Significance
Stem-cell microenvironment has been identified as an important modulator of plasticity, self-renewal, and differentiation. This work details the development of a hydrogel system tailored to promote human pluripotent stem cell (HPSC) self-renewal with a simple chemical microenvironmental switch to direct differentiation. Furthermore, the timing of switching post hydrogel fabrication can promote specific lineage differentiation as in vivo. This system highlights the role of microenvironment on fate choices of pluripotent cells and demonstrates that it may be tailored to control differentiation in vitro. Importantly, this approach may improve the generation of fully differentiated tissues, as demonstrated for cardiogenic differentiation. Our combination of hydrogels allows dense tissue structures to be produced from HPSCs by using a single-step process inaccessible to any current methodology.
Keywords: human embryonic stem cells, cardiomyocyte
Abstract
The ability of materials to define the architecture and microenvironment experienced by cells provides new opportunities to direct the fate of human pluripotent stem cells (HPSCs) [Robinton DA, Daley GQ (2012) Nature 481(7381):295–305]. However, the conditions required for self-renewal vs. differentiation of HPSCs are different, and a single system that efficiently achieves both outcomes is not available [Giobbe GG, et al. (2012) Biotechnol Bioeng 109(12):3119–3132]. We have addressed this dual need by developing a hydrogel-based material that uses ionic de-cross-linking to remove a self-renewal permissive hydrogel (alginate) and switch to a differentiation-permissive microenvironment (collagen). Adjusting the timing of this switch can preferentially steer the HPSC differentiation to mimic lineage commitment during gastrulation to ectoderm (early switch) or mesoderm/endoderm (late switch). As an exemplar differentiated cell type, we showed that directing early lineage specification using this single system can promote cardiogenesis with increased gene expression in high-density cell populations. This work will facilitate regenerative medicine by allowing in situ HPSC expansion to be coupled with early lineage specification within defined tissue geometries.
Human pluripotent stem cells (HPSCs) comprise human embryonic stem cells (HESCs) and human induced pluripotent stem cells (1). The ability to couple expansion and differentiation of these cells underpins current efforts in regenerative medicine (2, 3). Initial efforts to direct the fate of HPSCs by recapitulating the developmental process of gastrulation [by using spontaneous differentiation of embryoid bodies (EBs)] have been refined to allow directed differentiation in two and three dimensions (3D) (4). This process includes coupling bioreactor expansion of HPSCs in 3D aggregates with differentiation to neural lineages (5). The differentiated cells from these processes can be harvested and then used to seed geometrically complex scaffolds. However, this two-stage process could be better controlled and streamlined by in situ HPSC expansion and differentiation within a single template. Furthermore, in situ tissue development more closely recapitulates embryogenesis (6) and could produce tissue with authentic cellular complexity and physiology (7).
To date, natural (8, 9) and synthetic (10) materials have been developed to retain the self-renewal phenotype of HPSCs. We (11) and others (12) have shown that hydrogel systems can instruct cell behavior by providing cell-adhesive or nonadhesive microenvironments. Extracellular matrix (ECM) hydrogels, such as collagen, have fibrous microstructures (13) and are suitable for cell adhesion, growth, and migration (14). This characteristic is unlike hydrogels such as alginate, which are nonadhesive and nanoporous and prevent migration. Collagen (type I) is cross-linked by neutralizing acidity and leads to fibril formation, whereas alginate gels are formed or disaggregated by regulating divalent cation availability—usually Ca2+ in the form of CaCl2 (15).
Importantly, HPSC self-renewal and triggering of gastrulation-like differentiation requires different culture and microenvironmental conditions (2). Therefore, the development of hydrogel systems that allow the modification of the structural and adhesive microenvironment after the initial cross-linking would be ideal to control cell behavior (16–18). A previous study explored this concept and used alginate switching from cross-linked to un-cross-linked states to demonstrate nonadhesive-to-adhesive tailoring of the microenvironment in the presence of somatic cell lines. This switch affected attributes such as rate of solute transport, gel mechanics, cell adhesion, morphology, and migration (12). Here we describe the development of a system that can direct HPSC fate from self-renewal to differentiation by using alginate cross-linked/de-cross-linked state as a microenvironmental switch (Fig. 1A).
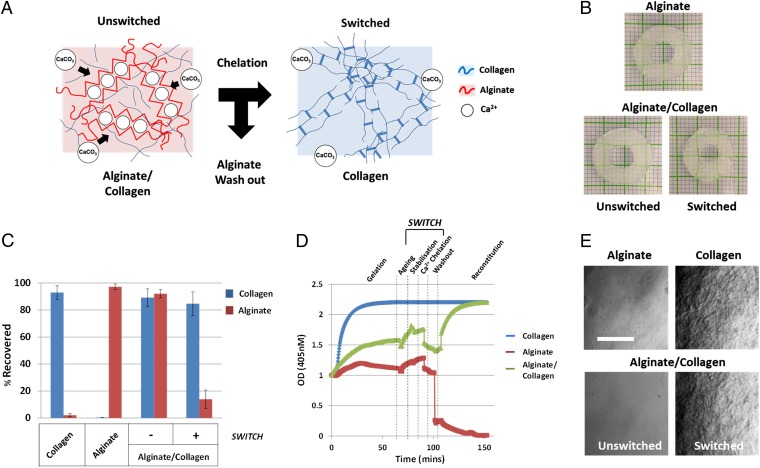
Fig. 1.
(A) Alginate serves as structural modulator to prevent the adhesive and fibrous network created by collagen. Upon switching (chelation of Ca2+ ions), alginate is removed, and collagen fibers are generated, forming an adhesive microenvironment. (B) The 3D-printed gels retain geometry when switched by chelation (grid: 1 mm). (C) Quantitation of alginate and collagen before and after switching (n = 3). (D) Spectroscopy measuring collagen fiber character during cross-linking and switching of hydrogels. Alginate prevents complete collagen network formation until chelation and washout (n = 6). (E) Confocal images of collagen fibers with transmission imaging. Collagen fiber formation is inhibited until alginate is removed. (Bar: 20 µm.)
Results
Creation of a Switching Hydrogel System.
We optimized gelation of our hydrogel system to aid fabrication into complex geometries. Using CaCO3 and d-glucono-δ-lactone (GDL) (19) we created a delayed setting formulation for alginate. CaCO3 and GDL addition allows alginate-containing suspensions, with or without cells, to be molded into complex geometric shapes before gelation. Using these approaches, we produced free-standing, uniform, and transparent cross-linked alginate gels within 5–10 min and complete cross-linking within 30 min at 37 °C [1.2% (wt/vol) alginate cross-linked with 34 mM CaCO3 and 42 mM GDL]. We confirmed complete and uniform cross-linking of alginate by dry/wet and rehydrated/wet weight comparisons of intact gels (Table S1) or gel slices (Fig. S1).
By combining collagen into this slow-setting alginate gel, we were able to construct combined matrices (alginate/collagen) that had the same gelation properties as alginate-only gels (Table S1 and Fig. S1). We demonstrated that these combined hydrogels could be de-cross-linked and the alginate component could be efficiently removed—a process we term “switching.” Switching converts the environment from alginate-dominated to collagen-dominated (Fig. 1A) and relies on Ca2+ ion chelation by using EDTA/sodium citrate-based treatment (12, 20). The material properties of this hydrogel system also allowed application in bioprinting technologies, which retained geometry after switching (Fig. 1B).
We determined that the switching process resulted in >85% removal of alginate but >80% retention of collagen in combined hydrogels (Fig. 1C). We also observed (Fig.1D) (21) that collagen fibril formation only occurred after alginate removal was complete, even when several days after fabrication (Fig. S2). Efficient removal of alginate was confirmed by 488-nm confocal microscopy (Fig. 1E) and environmental SEM (E-SEM) (Fig. S3). Alginate removal occurred with <10 min of chelation corroborated by assessing the dry/wet and rehydrated/wet weights, which showed that hydration characteristics changed during switching (Fig. S1).
Furthermore, the combination hydrogel mechanical properties changed from ∼21.37 ± 5.37 kPa (alginate-only being ∼19.37 ± 6.98 kPa) to ∼4.87 ± 1.64 kPa (collagen-only being ∼6.28 ± 2.83 kPa), representing a switch from alginate- to collagen-dominated character. Using ultrasound, we were also able to demonstrate a density and bulk mechanical change after switching relative to acoustic impedance of the material (Fig. S4). Overall, these data demonstrate that alginate cross-linking prevented the majority of collagen fibril formation. Switching does not lead to an extensive loss of collagen, triggers fibrillogenesis, and changes the bulk mechanical properties of the hydrogel.
To test the compatibility of the switching process with HPSC culture, we treated HPSCs maintained on tissue culture plastic with the chemical regime used to switch the hydrogels (Fig. S5). We thought this method would be a valid test because monolayers of HPSCs are considered to be exquisitely sensitive to changes in culture conditions (22). Treatments required for switching were compatible with survival, proliferation, and alkaline phosphatase (AP) activity of HPSCs (Fig. S5).
We tested how HUES7 HESCs responded when seeded within composite hydrogels formulated as disk shapes (Fig. 2) or injection-molded to create 3D structures, such as tubes (Fig. S6). Disc constructs were thin enough (∼25 µm) for adequate mass transport of nutrients/metabolic by-products (11) and were loaded with high cell densities (up to 5 × 106 HUES7 cells per milliliter) in mouse embryonic fibroblast-conditioned medium (CM) (23). After 21 d, HPSCs were uniformly distributed and sustained metabolic and AP activity. Similar levels of metabolic and AP activity were seen in alginate-only hydrogels but were considerably reduced in collagen-only hydrogels (Fig. S7A). Combination hydrogels increased in size (diameter of 3.21 ± 0.34 mm vs. 4.38 ± 0.57 mm on days 0 and 14, respectively) as HPSCs proliferated.
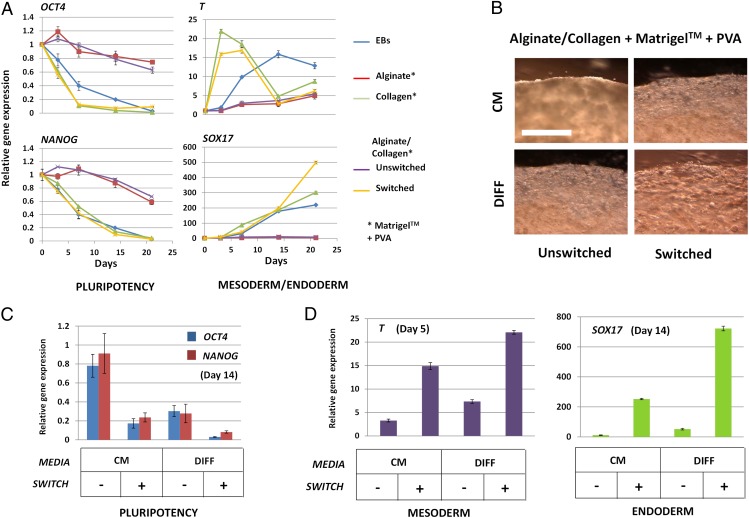
Fig. 2.
Switching HPSC self-renewal to differentiation. (A) Quantitative gene expression of pluripotency markers OCT4 and NANOG in HUES7 HESCs cultured in hydrogels (containing Matrigel and PVA) compared with those grown as conventional monolayers or as EBs (23). Relative expression levels for constructs over a 21-d period cultured in HPSC maintenance medium (CM). (Bars are SE; n = 6.) (B) Light microscopy of HUES7 HESCs cultured in composite hydrogels (containing Matrigel and PVA) with or without switching at day 0 (post-cross-linking of hydrogel) cultured in CM or DIFF medium (23). HUES7 cells within unswitched hydrogels cultured in CM retained HESC morphology and proliferated to fill the gel volume, which was inhibited if cultured in DIFF medium. HUES7 cells in switched hydrogels, especially those cultured in DIFF medium, rapidly lost HESC morphology and proliferation. Images were taken at 14 d post-cross-linking. (Bar: 50 µm.) (C and D) Quantitative gene expression analyses for the culture conditions described in B. OCT4 and NANOG (at day 14) (C) and T (at day 5; D, Left) and SOX17 (at day 14; D, Right) gene expression is shown. (Bars are SE; n = 6.)
Optimization of Switching Hydrogels for HPSC Self-Renewal.
Because poly(vinyl alcohol) (PVA) and the ECM Matrigel have been shown to have positive effects on maintenance of HESCs in monolayers (4, 24), we tested whether these substances could further facilitate HPSC pluripotency in the combined hydrogels (Fig. S7B).
First, we determined that addition of PVA and Matrigel did not prevent the alginate-mediated inhibition of collagen fibrillogenesis in combined hydrogels (Fig. S2A). Inclusion of PVA at a concentration of 1 mg/mL and Matrigel at 25% (vol/vol) enhanced expression of stem cell markers (transcription factors OCT4, NANOG, and AP) in the absence of differentiation marker expression (T for mesoderm and SOX17 for endoderm) (Fig. 2A and Fig. S7B) and thus enhanced the self-renewal phenotype of HPSCs. Therefore, Matrigel and PVA were included in subsequent hydrogel formulations. Microscopy of cells within optimized composite hydrogels showed a rounded morphology with an average cell diameter of 10 ± 0.32 µm, consistent with published sizes for undifferentiated cells (25) (Fig. S7C). Cells proliferated as isolated aggregates until they impinged on their neighbors and the aggregates merged (Figs. S7C and S8).
We next assessed the effect on cell behavior, self-renewal, and differentiation of conversion to the collagen-rich switched form. We seeded cells on top of gels to more clearly observe differences in cell–matrix interaction (Fig. S9). HPSCs on alginate-only or unswitched composite gels were rounded, had lower perimeter length and cell body area, and were loosely attached (Fig. S9A), whereas cells on collagen or switched composite gels were adherent (Fig. S9B).
Gene expression by quantitative PCR (QPCR) of HPSCs cultured in CM for 21 d within switched combined hydrogels showed significant down-regulation of pluripotency markers (P < 0.05 for both OCT4 and NANOG). Decreases in expression were observed 1 d after switching, with expression completely lost by day 6 for OCT4 and day 12 for NANOG (Fig. 3A). Conversely, differentiation markers (T for mesoderm and SOX17 for endoderm) were up-regulated directly after switching (Fig. 2A). Therefore, the microenvironment within switched combined gels has a dominant effect and “primes” differentiation of HPSCs, even in pluripotency-maintaining CM conditions.
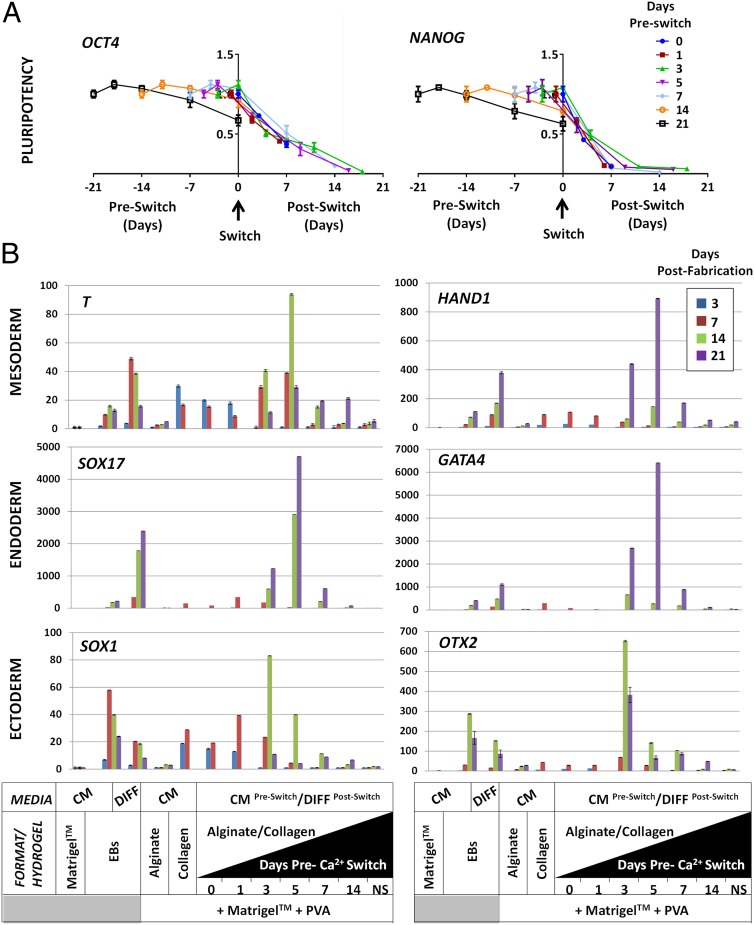
Fig. 3.
Switch timing influences HPSC fate. (A) Quantitative gene expression of pluripotency markers OCT4 (Left) and NANOG (Right) in HUES7 HESCs cultured with a variable switching time. Switching was undertaken on day 0 post-cross-linking or at day 1, 3, 5, 7, or 14 or not switched within the 21-d culture period. HUES7 HESCs cultured in CM with longer time before switching retained pluripotent gene expression at higher levels. Early switching caused rapid down-regulation of both OCT4 and NANOG. (Bars are SE; n = 6.) (B) Quantitative gene-expression characterization of mesodermal (T, Top Left; and HAND1, Top Right), endodermal (SOX17, Middle Left; and GATA4, Middle Right) and ectodermal (SOX1, Bottom Left; and OTX2, Bottom Right) differentiation with variable switching time. The timing of the switch appears to direct the differentiation process, with very early switching generating more ectodermal and later switching generating more endodermal gene expression. (Bars are SE; n = 6.)
As expected, substitution of CM for differentiation-inducing medium (DIFF medium; Fig. 2B) accelerated down-regulation of OCT4 and NANOG in switched hydrogels (Fig. S10). Furthermore, DIFF medium induced differentiation without switching, demonstrating that unswitched combination gels cannot override extrinsic influences from the culture medium. The switching of combined hydrogel microenvironment had a more profound effect on HPSC fate than changing medium conditions alone. Furthermore, if these parameters were changed together, there was a combined synergistic effect to more efficiently induce HPSC differentiation (Fig. 2 C and D).
The Effect of Switching on Early HPSC Lineage Specification.
Because the switching of combined hydrogels from alginate- to collagen-dominated character had profound influence on HPSC self-renewal, we assessed whether the timing of switching between states could direct early lineage specification during differentiation (Fig. 3). It has been suggested that the microenvironmental history of HPSCs could skew the induction of specific lineages as for embryonic gastrulation (26). Here, when switching hydrogels, we simultaneously swapped CM to DIFF medium to promote the priming of differentiation by switching (Fig. 3 C and D). We determined that NANOG and OCT4 are rapidly down-regulated upon switching at any time after gelation (P < 0.01 at 7 d after switching; n = 6) (Fig. 3A). We confirmed that switch timing can direct the efficient induction of the specific germ layers (mesoderm, endoderm, and ectoderm), as assessed with QPCR (Fig. 3B). We demonstrated that early switching generates more ectodermal differentiation (peaking with the switch on day 3 with SOX1 and OTX2 expression) (∼81- and ∼642 -fold increase over monolayer cultures, respectively; P < 0.05), whereas mesodermal commitment (T and HAND1 expression; P < 0.01) and endodermal commitment (SOX17 and GATA4 expression; P < 0.005) were highest with a day-5 switch (∼92/∼893-fold and ∼4,750/∼6,454 -fold increase over monolayer cultures, respectively; n = 6) (Fig. 3B). Therefore, we show that early HPSC lineage commitment can be skewed by the time of switching microenvironmental states in combined hydrogels. This finding indicates that with further optimization, this approach could allow precise fine-tuning of HPSC germ-layer differentiation.
Using Switching Hydrogels to Create Terminally Differentiated Tissues from HPSCs.
We wanted to determine whether switching of germ-layer specification could improve the generation of terminally differentiated tissues in situ, which is the ultimate goal for any regenerative medicine application (Fig. 4A). First, we used a transcription-factor-driven method by directly programming gene-regulatory networks (23). We transduced HUES7 HESCs with GATA4/TBX5 lentiviruses, loaded cells into combined hydrogels, and varied the time of switching (Fig. 4B). This approach induced mesoderm (T; P < 0.01), cardiac mesoderm (NXK2.5; P < 0.05), and a terminal cardiac marker (MYH6; P < 0.001). Although cardiac differentiation was apparent in unswitched hydrogels, switching promoted cardiogenesis (∼48,000-fold relative to nonprogrammed monolayer cultures). Day of switching also influenced their developmental position within the lineage, with cells toward a cardiac progenitor (day 9 switch) or specified cardiomyocyte identity (day 5 switch) (Fig. 4B).
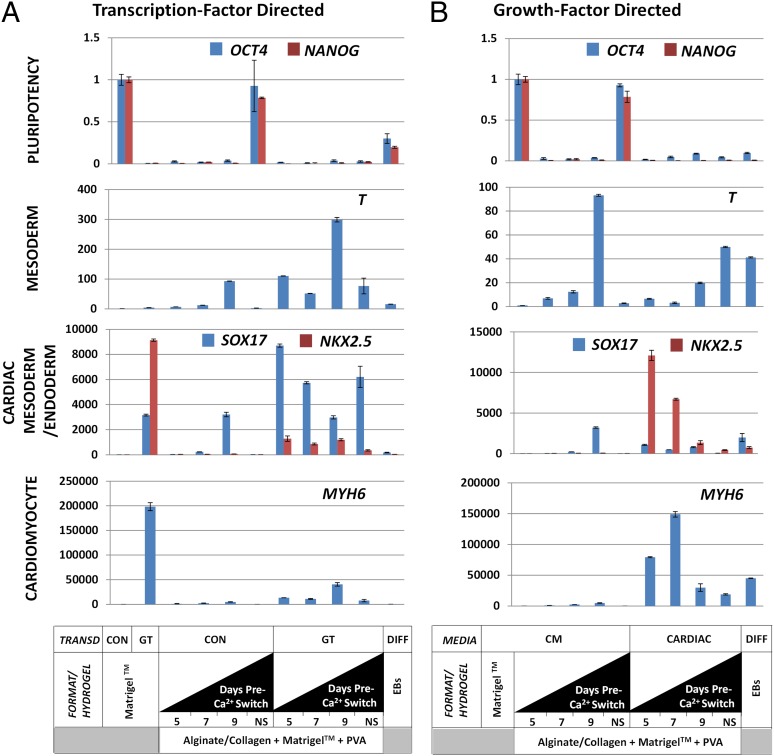
Fig. 4.
Promoting directed cardiac differentiation of HPSCs by transcription or growth factors using optimal switching time. (A) Quantitative gene expression of transcription-factor-driven differentiation after 21-d culturing with variable switching time (day 5, 7, or 9 or no switch). Analyses used pluripotency (OCT4 and NANOG), mesoderm (T, BRACHYURY), endoderm (SOX17), cardiac mesoderm (NXK2.5), and terminally differentiated cardiomyocyte (MYH6) markers. Control- transduced (CON) constructs (eGFP-expressing lentivirus) possessed no significant cardiac differentiation, whereas those transduced with GT significantly up-regulated cardiac mesoderm and cardiomyocyte markers (P < 0.05). This result was more efficient for cardiac mesoderm than for terminal differentiation (P < 0.05) compared with monolayer differentiation as described (23). Switching time significantly affected specification and terminal differentiation with 7 d before switching the most efficient at specifying cardiac mesoderm (P < 0.05). (Bars are SE; n = 6.) (B) Quantitative gene-expression characterization of growth-factor-driven differentiation. Control constructs cultivated in CM showed some differentiation depending on switching time but no significant cardiac gene expression. Hydrogels cultivated with the growth-factor regime showed significant cardiac gene expression, even in unswitched constructs (P < 0.05). Those switched at day 7 or 9 showed the highest specification and terminal differentiation beyond that produced by the transcription-factor-directed system. (Bars are SE; n = 6.)
To test whether cardiomyocyte differentiation could be induced within switched combined hydrogels by nontransgenic methods, we used a protocol (27) that relies of sequential addition of growth factors (BMP4 and FGF2; Fig. S11). This protocol induced significant up-regulation of cardiac markers NKX2.5 and MYH6 relative to switched hydrogels seeded with HPSCs cultured in CM or with HPSCs following the transcription-factor-driven protocol (P < 0.05) (Fig. 4 B and C). Growth factor induction of cardiogenesis in the switched hydrogels also enhanced expression of cardiac markers relative to a conventional EB differentiation protocol (P < 0.01). The optimal time to generate mature cardiomyocyte gene expression (MYH6) was achieved by day-7 switching (∼150,000-fold over nonprogrammed monolayer cultures), and enhanced cardiac mesoderm gene expression was achieved by day 5 switching (∼12,000-fold over nonprogrammed monolayer cultures) (P < 0.05; n = 6). By comparison, in unswitched combined hydrogels, expression of cardiac markers was ∼12-fold less efficient for transcription-factor-mediated differentiation and approximately eightfold less for growth-factor-mediated differentiation (P < 0.05; n = 6) (Fig. 4 B and C). These experiments demonstrate that extrinsic programming of HPSCs along with control of microenvironment to direct specification can more efficiently produce terminally differentiated cell types.
Discussion
HPSCs represent an attractive approach to generate any genetically matched tissue type (2). However, with unlimited potential, HPSCs are the furthest developmentally from differentiated tissues. Therefore, efficient and faithful control of expansion and differentiation must be achieved (22, 28). Previous work has used prefabricated chitosan/alginate scaffolds to maintain HPSC self-renewal (8). This approach requires chemical modification, heating, and lyophilization to create chemically bonded chitosan-alginate and produce porous sponges.
During all stages of embryogenesis, but especially at gastrulation/lineage commitment (between days 14 and 16 postovulation), changes in 3D microenvironments affect cell migration, growth, apoptosis, and identity (6). HESCs are derived from preimplantation embryos (29) but resemble the pluripotent cells of an older pregastrulation epiblast-stage embryo (30). Therefore, we hypothesized that by manipulating HPSC culture microenvironment, it could be possible to efficiently direct cell fate toward the cell type of choice (3, 31, 32). We combined existing technologies using a mixture of two natural hydrogels with divergent influence on cells (33). By tailoring these gels for initial self-renewal of HPSCs, we were able to achieve high cell densities (∼2 × 107 per milliliter; Fig. S7B) before switching the properties of the hydrogel to promote early lineage specification. The switching process had no direct negative effect on self-renewal of HPSCs and could be completed within 40 min with nontoxic chemicals (Fig. S5). In contrast to other studies (12), our system also has the advantage that construct geometry was retained after removal of alginate (Fig.1B). We performed an extensive study of proliferation and differentiation using germ-layer specification and cardiomyogenesis as an exemplar. By using published methods to direct differentiation—either by growth-factor regimes (27) or by transcription-factor regulation (23)—we demonstrated efficient cardiac differentiation depending on the timing of the switch, thereby showing that microenvironmental control significantly influences the cell fate outcomes.
Our material could be optimized with matrices that stimulate specific cell outcomes (34). For example, further work could directly replace collagen with decellularized ECM (dECM) gels to more closely recapitulate the microenvironment of the target tissue (e.g., heart dECM for cardiac differentiation) (35). Mechanistically, it is likely that cell adhesion, degradation of the hydrogel, and elasticity all influence cell behavior when hydrogels are switched.
This study has demonstrated that the self-renewal and differentiation of HPSCs can be controlled in situ within a single combination scaffold system. Furthermore, our work demonstrates that combined hydrogels when switched yield in situ tissue development that more closely recapitulates gene expression observed during embryogenesis (6, 7) and the process of lineage commitment in gastrulation. Tailoring microenvironmental changes as well as growth factor-directed and small-molecule-directed manipulation of cells will be an important parameter when devising methods to produce human engineered tissues for regenerative medicine applications.
Materials and Methods
Detailed information is provided in SI Materials and Methods. Combined hydrogels [1.2% (wt/vol) alginate and 2 mg/mL collagen type I] were cross-linked by CaCO3 and GDL (34 and 42 mM, respectively). Switching (removal of alginate) was achieved by first stabilizing gels with l-lysine and chelating Ca2+ with sodium citrate and EDTA (200 and 30 mM, respectively). HUES7 cells were grown on Matrigel in CM before fabrication of hydrogels. Hydrogels were grown in CM or in DIFF medium [DMEM containing FCS as described (24)]. Viability assays used AlamarBlue and Live/Dead assays. Microscopy of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate cell staining used macroconfocal analyses to assess HPSC growth in hydrogels. Cell proliferation and collagen fibrillogenesis was assessed by SEM and E-SEM. Mechanical and gel property analyses used protein and 1,9-dimethyl methylene blue assays for collagen and alginate, respectively. Wet/dry-weight assessments and ultrasound of hydrogels were used to assess switching efficiency and gel mechanics, respectively. Assessment of pluripotency was achieved by AP staining/assays and QPCR. HUES7 cells were transduced with GATA4/TBX5 lentiviruses to direct cardiogenesis as described (23). Growth-factor cardiac differentiation was performed as described by using FGF2 and BMP4 (27).
Supplementary Material
Acknowledgments
Work with HESCs was funded by the Engineering and Physical Sciences Research Council Centre for Innovative Manufacturing in Regenerative Medicine. The research leading to results with induced pluripotent stem cells has been supported by the European Research Council (ERC) under the European Community’s Seventh Framework Programme FP7/2007-2013/ERC Grant Agreement 227845.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1319685111/-/DCSupplemental.
References
- 1.Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481(7381):295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora N, Daley GQ. Pluripotent stem cells in research and treatment of hemoglobinopathies. Cold Spring Harb Perspect Med. 2012;2(4):a011841. doi: 10.1101/cshperspect.a011841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giobbe GG, et al. Confined 3D microenvironment regulates early differentiation in human pluripotent stem cells. Biotechnol Bioeng. 2012;109(12):3119–3132. doi: 10.1002/bit.24571. [DOI] [PubMed] [Google Scholar]
- 4.Vallier L, Pedersen RA. Human embryonic stem cells: An in vitro model to study mechanisms controlling pluripotency in early mammalian development. Stem Cell Rev. 2005;1(2):119–130. doi: 10.1385/SCR:1:2:119. [DOI] [PubMed] [Google Scholar]
- 5.Steiner D, et al. Derivation, propagation and controlled differentiation of human embryonic stem cells in suspension. Nat Biotechnol. 2010;28(4):361–364. doi: 10.1038/nbt.1616. [DOI] [PubMed] [Google Scholar]
- 6.Davidson LA. Integrating morphogenesis with underlying mechanics and cell biology. Curr Top Dev Biol. 2008;81:113–133. doi: 10.1016/S0070-2153(07)81003-9. [DOI] [PubMed] [Google Scholar]
- 7.Fisher OZ, Khademhosseini A, Langer R, Peppas NA. Bioinspired materials for controlling stem cell fate. Acc Chem Res. 2010;43(3):419–428. doi: 10.1021/ar900226q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z, Leung M, Hopper R, Ellenbogen R, Zhang M. Feeder-free self-renewal of human embryonic stem cells in 3D porous natural polymer scaffolds. Biomaterials. 2010;31(3):404–412. doi: 10.1016/j.biomaterials.2009.09.070. [DOI] [PubMed] [Google Scholar]
- 9.Tang M, Chen W, Weir MD, Thein-Han W, Xu HH. Human embryonic stem cell encapsulation in alginate microbeads in macroporous calcium phosphate cement for bone tissue engineering. Acta Biomater. 2012;8(9):3436–3445. doi: 10.1016/j.actbio.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li YJ, Chung EH, Rodriguez RT, Firpo MT, Healy KE. Hydrogels as artificial matrices for human embryonic stem cell self-renewal. J Biomed Mater Res A. 2006;79(1):1–5. doi: 10.1002/jbm.a.30732. [DOI] [PubMed] [Google Scholar]
- 11.Bayoussef Z, Dixon JE, Stolnik S, Shakesheff KM. Aggregation promotes cell viability, proliferation, and differentiation in an in vitro model of injection cell therapy. J Tissue Eng Regen Med. 2012;6(10):e61–e73. doi: 10.1002/term.482. [DOI] [PubMed] [Google Scholar]
- 12.Gillette BM, Jensen JA, Wang M, Tchao J, Sia SK. Dynamic hydrogels: Switching of 3D microenvironments using two-component naturally derived extracellular matrices. Adv Mater. 2010;22(6):686–691. doi: 10.1002/adma.200902265. [DOI] [PubMed] [Google Scholar]
- 13.Yang YL, Kaufman LJ. Rheology and confocal reflectance microscopy as probes of mechanical properties and structure during collagen and collagen/hyaluronan self-assembly. Biophys J. 2009;96(4):1566–1585. doi: 10.1016/j.bpj.2008.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drury JL, Mooney DJ. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials. 2003;24(24):4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 15.Mørch YA, Donati I, Strand BL, Skjåk-Braek G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules. 2006;7(5):1471–1480. doi: 10.1021/bm060010d. [DOI] [PubMed] [Google Scholar]
- 16.Hahn MS, et al. Photolithographic patterning of polyethylene glycol hydrogels. Biomaterials. 2006;27(12):2519–2524. doi: 10.1016/j.biomaterials.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 17.Freed LE, Engelmayr GC, Jr, Borenstein JT, Moutos FT, Guilak F. Advanced material strategies for tissue engineering scaffolds. Adv Mater. 2009;21(32-33):3410–3418. doi: 10.1002/adma.200900303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tayalia P, Mazur E, Mooney DJ. Controlled architectural and chemotactic studies of 3D cell migration. Biomaterials. 2011;32(10):2634–2641. doi: 10.1016/j.biomaterials.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuo CK, Ma PX. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22(6):511–521. doi: 10.1016/s0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 20.Tsai SW, Jeng MJ, Tsay RY, Wang YJ. Gel beads composed of collagen reconstituted in alginate. Biotechnol Tech. 1998;12(1):21–23. [Google Scholar]
- 21.Sims TJ, Avery NC, Bailey AJ. Quantitative determination of collagen crosslinks. Methods Mol Biol. 2000;139:11–26. doi: 10.1385/1-59259-063-2:11. [DOI] [PubMed] [Google Scholar]
- 22.Moon SY, Park YB, Kim DS, Oh SK, Kim DW. Generation, culture, and differentiation of human embryonic stem cells for therapeutic applications. Mol Ther. 2006;13(1):5–14. doi: 10.1016/j.ymthe.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Dixon JE, Dick E, Rajamohan D, Shakesheff KM, Denning C. Directed differentiation of human embryonic stem cells to interrogate the cardiac gene regulatory network. Mol Ther. 2011;19(9):1695–1703. doi: 10.1038/mt.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burridge PW, et al. Improved human embryonic stem cell embryoid body homogeneity and cardiomyocyte differentiation from a novel V-96 plate aggregation system highlights interline variability. Stem Cells. 2007;25(4):929–938. doi: 10.1634/stemcells.2006-0598. [DOI] [PubMed] [Google Scholar]
- 25.Velugotla S, et al. Dielectrophoresis based discrimination of human embryonic stem cells from differentiating derivatives. Biomicrofluidics. 2012;6(4):44113. doi: 10.1063/1.4771316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4(6):487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Burridge PW, et al. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS ONE. 2011;6(4):e18293. doi: 10.1371/journal.pone.0018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cherry AB, Daley GQ. Reprogrammed cells for disease modeling and regenerative medicine. Annu Rev Med. 2013;64:277–290. doi: 10.1146/annurev-med-050311-163324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gilbert SF (2010) Developmental Biology (Sinauer Associates, Sunderland, MA), 9th Ed.
- 30.Giritharan G, Ilic D, Gormley M, Krtolica A. Human embryonic stem cells derived from embryos at different stages of development share similar transcription profiles. PLoS ONE. 2011;6(10):e26570. doi: 10.1371/journal.pone.0026570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 32.Khetan S, et al. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater. 2013;12(5):458–465. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 34.Engler AJ, et al. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: Scar-like rigidity inhibits beating. J Cell Sci. 2008;121(Pt 22):3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilbert TW, Stolz DB, Biancaniello F, Simmons-Byrd A, Badylak SF. Production and characterization of ECM powder: Implications for tissue engineering applications. Biomaterials. 2005;26(12):1431–1435. doi: 10.1016/j.biomaterials.2004.04.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.