A long-standing paradigm in ecology has been that contemporary biodiversity requires a diverse array of life to have survived through repeated Pleistocene glaciations when much of the higher latitudes on earth were covered in thick ice sheets. The notion that isolated pockets of ice-free land or “cryptic refugia” also persisted within glaciated areas has been postulated to explain this (1). Under the cryptic refugia scenario, animal and plant life survived, albeit in a scattered distribution and at low densities, in ice-free areas determined largely by topography. These have been proposed to include mountainous areas and deeply incised valleys in terrestrial biomes and marine trenches in ocean biomes. The existence of cryptic refugia is supported by multiple threads of evidence from paleontological, biogeographic, and phylogeographic research (2, 3). They are envisaged to have played a key role in the maintenance of biodiversity through major severe climate events. In PNAS, Fraser et al. (4) present empirical support for the hypothesis that geothermal areas may also have been important as cryptic refuges. The authors apply spatial modeling to a comprehensive terrestrial biodiversity dataset for Antarctica. They identify compelling evidence that geothermal refugia have left indelible and measurable patterns on contemporary biogeography for this continent.
Since life first emerged more than 3.5 Gya, the Earth has experienced repeated glaciation events, with some models proposing total coverage with ice in a “snowball earth” scenario (5). Microbial life and some primitive metazoans survived these extreme glaciation events, but conditions would likely have been catastrophic for more complex metazoan life. Several glacial cycles have occurred since the Cambrian explosion of metazoan diversity, and these presented extreme challenges to life from thermal, spatial, oligotrophic, and other stressors. Nonetheless, diverse life persisted and later recolonized terrestrial and aquatic biomes (6). The European continent has been a focus for much of the research into the role of refugia in shaping contemporary species distributions. An historical view was that an ice-free swathe in the southern part of the continent served as a source for subsequent postglacial recolonization. However, the extant distribution of European trees revealed that contemporary biogeography could not be explained by this northward radiation alone. This became known as Reid’s paradox (7), and an explanation required additional unknown cryptic refugia. Paleo-records for trees, pollen, and mammals supported the notion of cryptic refugia (1, 8), and more recently, phylogeographic studies of terrestrial plants and vertebrates, plus marine macroalgae, have provided strong support for their role in shaping the observed distributions (3).
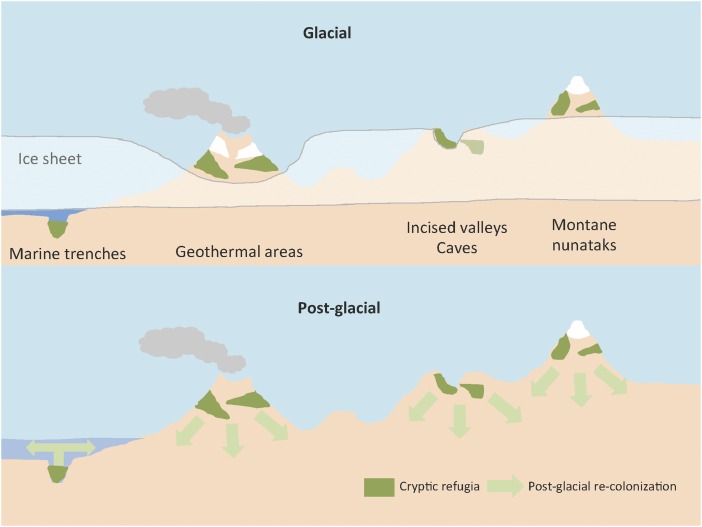
The idea that geothermal regions could also provide cryptic refugia is intriguing. Ice-free land could persist around volcanic craters and in geothermally heated ground and waters. Steam vents on ice-covered ground can also form subterranean ice caves. Although temperatures in some areas may exceed the thresholds for metazoan life, steep thermal gradients toward a more benign range of temperatures occur. This conceivably created a buffer zone between temperatures too high for life and the ice sheet and could have formed thermal oases for life during glacial periods. Such geothermal activity usually persists over extended time scales that could span glacial cycles and therefore could plausibly provide refugia for life. The global distribution of geothermal activity makes this an attractive model for explaining important aspects of global biogeography. We present a synthesis of how this potentially expands the range of cryptic refugia for life during glacial cycles in Fig. 1.
Fig. 1.
The diversity of cryptic refugia implicated in maintaining diverse life through glacial cycles and as nuclei for postglacial recolonization. In marine environments, deep trenches are postulated to have allowed persistence of macroalgae through maintenance of aqueous environments (3). In terrestrial biomes, geothermally heated areas (4), as well as high altitude mountainous terrain including equatorially oriented and deeply incised valleys, and subterranean caves are envisaged to have acted as refugia (3).
The work by Fraser et al. in PNAS (4) uses Antarctica as a natural model system against which to test the geothermal refugia hypothesis. Antarctica is broadly influenced by geothermal activity from volcanoes and subsurface radiogenic decay, and this has persisted in several areas since before the last glacial maximum (LGM). It is tractable for ecological investigations because environmental stress limits biodiversity to microorganisms (9), lower plants, and invertebrates (10). A comprehensive terrestrial database that described the occurrence of more than 30 phyla was used, and this was interfaced with landscape, climate, and geothermal data using a geographic information system (GIS). The use of GIS in ecology adds new spatial and temporal dimensions to statistical and simulation modeling by providing realistic, multilayered models of ecosystems (11). They are easily linked to a wide range of satellite, airborne, and other remote sensors and provide the ideal tool for visualizing and analyzing the spatial diversity gradient away from Antarctic hot spots. By analyzing spatial patterns using a range of models, Fraser et al. (4) identify strong support for the role of geothermal heating in explaining contemporary patterns of biogeography. Some differences between higher taxonomic ranks were evident. Plant species richness was significantly elevated on and around geothermal sites compared with nongeothermal areas. Similar patterns were also observed for fungi, although with slightly less support. For invertebrates, the geothermal effect was only observed in continental regions but not coastal locations. This was attributed in part to sampling bias, but also to topography where nunataks (ice-free rocky areas) in continental Antarctica—absent around coastal sites—are thought to have also acted as cryptic refugia. These findings may help explain why the biota of Antarctica appears to depart from the latitudinal gradient in species richness and exhibits strong endemic signals (12, 13).
At a broader level, the study provides an interesting examination of species richness gradients away from centers of elevated temperature. The Antarctic centers of biodiversity clustered within areas that encountered elevated temperature through glacial cycles provides an excellent template for empirical testing of diversity theory. They imitate the global latitudinal gradient with
Fraser et al. identify strong support for the role of geothermal heating in explaining contemporary patterns of biogeography.
important exceptions that provide an opportunity to gain useful insights into the mechanisms underpinning global diversity gradients. Unlike global gradients, those in the study by Fraser et al. (4) in PNAS did not conform to a decrease in diversity along a gradual decrease in contemporary temperature, but instead were better characterized by a gradual decrease in diversity across a step change in temperature. Furthermore, diversity fell with increasing distance from the hot spots in a nested fashion. This is important because it suggests that diversity is not currently limited by contemporary climate, nor is it limited by the rate of adaptation to cooler environments. Instead, the results suggest differential dispersal limitations among taxa inhabiting long-term refugia.
The work by Fraser et al. (4) in PNAS concludes that, because species richness does not correlate with contemporary climate, the hot spots are refugia where taxa have survived previous glacial periods, thus providing an Antarctic parallel to the notion of the tropics as a “museum” rather than a “cradle” for diversity (14). The authors reject the metabolic theory of diversity (15) as an explanation for their results on the grounds that diversity is distance related rather than related to contemporary climate. However, the metabolic theory and other theories of evolutionary speed (16, 17) do not necessarily predict an alignment between contemporary climate and diversity. Instead, they attribute diversity patterns to differential rates of origination mediated by the climatic history encountered over evolutionary time, and they invoke dispersal limitations to account for the maintenance of diversity gradients. The results in this study are therefore entirely consistent with predictions of evolutionary speed hypotheses. The nested diversity gradient away from the Antarctic hot spots is consistent with the “out of the tropics model” of global diversity (18), in which the tropics act as both cradles and museums of diversity; taxa preferentially originate in the tropics, or in this case, volcanic hot spots, and expand toward cooler regions without losing their warm climate component. The application of GIS used by Fraser et al. (4) to explore spatial relationships in Antarctic systems opens up exciting new opportunities for further investigation of Antarctic hot spots as both cradles and museums of diversity.
Footnotes
The authors declare no conflict of interest.
See companion article on page 5634.
References
- 1.Stewart JR, Lister AM. Cryptic northern refugia and the origins of the modern biota. Trends Ecol Evol. 1991;16(11):608–613. [Google Scholar]
- 2.Convey P, et al. Antarctic terrestrial life—challenging the history of the frozen continent? Biol Rev Camb Philos Soc. 2008;83(2):103–117. doi: 10.1111/j.1469-185X.2008.00034.x. [DOI] [PubMed] [Google Scholar]
- 3.Provan J, Bennett KD. Phylogeographic insights into cryptic glacial refugia. Trends Ecol Evol. 2008;23(10):564–571. doi: 10.1016/j.tree.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Fraser CI, Terauds A, Smellie J, Convey P, Chown SL. Geothermal activity helps life survive glacial cycles. Proc Natl Acad Sci USA. 2014;111:5634–5639. doi: 10.1073/pnas.1321437111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman PF, Schrag DP. The snowball Earth hypothesis: Testing the limits of global change. Terra Nov. 2002;14(3):129–155. [Google Scholar]
- 6.Hewitt GM. Genetic consequences of climatic oscillations in the Quaternary. Philos Trans R Soc Lond B Biol Sci. 2004;359(1442):183–195. doi: 10.1098/rstb.2003.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark J, et al. Reid’ s paradox of rapid dispersal theory and interpretation of paleoecological records. Bioscience. 1998;48(1):13–24. [Google Scholar]
- 8.Willis KJ, Rudner KJ, Sümegi P. The full-glacial forests of central and southeastern Europe. Quat Res. 2000;53(2):203–213. [Google Scholar]
- 9.Pointing SB, et al. Highly specialized microbial diversity in hyper-arid polar desert. Proc Natl Acad Sci USA. 2009;106(47):19964–19969. doi: 10.1073/pnas.0908274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams BJ, et al. Diversity and distribution of Victoria Land biota. Soil Biol Biochem. 2006;38(10):3003–3018. [Google Scholar]
- 11.Kerr JT, Ostrovsky M. From space to species: Ecological applications for remote sensing. Trends Ecol Evol. 2003;18(6):299–305. [Google Scholar]
- 12.Cannone N, Convey P, Guglielmin M. Diversity trends of bryophytes in continental Antarctica. Polar Biol. 2012;36(2):259–271. [Google Scholar]
- 13.Bahl J, et al. Ancient origins determine global biogeography of hot and cold desert cyanobacteria. Nat Commun. 2011;2:163. doi: 10.1038/ncomms1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stebbins GL. Flowering Plants: Evolution Above the Species Level. Cambridge, MA: Harvard Univ Press; 1974. [Google Scholar]
- 15.Allen AP, Gillooly JF, Savage VM, Brown JH. Kinetic effects of temperature on rates of genetic divergence and speciation. Proc Natl Acad Sci USA. 2006;103(24):9130–9135. doi: 10.1073/pnas.0603587103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillman LN, Wright SD. Species richness and evolutionary speed: The influence of temperature, water and area. J Biogeogr. 2014;41(1):39–51. [Google Scholar]
- 17.Rohde KL. Latitudinal gradients in species diversity: The search for the primary cuase. Oikos. 1992;65(3):514–527. [Google Scholar]
- 18.Jablonski D, Roy K, Valentine JW. Out of the tropics: Evolutionary dynamics of the latitudinal diversity gradient. Science. 2006;314(5796):102–106. doi: 10.1126/science.1130880. [DOI] [PubMed] [Google Scholar]