus eradication. We have previously shown that the heat shock protein 90 (Hsp90) is required for HIV-1 gene expression and mediates greater HIV-1 replication in conditions of hyperthermia. Here we report that specific inhibitors of Hsp90 such as 17-(N-allylamino)-17-demethoxygeldanamycin and AUY922 prevent HIV-1 reactivation in CD4+ T cells. A single modification at position 19 in the Hsp90 inhibitors abolished this activity, supporting the specificity of the target. We tested the impact of Hsp90 on known pathways involved in HIV-1 reactivation from latency; they include protein kinase Cs(PKCs), mitogen activated protein kinase/extracellular signal regulated kinase/positive transcriptional elongation factor-b and NF-κB. We found that Hsp90 was required downstream of PKCs and was not required for mitogen activated protein kinase activation. Inhibition of Hsp90 reduced degradation of IkBα and blocked nuclear translocation of transcription factor p65/p50, suppressing the NF-κB pathway. Coimmunoprecipitation experiments showed that Hsp90 interacts with inhibitor of nuclear factor kappa-B kinase (IKK) together with cochaperone Cdc37, which is critical for the activity of several kinases. Targeting of Hsp90 by AUY922 dissociated Cdc37 from the complex. Therefore, Hsp90 controls HIV-1 reactivation from latency by keeping the IKK complex functional and thus connects T-cell activation with HIV-1 replication. AUY922 is in phase II clinical trial and, in combination with a PKC-ϑ inhibitor in phase II clinical trial, almost completely suppressed HIV-1 reactivation at 15 nM with no cytotoxicity. Selective targeting of the Hsp90/Cdc37 interaction may provide a powerful approach to suppress HIV-1 reactivation from latency.
Combination antiretroviral therapy (cART) has significantly reduced mortality in HIV-1 infected individuals (1), but requires continuous long-term administration to maintain an undetectable viral load. cART must be administered chronically because of HIV-1 latency. The virus can become transcriptionally inactive in resting memory CD4+ T cells (and other cell types), which are long-lived, thus generating a reservoir undetectable by the immune system (2). When cART is stopped, the latent viral reservoir is activated and viral load rebounds to pretreatment levels within a few weeks (2). The long-lived latent viral reservoir prevents HIV-1 eradication and a cure.
Questions remain on how the latent reservoir is established and maintained. It is accepted that there is very low viral production even under cART (3). However, it is unclear if this residual viremia is due to ongoing replication in cryptic sites (mainly the gastrointestinal lymphatic system, GALT) where cART may diffuse at suboptimal concentrations, or to a long-lived reservoir that is stochastically activated. Addition of a new antiretroviral drug to an existing cART regimen, also called “intensification,” reduces residual viremia (4, 5). With time, cART intensification should reduce a reservoir maintained by continuous low-level viral replication. Clinical trials with cART intensification have yielded contradictory results (4–6) and phylogenetic studies showed that there is little evolution of the virus population constituting the reservoir, suggesting that residual viremia comes from a stable source rather than ongoing replication (7). It is likely that the latent HIV-1 reservoir is established relatively soon after infection. Initiation of cART during the acute phase of infection may reduce the size of the latent reservoir or even prevent its establishment. Indeed a small but significant proportion of individuals treated early do not show viral rebound after therapy interruption (so called posttherapy controllers) (8). Therefore, it may be possible to clear a small viral reservoir, provided effective treatment is initiated early enough.
This debate has important therapeutic implications. In the case of a large and long-lived reservoir that is maintained in the absence of ongoing viral replication, the only possible therapeutic strategy is to purge the latently infected cells. “Shock and kill” approaches are designed to induce HIV-1 reactivation in latently infected cells (shock), which will be killed either by cytopathic effects or by the immune system (9). HIV-1 reactivation can be achieved in vivo (10); however, several obstacles remain. First, there is no reliable assay yet to measure the effectiveness of HIV-1 reactivation in vivo. Second, there is little evidence so far that HIV-1 reactivation results in killing of (no longer latently) infected cells. Third, histone deacetylase inhibitor drugs used to reactivate HIV-1 are toxic, which may be a limitation if multiple administration cycles are required. In the case of a small reservoir that depends on some level of ongoing replication for its maintenance, the logical therapeutic approach is to prevent virus replication as far as possible, including its reactivation from latency, which will lead to progressive loss of latently infected cells due to their natural turnover over several years. Most likely, this therapeutic strategy can be implemented only within a short window of time in the early stages of infection.
Therefore, different therapeutic strategies might achieve a functional cure, depending on the stage of disease, time of therapy initiation, and size of the reservoir. Critical for the success of both strategies is a detailed knowledge of the mechanisms controlling HIV-1 reactivation. HIV-1 latency is a multifactorial process involving chromatin modifications, low levels of specific transcription factors, integration site selection, and cell activation (11–13). We have discovered that the heat shock protein 90 (Hsp90) is required for HIV-1 gene expression and that it localizes at the viral promoter DNA (14). Furthermore, we reported that Hsp90 is required for enhanced HIV-1 replication in conditions of hyperthermia (fever) by stimulating transcriptional activity of the viral promoter (15). Hyperthermia also stimulated HIV-1 reactivation from latency, and localization of Hsp90 at the viral transcriptional site increased from 30% in cells grown at 37 °C to 70% in cells grown at 39.5 °C. These observations suggested that Hsp90 might be important for HIV-1 reactivation from latency. Here we report that Hsp90 controls HIV-1 reactivation from latency by modulating the NF-κB pathway, and hence is a key molecule coupling T-cell activation to HIV-1 reactivation. Inhibitors of Hsp90 are in phase II clinical trials to treat cancer and are being considered to treat neurodegenerative diseases and cystic fibrosis (16). On the other hand, physiological processes, such as fever, can induce Hsp90. Thus, Hsp90, or specific Hsp90 client proteins, may be an important target for different therapeutic strategies aimed at a functional cure for HIV-1 infection.
Results
Hsp90 Regulates HIV-1 Reactivation from Latency.
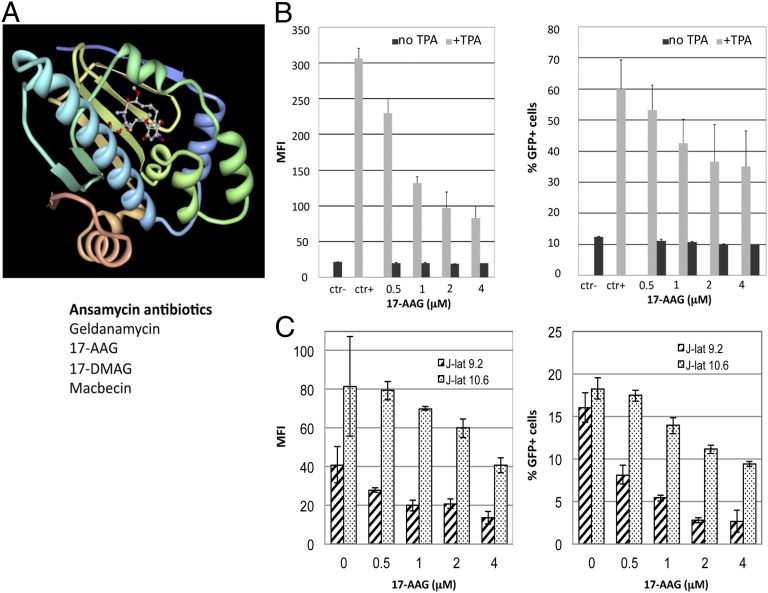
Preliminary evidence suggested that Hsp90 might be important for HIV-1 reactivation from latency in conditions of hyperthermia (15). To further examine the role of Hsp90 on HIV-1 reactivation, we used J-Lat cells, a well-established and widely used model of HIV-1 latency (17). In these cells, HIV-1 is latently integrated and can be reactivated by various stimuli, including phorbol esters [12-O-tetradecanoylphorbol-13-acetate (TPA)], prostratin, and TNFα (17, 18). The integrated viral genome encodes GFP, which affords precise quantification of HIV-1 reactivation from latency by flow cytometry. We used small molecules derivatives of ansamycin antibiotics to selectively inhibit Hsp90 (19). Their prototype is geldanamycin (GA) and its derivatives include 17-(N-allylamino)-17-demethoxygeldanamycin (17-AAG), 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG), and macbecin. These inhibitors all bind to the ATPase pocket of Hsp90 and compete with ATP, inactivating the chaperone (19–21) (Fig. 1A). J-Lat A2 cells, which carry a latent HIV-1 vector, were stimulated with TPA for 24–36 h in the presence of the compounds and then analyzed by flow cytometry. TPA induced significant HIV-1 reactivation, which translated into higher GFP mean fluorescent intensity (MFI) and a greater percentage of GFP+ cells (Fig. 1B). The 17-AAG reduced HIV-1 reactivation in a dose-dependent manner but showed no effect on basal levels of GFP expression in unstimulated cells (Fig. 1B). Similar results were obtained within J-Lat clones 9.2 and 10.6, which carry a full-length HIV-1 genome (17) (Fig. 1C). Given the similar results, we used J-Lat A2 cells in subsequent experiments.
Fig. 1.
Ansamycin antibiotics repress HIV-1 reactivation from latency. (A) Crystal structure of GA bound to the N-terminal ATPase pocket of Hsp90 (Protein Data Bank: 1YET). (B) J-Lat cells (A2 clone) were stimulated with 5 nM TPA for 24 h to induce HIV-1 reactivation in the presence of the indicated concentrations of 17-AAG and analyzed by FACS; ctr−, DMSO only. Bars show average values ± SD, n = 3. (C) Same as B, but J-Lat clones 9.2 and 10.6, harboring a full-length HIV-1 provirus, were used. Bars show average values less background (no TPA) ± SD, n = 3.
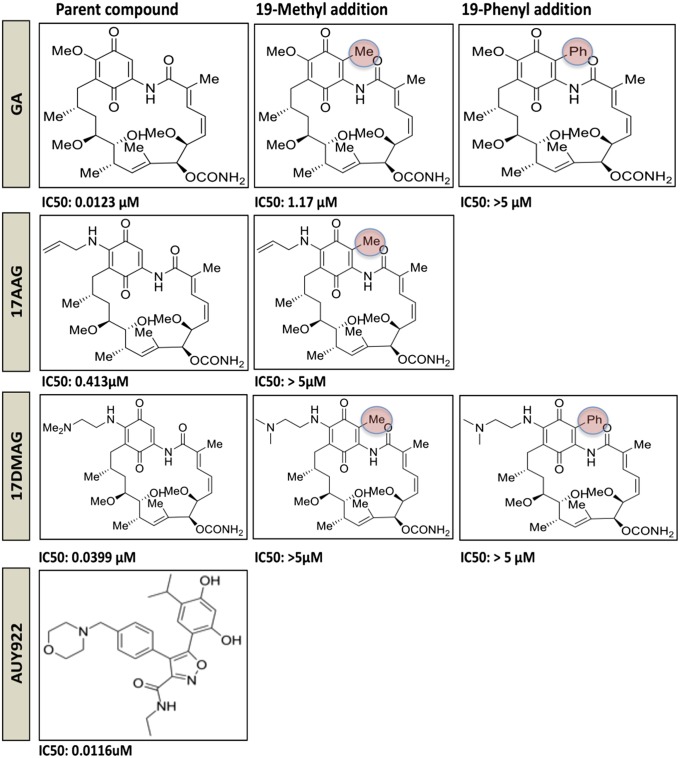
To test the specificity of this result, several derivatives of GA, 17-AAG, and 17-DMAG with different substitutions were used in the same experimental system. Notably, addition of a methyl or phenyl group at position 19 (22, 23) made the compounds significantly weaker suppressors of HIV-1 reactivation (Fig. 2). This effect was detected with GA, 17-AAG, and 17-DMAG, which share a common chemical structure but differ in the group at position 17 (19) (Fig. 2). In agreement with these results, compounds with an extra group at position 19 have lower affinity for Hsp90 due to a structural clash at the edge of the ATPase pocket (22). We also tested AUY922, a small molecule inhibitor of Hsp90, currently in phase II clinical trials (19). This compound is a derivative of radicicol and has a different chemical structure from 17-AAG, yet similarly targets the ATPase pocket of HSp90 (24). AUY922 potently inhibited HIV-1 reactivation from latency, with an IC50 of ∼12 nM (Fig. 2). The compounds did not show any significant cytotoxicity at the doses used, with the exception of GA, which was somewhat toxic at doses greater than 2 μM (Fig. S1). Therefore, structurally unrelated small compounds targeting Hsp90 can repress HIV-1 reactivation. Conversely, structurally related small compounds targeting Hsp90 lose activity by a single substitution at position 19.
Fig. 2.
Substitutions at position 19 reduce potency of Hsp90 inhibitors that repress HIV-1 reactivation. Chemical structures of different Hsp90 inhibitors are shown, with the specific substitutions at position 19 highlighted (red circles). The IC50 is indicated below each compound structure. IC50 was calculated in J-Lat A2 cells by ExcelFit as the concentration of drug, reducing by 50% the MFI values relative to cells treated with TPA only.
The Role of Hsp90 in the Activation of Mitogen Activated Protein Kinase/Extracellular Signal Regulated Kinase and PKC Pathways.
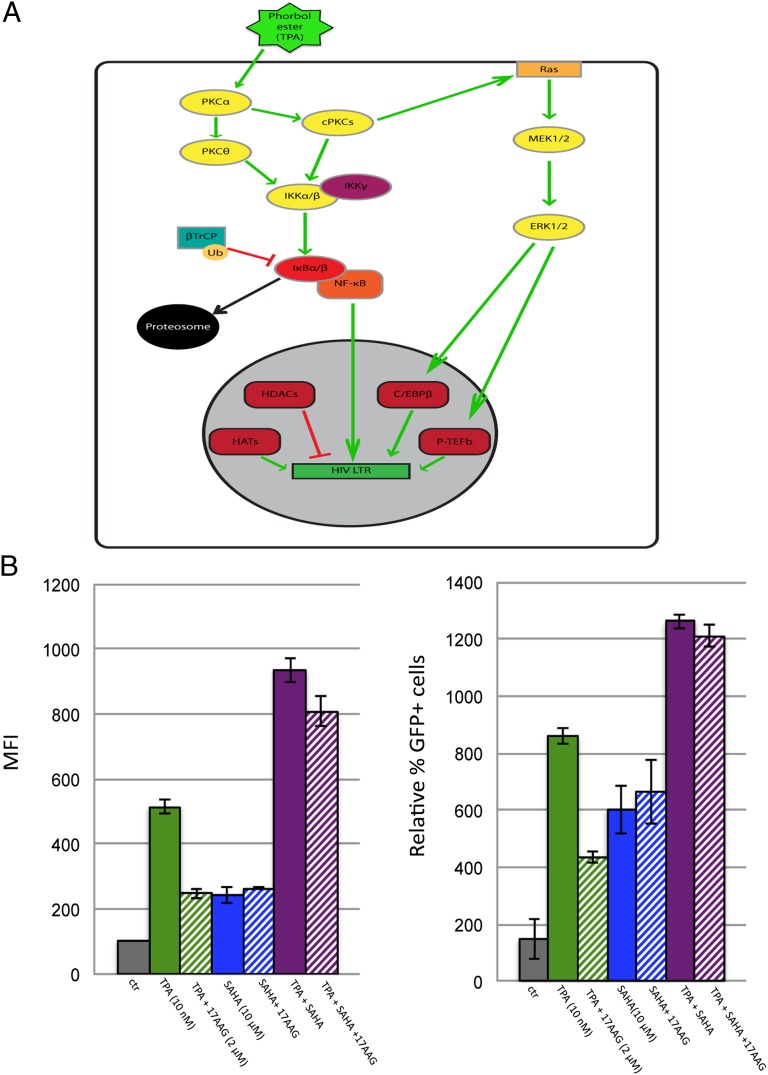
The results shown in Figs. 1 and 2 indicate that Hsp90 is a target controlling HIV-1 reactivation from latency, prompting us to investigate the mechanism. Several pathways induce HIV-1 reactivation from latency, including chromatin remodelling, signaling through the mitogen activated protein kinase/extracellular signal regulated kinase/positive transcriptional elongation factor-b (MEK/MAPK/P-TEFb) pathway, PKCs and the NF-κB pathway (11, 12). TPA activates several of these pathways; hence we examined the role of Hsp90 in each (Fig. 3A). Histone deacetylation of chromatin at the viral promoter (LTR) represses viral gene expression, whereas histone deacetylase (HDAC) inhibitors induce HIV-1 transcriptional activation and reactivation from latency (10, 25). To test if HDAC inhibitors required Hsp90 to reactivate HIV-1, TPA, or the HDAC inhibitor suberoylanilidehydroxamic acid (SAHA) were used to stimulate J-Lat cells in the presence of 17-AAG or DMSO. SAHA reactivated HIV-1 in J-Lat A2 cells, albeit less than TPA, but conversely to TPA, was insensitive to 17-AAG treatment (Fig. 3B). The 17-AAG also marginally repressed HIV-1 reactivation induced by the combination of TPA and SAHA (Fig. 3B). These results suggest that HDAC inhibitors stimulate HIV-1 reactivation independently of Hsp90.
Fig. 3.
Phorbol esters induce several pathways regulating HIV-1 reactivation. (A) Schematic representation of the pathways activated by TPA or prostratin (PS) leading to HIV-1 reactivation. (B) J-Lat cells (A2 clone) were stimulated with 5 nM TPA for 24 h to induce HIV-1 reactivation in the presence of the indicated concentrations of 17-AAG or SAHA or a combination of the two and analyzed by FACS; ctr−, DMSO only. Bars show average values ± SD, n = 3.
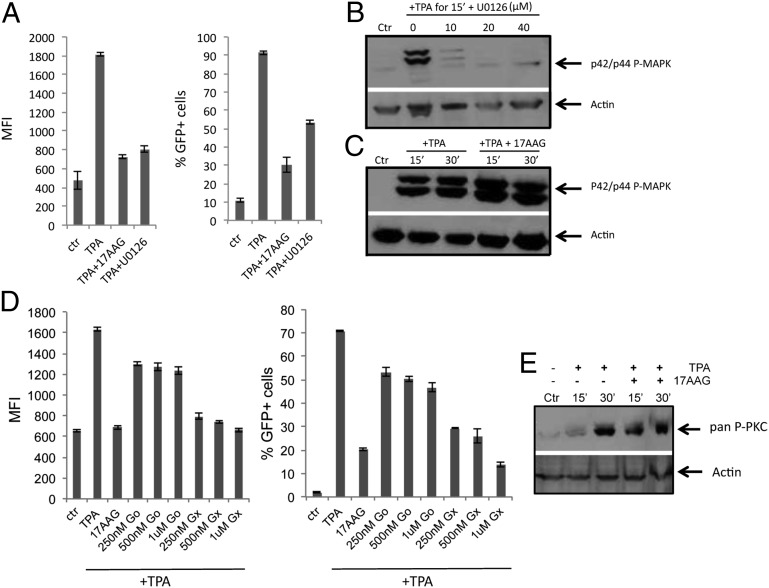
To test the involvement of Hsp90 in the MEK/MAPK/P-TEFb pathway, J-Lat cells were stimulated with TPA as previously described. TPA induced significant HIV-1 reactivation, which was repressed by the specific MEK1/2 inhibitor U0126 (26) (Fig. 4A). This result is consistent with previous studies showing the critical role of the MEK/MAPK/P-TEFb pathway in HIV-1 reactivation (13). Samples were analyzed by Western blot to detect phosphorylated MAPK p42/p44. TPA induced robust MAPK phosphorylation, which was repressed by U0126 in a dose-dependent manner (Fig. 4B). Notably, 17-AAG did not inhibit MAPK phosphorylation induced by TPA (Fig. 4C), suggesting that Hsp90 was not involved in this pathway either.
Fig. 4.
The MEK/MAPK pathway and PKCs are not targeted by Hsp90 inhibitors. (A) J-Lat cells (A2 clone) were stimulated with 10 nM TPA for 24 h to induce HIV-1 reactivation in the presence of 17-AAG (2 μM) and U0126 (20 μM) and analyzed by FACS. Bars show average values ± SD, n = 3. (B) Cells were stimulated with TPA (10 nM) for 15 min in the presence of the indicated concentrations of U0126. Samples were collected and analyzed by Western blot to detect phosphorylated p42/p44 MAPK. (C) Cells were stimulated with TPA (10 nM) for 15 or 30 min in the presence of 2 μM 17-AAG and analyzed by Western blot as above. (D) J-Lat cells (A2 clone) were stimulated with 10 nM TPA for 24 h to induce HIV-1 reactivation in the presence of 17-AAG (2 μM) and the indicated concentrations of Go or Gx. Bars show average values ± SD, n = 3. (E) Cells were stimulated with TPA (10 nM) for 15 or 30 min in the presence of 17-AAG (2 μM) and analyzed by Western blots with an anti-pan phosphorylated PKC antibody.
Next, we investigated the role of Hsp90 in HIV-1 reactivation mediated by PKCs. J-Lat cells were stimulated by TPA, a potent inducer of PKCs (27), in the presence of 17-AAG or two different PKC inhibitors: Gö6976 (Go), which targets conventional PKCs and was previously shown to repress HIV-1 reactivation (28), or GF109203X (Gx), which targets both conventional and novel PKCs (29) (Fig. 4D). Inhibition of PKCs repressed HIV-1 reactivation induced by TPA (Fig. 4D). Go was a weaker inhibitor of reactivation than Gx (Fig. 4D), consistent with the notion that both conventional and novel PKC isoforms are important in mediating HIV-1 reactivation (30). Samples were analyzed by Western blot to detect PKC phosphorylation as a measure of their induction. Robust PKC phosphorylation was detected 30 min after TPA addition; however, 17-AAG had no effect on this response (Fig. 4E). This suggested that Hsp90 was not directly required for PKC activation but most likely acted downstream of this step, although we cannot exclude that phosphorylation of a less abundant PKC isoform was inhibited but was masked by more abundant isoforms in our Western blot analysis.
Hsp90 and the NF-κB Pathway.
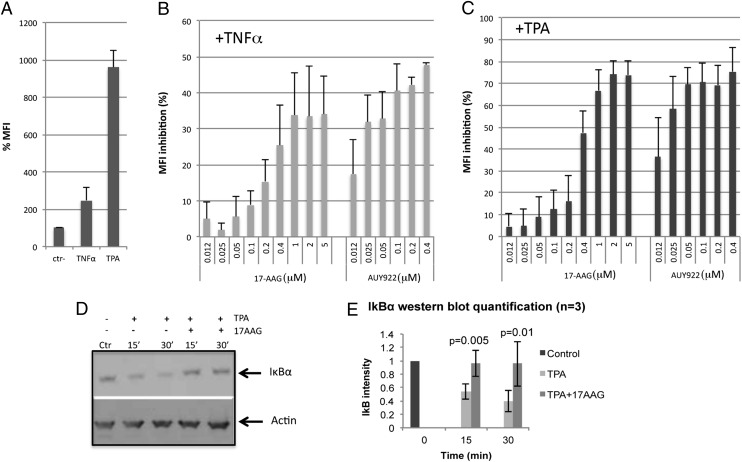
TPA activates the NF-κB pathway via the PKC pathway (31). PKC phosphorylation appeared functional in the presence of 17-AAG (Fig. 4 D and E), suggesting that 17-AAG may directly affect the NF-κB pathway, which is critical because the HIV-1 promoter has two binding sites for the transcription factor p65/p50 (RelA/p50) complex (12). The classical NF-κB pathway is regulated by the inhibitor of nuclear factor kappa-B kinase (IKK) complex (IKKα/IKKβ/IKKγ), which phosphorylates IkBα in complex with RelA and p50. Upon phosphorylation, IkBα is ubiquitinated and degraded, exposing a nuclear localization signal in RelA, promoting nuclear migration of the RelA/p50 complex and subsequent activation of NF-κB–responsive genes (31) (Fig. 3A). To test the involvement of Hsp90 in this pathway, J-Lat cells were stimulated with TNFα or TPA as described, in the presence of 17-AAG or AUY922. TNFα is an inducer of the NF-κB pathway and was indeed able to stimulate HIV-1 reactivation in J-Lat cells, albeit with less potency than TPA (Fig. 5A). Both 17-AAG and AUY922 significantly repressed HIV-1 reactivation induced by either TNFα or TPA (Fig. 5 B and C), with little or no cell toxicity (Fig. S2), suggesting that the Hsp90 inhibitors might directly target the NF-κB pathway. To further confirm this result, cells were analyzed by Western blot to detect degradation of IkBα, a critical step in the induction of the NF-κB pathway (31, 32). Treatment of cells with TPA for 30 min resulted in clear degradation of IkBα. However, when cells were also treated with 17-AAG there was no loss of IkBα (Fig. 5 D and E). This experiment was repeated using prostratin, a different phorbol ester that potently activates the NF-κB pathway (18), and obtained very similar results (Fig. S3).
Fig. 5.
Hsp90 inhibitors target the NF-κB pathway to block HIV-1 reactivation from latency. (A) J-Lat cells (A2 clone) were stimulated with TNFα (5 ng/mL) or TPA (5 nM) for 24 h to induce HIV-1 reactivation. (B) Cells stimulated with TNFα (5 ng/mL) or (C) TPA (5 nM) for 24 h in the presence of the indicated concentrations of AUY922 or 17-AAG were analyzed by flow cytometry. Bars in A–C show average values ± SD, n = 3. (D) Cells were stimulated with TPA (5 nM) for 15 or 30 min in the presence of 17-AAG (2 μM) and analyzed by Western blot with an antibody against IkBα. (E) Image-J quantification of IkBα band intensity relative to actin from three independent Western blots. Bars show average values ± SD, n = 3; control, no TPA, no 17-AAG.
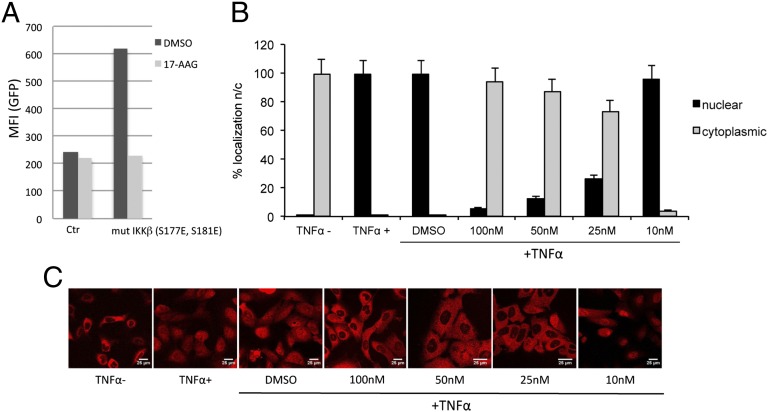
Next, we assessed the role of Hsp90 on the different components of the NF-κB pathway. The IKK complex was tested first. To this end, we nucleofected J-Lat cells with a combination of a plasmid encoding mCherry and another encoding an hyperactive IKKβ mutant (IKKβ S177E, S181E) (33) at a 1:2 ratio, then measured HIV-1 reactivation in the population of mCherry+ cells by two color flow cytometry. Control cells were nucleofected with the same total amount of mCherry plasmid only. The cotransfection protocol ensured that we only analyzed the cell population that was effectively transduced. The IKKβ mutant plasmid induced HIV-1 reactivation and 17-AAG blocked it (Fig. 6A), indicating that Hsp90 was important for the correct functioning of the IKK complex.
Fig. 6.
Hsp90 inhibitors act on the NF-κB pathway. (A) J-Lat cells were nucleofected with a 1:2 combination of a plasmid encoding mCherry and a plasmid encoding an hyperactive IKK mutant (mut IKKβ S177E, S181E) or the same total amount of mCherry plasmid only (Ctr). Twenty-four hours after nucleofection, cells were analyzed by two color FACS to detect the level of HIV-1 reactivation (GFP channel) within the mCherry+ cell population. Nucleofection of mut IKKβ S177E, S181E plasmid induced HIV-1 reactivation, which was repressed by 17-AAG (2 μM). (B) U2OS_exo cells were incubated with AUY922 for 20 h at the indicated concentrations before stimulation with TNFα (30 ng/mL) for 30 min and immunostaining to detect NF-κB (p65). Nuclear and cytoplasmic localization were scored by manual counting of at least 350 cells in triplicate. DMSO 1%, cells stimulated by TNFα in the absence of AUY922. (C) Representative pictures used to quantify nuclear and cytoplasmic localization of NF-κB.
Upon IKK activation and degradation of IkBα, the RelA/p50 complex is released and rapidly migrates into the nucleus (31). Using immunofluorescence confocal microscopy, we tested the nucleocytoplasmic distribution of RelA/p50 upon stimulation with TNFα in the presence of AUY922. To make the analysis by confocal microscopy easier and more reliable, adherent U2OS_exo cells were used for these experiments (34). RelA/p50 was cytoplasmic in control, untreated cells and almost exclusively nuclear in cells treated with TNFα (Fig. 6 B and C). AUY922 blocked nuclear migration of RelA/p50 induced by TNFα in a dose-dependent manner (Fig. 6 B and C), consistent with the notion that Hsp90 is required for activation of the NF-κB pathway.
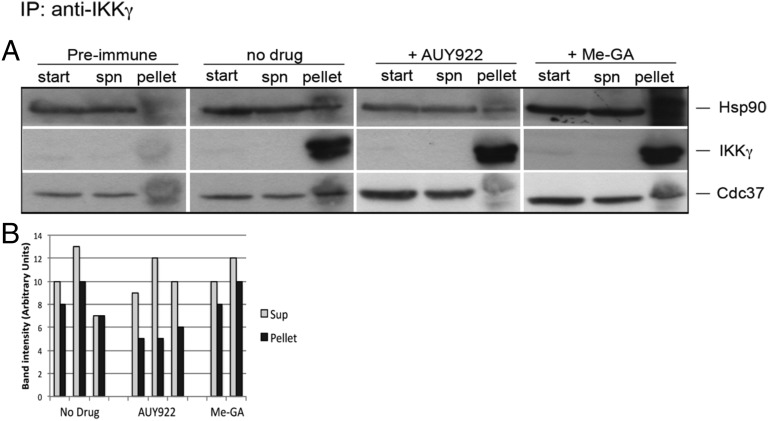
Taken together, these data strongly suggested that Hsp90 is directly required for activation of the NF-κB pathway at the level of IKK function; therefore, we wanted to investigate how AUY922 acted on IKK. Interestingly, two previous reports showed that Hsp90 was recruited into the IKK complex by binding to IKKγ (35, 36). We therefore explored possible interactions between Hsp90 and IKKγ by immunoprecipitation. J-Lat cells were stimulated by TPA for 5 min, and extracts were prepared, immunoprecipitated with anti-IKKγ antibodies, and analyzed by Western blot with antibodies specific for IKKγ, Hsp90, and the cochaperone Cdc37. Cdc37 is a kinase-specific cochaperone, which was shown to associate with IKKγ (35, 37) (Fig. 7). We detected an interaction between Hsp90 and IKKγ, which was specific because preimmune sera did not precipitate either of them (Fig. 7A). Addition of AUY922 did not dissociate Hsp90 from IKKγ; however, it dissociated Cdc37 from the complex (Fig. 7A). Notably, GA with a methyl group substitution at position 19, which was a much weaker repressor of HIV-1 reactivation (Fig. 2), did not displace Cdc37 from the IKKγ/Hsp90 complex (Fig. 7 A and B). These results indicate that Hsp90 and its cochaperone Cdc37 are part of the functional IKK complex. Targeting Hsp90 with small molecule inhibitors such as AUY922 dissociates Cdc37 and inactivates IKK, inhibiting IkBα degradation, RelA/p65 nuclear translocation, and HIV-1 reactivation from latency.
Fig. 7.
AUY922 dissociates Cdc37 from the Hsp90/IKK complex. (A) J-Lat cells were stimulated with TPA (10 nM) for 15 min and cell extracts used to perform immunoprecipitations with an anti-IKKγ polyclonal antibody or preimmune serum in the presence of 4 μM AUY922 or 4 μM 19-Me-GA. Samples were analyzed by Western blot with monoclonal antibodies against IKKγ, Hsp90, and Cdc37. (B) Image-J quantification of the Cdc37 band intensity detected in the supernatant and the pellet fractions. Each bar represents an individual experiment.
Drug Combination to Repress HIV-1 Reactivation from Latency.
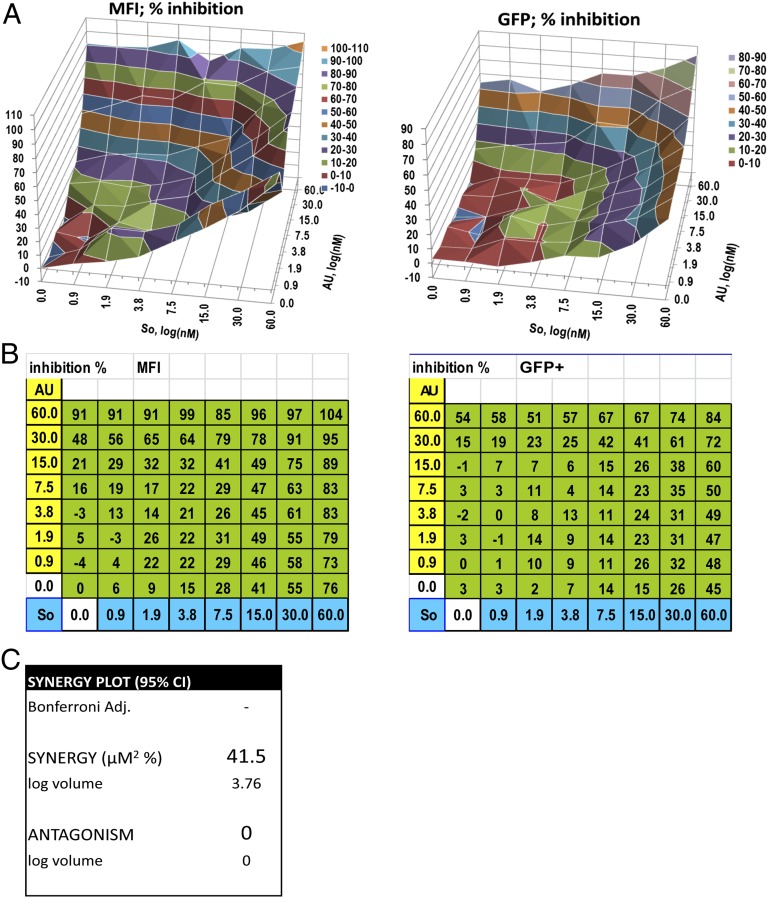
Because Hsp90 is required for NF-κB activation downstream of PKCs, we reasoned that combining AUY922 with a specific PKC inhibitor should have an additive effect on HIV-1 reactivation from latency. This might be advantageous, allowing the use of lower concentrations of each compound to achieve potent suppression of HIV-1 reactivation. We tested sotrastaurin, a small molecule PKCθ inhibitor, which was tested in phase II clinical trials in kidney transplantation to control immunorejection at the dose of 20–40 μM (38). PKCθ is a T-cell–specific isoform previously implicated in HIV-1 reactivation from latency (30). In agreement with our prediction, AUY922 and sotrastaurin had an additive or modest synergistic effect on HIV-1 reactivation, such that AUY922 at a concentration of 15 nM reached an IC90 in combination with as little as 60 nM sotrastaurin, which corresponds to 1/330th of the concentration of sotrastaurin used in the phase II trial (Fig. 8).
Fig. 8.
Combining AUY922 and sotrastaurin to repress HIV-1 reactivation. J-Lat A2 cells were stimulated by TPA (10 nM) for 24 h in the presence of the indicated concentrations of AUY922 and sotrastaurin, alone or in combination. Samples were analyzed by FACS and data plotted using MacSynergy II software. (A) Representative McSynergy II plot: areas of the graph above zero indicate an additive or synergistic effect. (B) Representative checkerboard grid used to calculate the plot shown in A. (C) Average McSynergy II score in μM2% of four independent experiments at 95% confidence interval. Values between 25 and 50 μM2% with log volumes of >2 and <5 indicate modest but significant synergy (50–52).
Discussion
We have previously shown that Hsp90 is required for HIV-1 gene expression (14, 15). Others have shown that the NF-κB pathway is an important regulator of HIV-1 latency reactivation (12) and that Hsp90 and Cdc37 bind to the IKK complex (35). Critically, we now unify in a coherent picture these separate observations, establishing a functional link between Hsp90 and HIV-1 reactivation from latency, which may have therapeutic implications. Furthermore, we provide evidence for an additional mechanism of action of AUY922, which relies on the displacement of Cdc37 from the Hsp90/IKK complex. Although with hindsight the connection between Hsp90, IKK, Cdc37, RelA/p65, and HIV-1 might appear obvious, Hsp90 is an abundant chaperone with many client proteins, and HIV-1 reactivation is a multifactorial process; hence, we expected multiple layers where Hsp90 might be involved. In fact our systematic analysis determined that the effect of Hsp90 on HIV-1 reactivation from latency primarily depends on the NF-κB pathway. Several lines of evidence support this conclusion. First, selective inhibition of Hsp90 did not affect activation of the MEK/MAPK pathway or PKCs, both of which induce HIV-1 reactivation from latency (13). Second, Hsp90 was not required for reactivation induced by HDAC inhibitors such as SAHA, suggesting that the chaperone is not directly involved in control of histone acetylation at the viral promoter. Third, Hsp90 was required for IkBα phosphorylation and degradation induced by TPA, and for RelA/p50 nuclear translocation, all critical steps of the NF-κB pathway (31). However, our results indicated that Hsp90 acts on the NF-κB pathway upstream of IkBα: by binding to IKKγ and recruiting Cdc37, Hsp90 appears critical for IKK function and hence HIV-1 reactivation. Indeed, 19-methyl GA, which was a much weaker repressor of HIV-1 reactivation, was unable to displace Cdc37 from the IKK/Hsp90 complex, whereas AUY922 could do so effectively, in agreement with its potent antireactivation activity. Cdc37 is an Hsp90 cochaperone essential for the function of several kinases (37), and our results suggest that its displacement by AUY922 impairs the activity of IKK. Thus, AUY922 may act in a double fashion: competing ATP binding to the Hsp90 ATPase pocket and displacing Cdc37 from Hsp90.
NF-κB is central to HIV-1 reactivation from latency, connecting activation of T cells with HIV-1 gene expression (12, 31). In most HIV-1 subtypes, the viral promoter has two binding sites for NF-κB that regulate transcription, particularly at the early stage postinfection, before significant amounts of Tat are produced (13, 39). The NF-κB pathway is activated by many stimuli in CD4+ cells, including T-cell receptor engagement, innate and intrinsic immune responses, and several cytokines (31). On the other hand, Hsp90 functions in many pathways that regulate cell homeostasis in response to external stimuli and stresses (40). This suggests that Hsp90 may be a key molecule linking HIV-1 reactivation from latency with different external stimuli. Within this framework, a good example is hyperthermia, which induces Hsp90 and HIV-1 reactivation from latency (15). Our results help interpret this interesting connection, because hyperthermia can activate the NF-κB pathway (41, 42). Hsp90 and Cdc37 were shown to modulate the innate immune response upon sensing of HIV-1 DNA (43), which depends, in part, on the NF-κB pathway. Hence our results also have relevance for HIV-1 reactivation induced by other invading pathogens.
Remarkably, a recent global analysis in Drosophila showed that Hsp90 is present at chromatin on promoters that must be rapidly activated or silenced, including IFN-induced genes (44). We reported previously that Hsp90 associates with the HIV-1 promoter at chromatin and that hyperthermia increases the frequency of localization of Hsp90 at the viral transcriptional site (14, 15). The integrated HIV-1 provirus may be considered as a cellular gene that needs to be rapidly activated. Therefore, it will be interesting to investigate if Hsp90 has a specific nuclear function in connection with HIV-1 reactivation from latency, in addition to its role in the activation of the NF-κB pathway. Hsp90 was also shown to aid the assembly of an active cyclinT1–CDK9 complex (also called P-TEFb complex), which is recruited by Tat to phosphorylate the C-terminal domain of RNA Pol II and promote transcriptional elongation (45). The P-TEFb complex is stable (>48 h) therefore the short-term Hsp90 inhibition in our experiments (24–36 h) is unlikely to reduce its levels significantly. Nonetheless, the Hsp90 inhibitors might affect P-TEFb complex localization or function at the viral promoter and hence repress HIV-1 reactivation, adding another potential target for Hsp90 inhibitors.
Hsp90 inhibitors are in phase II clinical trials (www.clinicaltrials.gov) to treat solid malignancies and lymphomas. It remains to be seen if such inhibitors have a safety profile good enough to be used to repress HIV-1 reactivation from latency in the clinical setting. One may envisage repressing HIV-1 reactivation in acutely infected individuals or at least in early stages postinfection, when the latent reservoir is still small. In combination with cART, blocking HIV-1 reactivation may lead to disappearance of the reservoir in a few years due to the natural turnover of latently infected cells (8). The safety profile of Hsp90 inhibitors might be improved by adding to the therapeutic regimen a PKCθ inhibitor with an additive effect on HIV-1 reactivation. Even if the safety profile of Hsp90 inhibitors will not be good enough for long-term use, it might be helpful to use the inhibitors for therapy of HIV-1+ lymphomas, which are rather frequent (46). Hodgkin and non-Hodgkin lymphomas in HIV-1+ individuals have a more adverse prognosis unless HIV-1 is suppressed during and shortly after treatment (47), yet concomitant administration of chemotherapy and cART can increase toxicity (46). Hsp90 inhibitors might produce a better outcome in these patients by treating the lymphoma and repressing HIV-1 replication at the same time. Interestingly, Hsp90 inhibitors have been shown to inhibit Kaposi sarcoma (KS) herpes virus and to induce regression of KS, another HIV-1–associated malignancy (48, 49).
Materials and Methods
Chemical Reagents.
TPA, prostratin, geldanamycin, 17-(N-allylamino)-17-demethoxygeldanamycin (17-AAG), 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG), were obtained from Sigma; Gö6976 and GF10920X from Calbiochem; U0126, AUY922, and sotrastaurin were obtained from Selleckchem; and SAHA was purchased from Cayman Chemical. The synthesis of 19-substituted geldanamycin derivatives was described previously (22, 23). Compounds diluted in DMSO were stored at −80 °C in the dark.
Cells and HIV-1 Reactivation.
J-Lat cells (5 × 105/mL) were cultured in Gibco RPMI-1640 media, supplemented with 10% (vol/vol) FCS and 5% (vol/vol) penicillin streptomycin at 37 °C, 5% CO2 under sterile conditions. For HIV-1 reactivation experiments, 106 cells/mL were mixed with TPA (10 nM final unless otherwise indicated) or TNFα (5 ng/mL final) and immediately 150 μL of cell mix was dispensed into 96-well plates. Drugs were added to the 96-well plates in serial dilutions to a total volume of 200 μL. Cells were incubated for 24 h before analysis by flow cytometry. To calculate IC50, activation of J-Lat cells was performed as described above using five serial drug dilutions. The MFI values were then plotted using ExcelFit.
Primary Antibodies.
The following antibodies were used for Western blotting: rabbit anti-IkBα [4812S; Cell Signal (1:1,000)]; mouse anti–NF-κB [sc-8008 F-6; Santa Cruz (1:200)]; rabbit anti-HSP90α/β [sc-7947; Santa Cruz (1:400)]; mouse anti-IKKγ [B-3; Santa Cruz (1:200)]; mouse anti-Cdc37 [C1; Thermo Scientific (1:200)]; rabbit anti-actin [A2668; Sigma (1:3,000)]; rabbit anti-pan P-PKC [beta II S660; Cell Signaling (1:2,000)]; and rabbit anti–P-p44/42 MAPK T202/Y204 [D13.14.4E; Cell Signaling (1:500)].
Western Blotting.
Cells were lysed in SDS sample buffer (0.5 M sucrose, 2 mM MgCl2, 140 mM Tris pH 8.0, 50 mM DTT, 2% SDS) and separated on 3–8% gradient SDS/PAGE (Novex). Proteins were transferred on nitrocellulose membrane that was subsequently incubated with primary antibodies described above. Secondary antibodies were goat anti-rabbit–HRP [Dako (1:3,000)] and goat anti-mouse–HRP [Dako (1:500)]. Detection was by cheminumilescence (ECL).
Immunoprecipitations.
Approximately 108 J-Lat cells were cultured in 500 mL media. Aliquots of 100 mL each were incubated with 5 nM TPA or DMSO in the presence of AUY922 (4 μM) or 19-Me-geldanamycin (4 μM) for 30 min. Following treatment, 1-mL aliquots were analyzed by FACS. The remaining samples were collected in 50-mL Falcon tubes, and centrifuged at 500 × g for 5 min in a benchtop centrifuge. The supernatant was removed and pellets were washed with 1 mL of ice-cold PBS twice. Subsequently, cells were gently resuspended in 1 mL lysis buffer [50 mM Hepes (pH 7.6), 150 mM NaCl, 1 mM EDTA, 0.1% Nonidet P-40, 10% (vol/vol) glycerol, 1 mM DTT, 0.5 mM PMSF, 20 mM β-glycerophosphate, and 1× complete protease inhibitor) before centrifuging at 18,000 × g for 10 min at 4 °C. The supernatant (which contains the cytoplasmic fraction) was collected and two aliquots of 25 μL stored (this was the START sample). The pellet (which contains the nuclear fraction) was washed with cold PBS twice before resuspension in 1 mL lysis buffer containing 10 μL benzonase (Sigma) and incubated at 37 °C for 40 min. Samples were incubated with 30 μL of rabbit preimmune serum or rabbit polyclonal IKKγ antibody (FL-419) (Santa Cruz) for 2 h at room temperature with rotation. Samples containing the cell lysates were incubated with magnetic Dynabeads Protein G, prepared according to the manufacturer’s instructions, for 1 h at 4 °C on a rotating platform. Samples were washed with 1 mL lysis buffer thrice and transferred into a clean tube. Samples were eluted in 25 μL elution buffer (50 mM glycine pH 2.8) before analysis by Western blot.
Immunofluorescence Confocal Microscopy.
U2OS_exo kept in DMEM 10% (vol/vol) FCS + Pen/Strep were transfected with plasmids encoding EYFP-MS2nls and HIV-1 Tat essentially as described previously (34). Cells where incubated with AUY922 for 20 h at the indicated concentrations before stimulation with TNFα (30 ng/mL) for 30 min. Cells were washed with PBS, fixed with 3.7% (vol/vol) paraformaldehyde for 15 min, incubated 5 min with 100 mM glycine, and permeabilized with 0.1% Triton X-100 for 5 min. Subsequently cells were incubated at 37 °C for 30 min with PBS, 1% BSA and 0.1% Tween 20 before incubation with the anti–NF-κB (p65) antibody (D14E12; Cell Signaling), diluted 1:200. The coverslips were rinsed three times with PBS 0.1% Tween 20 (washing solution) and incubated for 1 h with the secondary antibody conjugated to Alexa 594 diluted 1:500. Coverslips were washed three times with washing solution and mounted on slides using Vectashield mounting medium (Vector Laboratories). Fluorescent images were captured on Zeiss LSM510 META confocal microscope with a 63× NA 1.4 Plan-Apochromat oil objective. Nuclear and cytoplasmic localization was scored by manual counting of an average of 350 cells for each condition in triplicate.
Nucleofection.
Nucleofection was carried out in a Nucleofector-I electroporator (Amaxa) according to the manufacturer's protocol (Nucleofection kit V protocol for Jurkat cells). In brief, approximately 10 mL of exponentially growing J-Lat cells (0.6 × 106/mL) were centrifuged and resuspended in 400 μL zap-buffer mixed with supplement, and then 100 μL of cell suspension was mixed with DNA [400 ng pCRSW-mCherry + 800 ng pCMV2-IKK2 [IKKβ(S177E, S181E)] or 1,200 ng pCRSW-mCherry only] and transferred into an electroporation cuvette. Electroporation was performed with program X-001, cells were kept at room temperature for 10 min, then 0.5 mL warm media was added, cells transferred into a 12-well plate, and incubated at 37 °C/5% CO2 for 24 h before fixation (1% paraformaldehyde in PBS) and flow cytometry (LSR Fortessa).
AUY922 and Sotrastaurin Combination Analysis.
J-Lat A2 cells (0.8 × 106/mL) were plated (200 μL/well) in 96-well plates, which were predispensed with TPA (10 nM final) and serial dilutions of AUY922 and sotrastaurin in a checkerboard grid fashion. Cells were fixed and analyzed by flow cytometry after 24 h incubation. MacSynergy II software was used to calculate additive/synergistic effects (50, 51). The concentration range of the individual drugs was chosen to ensure that the inhibition by each drug remained <95%. This is because when a single drug reaches >95% inhibition, any additive or synergistic effect of drug combination can no longer be detected (51). McSynergy scores were calculated from four independent experiments using a 95% confidence interval according to the software instructions (52). A synergy volume was calculated by adding all of the positive values for each drug combination. These volumes were then statistically evaluated using the 95% confidence level and expressed in percentage of μM2.
Supplementary Material
Acknowledgments
We thank Mary Collins and Mehdi Baratchian for helpful discussions and reagents. This work was supported by a Medical Research Centre UK Grant and the European Union Framework Program 7 HIVINNOV (Grant 305137) (to A.F.), Parkinson’s UK (R.A.K. and C.J.M.), and the AIDS Program of Istituto Superiore di Sanità, Italy (A.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1320178111/-/DCSupplemental.
References
- 1.Palella FJ, Jr, et al. HIV Outpatient Study Investigators Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Finzi D, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278(5341):1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 3.Palmer S, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci USA. 2008;105(10):3879–3884. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramratnam B, et al. The decay of the latent reservoir of replication-competent HIV-1 is inversely correlated with the extent of residual viral replication during prolonged anti-retroviral therapy. Nat Med. 2000;6(1):82–85. doi: 10.1038/71577. [DOI] [PubMed] [Google Scholar]
- 5.Buzón MJ, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16(4):460–465. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 6.Dinoso JB, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci USA. 2009;106(23):9403–9408. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joos B, et al. Swiss HIV Cohort Study HIV rebounds from latently infected cells, rather than from continuing low-level replication. Proc Natl Acad Sci USA. 2008;105(43):16725–16730. doi: 10.1073/pnas.0804192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sáez-Cirión A, et al. ANRS VISCONTI Study Group Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9(3):e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durand CM, Blankson JN, Siliciano RF. Developing strategies for HIV-1 eradication. Trends Immunol. 2012;33(11):554–562. doi: 10.1016/j.it.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Archin NM, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487(7408):482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hakre S, Chavez L, Shirakawa K, Verdin E. Epigenetic regulation of HIV latency. Curr Opin HIV AIDS. 2011;6(1):19–24. doi: 10.1097/COH.0b013e3283412384. [DOI] [PubMed] [Google Scholar]
- 12.Chan JK, Greene WC. NF-κB/Rel: Agonist and antagonist roles in HIV-1 latency. Curr Opin HIV AIDS. 2011;6(1):12–18. doi: 10.1097/COH.0b013e32834124fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Lint C, Bouchat S, Marcello A. HIV-1 transcription and latency: An update. Retrovirology. 2013;10:67. doi: 10.1186/1742-4690-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vozzolo L, et al. Gyrase B inhibitor impairs HIV-1 replication by targeting Hsp90 and the capsid protein. J Biol Chem. 2010;285(50):39314–39328. doi: 10.1074/jbc.M110.155275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roesch F, et al. Hyperthermia stimulates HIV-1 replication. PLoS Pathog. 2012;8(7):e1002792. doi: 10.1371/journal.ppat.1002792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandt GE, Blagg BS. Alternate strategies of Hsp90 modulation for the treatment of cancer and other diseases. Curr Top Med Chem. 2009;9(15):1447–1461. doi: 10.2174/156802609789895683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan A, Bisgrove D, Verdin E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 2003;22(8):1868–1877. doi: 10.1093/emboj/cdg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams SA, et al. Prostratin antagonizes HIV latency by activating NF-kappaB. J Biol Chem. 2004;279(40):42008–42017. doi: 10.1074/jbc.M402124200. [DOI] [PubMed] [Google Scholar]
- 19.Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: Are we there yet? Clin Cancer Res. 2012;18(1):64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prodromou C, et al. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90(1):65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 21.Stebbins CE, et al. Crystal structure of an Hsp90-geldanamycin complex: Targeting of a protein chaperone by an antitumor agent. Cell. 1997;89(2):239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 22.Kitson RR, et al. Synthesis of 19-substituted geldanamycins with altered conformations and their binding to heat shock protein Hsp90. Nat Chem. 2013;5(4):307–314. doi: 10.1038/nchem.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitson RR, Moody CJ. An improved route to 19-substituted geldanamycins as novel Hsp90 inhibitors—potential therapeutics in cancer and neurodegeneration. Chem Commun (Camb) 2013;49(76):8441–8443. doi: 10.1039/c3cc43457e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brough PA, et al. 4,5-diarylisoxazole Hsp90 chaperone inhibitors: Potential therapeutic agents for the treatment of cancer. J Med Chem. 2008;51(2):196–218. doi: 10.1021/jm701018h. [DOI] [PubMed] [Google Scholar]
- 25.Van Lint C, Emiliani S, Ott M, Verdin E. Transcriptional activation and chromatin remodeling of the HIV-1 promoter in response to histone acetylation. EMBO J. 1996;15(5):1112–1120. [PMC free article] [PubMed] [Google Scholar]
- 26.Favata MF, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273(29):18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 27.Dorn GW, 2nd, Mochly-Rosen D. Intracellular transport mechanisms of signal transducers. Annu Rev Physiol. 2002;64:407–429. doi: 10.1146/annurev.physiol.64.081501.155903. [DOI] [PubMed] [Google Scholar]
- 28.Qatsha KA, Rudolph C, Marmé D, Schächtele C, May WS. Gö 6976, a selective inhibitor of protein kinase C, is a potent antagonist of human immunodeficiency virus 1 induction from latent/low-level-producing reservoir cells in vitro. Proc Natl Acad Sci USA. 1993;90(10):4674–4678. doi: 10.1073/pnas.90.10.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toullec D, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266(24):15771–15781. [PubMed] [Google Scholar]
- 30.Trushin SA, et al. Human immunodeficiency virus reactivation by phorbol esters or T-cell receptor ligation requires both PKCalpha and PKCtheta. J Virol. 2005;79(15):9821–9830. doi: 10.1128/JVI.79.15.9821-9830.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 32.Traenckner EB, et al. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J. 1995;14(12):2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mercurio F, et al. IKK-1 and IKK-2: Cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278(5339):860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 34.Maiuri P, et al. Fast transcription rates of RNA polymerase II in human cells. EMBO Rep. 2011;12(12):1280–1285. doi: 10.1038/embor.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen G, Cao P, Goeddel DV. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol Cell. 2002;9(2):401–410. doi: 10.1016/s1097-2765(02)00450-1. [DOI] [PubMed] [Google Scholar]
- 36.Field N, et al. KSHV vFLIP binds to IKK-gamma to activate IKK. J Cell Sci. 2003;116(Pt 18):3721–3728. doi: 10.1242/jcs.00691. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Soroka J, Buchner J. The Hsp90 chaperone machinery: Conformational dynamics and regulation by co-chaperones. Biochim Biophys Acta. 2012;1823(3):624–635. doi: 10.1016/j.bbamcr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Friman S, et al. Sotrastaurin, a novel small molecule inhibiting protein-kinase C: Randomized phase II study in renal transplant recipients. Am J Transplant. 2011;11(7):1444–1455. doi: 10.1111/j.1600-6143.2011.03538.x. [DOI] [PubMed] [Google Scholar]
- 39.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 40.Pearl LH, Prodromou C, Workman P. The Hsp90 molecular chaperone: An open and shut case for treatment. Biochem J. 2008;410(3):439–453. doi: 10.1042/BJ20071640. [DOI] [PubMed] [Google Scholar]
- 41.Stanley SK, Bressler PB, Poli G, Fauci AS. Heat shock induction of HIV production from chronically infected promonocytic and T cell lines. J Immunol. 1990;145(4):1120–1126. [PubMed] [Google Scholar]
- 42.Lee CT, Zhong L, Mace TA, Repasky EA. Elevation in body temperature to fever range enhances and prolongs subsequent responsiveness of macrophages to endotoxin challenge. PLoS ONE. 2012;7(1):e30077. doi: 10.1371/journal.pone.0030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee MN, et al. Identification of regulators of the innate immune response to cytosolic DNA and retroviral infection by an integrative approach. Nat Immunol. 2013;14(2):179–185. doi: 10.1038/ni.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sawarkar R, Sievers C, Paro R. Hsp90 globally targets paused RNA polymerase to regulate gene expression in response to environmental stimuli. Cell. 2012;149(4):807–818. doi: 10.1016/j.cell.2012.02.061. [DOI] [PubMed] [Google Scholar]
- 45.O’Keeffe B, Fong Y, Chen D, Zhou S, Zhou Q. Requirement for a kinase-specific chaperone pathway in the production of a Cdk9/cyclin T1 heterodimer responsible for P-TEFb-mediated tat stimulation of HIV-1 transcription. J Biol Chem. 2000;275(1):279–287. doi: 10.1074/jbc.275.1.279. [DOI] [PubMed] [Google Scholar]
- 46.Deeken JF, et al. The rising challenge of non-AIDS-defining cancers in HIV-infected patients. Clin Infect Dis. 2012;55(9):1228–1235. doi: 10.1093/cid/cis613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bower M, et al. Immunologic recovery in survivors following chemotherapy for AIDS-related non-Hodgkin lymphoma. Blood. 2008;111(8):3986–3990. doi: 10.1182/blood-2007-10-115659. [DOI] [PubMed] [Google Scholar]
- 48.Chen W, Sin SH, Wen KW, Damania B, Dittmer DP. Hsp90 inhibitors are efficacious against Kaposi Sarcoma by enhancing the degradation of the essential viral gene LANA, of the viral co-receptor EphA2 as well as other client proteins. PLoS Pathog. 2012;8(11):e1003048. doi: 10.1371/journal.ppat.1003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nayar U, et al. Targeting the Hsp90-associated viral oncoproteome in gammaherpesvirus-associated malignancies. Blood. 2013;122(16):2837–2847. doi: 10.1182/blood-2013-01-479972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prichard MN, Prichard LE, Shipman C., Jr Strategic design and three-dimensional analysis of antiviral drug combinations. Antimicrob Agents Chemother. 1993;37(3):540–545. doi: 10.1128/aac.37.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng JY, et al. The triple combination of tenofovir, emtricitabine and efavirenz shows synergistic anti-HIV-1 activity in vitro: A mechanism of action study. Retrovirology. 2009;6:44. doi: 10.1186/1742-4690-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prichard MN, Aseltine KR, Shipman C. MacSynergy II. Ann Arbor, MI: University of Michigan; 1993. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.