Significance
We find that most terrestrial ectotherms are insufficiently tolerant of high temperatures to survive the warmest potential body temperatures in exposed habitats and must therefore thermoregulate by using shade, burrows, or evaporative cooling. Our results reveal that exposure to extreme heat can occur even at high elevations and latitudes and show why heat-tolerance limits are relatively invariant in comparison with cold limits. To survive climate warming, ectotherms in most areas may need to rely on behaviors—and have access to habitats—that provide a reprieve from extreme operative temperatures.
Keywords: macrophysiology, operative temperature, climate sensitivity
Abstract
Physiological thermal-tolerance limits of terrestrial ectotherms often exceed local air temperatures, implying a high degree of thermal safety (an excess of warm or cold thermal tolerance). However, air temperatures can be very different from the equilibrium body temperature of an individual ectotherm. Here, we compile thermal-tolerance limits of ectotherms across a wide range of latitudes and elevations and compare these thermal limits both to air and to operative body temperatures (theoretically equilibrated body temperatures) of small ectothermic animals during the warmest and coldest times of the year. We show that extreme operative body temperatures in exposed habitats match or exceed the physiological thermal limits of most ectotherms. Therefore, contrary to previous findings using air temperatures, most ectotherms do not have a physiological thermal-safety margin. They must therefore rely on behavior to avoid overheating during the warmest times, especially in the lowland tropics. Likewise, species living at temperate latitudes and in alpine habitats must retreat to avoid lethal cold exposure. Behavioral plasticity of habitat use and the energetic consequences of thermal retreats are therefore critical aspects of species’ vulnerability to climate warming and extreme events.
Predicting the organismal responses to climate change—a global priority—requires an understanding of the physiological, behavioral, ecological, and evolutionary factors that constrain where species can live (1, 2). Macrophysiological analyses that predict large-scale patterns in the vulnerability of ectotherms to climate warming often invoke the concept of the “thermal-safety margin” (3–6), which measures the difference between a species’ maximum tolerance to heat and the warm air temperatures it regularly experiences. Such heat safety margins often increase markedly with latitude, implying that tropical species might be relatively more vulnerable to climate warming than are species living at higher latitudes (refs. 3–6; but see ref. 7), even though the rate of climate warming is lower in the tropics (8). Indeed, many temperate ectotherms appear to have maximum thermal tolerances that are 10–20 °C higher than required to withstand the average summer air temperatures where they live (3).
Comparative physiology offers three reasons to be skeptical about such high thermal-safety margins. First, to index environmental temperatures, prior studies often used mean annual or seasonal air temperatures—measures that may have little ecological relevance in more variable and seasonal environments (5, 9, 10). Indeed, rare extreme temperatures—not average ones—may be more important for long-term species persistence (11, 12).
Second, studies generally use air temperatures (Ta, taken in shade at 1- to 2-m height) to index thermal environments: These temperatures are readily available but poorly characterize the thermal environment from an ectotherm's perspective. An ectotherm's body temperature can differ strikingly from local Ta because heat exchange is affected not merely by convection, but also by radiation, conduction, evaporation, and metabolism (7, 13–15). For example, an Andean lizard basking at 4,450 m had a body temperature of 31 °C even though air temperature was only ∼0 °C (16). Operative temperatures (Te), which estimate an ectotherm's steady-state body temperature, are more biophysically accurate indices of microclimates experienced by ectotherms. Te can be estimated either with physical models placed in the environment (14) or by mathematical models (17, 18) (Methods).
Third, most terrestrial ectotherms are mobile and can behaviorally exploit local heterogeneity in Te to regulate body temperatures somewhat independently of local environmental temperatures (“Bogert effect”) (19). For example, merely by shifting time (e.g., day/night) or place of activity (e.g., open habitat, shade, or burrows), many ectotherms can have a body temperature that is markedly different from air temperature (20, 21). In addition, wet-skinned ectotherms such as amphibians cool their bodies evaporatively and thus have a lower Te than otherwise-comparable dry-skinned ectotherms (13).
Because Ta and Te are thus fundamentally different metrics of environmental heat loads (with Te varying according to microhabitat), thermal-safety margins based on these alternative metrics must also differ. Here, we estimate global patterns of thermal-safety margins based on maximum and minimum Te, instead of Ta.
Global patterns in thermal-safety margins are useful for understanding not only species’ vulnerabilities to climate warming, but also the historical role of physiology and behavior in protecting species from temperature extremes; we therefore also consider cold thermal-safety margins, which represent the offset between cold temperature tolerance and minimum Te. We also explore variation in thermal tolerance and thermal-safety margins with elevation, to compare patterns along elevational and latitudinal gradients.
We start by expanding a global, empirical dataset on physiological heat and cold tolerance limits in amphibians, reptiles, and insects from diverse latitudes and elevations (22). We next use a global climate database and a biophysical model to calculate maximum and minimum air (Ta) and operative temperatures (Te, as described in ref. 21) at the location of collection for both the dry and the wet-skinned ectotherms for which we have thermal-tolerance estimates. We then calculate and compare thermal-safety margins based on maximum and minimum Te for individual species versus “traditional” margins based on Ta (3–5) and evaluate patterns across latitude and elevation. We find that safety margins based on Te are much more likely to be negative than those based on Ta. This result implies that terrestrial ectotherms do not have sufficient physiological tolerance to protect them from dangerously extreme operative temperatures. Consequently, terrestrial ectotherms in almost all localities must rely on behavioral adjustments to survive the warmest times of year (17, 23–25). Finally, we estimate Te in a range of microhabitats (e.g., full sun, shade, burrows) and show that behavioral shifts in microhabitat use can provide the refugia necessary (24).
Our findings force a reevaluation of latitudinal and elevational patterns of thermal danger, revealing that exposure to extreme heat can occur even at high elevations and latitudes (7) and giving insight into why heat-tolerance limits are relatively invariant in comparison with cold limits (22, 26, 27). Moreover, we uncover taxon-specific patterns in biophysically based thermal-safety margins and behavioral options necessary and sufficient to evade dangerous thermal environments.
Results
Extreme Air and Operative Temperatures.
Maximum air temperatures [highest monthly mean of daily maximum air temperature (Ta,max)] were nonlinear with latitude, increasing slightly toward midlatitudes (∼30°) and then decreasing toward the poles (top of gray region in Fig. 1A) (Table S1) (see individual data in Fig. S1). Maximum air temperatures also declined with elevation, and this rate of decline was similar at all latitudes (nonsignificant negative interaction between latitude and elevation) (Table S1 and Fig. 1 B–D). Estimated maximum hourly operative temperatures of dry-skinned ectotherms exposed to the full sun (Te,max) generally followed these same patterns with latitude and elevation (r between Te,max and Ta,max = 0.46 ± 0.10, 95% confidence interval) but exceeded maximum air temperatures by an average of 21.7 °C ± 6.1 SD (indicated by the difference between the top of the yellow region and the top of the gray region in Fig. 1). Te,max in the full sun changes little with elevation at low latitudes but shows a progressively steeper negative relationship with elevation at higher latitudes (Fig. 1 B–D and negative interaction in Table S1).
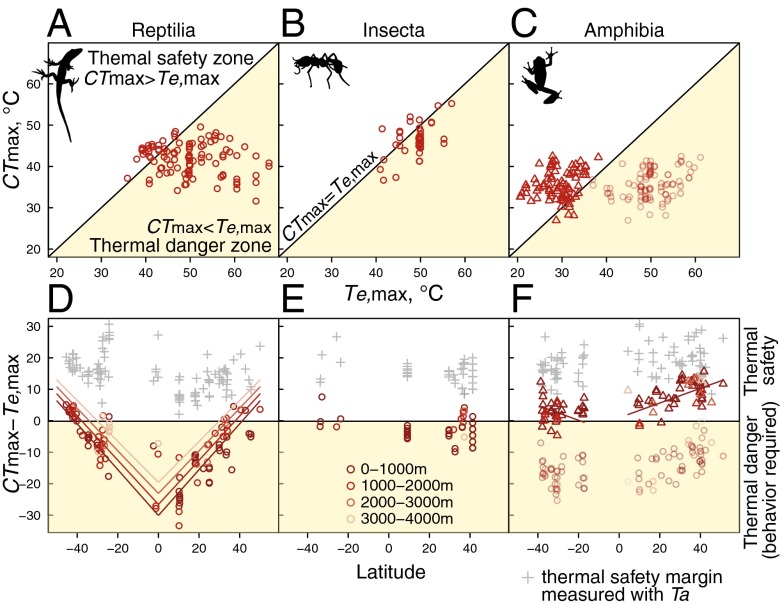
Fig. 1.
Maximum and minimum thermal-tolerance limits and range of annual extreme air and operative body temperatures as a function of latitude (A) and elevation (B–D). Warm and cool color points indicate upper and lower thermal-tolerance limits, respectively, after correcting for different acclimation temperatures. Lines indicate relationships from best-fit linear models of thermal tolerance, which take into account taxonomy and different metrics of cold tolerance. The gray region shows the range of hourly air temperatures across the year, and the light yellow region shows the range of extreme operative temperatures across the year, based on local regressions of lowland temperature data as a function of latitude (A) and on linear models of temperature as a function of latitude and elevation (B–D).
For hourly cold extremes, we found little difference between minimum air temperatures (Ta,min) and minimum operative temperatures (Te,min) of ectotherms (mean absolute difference of 1.19 °C ± 1.90 SD) (Fig. 1, bottom of gray and yellow regions). Both Ta,min and Te,min declined linearly with latitude and elevation (Fig. 1 and Table S1), and, for both thermal minimum measures, the rate of decline with elevation was marginally more shallow at higher latitudes (significant positive interaction) (Table S1).
Physiological Thermal-Tolerance Limits.
Physiological heat tolerance (CTmax) varied little with latitude but showed a slight dome-shaped relationship, peaking between 20° and 40° (Fig. 1 and Table 1) (the model with a quadratic term for latitude had a better fit than a linear relationship) (Table S2). CTmax declined slightly with elevation, and this relationship was consistent at all latitudes (no significant interaction between latitude and elevation) (Table 1 and Fig. 1). Upper thermal limits were on average higher than maximum air temperatures (by 12.2 °C ± 6.0 SD) but lower than maximum operative temperatures in full sun (by 9.3 °C ± 8.2 SD) (Fig. 1) (see Fig. S2 for equivalent relationships within major taxonomic groups).
Table 1.
Best-fit model results of CTmax and CTmin as a function of latitude and elevation
| Fixed effect | coefficient | SE | t-value | p-value |
| CTmax | ||||
| intercept | 39.26 | 3.08 | 12.75 | <0.0001 |
| absolute latitude | 0.318 | 0.077 | 4.13 | 0.0001 |
| elevation (km) | −1.02 | 0.45 | −2.26 | 0.0253 |
| absolute latitude2 | −0.0069 | 0.0014 | −5.02 | <0.0001 |
| latitude:elevation | −0.01 | 0.02 | −0.90 | 0.369 |
| CTmin | ||||
| intercept | 14.04 | 1.70 | 8.24 | <0.0001 |
| absolute latitude | −0.41 | 0.04 | −11.0 | <0.0001 |
| elevation (km) | −4.22 | 0.58 | −4.56 | <0.0001 |
| latitude:elevation | 0.052 | 0.020 | 2.55 | 0.0124 |
| cold limit metric (lethal) | −2.78 | 1.02 | −2.72 | 0.0076 |
A quadratic term for absolute latitude is included in the CTmax model based on Akaike Information Criterion (AIC) results (Table S2). For CTmin, a cold-tolerance limit metric is included to account for possible differences between critical thermal limits (base level) and lethal thermal limits (contrast shown). Model results are for thermal limits corrected for acclimation temperature; see Table S3 for model results on uncorrected data.
Cold-tolerance (CTmin) limits declined linearly with both latitude and elevation (Table 1 and Fig. 1) (see Fig. S2 for patterns within major taxonomic groups). On average, cold-tolerance limits were slightly lower than both the average lowest air temperatures and operative body temperatures although variation was considerable among species (Fig. 1).
Thermal Safety Versus Danger.
We directly compared thermal-tolerance limits (CTmax, CTmin) to extreme exposed operative temperatures (Te,max, Te,min) at each site of collection, to determine whether animals in open habitats have positive thermal-safety margins (i.e., CTmax > Te,max and CTmin < Te,min) or lack sufficient thermal tolerance to cope with extreme temperatures (CTmax < Te,max or CTmin > Te,min, “thermal danger”) (Figs. 2 and 3). Species with negative thermal-safety margins (thermal danger) must rely on access to refugia to survive during seasonal and daily extremes.
Fig. 2.
(A–C) Phase diagrams of heat-tolerance limits and maximum operative body temperature for reptiles, insects, and amphibians. The white region shows where species have a physiological thermal-safety margin even in open habitats, and the light yellow region shows where species are dependent on behavior or microhabitats to avoid maximum operative temperatures in open habitats. (D–F) Warm thermal-safety margins as a function of latitude based on maximum exposed operative temperatures (CTmax - Te,max; circles). Colors indicate elevation, and lines show best-fit regressions from linear models that had a slope significantly different from zero. Gray crosses indicate thermal-safety margins based on maximum air temperature (CTmax – Ta,max). Positive values indicate physiological thermal safety whereas negative values represent thermal danger and reliance on cooling habitats and behaviors.
Fig. 3.
(A–C) Phase diagrams of cold-tolerance limits and maximum operative body temperature for reptiles, insects, and amphibians. The white region shows where species have a physiological thermal-safety margin even in the coldest operative temperatures whereas the gray region shows where species are dependent on behavior or microhabitat use to avoid minimum operative temperatures in open habitats. (D–F) Cold thermal-safety margins as a function of latitude based on minimum exposed operative temperatures (Te,min − CTmin; circles). Positive values indicate physiological thermal safety whereas negative values represent thermal danger and reliance on cold-buffering habitats and behaviors. Colors indicate elevation, and lines show best-fit regression from linear models that were significantly different from zero.
Three major taxa reveal fundamentally different patterns. Most reptiles (84%) have heat-tolerance limits that are lower than the highest operative temperatures in the sun (negative thermal-safety margins) and thus must use behavioral thermoregulation to avoid heat death in the warmest times (Fig. 2A). This behavioral necessity is greatest both in the tropical lowlands and at midlatitude lowlands, where Te,max is much higher than CTmax (i.e., highest thermal danger) (Fig. 2D). This necessity for seeking cooler microhabitats—at least at midday in the warmest season—decreases with increasing latitude and elevation (Fig. 2D and Table S3).
In marked contrast to reptiles, insects have physiological heat-tolerance limits that fall near maximum operative temperatures (Fig. 2B). Moreover, neither latitude nor elevation had any effect on the degree of offset from the 1:1 relationship between CTmax and Te,max (Fig. 2E and Table S3).
Because most amphibians maintain wet skin when active, evaporative cooling guarantees that wet-skinned Te,max will be lower than dry-skinned Te,max at the same site (13). To demonstrate this cooling effect, we compared operative body temperatures for both dry- and wet-skinned conditions (Fig. 2C). Most amphibians have CTmax greater than wet-skin Te,max (Fig. 2C), but lower than dry-skin Te,max. Thus, wet-skinned amphibians maintain positive thermal-safety margins, which increase slightly with latitude but not with elevation (Fig. 2F and Table S3). Of course, without access to environmental water, amphibians quickly dry and experience thermal danger (13).
Behavioral Options for Avoiding Thermal Danger.
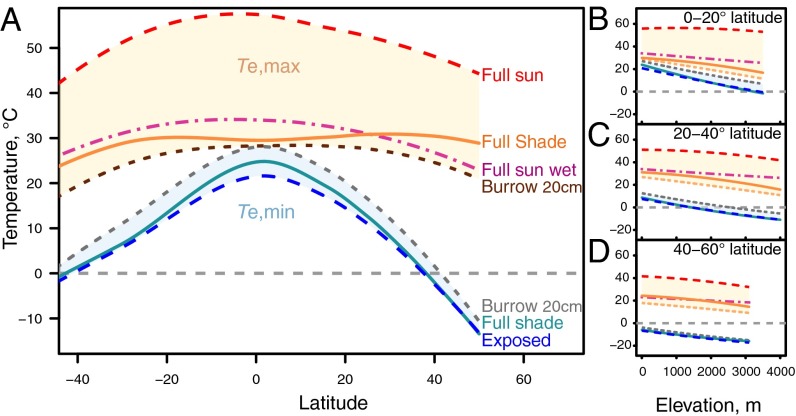
When the range of operative temperatures among potential microhabitats at a given site is considered, behavioral options for avoiding heat stress in the open become evident. An ectotherm that positions itself in full shade at any latitude and elevation (at 2 m height) would have an equilibrated body temperature close to air temperature, and therefore a much cooler Te,max than in the full sun (Fig. 4) (see Fig. S1 for individual Te estimates). Taking refuge underground allows a similar cooling effect on Te,max but a warming effect on Te,min (Fig. 4) (see Fig. S3 for Te patterns at multiple burrowing depths). Te,max and Te,min at a given burrowing depth both decline with latitude (Fig. 4); therefore, species in the tropics must burrow to a greater depth than those at higher latitudes to achieve the same body temperature at midday in the summer, and those at high latitudes must burrow to a greater depth to achieve the same body temperature at night in the winter (Fig. S3). Evaporation provides a physiological cooling mechanism (13): Wet-skin Te,max, even in the full sun, allows almost as much reduction in Te,max as shade-use (Fig. 4), compensating almost entirely for radiative heat gain as long as hydration is possible (18).
Fig. 4.
Operative body temperatures under various strategies of microhabitat use. (A) Operative temperature as a function of latitude. Lines show local regressions of lowland temperature data as a function latitude (A) and linear models of temperature as a function of latitude and elevation (B–D) (see Fig. S1 for Te estimates by location).
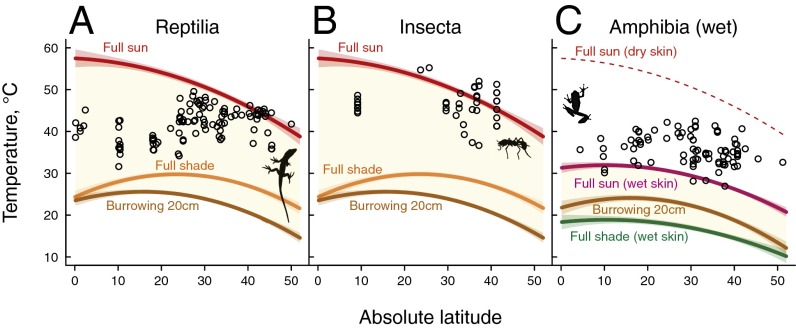
Directly comparing operative temperatures with thermal tolerance limits reveals that shaded habitats or burrows can provide necessary refugia from extreme heat for all taxa in our dataset (Fig. 5). For amphibians, if evaporative cooling is unavailable, shade use and burrows can also compensate (Fig. 5C). To reduce cold exposure, burrowing offers one potential behavioral retreat (Fig. S4).
Fig. 5.
Microhabitat use strategies available to ectotherms for maintaining operative body temperatures within tolerable heat limits. Curves bounding the light yellow region show operative body temperatures in full sun, shade, and burrowing to 20 cm, as a function of latitude (at a fixed mean elevation of 800 m), based on linear models (Table S1). CTmax (black points) are within the range of maximum operative body temperatures (light yellow region) for most reptiles (A) and insects (B), indicating the necessity for microhabitat use. For amphibians (C), CTmax exceeds the range of maximum operative temperatures if skin is wet (yellow region) but not if skin is dry (dotted red line).
Discussion
Heat-tolerance limits of terrestrial ectotherms vary little with latitude whereas cold-tolerance limits decline steadily with increasing latitude (4, 22, 26, 28–30). Here, we show that this pattern is mirrored globally across elevational gradients (as shown regionally in ref. 31). By comparing these physiological limits to operative body temperatures, we determined that most ectotherms are incapable of surviving in open habitats through physiological thermal tolerance alone and thus must have access to thermal refugia to survive. This requirement is greatest in the tropics, empirically corroborating the theoretical findings of Kearney et al. (21), who used generic thermal-tolerance limits and Te to show that behavioral avoidance of high body temperatures is necessary in the tropics. We build upon this work by analyzing empirical physiological data (CTmax, CTmin), providing species-specific estimates of both warm and cold thermal-safety margins across latitude and elevation and discussing the implications for species’ vulnerabilities to climate warming and extreme events.
Most prior studies of thermal-safety margins, which compared upper thermal limits with air temperatures (maxima or means), found that the majority of species have positive warm safety margins, except for some tropical species or midlatitude desert species (3–6). This pattern suggested that high-latitude species can physiologically tolerate future warming, at least for a while. However, when thermal-safety margins for open habitats are appropriately recalculated using maximum operative temperatures, which average 22 °C warmer than maximum air temperatures, most safety margins switch to negative. Thus, using maximum air temperature (gray crosses in Fig. 2 D–F) overestimates true thermal-safety margins and underestimates the importance of behavioral thermoregulation to cope with both contemporary and future conditions.
Thermal-Safety Margins by Taxon, Latitude, and Elevation.
Our findings suggest that reptiles, insects, and amphibians rely differently on cool microhabitats to avoid maximum operative temperatures. To avoid heat death, most reptiles must move to shaded habitats or burrows during the warmest hours of the year. This requirement is critical for species in tropical lowlands but less so for species living at higher latitudes and elevations. Previous observations of latitudinal differences in habitat use and empirical body temperatures in reptiles confirm this pattern in nature (4, 15, 21, 32) (see range of empirical body temperatures as a function of latitude in Fig. S5). Also, reptiles at higher elevations can tolerate more time in open sunny habitats than their lowland counterparts.
Our results suggest that variation in behavior and habitat use among reptiles has been more important than variation in heat tolerance in allowing species to exploit different climates. This pattern is illustrated in Fig. 2, which shows that variation in maximum operative temperatures in reptiles is not at all matched by parallel variation in upper thermal limits (observe the deviation from the 1:1 line in Fig. 2A). Therefore, for reptiles, thermoregulatory behavior has either buffered selection upon heat tolerance (4, 19–21, 29) or compensated for an invariability or “hard upper bound” in heat tolerance (27, 33).
Insects show a different pattern. They generally have higher CTmax than do reptiles (+3.6 °C on average), and their upper thermal-tolerance limits generally match maximum operative temperatures. This pattern implies that insects are less likely than reptiles to rely on behavior to avoid exposure to high temperatures. We caution that body size and shape were not varied from a 5-g lizard in our simulations of Te for insects, and this discrepancy influences Te values in complex ways (34). First, smaller size means a greater relative influence of convective heat over direct solar radiation on Te (34); thus, insect Te,max may be lower than our models indicate. However, small insects may experience a greater influence of radiative heat from the substrate if they are located deeper in the boundary layer; thus, they could have warmer Te,max than in our models (35). Smaller-bodied insects will also equilibrate faster to the steady-state Te estimated in our models, which would decrease the effectiveness of shuttling behavior to moderate body temperature (34). However, insects may also have greater access to small patches of shade than larger animals, such as cool microenvironments in crevices or the undersides of leaves where transpirational cooling may further reduce temperatures (36).
Amphibians show an altogether different pattern because evaporative cooling from their wet skin offsets radiative heat gain: In fact, Te of an evaporating amphibian, even in sun, is equivalent to that of a dry-skinned animal in shade (13). Upper thermal-safety margins of wet-skinned amphibians are lowest toward the tropics, as previously observed in studies using air temperatures (3), suggesting that tropical and subtropical amphibians will be more sensitive than amphibians at higher latitudes to given increases in temperature. Although evaporative cooling may theoretically liberate hydrated amphibians from needing access to shade or burrows during the hottest summer temperatures, amphibians nevertheless require access to wet or moist habitats to rehydrate. Thus, water balance and temperature are not easily dissociated in this group, and safety margins of amphibians are dependent on hydration. Our estimates of sensitivity to warming based on site-specific thermal-safety margins among amphibians gives a very different estimate of warming vulnerability than approaches using species-level variables such as climate occupancy or habitat specialization (2).
Are Species’ Heat Tolerances Maxed-Out?
For the species in thermal danger, sensitivity to new temperature extremes associated with climate warming will be mostly contingent on the availability of cool microhabitats, species’ ability to modify their behavior (such as switching habitats or timing of activity), and the fitness costs associated with these changes, such as narrower foraging windows or increased predation risk. These abilities and their costs are likely to vary with habitat (4, 21, 32, 37). For example, reptiles and insects that require access to shaded habitats and burrows likely have the behavioral plasticity to increase the time spent in these habitats as the climate warms although they must have the energetic stores and sources to offset any costs of spending more time in such habitats (38). We have shown that tropical lowland reptiles are already more dependent on finding cool microhabitats than are most reptile species at higher latitudes and elevations and that some tropical species have heat-tolerance limits that are only slightly above the coolest Te possible even in full shade. This finding reinforces the suggestion (4) that tropical-forest reptiles may be highly vulnerable to climate warming. However, for all taxa at any latitude, the ability to increase exploitation of habitats that protect from radiative heat may be limited, even if such habitats are available. Indeed, Sinervo et al. (38) suggested that Mexican lizards (Sceloporus spp.) forced to restrict activity by more than 4 h per day also have restricted net energy gain and are thus vulnerable to local extinction.
Cold Tolerance.
Our findings also highlight the importance of behavior in extreme cold conditions at high latitude and elevation. In the lowland tropics, most taxa can physiologically tolerate lower temperatures than they currently face. This safety margin may perhaps be a selective legacy of the Last Glacial Maximum (LGM), which ended only 20 ka ago, during which temperatures in the lowland tropics averaged 5 °C cooler than present (39), a mean temperature currently characteristic of 30° N or S latitude (40). Alternatively, some tropical lowland lineages may have perished in the LGM (or earlier glacial maxima) (40) and were later replaced by lineages from subtropical latitudes that retain their tolerance to cooler temperatures. Both options suggest conservatism of excess cold tolerance.
In contrast, cold-tolerance limits for about half of the higher latitude species in our dataset lie above minimum exposed operative temperatures, implying that such species will need to find protected retreats. For example, burrowing to 200 cm should buffer extreme cold temperatures at all locations (Fig. S4) although most animals wouldn't have to burrow that deep. Access to suitable winter retreats will become even more critical if environmental change reduces winter snowpacks, leading to a decline in soil temperature (41). Physiological acclimation (cold hardening) may provide complementary protection, especially among insects (26, 42, 43).
Conservation Implications.
We have shown that the vast majority of terrestrial ectotherms are currently living at or beyond the limits of their physiological tolerance and thus do not have unrestricted movement in full sun during the hottest times of the year. This finding has two implications, one for vulnerability prediction and another for conservation.
There is limited geographic variation in CTmax (Fig. 5) (27, 44) and in physiologically optimal temperatures (4)—especially in comparison with the variation in Te. This implies that predictions of ectotherm vulnerability that are based on the environmental temperatures of their present distributions (2), or even on comparisons of physiological limits to environmental temperatures (3, 45), likely miss the full story. More accurate species-level predictions may be possible by incorporating opportunities for behavioral thermoregulation (46) as well as estimating the energetic consequences of extended thermal retreats (10, 38).
As the world warms, most ectotherms will rely increasingly on behavioral thermoregulation and the availability of cool habitats to avoid heat stress. This prediction leads to specific conservation recommendations that reduce organisms’ exposure to thermal danger (47); specifically, high priority must be given to protecting habitats and migration corridors (e.g., forests) that provide shade, especially in the lowland tropics where many species already rely on such habitats.
Methods
Thermal-Tolerance Data.
We expanded a previously collated dataset by Sunday et al. (22) of paired upper and lower tolerance by adding studies that reported only an upper or lower tolerance estimate, thus increasing our sample of latitudes and elevations. We restricted our new database to studies that reported preexperimental acclimation or collection temperatures (n = 300). For upper thermal limits, we compiled critical maximum temperature (CTmax), the ramping temperature at which motor function is lost (48). To estimate lower thermal limits, which are measured less often than is CTmax, we included data for both CTmin (the declining temperature at which individuals lose critical motor function) and lethal temperature [a fixed temperature at which a given percentage (usually 50%) of individuals survive a predetermined duration of exposure (usually 24 h)]. CTmin were available for most reptile studies and about half of the insect studies whereas most amphibian studies used lower lethal limits (Fig. S2 and Dataset S1). To account for differences between these metrics, we incorporated experimental method (critical vs. lethal limits) as a factor in our statistical analyses.
Acclimation Correction.
Many studies used somewhat arbitrary and varying acclimation temperatures that are far from seasonal extremes (Fig. S6). To facilitate comparison of thermal limits with respect to seasonal extremes, we used the relationships between acclimation temperature and thermal limits within our dataset (using separate models for CTmax and CTmin) to adjust observed thermal limits to those expected for a seasonally more appropriate acclimation temperature (see SI Methods for details). Importantly, model results using acclimation-corrected CTmax and CTmin were quantitatively similar to those in which raw CTmax and CTmin were used and acclimation temperature was included as a fixed effect (Table S4). We show results using acclimation-corrected CTmax and CTmin as they more clearly illustrate relations among thermal tolerance, latitude, and elevation.
Operative Body Temperatures and Thermal-Safety Margins.
For each species, we used the biophysical modeling software “Niche Mapper” (21), monthly means of daily maximum and minimum temperatures, and other climate variables to simulate Te of nonthermoregulating, 5-g lizard-shaped objects with 90% solar absorptivity with a midpoint 1 cm above the ground. We simulated Te in open habitats, in full shade cover at 1cm above the surface, at fixed positions in the soil profile to a depth of 200 cm, and for both dry and wet skin. We extracted the maximum and minimum hourly Te across months. See SI Methods for expanded methods.
To quantify the direction and scale of mismatches between thermal-tolerance limits and extreme Te (thermal-safety margins), we calculated the difference between upper thermal limits and maximum operative temperatures (CTmax − Te,max) and the difference between minimum Te and lower thermal limits (Te,min − CTmin). Positive values indicate that the tolerance limit of a species exceeds the warmest (or coldest) Te it will encounter at its collection site, such that the organism has a physiological safety margin (3). Negative values indicate that the animal must sometimes avoid the warmest (or coldest) microhabitats or risk overheating (or freezing). For reptiles and insects, we analyzed dry-skinned estimates of Te, but, for amphibians, we used both dry- and wet-skinned estimates of Te.
Analysis.
Air and operative temperatures in relation to latitude and elevation.
We fitted linear models for each type of estimated Te (dry skin open; dry skin full shade; dry skin burrowing; wet skin open; wet skin full shade; wet skin burrowing) as a function of latitude and elevation across all collection sites. We included an interaction term between latitude and elevation to test whether the rate at which Te declines with elevation differs across latitude (49), and quadratic terms to allow for nonlinear relationships between Te and both latitude and elevation. To visualize nonlinear patterns of Ta and Te with latitude, we fitted local regression (loess) curves using default settings (2 degrees and alpha = 0.75) in R v. 2.15.2 (50) (see Fig. S1 for individual data across latitude and elevation).
Thermal-tolerance limits and safety margins with latitude and elevation.
We analyzed linear mixed-effects models fitted using maximum likelihood to evaluate patterns in thermal-tolerance limits and thermal-safety margins as a function of latitude, elevation, and their interaction. In all models, we included a hierarchical random effect of taxonomy to account for the nonrandom sampling structure across taxonomic groups. For models of thermal-tolerance limits, we compared models with and without the quadratic terms for latitude and elevation and reported that with the lowest Akaike Information Criterion. For cold tolerances, we included a factor for the thermal limit metric used (CTmin or lethal). For thermal-safety margins, we fit models separately for each ectotherm class based on the observation from our data that patterns differed.
Supplementary Material
Acknowledgments
We thank the members of the Earth to Ocean research group at Simon Fraser University for feedback and discussions. J.M.S. was funded by the Natural Sciences and Engineering Research Council of Canada and the University of British Columbia Biodiversity Research Centre. N.K.D. was funded by the Natural Sciences and Engineering Research Council of Canada and by the Canada Research Chairs program. The US National Science Foundation provided funding to R.K.C. (DEB 0639979 and DBI 0851245), J.T.L. (DEB-1157383; Project LLAMA), and R.B.H. (1038016). M.R.K. was funded by an Australian Research Fellowship (DP110102813) from the Australian Research Council. Thanks also to G. Quinn, who allocated salary to A.E.B. for contribution to this study.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 5450.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316145111/-/DCSupplemental.
References
- 1.Williams SE, Shoo LP, Isaac JL, Hoffmann AA, Langham G. Towards an integrated framework for assessing the vulnerability of species to climate change. PLoS Biol. 2008;6(12):2621–2626. doi: 10.1371/journal.pbio.0060325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foden WB, et al. Identifying the world’s most climate change vulnerable species: A systematic trait-based assessment of all birds, amphibians and corals. PLoS ONE. 2013;8(6):e65427. doi: 10.1371/journal.pone.0065427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deutsch CA, et al. Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA. 2008;105(18):6668–6672. doi: 10.1073/pnas.0709472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huey RB, et al. Why tropical forest lizards are vulnerable to climate warming. Proc R Soc Lond Ser B Biol Sci. 2009;276:1939–1948. doi: 10.1098/rspb.2008.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clusella-Trullas S, Blackburn TM, Chown SL. Climatic predictors of temperature performance curve parameters in ectotherms imply complex responses to climate change. Am Nat. 2011;177(6):738–751. doi: 10.1086/660021. [DOI] [PubMed] [Google Scholar]
- 6.Diamond SE, et al. Who likes it hot? A global analysis of the climatic, ecological, and evolutionary determinants of warming tolerance in ants. Glob Change Biol. 2013;18:448–456. [Google Scholar]
- 7.Buckley LB, Miller EF, Kingsolver JG. Ectotherm thermal stress and specialization across altitude and latitude. Integr Comp Biol. 2013;53(4):571–581. doi: 10.1093/icb/ict026. [DOI] [PubMed] [Google Scholar]
- 8.IPCC 2007. Climate Change 2007: The Physical Science Basis. Working Group 1 Contribution to the Fourth Assessment Report of the IPCC (Cambridge Univ Press, Cambridge, UK)
- 9.Porter WP, Mitchell JW, Beckman WA, DeWitt CB. Behavioral implications of mechanistic ecology: Thermal and behavioral modeling of desert ectotherms and their microenvironment. Oecol Aquat. 1973;13:1–54. doi: 10.1007/BF00379617. [DOI] [PubMed] [Google Scholar]
- 10.Buckley LB, Kingsolver JG. The demographic impacts of shifts in climate means and extremes on alpine butterflies. Funct Ecol. 2012;26:969–977. [Google Scholar]
- 11.Parmesan C, Root TL, Willig MR. Impacts of extreme weather and climate on terrestrial biota. Bull Am Meteorol Soc. 2000;81:443–450. [Google Scholar]
- 12.Hoffmann AA. Physiological climatic limits in Drosophila: Patterns and implications. J Exp Biol. 2010;213(6):870–880. doi: 10.1242/jeb.037630. [DOI] [PubMed] [Google Scholar]
- 13.Tracy CR. Model of dynamic exchanges of water and energy between a terrestrial amphibian and its environment. Ecol Monogr. 1976;46:293–326. [Google Scholar]
- 14.Bakken GS. Measurement and application of operative and standard operative temperatures in ecology. Integr Comp Biol. 1992;32:194–216. [Google Scholar]
- 15.Meiri S, et al. Are lizards feeling the heat? A tale of ecology and evolution under two temperatures. Glob Ecol Biogeogr. 2013;22:834–845. [Google Scholar]
- 16.Pearson OP. Habits of the lizard Liolaemus multiformis multiformis at high altitudes in southern Peru. Copeia. 1954;1954:111–116. [Google Scholar]
- 17.Kearney M, Porter W. Mechanistic niche modelling: Combining physiological and spatial data to predict species’ ranges. Ecol Lett. 2009;12(4):334–350. doi: 10.1111/j.1461-0248.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- 18.Bakken GS, Angilletta MJ. How to avoid errors when quantifying thermal environments. Funct Ecol. 2013;28(1):96–107. [Google Scholar]
- 19.Huey RB, Hertz PE, Sinervo B. Behavioral drive versus behavioral inertia in evolution: A null model approach. Am Nat. 2003;161(3):357–366. doi: 10.1086/346135. [DOI] [PubMed] [Google Scholar]
- 20.Bogert CM. Thermoregulation in reptiles: A factor in evolution. Evolution. 1949;3(3):195–211. doi: 10.1111/j.1558-5646.1949.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 21.Kearney M, Shine R, Porter WP. The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming. Proc Natl Acad Sci USA. 2009;106(10):3835–3840. doi: 10.1073/pnas.0808913106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sunday JM, Bates AE, Dulvy NK. Global analysis of thermal tolerance and latitude in ectotherms. Proc Biol Sci. 2011;278(1713):1823–1830. doi: 10.1098/rspb.2010.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grant BW, Dunham AE. Thermally imposed time constraints on the activity of the desert lizard Sceloporus merriami. Ecology. 1988;69:167–176. [Google Scholar]
- 24.Adolph SC. Influence of behavioral thermoregulation on microhabitat use by 2 Sceloporus lizards. Ecology. 1990;71:315–327. [Google Scholar]
- 25.Sears M, Angilletta M. Evaluating the costs of thermoregulation: Simulating animal movements through spatially-structured environments define cost curves for small lizards. Integr Comp Biol. 2007;47:E228. [Google Scholar]
- 26.Addo-Bediako A, Chown SL, Gaston KJ. Thermal tolerance, climatic variability and latitude. Proc R Soc Lond, Ser B Biol Sci. 2000;267:739–745. doi: 10.1098/rspb.2000.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Araújo MB, et al. Heat freezes niche evolution. Ecol Lett. 2013;16(9):1206–1219. doi: 10.1111/ele.12155. [DOI] [PubMed] [Google Scholar]
- 28.Snyder GK, Weathers WW. Temperature adaptations in amphibians. Am Nat. 1975;109:93–101. [Google Scholar]
- 29.van Berkum FH. Latitudinal patterns of the thermal sensitivity of sprint speed in lizards. Am Nat. 1988;32(3):327–343. [Google Scholar]
- 30.Cruz FB, Fitzgerald LA, Espinoza RE, Schulte JA. The importance of phylogenetic scale in tests of Bergmann's and Rapoport's rules: Lessons from a clade of South American lizards. J Evol Bio. 2005 doi: 10.1111/j.1420-9101.2005.00936.x. 18:1559%1574. [DOI] [PubMed] [Google Scholar]
- 31.Gaston KJ, Chown SL. Elevation and climatic tolerance: A test using dung beetles. Oikos. 1999;86:584–590. [Google Scholar]
- 32.Ruibal R. Thermal relations of five species of tropical lizards. Evolution. 1961;15:98–111. [Google Scholar]
- 33.Hamilton WJ. Life's Color Code. New York: McGraw Hill; 1973. [Google Scholar]
- 34.Stevenson RD. The relative importance of behavioral and physiological adjustments controlling body temperature in terrestrial ectotherms. Am Nat. 1985;126(3):362–386. [Google Scholar]
- 35.Willmer PG, Unwin DM. Field analyses of insect heat budgets: Reflectance, size and heating rates. Oecologia. 1981;50:250–255. doi: 10.1007/BF00348047. [DOI] [PubMed] [Google Scholar]
- 36.Pincebourde S, Woods HA. Climate uncertainty on leaf surfaces: The biophysics of leaf microclimates and their consequences for leaf-dwelling organisms. Funct Ecol. 2012;26:844–853. [Google Scholar]
- 37.Huey RB, Slatkin M. Cost and benefits of lizard thermoregulation. Q Rev Biol. 1976;51(3):363–384. doi: 10.1086/409470. [DOI] [PubMed] [Google Scholar]
- 38.Sinervo B, et al. Erosion of lizard diversity by climate change and altered thermal niches. Science. 2010;328(5980):894–899. doi: 10.1126/science.1184695. [DOI] [PubMed] [Google Scholar]
- 39.Bush MB, Silman MR, Urrego DH. 48,000 years of climate and forest change in a biodiversity hot spot. Science. 2004;303(5659):827–829. doi: 10.1126/science.1090795. [DOI] [PubMed] [Google Scholar]
- 40.Colwell RK, Brehm G, Cardelús CL, Gilman AC, Longino JT. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science. 2008;322(5899):258–261. doi: 10.1126/science.1162547. [DOI] [PubMed] [Google Scholar]
- 41.Groffman PM, et al. Colder soils in a warmer world: A snow manipulation study in a northern hardwood forest ecosystem. Biogeochemistry. 2001;56:135–150. [Google Scholar]
- 42.Sinclair BJ. Insect cold tolerance: How many kinds of frozen? Eur J Entomol. 1999;96:157–164. [Google Scholar]
- 43.Bale JS. Insects and low temperatures: From molecular biology to distributions and abundance. Philos Trans R Soc Lond B Biol Sci. 2002;357(1423):849–862. doi: 10.1098/rstb.2002.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grigg JW, Buckley LB. Conservatism of lizard thermal tolerances and body temperatures across evolutionary history and geography. Biol Lett. 2013;9(2):20121056. doi: 10.1098/rsbl.2012.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sunday JM, Bates AE, Dulvy NK. Thermal tolerance and the global redistribution of animals. Nat Clim Change. 2012;2:686–690. [Google Scholar]
- 46.Huey RB, et al. Predicting organismal vulnerability to climate warming: Roles of behaviour, physiology and adaptation. Philos Trans R Soc Lond B Biol Sci. 2012;367(1596):1665–1679. doi: 10.1098/rstb.2012.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dawson TP, Jackson ST, House JI, Prentice IC, Mace GM. Beyond predictions: Biodiversity conservation in a changing climate. Science. 2011;332(6025):53–58. doi: 10.1126/science.1200303. [DOI] [PubMed] [Google Scholar]
- 48.Lutterschmidt WI, Hutchison VH. The critical thermal maximum: History and critique. Can. J. Zool. Rev. Can. Zool. 1997;75:1561–1574. [Google Scholar]
- 49.Dillon ME, Wang G, Huey RB. Global metabolic impacts of recent climate warming. Nature. 2010;467(7316):704–706. doi: 10.1038/nature09407. [DOI] [PubMed] [Google Scholar]
- 50.R Core Team 2009. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.