Significance
The assembly of ecological communities results from multiple mechanisms acting concurrently. Disentangling their relative importance represents a major challenge. Our study quantifies the relative importance of four trait-mediated mechanisms and associated changes in functional diversity over succession. We leverage the rapid community assembly in tropical successional forests and the rich information in functional trait data and spatially explicit long-term tree demographic data. Trait correlations with average species survival rates were the most important processes affecting survival and linked to a decrease in wood trait diversity during succession. Simultaneously, evidence for stabilizing niche differences associated with leaf traits was reflected in increasing leaf functional diversity. Our results suggest mechanisms by which individual performance affects successional change in community-wide diversity.
Keywords: community turnover, hierarchical Bayes, individual variation, secondary forest, species interactions
Abstract
Interspecific differences in relative fitness can cause local dominance by a single species. However, stabilizing interspecific niche differences can promote local diversity. Understanding these mechanisms requires that we simultaneously quantify their effects on demography and link these effects to community dynamics. Successional forests are ideal systems for testing assembly theory because they exhibit rapid community assembly. Here, we leverage functional trait and long-term demographic data to build spatially explicit models of successional community dynamics of lowland rainforests in Costa Rica. First, we ask what the effects and relative importance of four trait-mediated community assembly processes are on tree survival, a major component of fitness. We model trait correlations with relative fitness differences that are both density-independent and -dependent in addition to trait correlations with stabilizing niche differences. Second, we ask how the relative importance of these trait-mediated processes relates to successional changes in functional diversity. Tree dynamics were more strongly influenced by trait-related interspecific variation in average survival than trait-related responses to neighbors, with wood specific gravity (WSG) positively correlated with greater survival. Our findings also suggest that competition was mediated by stabilizing niche differences associated with specific leaf area (SLA) and leaf dry matter content (LDMC). These drivers of individual-level survival were reflected in successional shifts to higher SLA and LDMC diversity but lower WSG diversity. Our study makes significant advances to identifying the links between individual tree performance, species functional traits, and mechanisms of tropical forest succession.
Quantifying the relative importance of mechanisms that drive community assembly remains a central challenge in ecology. Interspecific variation in ecological strategies is considered a major driver of community assembly and has been classified by Chesson (1) into relative fitness (i.e., per capita population growth rate) differences and stabilizing niche differences (2). These processes act concurrently, and their importance may vary over space and time (3). However, the simultaneous effects of different assembly mechanisms on community dynamics have not been well-characterized, particularly in diverse communities such as tropical forests. High dynamism of vegetation composition during tropical forest succession creates an ideal opportunity to investigate the drivers of community assembly.
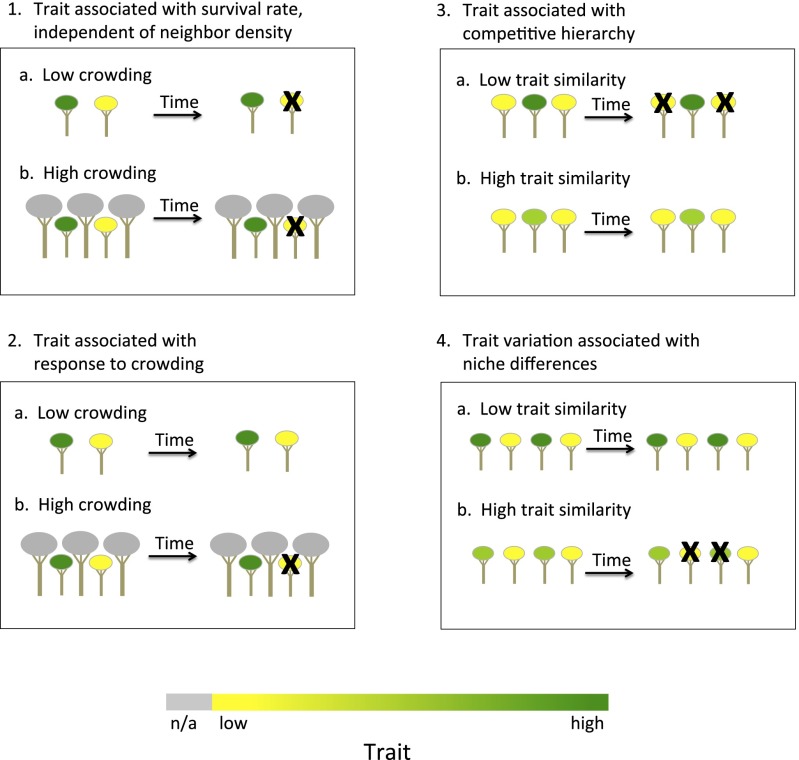
Interspecific niche and fitness differences can be characterized through the lens of interspecific functional trait variation, an approach that can shed light on drivers of community functional composition and dynamics (2, 4, 5). Traits may mediate niche and fitness differences in at least four ways (Fig. 1). First, interspecific trait variation can be correlated with fitness independent of neighbor density, such that species possessing traits associated with the highest fitness should exclude species with unfavorable traits (1, 2) (1 in Fig. 1). Second, traits may be associated with a species’ sensitivity to neighbor density (i.e., a trait × crowding interaction effect on fitness). As a result, certain trait values may have relatively low fitness under high crowding (6–9) (2 in Fig. 1). For example, species with low wood specific gravity (WSG) might have higher fitness at the initiation of succession but be disadvantaged later in succession because of sensitivity to crowding. Third, negative density effects of neighbors may be asymmetric between species and dependent on hierarchies (i.e., species with favorable traits experience weaker negative density effects of neighbors with unfavorable traits, generating relative fitness differences) (10) (3 in Fig. 1). For example, species with high WSG may have stronger negative density effects on neighbors with low WSG than vice versa (10). Fourth, trait variation may be related to local niche differences between neighbors, such that greater trait differences weaken negative density effects of neighbors and promote stable coexistence of functionally diverse neighbors (11, 12) (4 in Fig. 1). Overall, traits associated with fitness differences (1–3 in Fig. 1) are expected to decrease in diversity over time, whereas traits associated with stabilizing niche differences (4 in Fig. 1) are expected to increase in diversity, assuming no trait bias in immigration. Gaining a more nuanced, complete, and predictive understanding of community assembly requires that we quantify the effects of these assembly processes on species demography and the associated changes at the community level (13, 14).
Fig. 1.
Illustration of the four mechanisms of trait-mediated assembly included in our models. Assembly may be driven by species differences in (1) survival independent of crowding by neighbors, (2) response to increased crowding, (3) competitive hierarchies, and (4) local niche. All four processes may be revealed through the lens of functional trait variation (the spectrum from yellow to green) and its effects on mortality (black Xs). Note that the color schemes are not meant to be mutually compatible. In 1, the green color is associated with greater fitness (e.g., because of higher survival) compared with yellow, independent of neighborhood crowding. (2) Green traits have lower sensitivity to crowding relative to yellow traits (irrespective of neighbors' traits, which are shown in gray). (3) Species have trait hierarchies. Here, green is dominant (i.e., greener species always have greater negative effect on the performance of yellower neighbors than vice versa). (a) Because their traits are very different, the green species has a stronger competitive effect on the yellow species compared with (b) the competitive effect of the yellow-green species on the yellow species. (4) In contrast, when trait variation is associated with stabilizing niche differences between neighbors, negative density effects are experienced similarly by both species. (a) Neighbors with greater trait-associated niche difference (green vs. yellow) have a weaker competitive effect relative to (b) species with less difference in traits (green vs. yellow-green).
Successional tropical forests exhibit rapid community assembly and thus, provide an ideal system for testing assembly theory (15). High species richness and elevated tree growth and mortality rates relative to old-growth forests contribute to rapid species turnover and increase the likelihood of detecting assembly processes across succession (16). As succession proceeds, increased crowding alters local resource availability, which may drive species turnover (17–19). Early successional forests are typically dominated by species with acquisitive traits, such as low WSG and high specific leaf area (SLA), which enable rapid growth, resource capture, and high fitness under conditions of high resource availability (17, 20, 21). In contrast, traits that tend to dominate older forests [e.g., dense wood and tough leaves (22, 23)] are thought to promote long-term survival and fitness under high crowding and low resource availability (17, 24). Trait diversity is also expected to change as succession proceeds, although the direction and mechanisms of diversity changes remain unclear (23). Despite considerable previous efforts, few researchers have tested these hypotheses using demographic data in diverse systems.
We use a long-term dataset of tropical forest succession to disentangle how trait-mediated processes drive community dynamics. Spatially explicit longitudinal studies of individual performance offer a powerful approach to reveal drivers of community dynamics (9, 12, 14, 25–27). Empirical studies of functional community assembly have typically compared static community patterns across sites using aggregated trait metrics (28–30). These studies assume that community patterns are the result of prior assembly processes. However, static and aggregated patterns provide limited capability to distinguish between multiple, often opposing assembly processes (14, 31).
Our novel approach simultaneously quantifies the relative importance of multiple assembly processes and links these processes to contrasting effects on functional diversity. We use trait data for over 200 tree species, and we develop spatially explicit statistical models of tree survival over 15 y across eight 1-ha forest plots at diverse stages of succession in lowland wet forests of Costa Rica (SI Appendix, Table S1). We ask three central questions about trait-mediated effects on tree survival rates, a major component of fitness, and successional community dynamics.
-
i)What are the simultaneous effects and relative importance of four trait-mediated assembly processes on tree survival? Specifically, we ask four subquestions about the processes.
-
i.i)How are functional traits related to interspecific variation in average survival?
-
i.ii)How do trait relationships with survival change with variation in neighborhood crowding?
-
i.iii)Do competitive interactions depend on trait hierarchy, indicating that crowding effects are based on dominance?
-
i.iv)Do competitive interactions with neighbors depend on absolute trait differences between neighbors, suggesting stabilizing niche differences?
-
i.i)
-
ii)
Is the demographic evidence for trait-mediated fitness and niche differences (i) reflected in community-level changes in trait diversity across successional stages? We expect that traits associated with relative fitness (i.e., associated with greater average survival, decreased sensitivity to crowding, and hierarchical dominance) will decrease in diversity over time, whereas traits associated with stabilizing niche differences will increase in diversity during forest succession.
-
iii)
Is the demographic evidence for trait-mediated fitness differences reflected in functional differences between second-growth specialists and old-growth specialists? We expect that traits associated with greater relative fitness will be more strongly associated with old-growth specialists compared with second-growth specialists.
To answer these questions, we built hierarchical Bayes models of community-wide stem survival, which include parameters for each assembly process in questions i.i–i.iv (12, 27) (details in Methods). We fit models for each of three functional traits associated with resource use and life history: SLA (defined as leaf area per unit dry mass), leaf dry matter content (LDMC; defined as leaf dry mass over leaf fresh mass), and WSG (defined as the density of wood relative to the density of water). These traits represent leading axes of ecological variation among tropical tree species that have been previously implicated in interspecific variation in resource use efficiency, species interactions, and life history strategies (32–34). SLA represents a major axis of variation between rapid resource acquisition for species with high SLA vs. conservative strategies with low tissue turnover for species with low SLA (32, 35). Species with high LDMC have lower leaf protein and reduced ability to exploit resource-rich environments but better performance under low resources and drought (7, 34, 36, 37). WSG is associated with a tradeoff between rapid growth rates for species with low WSG and high structural support and resistance to natural enemies for species with high WSG (9, 33).
Results
Trait Relationships with Average Survival.
Consistent with expectations, average survival rates (independent of neighborhood) were significantly higher for species with high LDMC and WSG compared with species with low LDMC and WSG (1 in Fig. 2 and SI Appendix, Fig. S1). Contrary to expectations, SLA was not significantly associated with survival.
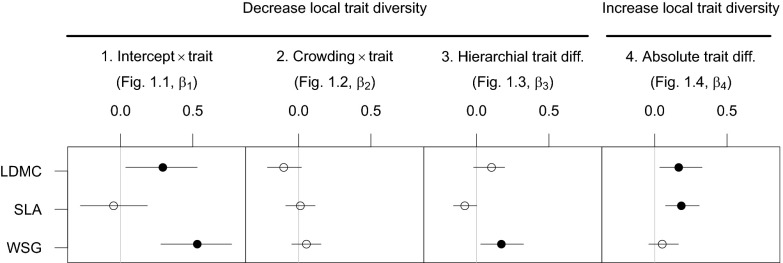
Fig. 2.
Standardized regression coefficients modeling the effects of trait-mediated assembly processes on survival. Numbered β-subscripts 1–4 correspond to research questions i.i–i.iv and similarly numbered processes 1–4 in Fig. 1, respectively. The first three modeled processes (1–3) promote lower trait diversity among neighbors, whereas the last process (4) promotes greater trait diversity. Circles show posterior medians of β-coefficients, and lines indicate 95% confidence intervals (filled circles indicate significant effects). Positive β indicates greater survival with increasing values of the covariate. For example, positive β1 for WSG for survival indicates that species with greater WSG had greater survival, independent of crowding effects. Negative β2 for LDMC for survival indicates that species with greater LDMC were more sensitive to crowding effects (NCI), although the finding was not significant. Positive β3 for WSG for survival indicates that trees with higher WSG had a greater negative impact on neighbors with relatively lower WSG than vice versa (NCIH). Positive β4 for LDMC and SLA indicates greater survival for focal trees with high absolute trait differences with neighbors (NCIS).
Standardized regression coefficients with greater absolute values are relatively more important in explaining survival. In this regard, our model offers a clear way of comparing the relative importance of the trait-based parameters that we studied. The regression coefficient that determines the relationship between traits and survival of each species independent of neighborhood variation (β1) had the greatest magnitude of the four types of trait effects considered (corresponding to 1–4 in Figs. 1 and 2).
Neighborhood Crowding and Trait Relationships with Survival.
On average, species survival decreased with increased neighborhood crowding, which was measured by the neighborhood crowding index (NCI) (Methods). However, interspecific variation in sensitivity to crowding was not significantly associated with any of the three traits (2 in Fig. 2, β2-parameter and SI Appendix).
Trait-Mediated Competitive Dominance Hierarchy.
The effects of trait-mediated hierarchical dominance interactions, measured by hierarchical trait differences with neighbors [signed trait difference weighted by NCI (NCIH)] (Methods), were only significant for WSG (3 in Fig. 2, β3-parameter and SI Appendix). We found that negative crowding effects on survival were lower for focal trees when their WSG was higher than that of neighbors. Note that hierarchical effects were dependent on the signed trait difference, size, and proximity of neighbors and thus, are distinct from WSG effects on species average survival rates (β1).
Traits and Stabilizing Niche Differences Among Neighbors.
Two of three traits exhibited significant evidence for niche differences in neighbor interactions. Focal trees had significantly greater survival when there were greater absolute differences with neighbors in LDMC and SLA [absolute trait difference weighted by NCI (NCIS)] (4 in Fig. 2, β4-parameter). Our findings suggest that, overall, increased trait difference with neighbors was associated with increased survival of focal trees.
Successional Changes in Trait Diversity.
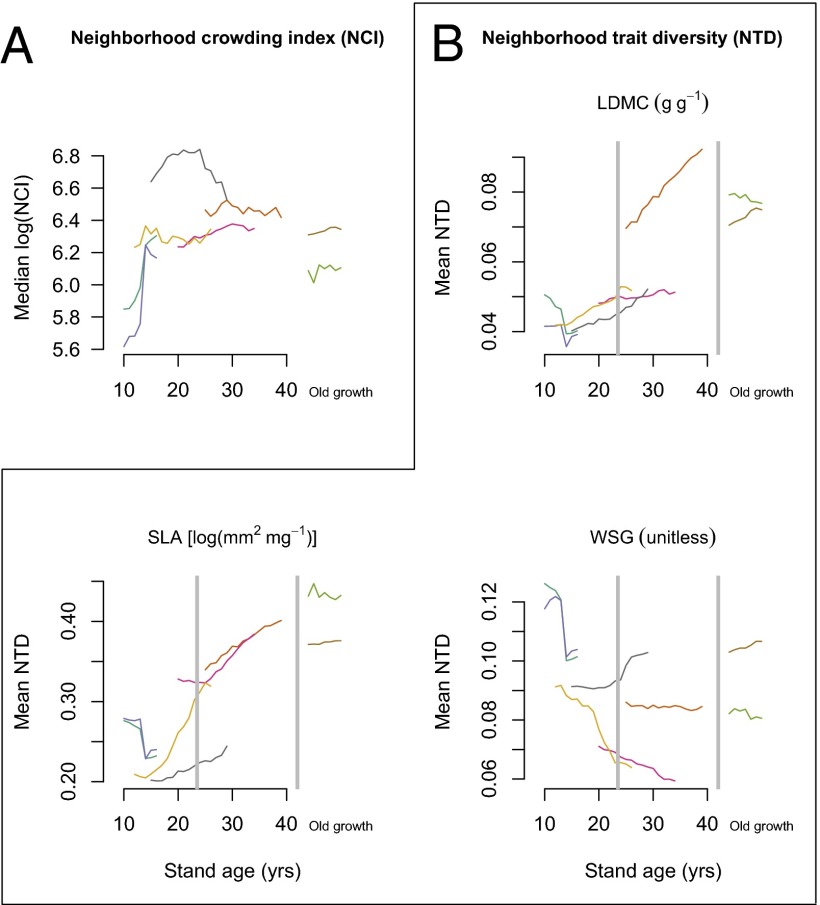
Median neighborhood crowding (NCI) among individuals in 10-m radius neighborhoods increased rapidly during early succession (10–14 y after abandonment) (Fig. 3). After 15–20 y, median crowding remained roughly constant but declined in old-growth stands. Neighborhood trait diversity (NTD) was measured as the average trait difference between focal trees and their neighbors weighted by neighbor size and proximity. NTD showed divergent changes with stand age depending on the functional trait (Fig. 3 and SI Appendix). NTD measured with LDMC (linear mixed effects model; t = 3.8, P = 0.0003) and SLA (t = 5.3, P < 0.0001) significantly increased from early-growth to midsuccessional to old-growth stands. In contrast, WSG showed a decreasing trend in NTD in older stands, although the trend was not significant (t = −1.2, P = 0.2291).
Fig. 3.
Change in stem neighborhoods with increasing stand age. (A) Change in median NCI [log(NCI)] (Methods). (B) Change in NTD, which is weighted by neighbor proximity and size. Gray lines indicate divisions between stand age categories used to test the effect of stand age on NTD. NTD represents the average trait difference between focal trees and their neighbors weighted by neighbor size and proximity calculated for each trait (Methods). Each of the eight 1-ha forest plots is represented by a differently colored line.
Trait Differences of Second-Growth vs. Old-Growth Specialists.
We used a recently developed multinomial model to classify 66 species as second-growth specialists and 133 species as old-growth specialists based on relative abundances in each habitat; the remaining 189 species were either generalists or too rare to classify (38). Old-growth specialists had significantly greater WSG (mean = 0.57) compared with second-growth specialists (mean = 0.44, Wilcoxon test: P < 10−7). In contrast, old-growth specialists did not have significantly different LDMC (mean = 0.35 g g−1) or log SLA (mean = 2.68 log mm2 mg−1) compared with second-growth specialists (mean LDMC = 0.34 g g−1 and mean log SLA = 2.71 log mm2 mg−1; P = 0.60 and P = 0.54, respectively).
Discussion
We coupled a long-term study of tree demography in successional forests with a novel modeling approach to reveal the relative importance of four trait-mediated, spatially explicit mechanisms underlying community assembly during tropical forest succession. Our models included trait-mediated effects associated with relative fitness differences—mean survival, responses to crowding, and dominance hierarchies—and effects associated with stabilizing niche differences within local neighborhoods.
Among these mechanisms, our evidence indicated that the most important were trait-mediated relative fitness differences among species (Fig. 2, β1). The link between traits and average survival is not surprising given that the traits that we studied are closely associated with resource acquisition, storage, and defense (32, 33, 35, 39–41). Among the traits considered, WSG was the strongest predictor of species average survival, independent of variation in neighborhood crowding. In accordance with other studies, we found that higher WSG was associated with higher survival (9, 40, 42, 43). WSG is closely related to resistance to physical damage but also correlates with other traits that may reduce mortality, such as resource conservation and high leaf toughness (44). Additionally, we found that average species survival was greater for species with high LDMC values. Like WSG, high LDMC is associated with conservative resource use strategies and drought resistance (34, 36, 37). Our study sites are closed canopy midsuccessional and old-growth stands with low light availability, which may favor persistence of species with high LDMC and WSG that can avoid mortality by withstanding low resource availability.
We did not find significant effects of species traits on sensitivity to crowding (Fig. 2, β2). The lack of crowding response effects may seem surprising given findings that traits associated with late-successional species typically confer higher relative fitness under low resource conditions (17, 24). Successional niche theory predicts a tradeoff between species having high fitness under low crowding and species with high fitness under high crowding (21). This tradeoff is thought to be a major empirical driver of community turnover (45). Our findings may be partly due to the fact that the youngest stands in our dataset were 10 y in age, and we had fewer young stands with low crowding compared with midsuccessional stands with high crowding. Additionally, high fitness of early-successional specialists may be caused by rapid growth and fecundity, vital rates that we did not include here (8). Thus, we may have been limited in our ability to characterize traits associated with fitness during initial stages of succession. Because our study was observational, we were unable to observe all species under a full range of crowding conditions, potentially limiting our power to detect interspecific variation in crowding responses. Nevertheless, our observations reflect the conditions that species encounter in natural stands and thus, reflect the importance of assembly mechanisms at work under realistic crowding variation.
Our study provides evidence for the importance of trait-associated stabilizing niche differences in succession. We found significant effects of trait similarity between focal trees and neighbors (β4), consistent with the hypothesis that species niche differences associated with functional traits mediate species interactions. SLA, a key indicator of ecological strategy (35), showed the strongest demographic evidence for stabilizing niche differences among neighbors (Fig. 2, β4). SLA is closely linked to a tradeoff between photosynthetic rate and cost of leaf construction or leaf lifespan (32). Species that differ in SLA may compete less strongly and be more likely to stably coexist compared with species with similar SLA. Additional studies from mature tropical forests have implicated SLA in environmental filtering and niche differentiation (27–29), although SLA-mediated interactions were less important in hurricane-prone forests in Puerto Rico (12). Differences in LDMC among neighbors also were associated with higher survival, although previous studies did not find an importance of LDMC for neighborhood niche differences among tropical trees (12, 28, 29). Additionally, effects of trait similarity between focal trees and neighbors (β4) were significant, whereas focal tree trait effects on crowding response (β2) were not, indicating that crowding effects are more dependent on the composition of neighborhoods than the traits of focal trees.
Our models showed weaker evidence for fitness differences associated with trait-mediated hierarchical interactions than trait-mediated stabilizing niche differences. However, hierarchical effects of high WSG species were evident in that species with greater WSG had stronger impacts on neighbors with lower WSG (Fig. 2, β3). This pattern may be the result of greater resource conservation in species with high WSG values (10). This result supports the findings by Kunstler et al. (10) in French forests, where neighborhood interactions were better modeled by hierarchical trait effects than effects of absolute trait differences. Although we found hierarchical effects only for WSG, the difference in our findings might be attributed to the fact that Kunstler et al. (10) did not incorporate trait associations with species average demography, which were the most important trait-mediated drivers in our study. Our results suggest that the most important differences among species in community assembly may be fitness differences independent of variation in neighbor density.
Another goal of our study was to link trait-mediated effects on survival rates to long-term changes in trait diversity at the community level. We expected that traits associated with stabilizing niche differences among neighbors would display increased trait diversity as stands age. Consistent with this expectation, absolute difference in neighbor SLA and LDMC was associated with significantly higher survival, suggesting niche differences among neighbors, and these same traits also showed greater neighborhood trait diversity in older stands. We previously observed increasing phylogenetic diversity with stand age in our plots (46), which assuming that traits are conserved, is likely influenced by trait-mediated niche differences. However, the demographic effects of trait-mediated niche differences were countered by strong trait correlations with average demography, suggesting that assembly simultaneously changed community trait means and variance. The only trait that exhibited decreased diversity over time was WSG. This result is consistent with the strong correlation between WSG and average survival rates and competitive hierarchies suggesting relative fitness differences, which are expected to reduce neighborhood trait diversity. Although other assembly processes can contribute to changes in neighborhood trait diversity, we did not observe a significant trend in neighborhood trait diversity caused by recruits replacing dead stems (SI Appendix).
We expected that traits associated with higher relative fitness would be more prevalent among old- vs. second-growth specialists. The trait–demographic relationships that we modeled were largely consistent with differences that we observed between second- and old-growth specialists and findings from previous studies. WSG is related to physical sturdiness and was significantly higher among old- vs. second-growth specialists, and it was also associated with higher species survival (Fig. 2 and SI Appendix, Fig. S1). Thus, higher survival of better defended and structurally sound species is likely a major driver of successional habitat specialization, consistent with previous observations of successional trait changes of other forests (10, 19, 22). Our results suggest that communities shift to functional types with the highest survival during succession. Additionally, it is possible that trait differences between second- and old-growth specialists were associated with competition colonization tradeoffs (21), although we did not quantify colonization ability.
Our results offer important insights into the mechanisms of successional community dynamics. Researchers have hypothesized that increased crowding drives community succession, because species with acquisitive functional traits that colonize and grow rapidly early in succession compete poorly under crowded conditions later in succession (18, 21, 45). We did not find evidence to support this hypothesis, because no trait was significantly associated with species response to crowding. However, additional traits that we did not study may be associated with crowding responses and other fitness and niche differences. Also, note that a given trait may be associated with variation in both fitness and niche (5). Our results suggest that second-growth specialists with low WSG have lower fitness than old-growth specialists, leading to declining populations during forest succession (22). The presence of second-growth specialists in successional habitats may be promoted by colonization abilities and rapid generation time, possibly trading off with competitive ability and long-term persistence (21). Finally, our results are consistent with other studies that show trait-dependent changes in functional diversity over succession (23, 30). Our results suggest that differences in successional trajectories among traits are partly caused by the degree to which traits are associated with fitness vs. niche differences.
Conclusions
We used the large amount of information contained in individual tree neighborhoods and long-term performance to elucidate multiple functional and demographic drivers of tropical forest community assembly. Our analysis revealed evidence of major assembly roles for (i) trait-based demographic differences that contribute to interspecific fitness differences and (ii) trait-based stabilizing niche differences that reduce interspecific competition and contribute to successional increases in functional diversity (1, 2). Specifically, we showed that traits likely linked with niche differences (SLA and LDMC) increased in diversity with succession, whereas a trait likely linked with only fitness differences (WSG) decreased in diversity with succession. Our long-term study indicates that higher WSG likely enhances species survival and fitness, supporting hypothesized links between functional traits, demography, and successional change (47). Our study makes significant advances to the goal of quantifying the mechanisms underlying community assembly and dynamics in natural communities. Future studies should include vital rates not modeled here to definitively link neighborhood interactions, per capita population growth rates, and species and functional diversity with community assembly.
Methods
Study Sites and Data.
We annually monitored woody stems ≥5 cm in diameter at breast height (DBH) in six successional and two mature forest 1-ha plots in the Caribbean lowlands (50–220 m above sea level) of Costa Rica (19, 46, 48, 49) (SI Appendix, Table S1). Four successional plots were monitored beginning in 1997, and the remaining plots were monitored beginning in 2005. Here, we include data through the 2011 census. All plots had been cleared and grazed lightly for several years before abandonment and had closed canopies from the initiation of vegetation monitoring (50). Each stem was identified to species, tagged, mapped, and annually measured for DBH. After excluding stems within 10 m of plot edges (4,870 stems), we modeled the survival of 5,265 unique stems across the 1997–2011 study period. Survival was not modeled for 440 stems belonging to species with unknown trait data.
We used standardized, published protocols for all functional trait measurements (more details in SI Appendix) (39, 51). We measured functional traits for 215 species of a total of 389 species, including 200 of 226 tree species that had four or more individuals in any of the annual censuses. Measurements were made on 1–62 individuals per sampled species. In addition to SLA, LDMC, and WSG, we measured leaf size (centimeters2), leaf thickness (millimeters), leaf toughness (N millimeter−1), and leaf density (milligrams millimeter−3). Results for leaf size, thickness, toughness, and density can be found in SI Appendix.
Demographic Models.
We separately fit survival models for each trait to manage model complexity and correlations among traits (SLA vs. WSG, r = −0.07; WSG vs. LDMC, r = 0.30; LDMC vs. SLA, r = 0.63). Each model included the species sampled for that trait. We excluded species with missing traits from analysis of survival, although their effects on focal stems were included as an additional term.
The model takes the form
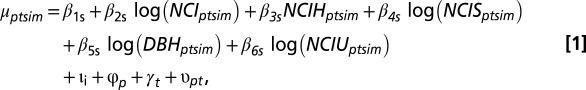 |
where 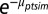 gives the scale parameter for a Weibull distribution, determining expected survival time. The subscripts refer to stem m of individual i (potentially multistemmed) of species s in plot p measured in census years t and t + 1. The first four terms in the regression are associated with the four studied ways in which functional trait variation affects community assembly (corresponding to 1–4 in Fig. 1). The first term of the regression, β1s, is a species-specific intercept, whereas the next three terms determine the effects of crowding by neighbors (NCI), crowding by neighbors dependent on trait hierarchy (NCIH), and crowding dependent on absolute trait differences between focal trees and neighbors (NCIS). Additional coefficients describe effects of size (DBH) and crowding by neighbors with unknown traits (NCIU). All coefficients in a given trait model were fit simultaneously, including second-level regressions (Eqs. 2 and 4). Expected survival time b(μptsim) was modeled with Weibull-distributed process error, where survival time was left- and right-censored from the recensus date (52). Covariate effects (e.g., DBH and NCI) are included as their state in year t. Normally distributed random effects for individual, plot, year, and year × plot are ιi, φp, γt, and υpt, respectively.
gives the scale parameter for a Weibull distribution, determining expected survival time. The subscripts refer to stem m of individual i (potentially multistemmed) of species s in plot p measured in census years t and t + 1. The first four terms in the regression are associated with the four studied ways in which functional trait variation affects community assembly (corresponding to 1–4 in Fig. 1). The first term of the regression, β1s, is a species-specific intercept, whereas the next three terms determine the effects of crowding by neighbors (NCI), crowding by neighbors dependent on trait hierarchy (NCIH), and crowding dependent on absolute trait differences between focal trees and neighbors (NCIS). Additional coefficients describe effects of size (DBH) and crowding by neighbors with unknown traits (NCIU). All coefficients in a given trait model were fit simultaneously, including second-level regressions (Eqs. 2 and 4). Expected survival time b(μptsim) was modeled with Weibull-distributed process error, where survival time was left- and right-censored from the recensus date (52). Covariate effects (e.g., DBH and NCI) are included as their state in year t. Normally distributed random effects for individual, plot, year, and year × plot are ιi, φp, γt, and υpt, respectively.
Trait relationships with average survival.
To address question i.i above, we modeled how species traits affect species average survival, β1s, in a second-level (i.e., species-level) regression:
where β1 gives the effect of functional trait Fs, ε1s is the normally distributed random effect of species s, and β1.0 is the intercept for all species. High values of |β1| indicate a close link between a trait and average survival.
Neighborhood crowding and trait relationships with survival.
To address question i.ii, we modeled how the traits of a focal tree mediate its response to crowding (β2s). First, we calculated NCI for each stem m based on the size and distance of its neighbors,
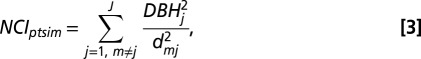 |
where dmj is the distance between stem m and neighbor j, of which there are J total neighbors within a specified radius. We used a radius of 10 m, which is sufficient to capture the great majority of neighborhood effects (25, 53). As noted above, to avoid edge effects, we excluded stems within 10 m of plot boundaries in our analysis.
Second, we modeled trait effects on species response to crowding, β2s (Eq. 1), in an additional second-level regression:
where β2 determines the influence of functional trait Fs on species response to NCI. High values of |β2| indicate a close association between interspecific variation in traits and sensitivity to crowding.
Traits and competitive dominance hierarchies.
To address question i.iii, we modeled crowding effects dependent on trait hierarchy between neighbors, where the competitive effects of a species pair are asymmetric (e.g., low trait value more negatively affected by crowding of neighbor with high trait value). These effects, NCIH, are calculated as
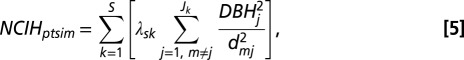 |
where s is the focal species and S is the total number of neighbor species k. The effect of crowding is mediated by the trait differences between neighbors, λsk, which are calculated as λsk = Fs − Fk, where Fs and Fk are the traits of focal species s and neighboring species k, respectively (10). The effect of NCIH is determined by the β3-coefficient (Eq. 1), which when positive, indicates that neighborhood crowding effects are relatively weaker when focal tree trait value Fs is greater than the neighbors’ trait value Fk. When β3 is negative, crowding effects are relatively weaker when focal tree trait value Fs is less than the neighbors’ trait value Fk. NCIH = 0 for conspecific neighbors (i.e., s = k).
Traits and niche variation among neighbors.
To address question i.iv, we assumed that crowding effects could depend on absolute symmetric trait differences between focal stem m and neighbors j (of Jk neighbors of species k) as follows:
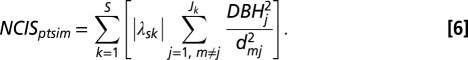 |
As opposed to NCIH, NCIS is based on the absolute value of trait differences, λsk (12). The sign of the β4-coefficient determines the effect of NCIS (Eq. 1), with positive values indicating that greater trait differences with neighbors lead to amelioration of crowding effects. NCIS = 0 for conspecific neighbors (i.e., s = k).
Trait-independent effects.
Ontogenetic changes in survival are modeled by the DBH parameter (β5s), which is species-specific and drawn from a normal distribution with mean β5. We lacked trait data for some rare species. To account for potential bias in their crowding effects, we calculated an additional term, NCIU, with coefficient β6s (Eq. 1). NCIU was not weighted by traits and was equal to NCI summed only for neighbors j belonging to species with missing trait data.
Interspecific variation in trait-mediated crowding responses was modeled such that species-specific parameters β3s, β4s, and β6s were drawn from normal hyperdistributions with means β3, β4, and β6, respectively (9, 27). For regression purposes, NCI and NCIS were transformed by adding one and taking the log, because metrics were highly right-skewed. Trait, NCI, NCIS, NCIH, and NCIU metrics were all standardized to mean zero and unit SD to facilitate interpretation and comparisons among parameters (54).
We fit separate survival models for each trait. All parameters were given proper diffuse priors, and posterior sampling was conducted by Markov Chain Monte Carlo using JAGS software (http://mcmc-jags.sourceforge.net/). β-Parameters were considered significant when 95% confidence intervals did not overlap with zero. We conducted predictive checks by simulating survival for each stem based on the underlying parameter posterior samples and calculating accuracy of predictions (54).
Successional differences in neighborhood trait diversity.
We also studied successional changes in NTD of each stem, which were the weighted average trait differences between a focal stem and its neighbors. We calculated NTD = NCIS/NCI to weight pairwise trait differences by the proximity and size of neighbors (i.e., the components of NCI). For each plot in each year, we calculated the mean NTD for each trait. We classified stand age as a categorical ordinal covariate [1 = early successional (<24 y old), 2 = midsuccessional (24–39 y old), 3 = old growth (unknown age)]. We then tested whether NTD for each trait changed linearly across the three stand age categories using a linear mixed effects model with plot as a random effect (implemented using the R package nlme) (55).
Trait differences between second- and old-growth specialists.
We tested for trait differences among species classified as second- vs. old-growth specialists. Classifications were taken from a multinomial analysis of species relative abundances in second- vs. old-growth stands (38). We tested for differences in species traits between the two categories using Wilcoxon rank sum tests.
Supplementary Material
Acknowledgments
We thank Bénédicte Bachelot, Frans Bongers, Robert Muscarella, Naomi Schwartz, and three anonymous reviewers for comments on this manuscript. We thank Martyn Plummer for assistance with JAGS. Financial support was provided by US National Science Foundation Award DEB-1050957 (to M.U.). Collection of trait data was made possible, in part, by US National Science Foundation Graduate Research Fellowship Program Grant DEB-1110722 (to V.K.B. and R.L.C.) and support to V.K.B. from the Organization for Tropical Studies, the Christiane and Christopher Tyson Fellowship, the Ronald Bamford Endowment Fund, the American Philosophical Society, and a Garden Club of America Award in Tropical Botany. Long-term monitoring of trees was supported by grants from the Andrew Mellon Foundation and the University of Connecticut Research Foundation, US National Science Foundation Awards 0424767, 0639393, 1147429, and 1110722 (to R.L.C.), and National Aeronautics and Space Administration Research Opportunities in Space and Earth Sciences Grant NNH08ZDA001N-TE.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1319342111/-/DCSupplemental.
References
- 1.Chesson P. Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst. 2000;31:343–366. [Google Scholar]
- 2.HilleRisLambers J, Adler PB, Harpole WS, Levine JM, Mayfield MM. Rethinking community assembly through the lens of coexistence theory. Annu Rev Ecol Evol Syst. 2012;43:227–248. [Google Scholar]
- 3.Cottenie K, De Meester L. Metacommunity structure: Synergy of biotic interactions as selective agents and dispersal as fuel. Ecology. 2004;85(1):114–119. [Google Scholar]
- 4.Weiher E, Keddy PA. Assembly rules, null models, and trait dispersion: New questions from old patterns. Oikos. 1995;74:159–164. [Google Scholar]
- 5.Adler PB, Fajardo A, Kleinhesselink AR, Kraft NJB. Trait-based tests of coexistence mechanisms. Ecol Lett. 2013;16(10):1294–1306. doi: 10.1111/ele.12157. [DOI] [PubMed] [Google Scholar]
- 6.Hubbell SP, Foster RB. In: Plant Ecology. Crawley MJ, editor. Oxford: Blackwell; 1986. pp. 77–96. [Google Scholar]
- 7.Grime JP, et al. Integrated screening validates primary axes of specialisation in plants. Oikos. 1997;79(2):259–281. [Google Scholar]
- 8.Goldberg DE, Landa K. Competitive effect and response: Hierarchies and correlated traits in the early stages of competition. J Ecol. 1991;79(4):1013–1030. [Google Scholar]
- 9.Rüger N, Wirth C, Wright SJ, Condit R. Functional traits explain light and size response of growth rates in tropical tree species. Ecology. 2012;93(12):2626–2636. doi: 10.1890/12-0622.1. [DOI] [PubMed] [Google Scholar]
- 10.Kunstler G, et al. Competitive interactions between forest trees are driven by species’ trait hierarchy, not phylogenetic or functional similarity: Implications for forest community assembly. Ecol Lett. 2012;15(8):831–840. doi: 10.1111/j.1461-0248.2012.01803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macarthur R, Levins R. The limiting similarity, convergence, and divergence of coexisting species. Am Nat. 1967;101(921):377–385. [Google Scholar]
- 12.Uriarte M, et al. Trait similarity, shared ancestry and the structure of neighbourhood interactions in a subtropical wet forest: Implications for community assembly. Ecol Lett. 2010;13(12):1503–1514. doi: 10.1111/j.1461-0248.2010.01541.x. [DOI] [PubMed] [Google Scholar]
- 13.Leibold MA, McPeek MA. Coexistence of the niche and neutral perspectives in community ecology. Ecology. 2006;87(6):1399–1410. doi: 10.1890/0012-9658(2006)87[1399:cotnan]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 14.Clark JS, et al. High-dimensional coexistence based on individual variation: A synthesis of evidence. Ecol Monogr. 2010;80(4):569–608. [Google Scholar]
- 15.Anderson KJ. Temporal patterns in rates of community change during succession. Am Nat. 2007;169(6):780–793. doi: 10.1086/516653. [DOI] [PubMed] [Google Scholar]
- 16.Chazdon RL, et al. Rates of change in tree communities of secondary Neotropical forests following major disturbances. Philos Trans R Soc Lond B Biol Sci. 2007;362(1478):273–289. doi: 10.1098/rstb.2006.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grime JP. Plant Strategies, Vegetation Processes, and Ecosystem Properties. New York: Wiley; 1979. [Google Scholar]
- 18.Guariguata MR, Ostertag R. Neotropical secondary forest succession: Changes in structural and functional characteristics. For Ecol Manage. 2001;148(1-3):185–206. [Google Scholar]
- 19.Chazdon RL. In: Tropical Forest Community Ecology. Carson WP, Schnitzer SA, editors. New York: Wiley-Blackwell; 2008. [Google Scholar]
- 20.Bazzaz FA, Pickett STA. Physiological ecology of tropical succession: A comparative review. Annu Rev Ecol Syst. 1980;11:287–310. [Google Scholar]
- 21.Pacala SW, Rees M. Models suggesting field experiments to test two hypotheses explaining successional diversity. Am Nat. 1998;152(5):729–737. doi: 10.1086/286203. [DOI] [PubMed] [Google Scholar]
- 22.Slik JWF, et al. Wood density as a conservation tool: Quantification of disturbance and identification of conservation-priority areas in tropical forests. Conserv Biol. 2008;22(5):1299–1308. doi: 10.1111/j.1523-1739.2008.00986.x. [DOI] [PubMed] [Google Scholar]
- 23.Lohbeck M, et al. Functional diversity changes during tropical forest succession. Perspect Plant Ecol Evol Syst. 2012;14(2):89–96. [Google Scholar]
- 24.Tilman D. Secondary succession and the pattern of plant dominance along experimental nitrogen gradients. Ecol Monogr. 1987;57(3):189–214. [Google Scholar]
- 25.Uriarte M, Canham CD, Thompson J, Zimmerman JK. A neighborhood analysis of tree growth and survival in a hurricane-driven tropical forest. Ecol Monogr. 2004;74(4):591–614. [Google Scholar]
- 26.Russo SE, Brown P, Tan S, Davies SJ. Interspecific demographic trade-offs and soil-related habitat associations of tree species along resource gradients. J Ecol. 2008;96(1):192–203. [Google Scholar]
- 27.Lasky JR, Sun I-F, Su S-H, Chen Z-S, Keitt TH. Trait-mediated effects of environmental filtering on tree community dynamics. J Ecol. 2013;101(3):722–733. [Google Scholar]
- 28.Kraft NJB, Valencia R, Ackerly DD. Functional traits and niche-based tree community assembly in an Amazonian forest. Science. 2008;322(5901):580–582. doi: 10.1126/science.1160662. [DOI] [PubMed] [Google Scholar]
- 29.Swenson NG, Enquist BJ. Opposing assembly mechanisms in a neotropical dry forest: Implications for phylogenetic and functional community ecology. Ecology. 2009;90(8):2161–2170. doi: 10.1890/08-1025.1. [DOI] [PubMed] [Google Scholar]
- 30.Raevel V, Violle C, Munoz F. Mechanisms of ecological succession: Insights from plant functional strategies. Oikos. 2012;121(11):1761–1770. [Google Scholar]
- 31.Kembel SW. Disentangling niche and neutral influences on community assembly: Assessing the performance of community phylogenetic structure tests. Ecol Lett. 2009;12(9):949–960. doi: 10.1111/j.1461-0248.2009.01354.x. [DOI] [PubMed] [Google Scholar]
- 32.Wright IJ, et al. The worldwide leaf economics spectrum. Nature. 2004;428(6985):821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- 33.Chave J, et al. Towards a worldwide wood economics spectrum. Ecol Lett. 2009;12(4):351–366. doi: 10.1111/j.1461-0248.2009.01285.x. [DOI] [PubMed] [Google Scholar]
- 34.Kursar TA, et al. The evolution of antiherbivore defenses and their contribution to species coexistence in the tropical tree genus Inga. Proc Natl Acad Sci USA. 2009;106(43):18073–18078. doi: 10.1073/pnas.0904786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. Plant ecological strategies: Some leading dimensions of variation between species. Annu Rev Ecol Syst. 2002;33:125–159. [Google Scholar]
- 36.Wilson PJ, Thompson K, Hodgson JG. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol. 1999;143(1):155–162. [Google Scholar]
- 37.Markesteijn L, Poorter L, Bongers F, Paz H, Sack L. Hydraulics and life history of tropical dry forest tree species: Coordination of species’ drought and shade tolerance. New Phytol. 2011;191(2):480–495. doi: 10.1111/j.1469-8137.2011.03708.x. [DOI] [PubMed] [Google Scholar]
- 38.Chazdon RL, et al. A novel statistical method for classifying habitat generalists and specialists. Ecology. 2011;92(6):1332–1343. doi: 10.1890/10-1345.1. [DOI] [PubMed] [Google Scholar]
- 39.Cornelissen JHC, et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot. 2003;51(4):335–380. [Google Scholar]
- 40.Wright SJ, et al. Functional traits and the growth-mortality trade-off in tropical trees. Ecology. 2010;91(12):3664–3674. doi: 10.1890/09-2335.1. [DOI] [PubMed] [Google Scholar]
- 41.Hérault B, et al. Functional traits shape ontogenetic growth trajectories of rain forest tree species. J Ecol. 2011;99(6):1431–1440. [Google Scholar]
- 42.Poorter L, et al. Are functional traits good predictors of demographic rates? Evidence from five neotropical forests. Ecology. 2008;89(7):1908–1920. doi: 10.1890/07-0207.1. [DOI] [PubMed] [Google Scholar]
- 43.Kraft NJB, Metz MR, Condit RS, Chave J. The relationship between wood density and mortality in a global tropical forest data set. New Phytol. 2010;188(4):1124–1136. doi: 10.1111/j.1469-8137.2010.03444.x. [DOI] [PubMed] [Google Scholar]
- 44.Westbrook JW, et al. What makes a leaf tough? Patterns of correlated evolution between leaf toughness traits and demographic rates among 197 shade-tolerant woody species in a neotropical forest. Am Nat. 2011;177(6):800–811. doi: 10.1086/659963. [DOI] [PubMed] [Google Scholar]
- 45.Rees M, Condit R, Crawley M, Pacala S, Tilman D. Long-term studies of vegetation dynamics. Science. 2001;293(5530):650–655. doi: 10.1126/science.1062586. [DOI] [PubMed] [Google Scholar]
- 46.Norden N, Letcher SG, Boukili V, Swenson NG, Chazdon R. Demographic drivers of successional changes in phylogenetic structure across life-history stages in plant communities. Ecology. 2012;93(Suppl 8):S70–S82. [Google Scholar]
- 47.Chazdon RL. Second-Chance: Tropical Forest Regeneration in an Age of Deforestation. Chicago: Univ of Chicago Press; 2014. [Google Scholar]
- 48.Capers RS, Chazdon RL, Brenes AR, Alvarado BV. Successional dynamics of woody seedling communities in wet tropical secondary forests. J Ecol. 2005;93(6):1071–1084. [Google Scholar]
- 49.Chazdon RL, et al. Composition and dynamics of functional groups of trees during tropical forest succession in northeastern Costa Rica. Biotropica. 2010;42(1):31–40. [Google Scholar]
- 50.Redondo BA, Vilchez AB, Chazdon RL. Estudio de la dinámica y composición de cuatro bosques secundarios en la region Huetar Norte, Sarapiquí–Costa Rica. Rev For Centroam. 2001;36:21–26. [Google Scholar]
- 51.Williamson GB, Wiemann MC. Measuring wood specific gravity...Correctly. Am J Bot. 2010;97(3):519–524. doi: 10.3732/ajb.0900243. [DOI] [PubMed] [Google Scholar]
- 52.Somers GL, Oderwald RG, Harms WR, Langdon OG. Predicting mortality with a Weibull distribution. For Sci. 1980;26(2):291–300. [Google Scholar]
- 53.Hubbell SP, Ahumada JA, Condit R, Foster RB. Local neighborhood effects on long-term survival of individual trees in a neotropical forest. Ecol Res. 2001;16(5):859–875. [Google Scholar]
- 54.Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge, UK: Cambridge Univ Press; 2007. [Google Scholar]
- 55.Pinheiro J, Bates D, DebRoy S, Sarkar D. R Core Team 2013. nlme: Linear and Nonlinear Mixed Effects Models (R Package), Version 3.1-115.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.