Significance
Deciphering the systemic signaling mechanisms that modulate metabolic activity has important implications owing to the central role that metabolism plays in regulating organismal adaptability and survival. Here, we show that loss of Drosophila TGF-β/Activin-like ligand Dawdle (Daw) causes major alterations in larval metabolic activity, including accumulation of tricarboxylic acid cycle intermediates, acidification of hemolymph pH, and misregulation of insulin signaling and nuclear-encoded mitochondrial gene expression. These metabolic defects lead to a food-dependent lethality phenotype, suggesting that Daw likely influences environmental adaptability via its modulation of several central metabolic processes. These observations, coupled with previous findings in mammals and Caenorhabditis elegans, highlight a potentially conserved role for TGF-β/Activin signaling in regulating important metabolic processes across the animal kingdom and may have clinical implications.
Keywords: dIlp2, hormone, acidosis
Abstract
The ability to maintain cellular and physiological metabolic homeostasis is key for the survival of multicellular organisms in changing environmental conditions. However, our understanding of extracellular signaling pathways that modulate metabolic processes remains limited. In this study we show that the Activin-like ligand Dawdle (Daw) is a major regulator of systemic metabolic homeostasis and cellular metabolism in Drosophila. We find that loss of canonical Smad signaling downstream of Daw leads to defects in sugar and systemic pH homeostasis. Although Daw regulates sugar homeostasis by positively influencing insulin release, we find that the effect of Daw on pH balance is independent of its role in insulin signaling and is caused by accumulation of organic acids that are primarily tricarboxylic acid (TCA) cycle intermediates. RNA sequencing reveals that a number of TCA cycle enzymes and nuclear-encoded mitochondrial genes including genes involved in oxidative phosphorylation and β-oxidation are up-regulated in the daw mutants, indicating either a direct or indirect role of Daw in regulating these genes. These findings establish Activin signaling as a major metabolic regulator and uncover a functional link between TGF-β signaling, insulin signaling, and metabolism in Drosophila.
Regulation of cellular metabolism and metabolic homeostasis is crucial for maintaining cellular and organismal physiology. Consequently, robust regulatory networks have evolved in most organisms to adapt and maintain a desired metabolic state depending on the environment and/or developmental stage (1, 2). Deciphering how components of these networks function to achieve homeostasis is essential for understanding normal physiology and the underlying mechanisms behind multifaceted disorders like metabolic syndrome and diabetes. Our understanding of these regulatory networks is largely confined to known metabolic pathways like the insulin signaling (IS) pathway, cellular target of rapamycin (TOR) pathway, and neuroendocrine signals. Nevertheless, additional extracellular signaling mechanisms that can regulate cellular and physiological metabolism must exist to allow integration of environmental, physiological, and developmental cues. In accordance with this possibility, involvement of classical developmental pathways in regulating physiological homeostasis has recently gained much attention (3, 4).
One such developmental pathway that has been shown to affect several aspects of metabolism, including nutrient and energy homeostasis, is TGF-β signaling (5, 6). TGF-β signaling has recently been proposed to regulate mitochondrial biogenesis in mammals based on the observation that aberrant Activin signaling in mice can lead to changes in energy metabolism and mitochondrial gene expression (7). However, the study used a gene-replacement strategy where the mature domain of Activin-A was replaced with that of Activin-B (InhibaBK), thereby changing the relative levels of these two ligands. TGF-β ligands signal through distinct combinations of type I and type II receptors that are specific to different classes of ligands (8). Although both Activin-A and -B can signal redundantly through the type I receptor ALK4, Activin-B can also signal through ALK7 initiating unique biological responses (9). Hence, studies involving the InhibaBK mouse fail to distinguish individual contributions of Activin-A and Activin-B in the manifestation of the energy metabolism defects. Additionally, both Activin-A and -B have been implicated in regulation of IS (10). Because IS is known to impinge on both the TOR and AMPK signaling pathways, aberrant Activin signaling can potentially affect mitochondrial metabolism by affecting IS. Parsing out these mechanistic details is crucial for understanding the role of TGF-β/Activin signaling in metabolism and devising strategies to manipulate this pathway for therapeutic gain.
In this study, we use Drosophila melanogaster to investigate the role of TGF-β/Activin signaling in regulation of metabolic homeostasis. Drosophila contains a highly conserved TGF-β signaling pathway. However, the number of signaling components in Drosophila is much smaller than vertebrates, allowing easy genetic manipulation of the pathway and reduces complications arising from functional redundancies between signaling molecules (8). Canonical TGF-β/Activin signaling in the fly is mediated by just three ligands, Activinβ (Actβ), Dawdle (Daw) [also called Alp (Activin-like peptide)], and Myoglianin (Myo), that signal through a single type I receptor, Babo, and a single intracellular R-Smad homolog, Smox (11). In addition, considerable parallels exist in the regulation of metabolic homeostasis between Drosophila and mammals providing a powerful genetic system to investigate the role of TGF-β signaling in metabolism and homeostasis (1, 2, 5).
Here, we demonstrate that canonical TGF-β/Activin signaling, mediated by the Drosophila Activin homolog Daw, is a central metabolic regulator that impacts both mitochondrial metabolism and IS. Interestingly, we find that although Daw regulates IS by positively regulating release of Drosophila insulin-like peptides (Dilps), the effect of Daw on mitochondrial metabolism is independent of insulin/insulin-like growth factor signaling (IIS) and may be mediated by changes in expression of nuclear-encoded mitochondrial genes.
Results
Absence of Daw Leads to Food-Dependent Lethality and Loss of Insulin Signaling.
Null daw mutants showed a food-dependent larval lethality phenotype where greater than 90% of mutant larvae died before the third instar wandering stage when grown on standard cornmeal food (CMF) (Fig. 1 A and B). Mutant larvae that did reach the pupal stage (<10%) showed significant developmental delay compared with yw controls (Fig. 1A), and the mutant pupae never reached the adult stage. Interestingly, both larval lethality and developmental delay were significantly rescued when mutants were grown on 60% yeast paste food (YPF) (Fig. 1 A and B). Moreover, when grown on YPF, a significant number of daw mutants eclosed as adults that were of comparable size to control animals (Fig. S1A). Similar to daw mutants, loss of babo or Smox also showed a food-dependent larval lethal phenotype. The majority of either babo or Smox mutant larvae also failed to reach the pupal stage on CMF but formed prepupae on YPF. Notably, unlike daw, null mutations in either myo or actβ do not significantly affect larval viability on CMF food. These results show that canonical TGF-β signaling, initiated by Daw, is necessary for survival on the CMF.
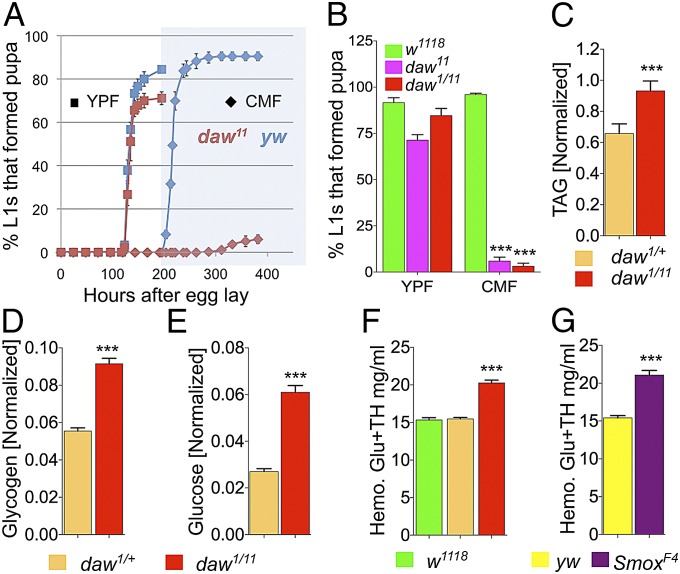
Fig. 1.
Loss of canonical Daw signaling leads to food dependent larval lethality and loss of IIS. (A) Developmental curves showing percentage of larvae that formed pupae and the time required to reach pupariation on CMF and YPF (n = 3–4; all viability assays contained 60 animals per sample). (B) Viability of homozygous (daw11) and transheterozygous (daw1/11) mutants and wild-type controls on CMF and YPF (n = 6). (C–E) Total TAG, glycogen, and glucose content (normalized to total protein) of daw mutant and control larvae grown on YPF (n = 20–36). (F and G) Hemolymph glucose plus trehalose (Glu+TH) concentration of daw and Smox mutant and control larvae (n = 36 for daw and controls and n = 8–12 for Smox mutants and controls). All results are reported as means ± SEM.
To determine the cause of this food-dependent lethality, we looked for metabolic defects in the daw mutants by measuring steady-state levels of key metabolites in feeding third-instar mutant larvae grown on YPF and comparing them to stage matched controls. daw mutant larvae showed a significant increase in total triacylglycerol (TAG), glucose, and glycogen stores compared with controls (Fig. 1 C–E). These observations indicate a role for Daw in IIS as a number of previous studies in Drosophila have shown an association between elevated TAG and glycogen with loss of IIS (12–14). Because IIS is also known to negatively regulate circulating sugar concentration in Drosophila larvae, we examined and measured the circulating sugar concentration of daw mutant and control larvae and found a significant increase in the daw mutants compared with relevant controls confirming a strong loss of IIS in daw mutants (Fig. 1F). daw mutant larvae also showed a strong reduction in phospho-Akt level and a strong increase in InR expression in the peripheral tissues, further supporting the loss of IIS in these mutants (Fig. S1 B and C). A similar increase in circulating sugar concentration (about 40%) was also observed in Smox null mutant larvae, indicating that Daw acts through the canonical Smad pathway to regulate IIS (Fig. 1G).
Loss of Daw Leads to Accumulation of Tricarboxylic Acid Cycle Intermediates and Systemic Acidosis.
To identify potential additional metabolic roles for Daw in larvae, we performed a high-throughput GC/MS-based metabolomics analysis of daw mutant and control larvae comparing abundances of small cellular metabolites. We found that loss of Daw led to a significant increase in the abundance of multiple intracellular metabolic acids. The majority of these organic acids are intermediates in the tricarboxylic acid (TCA) cycle, indicating a role of Daw in the regulation of intracellular sugar/mitochondrial metabolism (Fig. 2A). Interestingly, some of these metabolites, such as citrate and succinate, are found in high concentrations in the hemolymph of Lepidopterans and are proposed to play an important role in regulating pH balance (15, 16). We therefore checked defects in pH homeostasis in the daw mutants. We found that accumulation of acidic metabolites in the daw mutants is indeed associated with significant acidification of daw larval hemolymph compared with controls (Fig. 2B). Interestingly, CMF is substantially more acidic than YPF owing to addition of propionic acid (PA) as a mold inhibitor. Because daw mutants exhibit internal acidosis, we explored the possibility that the mutants may be vulnerable to acidic food conditions. We found that PA-induced acidity is one of the major components of CMF that leads to lethality of daw mutants on this food (Figs. S2 and S3 and SI Results and Discussion).
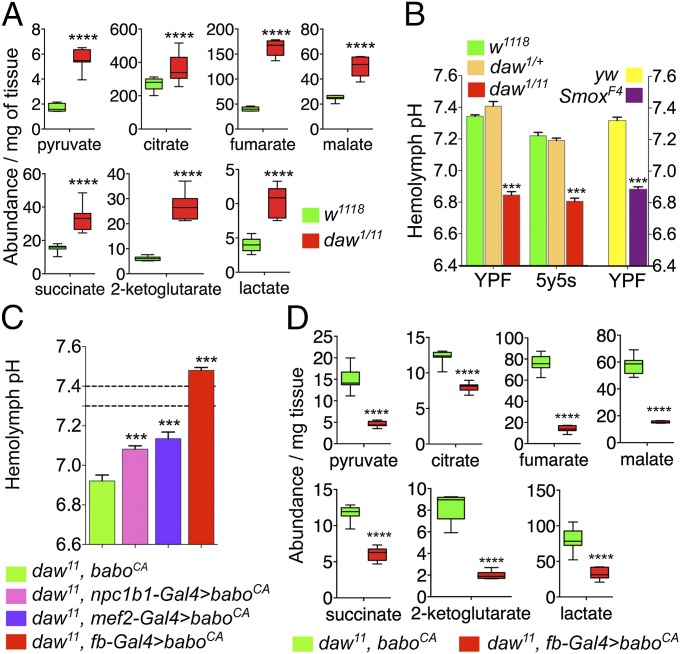
Fig. 2.
Loss of canonical Daw signaling leads to cellular metabolic defects and systemic acidosis. (A) High-throughput GC/MS-based measurement of cellular metabolic intermediates show significant increase in multiple sugar metabolism and TCA cycle intermediates (n = 8.) (The experiment was repeated twice. Results remained consistent and box plots from only one set of data are shown here.) (B) Hemolymph pH of daw mutant, Smox mutant, and control larvae grown on YPF and a semidefined 5y5s food (n = 8–12). (C) Hemolymph pH of daw mutant expressing baboCA in the gut (npc1b1-Gal4), muscle (mef2-Gal4), and FB (fb-Gal4) and relevant control larvae (n = 8–12). (Dashed lines indicate range for hemolymph pH of wild-type animals YPF.) (D) Relative abundance of metabolites that were up-regulated in daw mutants in control (daw mutant) larvae and mutant larvae overexpressing baboCA in the larval FB (box plot; n = 8 per value; with 15 animals per sample).
The accumulation of acidic metabolites in daw mutants indicates that the observed acidosis is likely metabolic in origin. We tested whether the acidosis phenotype of daw mutants can be rescued by activating TGF-β signaling in major metabolic tissues of the larvae. We found that expression of an activated type I receptor, baboCA, in large metabolically active tissues like the gut, muscle, or fat body (FB), was able to significantly rescue the acidosis phenotype (Fig. 2C). Interestingly, overexpressing baboCA in the FB most strongly affected hemolymph pH, making the hemolymph significantly more basic than normal, and also caused a significant reduction in levels of metabolic acids in the daw mutants (Fig. 2 C and D). These observations indicate that Daw affects hemolymph pH and production of metabolic acids by primarily targeting the FB and also indicates a direct correlation between acidosis and the level of metabolic acids.
Daw Dose-Dependently Regulates Hemolymph Sugar Concentration and pH.
TGF-β signaling in Drosophila is mediated by three ligands, Daw, Actβ, and Myo. Because loss of Daw alone leads to the acidosis phenotype, we checked whether Daw is the primary TGF-β ligand acting on the larval FB. Western blots performed using FB and muscle/epidermis tissue showed that endogenous p-Smox signal is specifically lost in the larval FB in absence of Daw, indicating that Daw is the primary TGF-β ligand acting on the FB (Fig. 3A). Consistently, overexpression of daw in the FB caused significant alkalinization of the daw mutant hemolymph, phenocopying the effect of FB-specific overexpression of BaboCA (Fig. 3B). Interestingly, FB overexpression of daw also completely rescued larval lethality of the daw mutants on CMF enabling them to eclose as adults (Fig. S4A) and also significantly rescued the diabetic phenotype observed in the daw mutants (Fig. 3C). The FB however is unlikely to be the target of daw signaling for the loss of IIS observed in the mutants because expression of smox RNAi in the FB does not induce a diabetic phenotype in wild-type larvae, nor does FB > BaboCA rescue the diabetic phenotype daw mutants (Fig. 4E). Therefore, we explored the possibility that Daw made by the FB can act on other tissues in the larvae in an endocrine fashion. To test this hypothesis, we tried rescuing the acidosis phenotype of the daw mutants by overexpressing daw in the insulin-producing cells (IPCs). Overexpression of daw in the IPCs was able to significantly rescue the acidosis phenotype of the daw mutants, indicating that Daw made by the IPCs can rescue TGF-β signaling in the FB that is responsible for the acidosis phenotype (Fig. 3D). We further confirmed the ability of Daw to function in an endocrine manner by overexpressing daw in a number of non-FB tissues (IPCs, oenocytes, and muscle) in daw mutants and by examining restoration of p-Smox in the FB using Western blots. Overexpression of daw in any of these tissues significantly restored p-Smox in the FB, and the strength of signal in the FB correlated with the size of the tissue in which daw was expressed (muscle > oenocytes> IPCs; Fig. 3E). Overexpression of daw in any of these tissues could also rescue lethality of daw mutants on CMF, and expression of the other two Drosophila TGF-β ligands, actβ and myo, did not increase in these rescued animals (Fig. S4 B and C). These results show that Daw expressed in one tissue can indeed signal systemically and likely in a dose-dependent manner much like a hormone. The dose-dependent effect of Daw on the strength of TGF-β signaling is also observed in our rescue experiments for metabolic phenotypes. Whereas expressing daw in the IPCs partially rescue both the acidosis and diabetic phenotype, expressing daw in the FB caused the hemolymph pH to become more alkaline than normal (Fig. 3B) and also caused the circulating sugar concentration to drop much below the normal range (Fig. 3D).
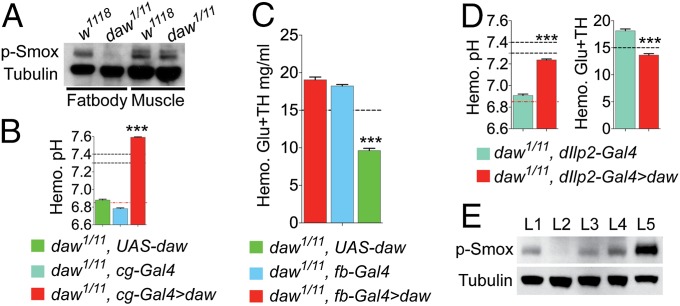
Fig. 3.
Dose-sensitive endocrine role of Daw in regulating sugar and pH homeostasis. Black dashed lines in the figures indicate normal value/range of hemolymph sugar concentration or pH in wild-type animals. (A) Endogenous p-Smox signal in wild-type and daw mutants larval FB and muscle. (B) Hemolymph pH of daw mutant larvae expressing daw under the regulation of cg-Gal4 driver and of relevant controls (n = 6). Red dashed line, hemolymph pH of daw mutants on YPF. (C) Hemolymph Glu+TH concentration of daw mutant larvae expressing a daw under the regulation of FB-specific driver fb-Gal4 and of relevant controls (n = 12–20). (D) Hemolymph pH and Glu+TH concentration of daw mutant larvae expressing daw in the larval IPCs (dilp2-Gal4 driver) and of relevant controls (n = 12–13 and 24, respectively). (E) Endogenous p-Smox levels in FB tissue dissected from wt, mutant control and rescued animals expressing daw in the IPCs, oenocytes, and muscle. L1: w1118; L2: daw1/11, UAS-daw/+; L3: daw1/11, dIlp2 > daw; L4: daw1/11, OK72 > daw; L5: daw1/11, mef2 > daw. All quantitative results are reported as means ± SEM.
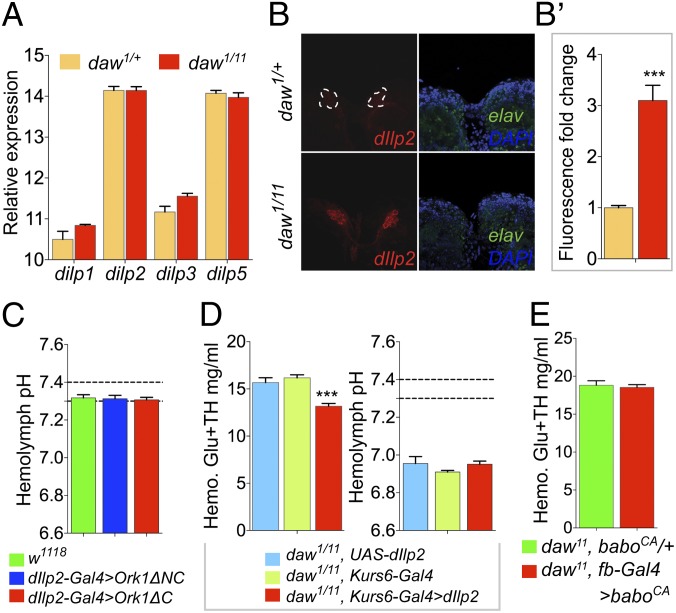
Fig. 4.
Independent roles of Daw in Dilp release and metabolic acidosis in the Drosophila larvae. (A) Expression level of dilp1, dilp2, dilp3, and dilp5 in dissected daw1/11 and control larval CNSs reported as abundance relative to rpl23 (n = 6). (B) Immunostaining of CNSs from stage matched feeding daw mutant and control larvae with anti-Dilp2 (red) antibody (stained and imaged under identical conditions). Anti-Elav (green) staining of same samples serves as internal control for staining intensity. (B′) Fluorescence-intensity quantification of Dilp2 staining of CNSs from daw mutant and control feeding larvae (n = 9 CNSs from three independent experiments). (C) Hemolymph pH of mid–third-instar larvae expressing a potassium channel Ork1ΔC in the IPCs (n = 8–11). (D) Hemolymph Glu+TH concentration and hemolymph pH of daw mutant larvae overexpressing dilp2 under regulation of the Kurs6-Gal4 driver and of relevant controls (n = 12 for both experiments). (E) Hemolymph sugar concentration of daw mutant larvae expressing baboCA under the regulation of fb-Gal4 driver and of mutant controls (n = 10–14). All results are reported as means ± SEM.
Daw Independently Regulates Dilp Release and Accumulation of Metabolic Acids in Peripheral Tissues.
Because daw mutants show metabolic phenotypes such as an elevated circulating sugar concentration and TAG accumulation, similar to that produced by a reduction in IS, we sought to elucidate the mechanism by which Daw affects IIS. To do so, we examined various aspects of IIS such as dilp expression, Dilp release, and signal reception in peripheral tissues of daw mutants. We found that loss of Daw did not affect mRNA levels of dilp1, dilp2, dilp3, or dilp5 in the IPCs of feeding mid–third-instar larvae (Fig. 4A). Under feeding conditions, larval IPCs release most of their Dilps into the hemolymph resulting in only faint signals within the neuron cell body when immunostained with anti-Dilp antibodies (17). However, immunostaining CNSs from feeding daw mutant larvae with anti-Dilp2 antibody showed significant residual Dilp2 peptide in the IPC cell bodies (Fig. 4 B and B′). In the absence of any change in mRNA levels of dilp2, this result likely indicates a deficiency in Dilp release from the IPCs. Similar accumulation was also observed for Dilp5 peptide when the ability of the mutant IPCs to release the peptide in response to feeding was assayed (Fig. S5A). These results suggest that Daw affects IIS by regulating release of Dilps from the larval IPCs.
Loss of IS is known to cause metabolic ketoacidosis in vertebrates. Therefore, we tested whether loss IIS is responsible for, or contributes to, the acidosis phenotype observed in the daw mutants. To this end, we generated chronically diabetic larvae by overexpressing a potassium channel Ork1ΔC in the larval IPCs thereby blocking Dilp release (Fig. S6A). However, these severely diabetic larvae showed normal hemolymph pH, indicating that loss of IIS alone does not induce metabolic acidosis in Drosophila larvae (Fig. 4C). To further test whether loss of IIS contributes to acidosis observed in the daw mutants, we rescued the diabetic phenotype of the daw mutants and checked whether acidosis was ameliorated. Overexpressing dilp2 in a group of noninsulin producing neurosecretory cells using the Kurs6-Gal4 driver has been shown previously to hasten developmental timing and produce smaller adults, as expected for Dilp2 overproduction (17). We found that overexpressing dilp2 using the Kurs6-Gal4 driver significantly reduced circulating sugar concentration in wild-type larvae (Fig. S6B) and also significantly rescued the diabetic phenotype of daw null mutants (Fig. 4D). However, Kurs6-Gal4–driven overexpression of dilp2 did not rescue the acidosis phenotype observed in the daw mutants (Fig. 4D), indicating that loss of IIS does not contribute to acidosis observed in daw mutants. Conversely, activation of TGF-β signaling in the FB, which can rescue both acidosis and accumulation of TCA cycle intermediates in the daw mutants (Fig. 2C), does not rescue the diabetic phenotype observed in the mutants (Fig. 4E). Taken together, these results show that the effect of Daw on acidosis and accumulation of TCA cycle intermediates is independent of its effect on IIS.
Expression of Cellular Metabolic and Nuclear-Encoded Mitochondrial Genes Is Significantly Altered in the daw Mutants.
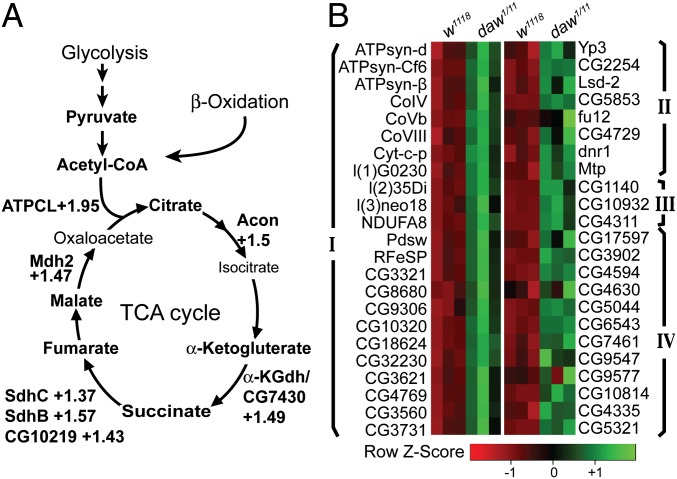
RNA-sequencing analysis of FB-specific RNA was used to examine the molecular basis of the metabolic changes that lead to the acidosis phenotype in daw mutants. A total of 986 genes deregulated ≥1.3-fold in the daw mutant FB were detected. A gene ontology (GO)-enrichment analysis shows that majority of the significantly enriched GO classes belongs to cellular metabolic processes (Fig. S7). We found that, consistent with acidosis observed in daw mutants, genes involved in carboxylic and organic acid metabolic processes were significantly more enriched among the up-regulated genes (Fig. S7). Notably, a large number of TCA cycle enzymes were significantly up-regulated in the daw mutants, indicating an increase in TCA cycle activity and output (Fig. 5A). Apart from TCA cycle enzymes, a large number of known and predicted proteins involved in the respiratory electron transport chain (ETC) were also significantly up-regulated (Fig. 5B and Fig. S8). Additionally, enzymes involved in β-oxidation, lipid mobilization, and ketogenesis were also up-regulated (Fig. 5C and Fig. S8). Because the TCA cycle, ETC, and β-oxidation are all housed in the mitochondrion, these results indicate a general increase in the expression of nuclear-encoded mitochondrial genes in the daw mutants. Up-regulation of β-oxidation and lipid mobilization genes also indicate an increased mobilization and degradation of fatty acids that can fuel the TCA cycle and mitochondrial metabolism by producing more acetyl-CoA. Consistently, a liquid chromatography/MS-based quantification of acetyl-CoA showed a significant increase in acetyl-CoA content of daw mutant larvae (Fig. S9B).
Fig. 5.
Daw is a major regulator of metabolic gene expression in the Drosophila larval FB. (A) Enzymes involved in the TCA cycle are up-regulated in the daw mutants. (B) Heat map of selected gene groups that were significantly up-regulated in the daw mutants. Group I, known and predicted ETC genes; group II, lipid mobilization; and group III, Ketogenesis and Group IV: β-oxidation.
Discussion
In this study, we investigated the involvement of the Drosophila Activin-like ligand Daw in regulating systemic and cellular metabolism. Our results indicate that Daw is a major regulator of cellular and mitochondrial metabolism and IIS in Drosophila larvae. We also find that loss of Daw leads to a significant increase in the expression of numerous known and predicted nuclear-encoded mitochondrial genes (heretofore referred to as mitochondrial genes) that are involved in the ETC, TCA cycle, and β-oxidation, indicating a potential role of Daw in regulating mitochondrial gene expression.
We report that loss of Daw leads to significant internal acidosis that is associated with increased abundance of metabolic acids like pyruvate and lactate along with citrate, malate, succinate, α-ketoglutarate, and fumarate that are intermediates of TCA cycle. Because activating TGF-β signaling in the FB could rescue both the acidosis phenotype and accumulation of metabolic acids in the daw mutants, our observations strongly suggest that acidosis observed in daw mutants is metabolic in nature and is caused by the accumulation of metabolic acids produced by the larval FB. Accumulation of TCA cycle intermediates is of particular interest because it indicates an involvement of TGF-β signaling in regulating either directly or indirectly the TCA cycle. We observe that loss of Daw leads to significant increase in the expression of multiple TCA cycle enzymes in the larval FB, leading to the possibility that overproduction of TCA cycle intermediates is caused by an increased TCA cycle activity. Similar effects of TCA cycle enzyme overexpression on overproduction of metabolic acids have been documented before (18–20). For instance, overexpression of mitochondrial malate dehydrogenase has been shown to increase both production and exudation of citrate, oxalate, malate, succinate, and acetate by up to 4.2-fold and 7.1-fold, respectively, in Alfalfa root tips (20). Similarly, overexpression of citrate synthase has been documented to increase citrate production in both Saccharomyces cerevisiae and Arabidopsis (18, 19). We checked whether a similar effect could be seen in Drosophila larvae by overexpressing Drosophila mitochondrial malate dehydrogenase (mdh2) using a tub-Gal4 driver. Pan larval overexpression of mdh2 was able to cause significant acidification of larval hemolymph, supporting the possibility that loss of daw leads to acidosis by up-regulating expression of TCA cycle enzymes (Fig. S9A). Nevertheless, our data do not rule out the possibility that overexpression of mitochondrial genes is rather an outcome and not the cause for the metabolic changes seen in daw mutants.
Although the effects of Daw on acidosis and accumulation of mitochondrial metabolites is caused primarily by its action on the larval FB, we could rescue these phenotypes by overexpressing daw in the IPCs, indicating that Daw can act in an endocrine fashion. This conclusion is further supported by our data showing that loss of endogenous p-Smox signal in daw mutant FB is rescued by overexpressing daw either in the IPCs, oenocytes, or muscle. Additionally, our finding that exogenously expressed Daw influences circulating sugar concentration, hemolymph pH, and FB-specific p-Smox signal in a tissue size (dose)-dependent manner further suggests that Daw is likely released into the hemolymph from tissues of origin and exerts its effect in an endocrine manner like a hormone. Consistent with this view, recent MS data reveal the presence of Daw in the hemolymph (21). Curiously, unlike most hormones whose expression is limited to one tissue or gland, daw is expressed in a wide array of tissues including muscle, FB, gut, imaginal discs, and surface glia of the CNS (22, 23). One possible purpose for such a wide distribution in hormone expression could be that, being a metabolic regulator, expression or release of Daw is regulated by local nutritional or stress responses and Daw in turn signals to other tissues to allow systemic integration of these stimuli.
In vertebrates Activin-A has been proposed to positively regulate insulin release based on in vitro experiments showing increased Ca2+ influx and insulin release from isolated β-cells upon stimulation with Activin-A (10, 24, 25). Whereas numerous receptor and TGF-β–antagonist loss/gain-of-function studies support a physiological role for TGF-β signaling in β-cell function and glucose tolerance (26, 27), evidence for any in vivo role of Activin-A in insulin release is lacking. Our finding, that Daw positively regulates Dilp release and IIS in Drosophila larvae, provides compelling evidence for the in vivo role of an Activin-like ligand in insulin release. Recent work in Caenorhabditis elegans suggests a similar role of TGF-β signaling in insulin release where loss of the worm R-Smad homolog daf-8 blocks release of a surrogate neuropeptide (human atrial natriuretic factor–GFP) from the insulin-producing ASI neurons (28). Mechanistically, DAF8 was shown to regulate polarity of the neurosecretory neurons by up-regulating expression of a potassium channel (exp-2). However, such a cell-autonomous mechanism may not be the case in Drosophila because neither SmoxRNAi nor baboCA overexpression in the IPCs affected circulating sugar concentration in the wild-type or daw mutants, respectively (Fig. S5 B and C). Moreover, the Drosophila exp-2 homolog, shab, did not show any expression change in daw mutants, indicating potential mechanistic differences in the way TGF-β signaling regulates insulin release in worms and flies (Fig. S5D). Nevertheless, our work demonstrates a functional conservation of the role of TGF-β/Activin signaling in regulating insulin release and provides previously unidentified direct evidence for an in vivo role of a TGF-β/Activin ligand in the process.
Intriguingly, although loss of IS is known to cause metabolic ketoacidosis in vertebrates, we found that chronic loss of IIS did not lead to acidosis in Drosophila larvae, nor did loss of IIS contribute to the acidosis observed in the daw mutants. Lack of a ketoacidosis response in Drosophila compared with vertebrates upon loss of IIS could be attributable to differences in the way IIS regulates metabolism in insect larvae. Unlike vertebrates, where loss of insulin leads to fat mobilization and production of ketone bodies, in Drosophila and Bombyx larvae, loss of insulin leads to accumulation of TAG, suggesting that the modes of alternative energy production upon loss of IIS are likely different in insect larvae and vertebrates (13, 14, 29).
Another interesting point is that we could also completely rescue acidosis in daw mutants without ameliorating the diabetic phenotype. This finding indicates that acidosis does not contribute to loss of IIS in the daw mutants, as has been proposed by a few vertebrate studies where metabolic acidosis seems to lead to insulin resistance (30, 31). It is important to note that acidosis always correlates with the relative amount of metabolic acids. Because we could reduce the output of metabolic acids without affecting the diabetic phenotype, we also conclude that Daw regulates peripheral cellular and mitochondrial metabolism independent of IIS.
Deregulation of mitochondrial metabolism and function is associated with a number of both inherited and acquired human disorders, including but not limited to type 2 diabetes, neurodegenerative disorders, and tumorigenesis (32, 33). Consequently, therapeutic interventions that can restore or increase mitochondrial activity are thought to be essential for our ability to treat these complex and often debilitating diseases (32). Our study showing that the Drosophila Activin-like ligand Daw is involved either directly or indirectly in regulating mitochondrial gene expression and TCA cycle activity suggests that TGF-β/Activin signaling could be a potential therapeutic target for modulating metabolism. Additionally, our results suggest the possibility that there could be a functional relationship between altered TGF-β signaling and the manifestation of certain human diseases. For instance, both acidosis and TGF-β signaling are known to play critical roles in tumor progression by facilitating angiogenesis and metastasis (34–38). Our observation that TGF-β signaling can affect production of metabolic acids, including lactate, presents a unique perspective for understanding the role of TGF-β signaling in tumor progression. The effect of Daw on TCA cycle activity and expression of oxidative phosphorylation genes also has implications for tumor growth. Reduction of TCA cycle activity and oxidative phosphorylation coupled with an increased flux through glycolysis (Warburg effect) is characteristic of most tumors and is considered essential for production of cellular building blocks required for sustaining rapid cellular growth (39, 40). Involvement of Daw in the regulation of the TCA cycle and oxidative phosphorylation, along with the strong reported association of TGF-β with tumor growth, raises the possibility that TGF-β affects tumor growth by impinging on Warburg metabolism. Interestingly, a recent paper demonstrating the involvement of TGF-β signaling in promoting Warburg metabolism in breast tumors is consistent with this view (41). In conclusion, our study showing the involvement of the Drosophila TGF-β/Activin ligand Daw in regulating metabolism and IS expands our understanding of the role of TGF-β signaling in physiology and disease. Additionally, our work opens up the possibility of using Drosophila genetics for better understanding the role of TGF-β signaling in regulating metabolism.
Materials and Methods
Fly Strains and Food.
daw alleles daw11 and dawaact1 (referred as daw1) and SmoxF4, baboFd4, and baboCA alleles are described elsewhere (22, 42–45). Other fly lines used were as follows: UAS-daw (22), UAS-dilp2 (46), UAS-Ork1ΔC and UAS-Ork1ΔNC [Bloomington Stock Center (BSC)], UAS-SmoxRNAi3B3 (43), cg-Gal4 (BSC), fb-Gal4 (T. Neufeld, University of Minnesota, Minneapolis), mef2-Gal4 (BSC), npc1b1-Gal4 (L. Pallanck, University of Washington, Seattle), UAS-mdh2 (47), and Kurs6-Gal4 (G. Korge, Freie University Berlin, Berlin).
For overexpression experiments, a single Gal4 element was used to drive a single UAS construct. Controls contained either a single copy of the Gal4 driver (Gal4 control) or a single copy of the UAS element (UAS control). Mutant and control larvae were grown on YPF or semidefined food from the time of hatching onwards, unless mentioned otherwise. Refer to SI Materials and Methods for more details on growth conditions; 5y15s [high-sugar diet (HSD)] food consisted of 5% (wt/vol) yeast, 10% (wt/vol) dextrose, and 5% (wt/vol) sucrose.
Metabolite and pH Assay.
TAG, glycogen, and hemolymph sugar assays were performed as described elsewhere (17, 48). For hemolymph pH assay, 3 μL of hemolymph was mixed with 2 μL of pyranine dye (Invitrogen; 1.2 mM final concentration). The mixture was briefly centrifuged, and the absorbance spectrum of the supernatant was measured using a multichannel nanodrop spectrophotometer (NanoDrop 8000). The ratio of absorbance at 450 nm and 405 nm was plotted against a standard curve to determine the pH. pH standards ranging between pH 6.6 and pH 7.6 were prepared by titrating 50 mM Tris⋅HCl. See SI Materials and Methods for more details.
Statistics.
All graphs and statistical analyses were generated using GraphPad Prism4. Standard Student t test or ANOVA followed by a post hoc Tukey’s honest significant difference test was used for statistical analysis. P values are designated using the following convention: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001.
Antibodies and Immunostaining.
Antibodies used were rat anti-Dilp2 (1/500), mouse anti-Dilp5 (1/800) (gift from the P. Leopold laboratory, Institut Valrose Biologie, Nice, France), and mouse anti-Elav (1/500) (9F8A9; Developmental Studies Hybridoma Bank). Antibody straining was performed as described elsewhere (17). Samples were imaged using a confocal laser-scanning microscope (Zeiss LSM710).
Fluorescence Quantification.
Confocal Z series of the IPCs were obtained using a 1-μm step size and identical laser power and scan settings. Zeiss Zen software was used to generate multiple intensity projection images. Subsequently, background signal was subtracted and fluorescence intensity was measured by drawing selections on the IPCs.
RNA Sequencing.
RNA sequencing (Illumina) of FB RNA was performed by Genewiz. Data analysis was done using the Galaxy tool set made available by the Minnesota Supercomputing Institute.
Supplementary Material
Acknowledgments
We thank Philip A. Jensen for initial observation of the nutrient-dependent viability of the daw mutants. We thank the metabolomics core facility at the University of Utah and Dr. James Cox (Director, metabolomics core facility) for GC/MS metabolomic analysis. We thank A. J. Peterson, N. Yamanaka, and M. S. O’Connor for comments on the manuscript. We thank the Vienna Drosophila RNAi Center and Bloomington Stock Center for providing Drosophila lines. A.C.G. was supported by an American Heart Association predoctoral fellowship. This work was also supported by National Institutes of Health Grant R01 GM095746 (to M.B.O.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1319116111/-/DCSupplemental.
References
- 1.Baker KD, Thummel CS. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab. 2007;6(4):257–266. doi: 10.1016/j.cmet.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Léopold P, Perrimon N. Drosophila and the genetics of the internal milieu. Nature. 2007;450(7167):186–188. doi: 10.1038/nature06286. [DOI] [PubMed] [Google Scholar]
- 3.Pajvani UB, et al. Inhibition of Notch signaling ameliorates insulin resistance in a FoxO1-dependent manner. Nat Med. 2011;17(8):961–967. doi: 10.1038/nm.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu H, et al. Wnt signaling regulates hepatic metabolism. Sci Signal. 2011;4(158):ra6. doi: 10.1126/scisignal.2001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballard SL, Jarolimova J, Wharton KA. Gbb/BMP signaling is required to maintain energy homeostasis in Drosophila. Dev Biol. 2010;337(2):375–385. doi: 10.1016/j.ydbio.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zamani N, Brown CW. Emerging roles for the TGF-β superfamily in regulating adiposity and energy expenditure. Endocr Rev. 2011;32(3):387–403. doi: 10.1210/er.2010-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, et al. Activin signaling: Effects on body composition and mitochondrial energy metabolism. Endocrinology. 2009;150(8):3521–3529. doi: 10.1210/en.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: Molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8(12):970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 9.Tsuchida K, et al. Activin isoforms signal through type I receptor serine/threonine kinase ALK7. Mol Cell Endocrinol. 2004;220(1-2):59–65. doi: 10.1016/j.mce.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Bertolino P, et al. Activin B receptor ALK7 is a negative regulator of pancreatic β-cell function. Proc Natl Acad Sci USA. 2008;105(20):7246–7251. doi: 10.1073/pnas.0801285105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker L, Stathakis DG, Arora K. Regulation of BMP and activin signaling in Drosophila. Prog Mol Subcell Biol. 2004;34:73–101. doi: 10.1007/978-3-642-18670-7_4. [DOI] [PubMed] [Google Scholar]
- 12.Broughton SJ, et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci USA. 2005;102(8):3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teleman AA. Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem J. 2010;425(1):13–26. doi: 10.1042/BJ20091181. [DOI] [PubMed] [Google Scholar]
- 14.Tatar M, et al. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292(5514):107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 15.Wyatt GR. The biochemistry of insect hemolymph. Annu Rev Entomol. 1961;6(1):75–102. [Google Scholar]
- 16.Harrison JF. Insect acid-base physiology. Annu Rev Entomol. 2001;46:221–250. doi: 10.1146/annurev.ento.46.1.221. [DOI] [PubMed] [Google Scholar]
- 17.Géminard C, Rulifson EJ, Léopold P. Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 2009;10(3):199–207. doi: 10.1016/j.cmet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Anoop VM, Basu U, McCammon MT, McAlister-Henn L, Taylor GJ. Modulation of citrate metabolism alters aluminum tolerance in yeast and transgenic canola overexpressing a mitochondrial citrate synthase. Plant Physiol. 2003;132(4):2205–2217. doi: 10.1104/pp.103.023903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koyama H, et al. Overexpression of mitochondrial citrate synthase in Arabidopsis thaliana improved growth on a phosphorus-limited soil. Plant Cell Physiol. 2000;41(9):1030–1037. doi: 10.1093/pcp/pcd029. [DOI] [PubMed] [Google Scholar]
- 20.Tesfaye M, Temple SJ, Allan DL, Vance CP, Samac DA. Overexpression of malate dehydrogenase in transgenic alfalfa enhances organic acid synthesis and confers tolerance to aluminum. Plant Physiol. 2001;127(4):1836–1844. [PMC free article] [PubMed] [Google Scholar]
- 21.Handke B, et al. The hemolymph proteome of fed and starved Drosophila larvae. PLoS ONE. 2013;8(6):e67208. doi: 10.1371/journal.pone.0067208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serpe M, O’Connor MB. The metalloprotease tolloid-related and its TGF-β-like substrate Dawdle regulate Drosophila motoneuron axon guidance. Development. 2006;133(24):4969–4979. doi: 10.1242/dev.02711. [DOI] [PubMed] [Google Scholar]
- 23.Parker L, Ellis JE, Nguyen MQ, Arora K. The divergent TGF-β ligand Dawdle utilizes an activin pathway to influence axon guidance in Drosophila. Development. 2006;133(24):4981–4991. doi: 10.1242/dev.02673. [DOI] [PubMed] [Google Scholar]
- 24.Florio P, et al. Activin A stimulates insulin secretion in cultured human pancreatic islets. J Endocrinol Invest. 2000;23(4):231–234. doi: 10.1007/BF03343713. [DOI] [PubMed] [Google Scholar]
- 25.Furukawa M, Nobusawa R, Shibata H, Eto Y, Kojima I. Initiation of insulin secretion in glucose-free medium by activin A. Mol Cell Endocrinol. 1995;113(1):83–87. doi: 10.1016/0303-7207(95)03617-g. [DOI] [PubMed] [Google Scholar]
- 26.Mukherjee A, et al. FSTL3 deletion reveals roles for TGF-β family ligands in glucose and fat homeostasis in adults. Proc Natl Acad Sci USA. 2007;104(4):1348–1353. doi: 10.1073/pnas.0607966104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smart NG, et al. Conditional expression of Smad7 in pancreatic beta cells disrupts TGF-beta signaling and induces reversible diabetes mellitus. PLoS Biol. 2006;4(2):e39. doi: 10.1371/journal.pbio.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park D, et al. Repression of a potassium channel by nuclear hormone receptor and TGF-β signaling modulates insulin signaling in Caenorhabditis elegans. PLoS Genet. 2012;8(2):e1002519. doi: 10.1371/journal.pgen.1002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satake S, et al. Bombyxin, an insulin-related peptide of insects, reduces the major storage carbohydrates in the silkworm Bombyx mori. Comp Biochem Physiol B Biochem Mol Biol. 1997;118(2):349–357. doi: 10.1016/s0305-0491(97)00166-1. [DOI] [PubMed] [Google Scholar]
- 30.Cuthbert C, Alberti KG. Acidemia and insulin resistance in the diabetic ketoacidotic rat. Metabolism. 1978;27(12 Sup2):1903–1916. doi: 10.1016/s0026-0495(78)80008-0. [DOI] [PubMed] [Google Scholar]
- 31.Mak RH. Insulin and its role in chronic kidney disease. Pediatr Nephrol. 2008;23(3):355–362. doi: 10.1007/s00467-007-0611-2. [DOI] [PubMed] [Google Scholar]
- 32.Andreux PA, Houtkooper RH, Auwerx J. Pharmacological approaches to restore mitochondrial function. Nat Rev Drug Discov. 2013;12(6):465–483. doi: 10.1038/nrd4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gasparre G, Porcelli AM, Lenaz G, Romeo G. Relevance of mitochondrial genetics and metabolism in cancer development. Cold Spring Harb Perspect Biol. 2013;5(2):pii: a011411. doi: 10.1101/cshperspect.a011411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhup S, Dadhich RK, Porporato PE, Sonveaux P. Multiple biological activities of lactic acid in cancer: Influences on tumor growth, angiogenesis and metastasis. Curr Pharm Des. 2012;18(10):1319–1330. doi: 10.2174/138161212799504902. [DOI] [PubMed] [Google Scholar]
- 35.Estrella V, et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013;73(5):1524–1535. doi: 10.1158/0008-5472.CAN-12-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain M, et al. Mitochondrial reactive oxygen species regulate transforming growth factor-β signaling. J Biol Chem. 2013;288(2):770–777. doi: 10.1074/jbc.M112.431973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342(18):1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 38.Baumann F, et al. Lactate promotes glioma migration by TGF-β2-dependent regulation of matrix metalloproteinase-2. Neuro-oncol. 2009;11(4):368–380. doi: 10.1215/15228517-2008-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330(6009):1340–1344. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- 41.Guido C, et al. Metabolic reprogramming of cancer-associated fibroblasts by TGF-β drives tumor growth: Connecting TGF-β signaling with “Warburg-like” cancer metabolism and L-lactate production. Cell Cycle. 2012;11(16):3019–3035. doi: 10.4161/cc.21384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng X, et al. TGF-β signaling activates steroid hormone receptor expression during neuronal remodeling in the Drosophila brain. Cell. 2003;112(3):303–315. doi: 10.1016/s0092-8674(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 43.Peterson AJ, et al. R-Smad competition controls activin receptor output in Drosophila. PLoS ONE. 2012;7(5):e36548. doi: 10.1371/journal.pone.0036548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brummel T, et al. The Drosophila activin receptor baboon signals through dSmad2 and controls cell proliferation but not patterning during larval development. Genes Dev. 1999;13(1):98–111. doi: 10.1101/gad.13.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gesualdi SC, Haerry TE. Distinct signaling of Drosophila Activin/TGF-β family members. Fly (Austin) 2007;1(4):212–221. doi: 10.4161/fly.5116. [DOI] [PubMed] [Google Scholar]
- 46.Brogiolo W, et al. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol. 2001;11(4):213–221. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 47.Wang L, Lam G, Thummel CS. Med24 and Mdh2 are required for Drosophila larval salivary gland cell death. Dev Dyn. 2010;239(3):954–964. doi: 10.1002/dvdy.22213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palanker L, Tennessen JM, Lam G, Thummel CS. Drosophila HNF4 regulates lipid mobilization and β-oxidation. Cell Metab. 2009;9(3):228–239. doi: 10.1016/j.cmet.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.