We thank Corbeil et al. for raising important points (1) regarding our recent article (2) describing the role of epithelial dedifferentiation in kidney repair. We agree that the forward primer we used to detect CD133 mRNA is in fact not directed against the CD133 transcript. This error resulted from the original primer order in which we mistakenly transcribed an unrelated sequence. Surprisingly, this primer pair did amplify a product with a single peak by quantitative PCR, and that is what we reported in figure 3f of our report (2). We sincerely regret this mistake and appreciate that Corbeil et al. detected it.
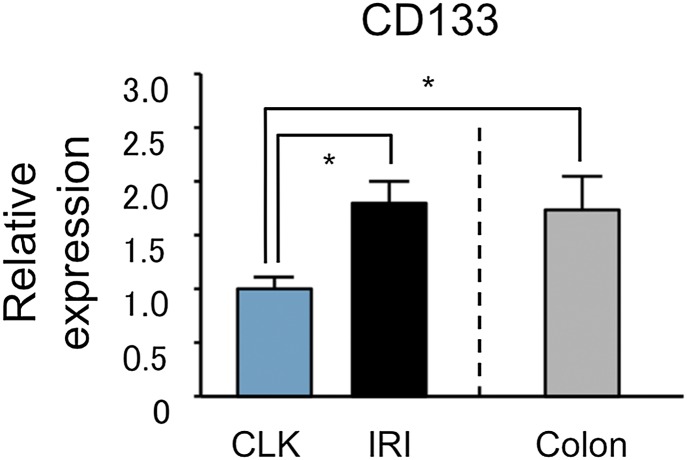
We have now repeated the quantitative PCR for CD133 on the same samples using specific primers, and the results are shown in Fig. 1. CD133 is up-regulated after ischemia, but to a lower degree than in our original report. Colon was used as a positive control because CD133 is abundantly expressed on colonic epithelia (3). We are submitting an erratum to PNAS to replace the incorrect results with these newer ones.
Fig. 1.
CD133 mRNA is up-regulated in differentiated epithelial cells after injury. Colon serves as a positive control for CD133 expression. *P < 0.05. CLK, contralateral uninjured kidney; IRI, ischemia reperfusion injury.
In human kidney, the AC133 mAb detects glycosylated CD133 protein in a scattered population of tubular cells and these have been proposed to be stem cells. This antibody does not cross-react with mouse, however, as we stated (4). The observation by Corbeil et al. (1) that the pan-CD133 antibody 13A4 does not detect the CD133 protein in the proximal convoluted tubules is intriguing. Note that the SLC34a1-CreERt2 driver we created is expressed primarily in the proximal convoluted tubule (S1 and S2 segments) with lower expression in the proximal straight tubule (S3 segment). Therefore, the up-regulation of CD133 mRNA in cells from the proximal convoluted tubule is consistent with up-regulation of CD133 in dedifferentiating epithelia.
We agree that strong expression of CD133 in healthy kidney is inconsistent with the notion that it exclusively marks a stem cell population. Indeed, a primary conclusion of our genetic lineage analysis is that there is no scattered population of preexisting epithelial stem cells but, rather, terminally differentiated cells repair injury through dedifferentiation (2). It has recently been shown that differentiated lung epithelial cells can dedifferentiate into functional stem cells, highlighting the plasticity of differentiated cells (5). Whether CD133 plays a role in this process in kidney or simply defines cells undergoing injury-induced dedifferentiation are important questions for future investigation.
Footnotes
The authors declare no conflict of interest.
References
- 1.Corbeil D, Fargeas CA, Jászai J. CD133 might be a pan marker of epithelial cells with dedifferentiation capacity. Proc Natl Acad Sci USA. 2014;111:E1451–E1452. doi: 10.1073/pnas.1400195111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD. Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci USA. 2014;111(4):1527–1532. doi: 10.1073/pnas.1310653110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shmelkov SV, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118(6):2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angelotti ML, Lazzeri E, Lasagni L, Romagnani P. Only anti-CD133 antibodies recognizing the CD133/1 or the CD133/2 epitopes can identify human renal progenitors. Kidney Int. 2010;78(6):620–621, author reply 621. doi: 10.1038/ki.2010.243. [DOI] [PubMed] [Google Scholar]
- 5.Tata PR, et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503(7475):218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]