Significance
Simple alkyl thiols such as methanethiol are widely speculated to spontaneously form in seafloor hot spring fluids and are implicated in facilitating the emergence of protometabolism and microbial life in early Earth hydrothermal systems, the complexation of hydrothermally derived metals, and as fuels for microbial ecosystems. Existing models suggest that methanethiol forms by nonbiological reduction of hydrothermal inorganic carbon (CO2 or CO). We demonstrate that methanethiol is actively produced in low-temperature mixing zones of hydrothermal systems, but our data suggest it is the thermal destruction of preexisting organic matter (likely subsurface microbial biomass) that is responsible. Formation of organosulfur compounds and other degradation products during subseafloor mixing may influence the biogeochemistry of low-temperature hydrothermal fluids inhabited by microbial life.
Keywords: methyl mercaptan, biogeochemistry, origin of life, prebiotic chemistry
Abstract
Simple alkyl thiols such as methanethiol (CH3SH) are widely speculated to form in seafloor hot spring fluids. Putative CH3SH synthesis by abiotic (nonbiological) reduction of inorganic carbon (CO2 or CO) has been invoked as an initiation reaction for the emergence of protometabolism and microbial life in primordial hydrothermal settings. Thiols are also presumptive ligands for hydrothermal trace metals and potential fuels for associated microbial communities. In an effort to constrain sources and sinks of CH3SH in seafloor hydrothermal systems, we determined for the first time its abundance in diverse hydrothermal fluids emanating from ultramafic, mafic, and sediment-covered midocean ridge settings. Our data demonstrate that the distribution of CH3SH is inconsistent with metastable equilibrium with inorganic carbon, indicating that production by abiotic carbon reduction is more limited than previously proposed. CH3SH concentrations are uniformly low (∼10−8 M) in high-temperature fluids (>200 °C) from all unsedimented systems and, in many cases, suggestive of metastable equilibrium with CH4 instead. Associated low-temperature fluids (<200 °C) formed by admixing of seawater, however, are invariably enriched in CH3SH (up to ∼10−6 M) along with  and low-molecular-weight hydrocarbons relative to high-temperature source fluids, resembling our observations from a sediment-hosted system. This strongly implicates thermogenic interactions between upwelling fluids and microbial biomass or associated dissolved organic matter during subsurface mixing in crustal aquifers. Widespread thermal degradation of subsurface organic matter may be an important source of organic production in unsedimented hydrothermal systems and may influence microbial metabolic strategies in cooler near-seafloor and plume habitats.
and low-molecular-weight hydrocarbons relative to high-temperature source fluids, resembling our observations from a sediment-hosted system. This strongly implicates thermogenic interactions between upwelling fluids and microbial biomass or associated dissolved organic matter during subsurface mixing in crustal aquifers. Widespread thermal degradation of subsurface organic matter may be an important source of organic production in unsedimented hydrothermal systems and may influence microbial metabolic strategies in cooler near-seafloor and plume habitats.
Since their discovery in 1977, seafloor hot spring fluids have been widely proposed as a potential source of organic molecules necessary for early life to emerge and thrive on a Hadean–Archaean Earth (1–5), and for metabolic energy and fixed carbon in modern hydrothermal systems (6, 7). Abiotic (nonbiological) reduction of inorganic carbon (CO2 or CO) to methanethiol (methyl mercaptan, CH3SH) is considered a crucial first step in the putative transition from prebiotic to primitive metabolic chemistry, leading to the emergence of hyperthermophilic microbial life (8–13). Specifically, methanethiol is the presumptive abiotic precursor of acetyl thioester (8, 12, 13)—the functional moiety of the Acetyl-CoA coenzyme central to many ancient metabolic pathways—and a sustainable abiotic source of acetyl thioesters is a key feature of models proposing the emergence of primordial metabolism in hydrothermal settings (5). Alkyl thiols are additionally implicated in the synthesis of the key metabolite pyruvate (10), which is speculated to have led to a primordial protometabolic network in a hydrothermal setting (14). In modern hot spring environments, hydrothermally produced thiols could constitute metabolic energy and carbon sources (15) for mesophilic and thermophilic microorganisms in subseafloor, near-vent, and plume settings given that such compounds are intensively cycled in sedimentary microbial habitats (16). Moreover, due to their strong metal-binding abilities, thiol functional groups are also increasingly implicated in the complexation and delivery of hydrothermally derived metals such as Fe and Cu to the deep ocean (17–19).
Abiotic reduction of inorganic carbon to CH3SH has been shown to occur under experimental hydrothermal conditions (8, 20, 21), and thermodynamic considerations indicate that the abundance of CH3SH in metastable equilibrium with inorganic carbon sources should increase strongly with dissolved hydrogen (H2) abundance (22). This has led to the assumption that CH3SH should be enriched in H2-rich fluids emanating from serpentinite-hosted hydrothermal settings, such as the Lost City hydrothermal field (13). Without evidence from analogous modern hydrothermal fluids, however, the assumption of widespread thiol production by inorganic carbon reduction in prebiotic seafloor hot springs lacks support.
It is generally assumed that low-molecular-weight organic compounds in hydrothermal fluids emanating from settings lacking significant sedimentary organic matter (unsedimented systems) are primarily derived from inorganic carbon (e.g., mantle-derived CO2) via abiotic reduction reactions, either by homogeneous reduction or involving heterogeneous mineral catalysts (23). CH4, C2+ hydrocarbons, and formate, for example, are postulated to be the products of abiotic carbon reduction in Lost City vent fluids (24, 25). However, potential contributions of thermogenic (i.e., derived from abiotic thermal decomposition of either preexisting biomass or biologically derived compounds) or biogenic (i.e., derived from metabolic activities of viable organisms) carbon compounds to hydrothermal fluids are poorly constrained at present (7, 26).
The fundamental physicochemical processes governing the formation of hydrothermal fluids and the abundances of presumptive inorganic substrates for abiotic synthesis (CO2/CO, H2, and H2S) have likely persisted through geologic time (3); hence, diverse modern analogs present an opportunity to assess the potential for abiotic synthesis of CH3SH on early Earth. Therefore, to clarify its production and origin, we investigated the distribution of CH3SH in 38 hot spring fluids from diverse geologic environments throughout the global midocean ridge system that span a range of temperatures and redox states. Isobaric gas-tight fluid samplers (27) were used to collect the hydrothermal fluids, and the citations for each system included here provide the most relevant or recent reported fluid compositions. Samples were taken from four unsedimented hydrothermal vent fields hosted in basaltic rock: Lucky Strike (28) and TAG (29) on the Mid-Atlantic Ridge (MAR), 9°50′N (30) on the East Pacific Rise, and Piccard (31, 32) on the Mid-Cayman Rise (MCR). Three diverse unsedimented vent fields where serpentinization of ultramafic rock is postulated to influence fluid compositions were sampled: Von Damm (33) on the MCR, and Lost City (24) and Rainbow (34) on the MAR. Fluids were also collected from the sediment-hosted Guaymas Basin vent field in the Gulf of California (35, 36), where extensive hydrothermal alteration of organic-rich sediment overburden is occurring. Our results indicate that production of abiotic CH3SH from inorganic carbon is not occurring to a significant extent and that abiotic degradation of preexisting organic matter may instead be the dominant source. The widespread production we observe during crustal mixing of hydrothermal fluids has numerous implications for the biogeochemistry of seafloor hydrothermal systems, which we discuss here.
Results and Discussion
Measured CH3SH concentrations vary from ∼10−9 to 10−6 M in unsedimented hydrothermal systems (Dataset S1). In general, low-temperature (<200 °C) fluids formed by subsurface mixing of high-temperature endmember fluids with seawater (see Endmember and Mixed Vent Fluid Compositions) are most enriched. For example, CH3SH concentrations in mixed fluids at Piccard are above 10−6 M, whereas associated endmember sources are ∼10−8 M. Cooler endmember fluids at the sediment-hosted Guaymas Basin system contain over 10−5 M CH3SH—the highest concentrations observed—whereas nearby hotter endmember fluids have abundances similar to most endmember fluids from unsedimented systems (∼10−8 M). Concentrations are lowest in endmember fluids (94–96 °C) of the Lost City hydrothermal field (1.4–1.9 × 10−9 M).
Thermodynamic Evaluation of CH3SH Abundances.
The abiotic production of CH3SH in hydrothermal solutions is typically described (13, 22) by the overall reaction of CO2, H2, and H2S according to the relationship
Although CO is also considered a possible substrate for reduction (12, 13, 22), it does not provide an alternate pathway to CH3SH that is independent of CO2 reduction in fluids emanating from unsedimented systems. CO is predicted to be maintained at very low abundances due to rapid equilibrium with CO2 and H2 (37). Equilibrium between CO2 and CO is confirmed by calculated chemical affinities near zero for the high-temperature vent fluids from unsedimented systems presented in this study (see Assessment of Metastable Equilibrium Using Chemical Affinities and Widespread CO2-H2-CO Equilibrium in Unsedimented Systems and Eqs. S1 and S2). The stoichiometry of reaction 1 indicates that for a given temperature and pressure, CH3SH abundance at metastable equilibrium should be highly sensitive to variations in dissolved H2 due to the third-power H2 dependence of the associated mass action expression. Using measured concentrations of CO2, H2, and H2S and thermodynamic data (22) for reaction 1 at measured vent temperatures and pressures, CH3SH abundances in unsedimented hydrothermal fluids are predicted to vary by over ten orders of magnitude for metastable chemical equilibrium (see Fig. 1 and Thermodynamic Prediction of Metastable CH3SH Abundances). Aqueous H2 concentrations that vary by almost three orders of magnitude notwithstanding, observed concentrations of CH3SH are relatively uniform (∼10−8 M) in high-temperature endmember fluids regardless of the geologic setting (Fig. 1 and Dataset S1). Although CH3SH concentrations below predicted values might suggest kinetic inhibition of reaction 1 in high-H2 fluids (e.g., at Rainbow, Piccard, and Von Damm), this does not explain the strongly enriched nature of very H2-poor fluids relative to predictions (e.g., TAG and Lucky Strike). It is possible that endmember fluids may cool during ascent from higher temperature and pressure subsurface reaction zones, where equilibrium according to reaction 1 might regulate CH3SH abundances. Such a scenario is unlikely, however, because predicted metastable equilibrium concentrations according to reaction 1 decrease strongly with increasing temperature (22), which would result in the apparent enrichments in low-H2 fluids becoming even more pronounced. Collectively, these observations suggest that CH3SH, CO2, H2S, H2, and H2O do not attain a state of metastable equilibrium at the diverse conditions encountered by fluids in modern hydrothermal systems. This implies that abiotic synthesis of CH3SH from inorganic carbon is unlikely and incapable of sustaining metastable equilibrium abundances.
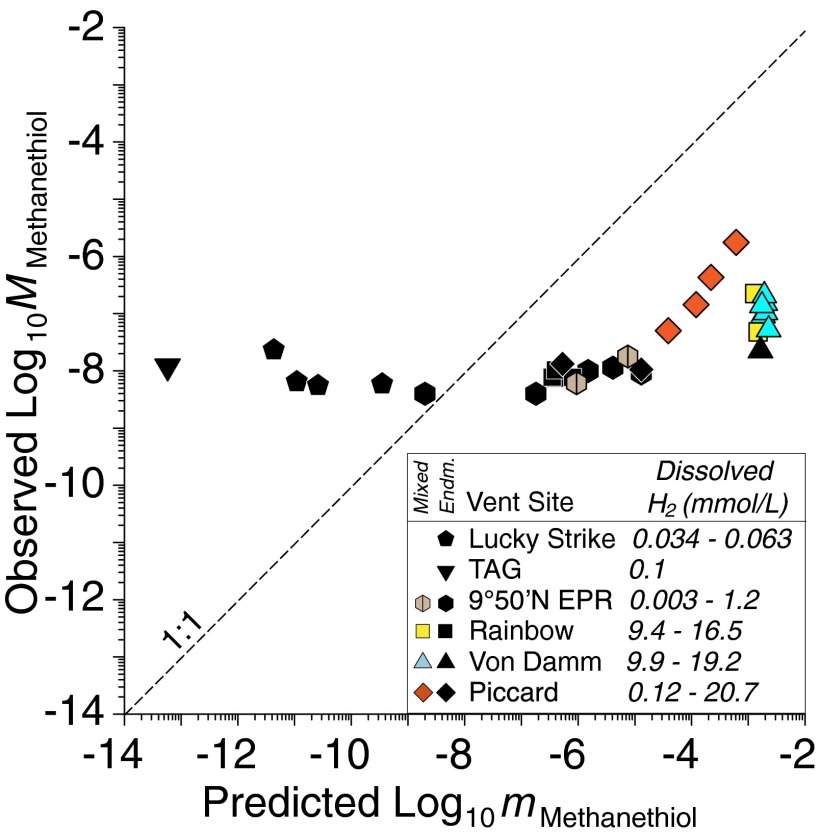
Fig. 1.
Plot of predicted concentrations (mMethanethiol, mol/kg H2O) of methanethiol (CH3SH) in metastable equilibrium with CO2, H2, and H2S according to reaction 1 versus observed concentrations (MMethanethiol, mol/L fluid) for endmember (black symbols) and mixed (colored symbols) hydrothermal fluids emanating from unsedimented mafic and ultramafic geologic settings, with respective ranges of dissolved H2 concentration shown in the legend (see Endmember and Mixed Vent Fluid Compositions and Thermodynamic Prediction of Metastable CH3SH Abundances, for further details). Predicted values were calculated assuming ideal behavior of neutral aqueous species. Observed concentrations of CH3SH in fluids with low dissolved H2 are in excess of predicted values and, conversely, are below predicted values in high-H2 fluids. Highest observed concentrations of CH3SH are in low-temperature mixed fluids at Piccard, Von Damm, and Rainbow (see text). The apparent trend of predicted and observed values at Piccard is a consequence of covarying H2 and CH3SH during subsurface mixing with seawater.
The relative uniformity of endmember abundances shown in Fig. 1, despite widely differing H2 abundances, suggests that CO2 reduction is not responsible for the production of CH3SH in high-temperature endmember fluids. Aside from CO2, CH4 is invariably the next largest stable pool of dissolved carbon in hydrothermal solutions, with all other aqueous single carbon species representing metastable states (7, 26). Indeed, models that propose reaction 1 as the source of CH3SH in hydrothermal fluids (5, 13, 22) inherently assume that metastable CH3SH is kinetically inhibited from destruction by further reduction to CH4. For many high-temperature endmember fluids presented here, however, calculated chemical affinities (see Assessment of Metastable Equilibrium Using Chemical Affinities and Eq. S1) show CH3SH is indeed at or close to metastable equilibrium with CH4 according to the reaction
For example, affinities for reaction 2 for all Rainbow endmember fluids (−3.8 to +0.5 kJ/mol) are within typical uncertainties of equilibrium at conditions of venting (see Assessment of Metastable Equilibrium Using Chemical Affinities). At Piccard (+6.4 to +14.6 kJ/mol) and Lucky Strike (−4.7 to −12.8 kJ/mol), endmembers are also close to equilibrium in several cases, as are some vents at 9°50′N (e.g., Tica, +5.5 kJ/mol). Thus, in contrast to reaction 1, measured CH3SH concentrations in endmember fluids are more consistent with predicted values according to reaction 2. Although we cannot exclude that the reverse of reaction 2 is occurring, there is to date no evidence to suggest that aqueous CH4 can react with H2S under hydrothermal conditions. On the contrary, CH3SH was observed to react to form small quantities of CH4 at 100 °C in the thioester synthesis experiments of Huber and Wächterhäuser (8). Thermodynamic data (22) indicate that reaction 2 would maintain CH3SH at low levels with respect to CH4 for most hydrothermal fluid compositions, with an inverse dependence on H2 abundance. This is incompatible with the notion of greater abiotic CH3SH production with increasing H2 abundance, as invoked in scenarios for prebiotic hydrothermal thioester production (8, 12, 13). Regardless of whether or not CH3SH forms by inorganic carbon reduction (reaction 1) or other biogenic or thermogenic processes during hydrothermal fluid circulation, the data presented here strongly suggest that metastable equilibrium with CH4 at high temperatures is sufficiently fast that it regulates CH3SH abundances in endmember fluids according to reaction 2.
Thermogenic CH3SH Production.
The highest CH3SH concentrations were observed in endmember vent fluids from the sediment-covered Guaymas Basin rift zone, where the influence of hydrothermal alteration of immature organic matter and biomass is readily apparent (Fig. 2). At Guaymas Basin, basaltic dikes and sills intrude into 0.5-km-thick organic-rich diatomaceous ooze overlaying the ridge axis, resulting in rapid and widespread hydrothermal alteration of immature sedimentary organic matter and expulsion of hydrothermal petroleum at the seafloor (36, 38). In addition to abundant  and dissolved CO2, multiple classes of thermogenic organic compounds are added to circulating fluids during this process (35, 36, 39). Alkyl thiols are considered to form predominantly at low thermal maturities during petroleum generation in slowly subsiding sedimentary basins (40), and their production in this setting is therefore not surprising. Cyclic polysulfide organosulfur compounds (thiolanes, thianes, and thiepanes) have previously been reported in fragments of an active smoker chimney from Guaymas Basin, indicating organosulfur production during hydrothermal petroleum generation (38). Production of CH3SH during hydrothermal alteration of sedimentary organic matter could reflect the removal of organosulfur moieties from macromolecular organic structures or the secondary reaction of thermogenic products such as CO. Indeed, fluids with abundant CH3SH at Guaymas also have excess CO relative to equilibrium with CO2 and H2 (see Dataset S1 and Widespread CO2-H2-CO Equilibrium in Unsedimented Systems).
and dissolved CO2, multiple classes of thermogenic organic compounds are added to circulating fluids during this process (35, 36, 39). Alkyl thiols are considered to form predominantly at low thermal maturities during petroleum generation in slowly subsiding sedimentary basins (40), and their production in this setting is therefore not surprising. Cyclic polysulfide organosulfur compounds (thiolanes, thianes, and thiepanes) have previously been reported in fragments of an active smoker chimney from Guaymas Basin, indicating organosulfur production during hydrothermal petroleum generation (38). Production of CH3SH during hydrothermal alteration of sedimentary organic matter could reflect the removal of organosulfur moieties from macromolecular organic structures or the secondary reaction of thermogenic products such as CO. Indeed, fluids with abundant CH3SH at Guaymas also have excess CO relative to equilibrium with CO2 and H2 (see Dataset S1 and Widespread CO2-H2-CO Equilibrium in Unsedimented Systems).
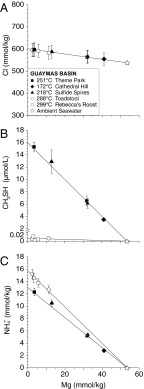
Fig. 2.
Plots of measured concentrations of chloride (Cl; A), methanethiol (CH3SH; B), and ammonium (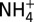 ; C) versus Mg in Guaymas Basin vent fluid samples, showing CH3SH and
; C) versus Mg in Guaymas Basin vent fluid samples, showing CH3SH and 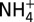 production. Mg is used as an index of mixing between seawater and vent fluid (see Endmember and Mixed Vent Fluid Compositions for further details). Cooler endmember fluids (Theme Park, Cathedral Hill, and Sulfide Spires; solid symbols) from areas of hydrothermal petroleum expulsion have higher CH3SH concentrations and lower C1/(C2+C3) ratios (<60) than hotter fluids from large flange structures (>124; Toadstool and Rebecca’s Roost; open symbols), implying thermal maturity differences (see text). Cl (A) suggests a common source endmember. All fluids have interacted with sedimentary organic matter under hydrothermal conditions, yielding elevated
production. Mg is used as an index of mixing between seawater and vent fluid (see Endmember and Mixed Vent Fluid Compositions for further details). Cooler endmember fluids (Theme Park, Cathedral Hill, and Sulfide Spires; solid symbols) from areas of hydrothermal petroleum expulsion have higher CH3SH concentrations and lower C1/(C2+C3) ratios (<60) than hotter fluids from large flange structures (>124; Toadstool and Rebecca’s Roost; open symbols), implying thermal maturity differences (see text). Cl (A) suggests a common source endmember. All fluids have interacted with sedimentary organic matter under hydrothermal conditions, yielding elevated 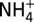 (C), but CH3SH (B) and C2+ hydrocarbons have likely decomposed to CH4 at the higher thermal stress of the hotter fluids. Uncertainties (2s) not shown are smaller than data symbols.
(C), but CH3SH (B) and C2+ hydrocarbons have likely decomposed to CH4 at the higher thermal stress of the hotter fluids. Uncertainties (2s) not shown are smaller than data symbols.
The abundance of CH3SH in fluids at Guaymas Basin is characterized by a bimodal distribution, with cooler endmember fluids being most enriched and hotter endmember fluids having similarly low abundances to fluids in unsedimented systems. CH3SH-depleted Rebecca’s Roost and Toadstool fluids (288–299 °C; Fig. 2) have substantially higher C1/(C2+C3) ratios (124–132 versus 55–60) than the CH3SH-rich cooler fluids (172–251 °C), suggesting higher thermal maturity in the hotter fluids and the conversion of longer-chain alkanes to shorter chains (39). These observations suggest that abundant CH3SH is produced predominantly during early hydrothermal alteration of immature organic matter, consistent with observations from conventional petroleum-producing systems (40). In a similar manner to C2+ hydrocarbons, but unlike  , CH3SH may have decomposed (e.g., by reaction 2) at the higher thermal stress of Rebecca’s Roost and Toadstool fluids before venting.
, CH3SH may have decomposed (e.g., by reaction 2) at the higher thermal stress of Rebecca’s Roost and Toadstool fluids before venting.
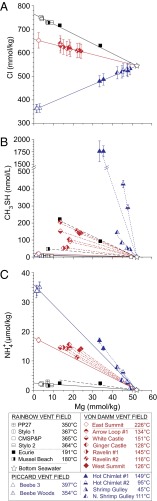
Although sedimentary organic matter is lacking at unsedimented spreading centers, the potential still exists for thermogenic production of thiols due to the presence of a putative subsurface biosphere and vent-associated biomass (26, 41–43). In low-temperature hydrothermal fluids (<200 °C) from numerous unsedimented settings, there is substantial evidence to support CH3SH production during mixing of endmember fluids with seawater within hydrothermal upflow zones or crustal aquifers. Linear mixing relationships between measured Cl and Mg concentrations for fluids sampled at Rainbow, Von Damm, and Piccard indicate that a common source fluid at each vent field feeds all of the overlying vents (Fig. 3A). That many of the replicate fluid samples collected at the low-temperature vents are characterized by nearly identical Mg concentrations implies that mixing of Mg-depleted endmember hydrothermal fluids with Mg-rich seawater occurred in the subsurface before sampling because stochastic admixing of ambient seawater during fluid collection at vent orifices would likely result in highly variable Mg concentrations (see Endmember and Mixed Vent Fluid Compositions). In contrast to the conservative behavior that characterizes Cl abundances during mixing, CH3SH concentrations in mixed fluids are all substantially greater than expected for conservative dilution of the associated high-temperature endmember (Fig. 3B and Fig. S1). Such enrichments require production of CH3SH during subsurface mixing and associated cooling. Although we cannot completely exclude that some component of the CH3SH in mixed fluids is derived from CO2 reduction or abiotic formation from other metastable intermediates, CH3SH abundances are far below values predicted for metastable equilibrium with respect to CO2, H2S, H2, and H2O (Fig. 1). It is difficult to argue that reaction 1 should only proceed at the lower temperatures and H2 abundances of mixed fluids, given the complete lack of evidence that inorganic carbon reduction is responsible for CH3SH production in endmember fluids, where substantially faster reaction rates would be expected due to higher temperatures and reactant concentrations.
Fig. 3.
Plots of measured concentrations of chloride (Cl; A), methanethiol (CH3SH; B), and ammonium (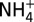 ; C) versus Mg in fluid samples from three unsedimented hydrothermal vent fields. Mg is used as an index of mixing between seawater and vent fluid (see Endmember and Mixed Vent Fluid Compositions for further details). Low-Mg samples from high-temperature endmember fluids (open symbols) and higher-Mg samples from low-temperature fluids (half filled/solid symbols) at each field all lie on common mixing lines with respect to Cl, indicating single common source fluids at each site. At all vent fields, the higher Mg, low-temperature fluids formed by predominantly subsurface mixing with seawater (dashed lines) are enriched in CH3SH and
; C) versus Mg in fluid samples from three unsedimented hydrothermal vent fields. Mg is used as an index of mixing between seawater and vent fluid (see Endmember and Mixed Vent Fluid Compositions for further details). Low-Mg samples from high-temperature endmember fluids (open symbols) and higher-Mg samples from low-temperature fluids (half filled/solid symbols) at each field all lie on common mixing lines with respect to Cl, indicating single common source fluids at each site. At all vent fields, the higher Mg, low-temperature fluids formed by predominantly subsurface mixing with seawater (dashed lines) are enriched in CH3SH and 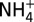 (and low-molecular-weight alkanes; see text) relative to conservative dilution of endmember fluids (solid lines) with seawater. Uncertainties (2s) not shown are smaller than the data symbols.
(and low-molecular-weight alkanes; see text) relative to conservative dilution of endmember fluids (solid lines) with seawater. Uncertainties (2s) not shown are smaller than the data symbols.
Elevated concentrations of other organically derived aqueous species in these low-temperature mixed fluids provide compelling support for thermogenic CH3SH production during mixing. As is evident at Guaymas, short-chain hydrocarbons and  are typically produced simultaneously during hydrothermal alteration of organic matter (35, 36, 39, 44). High CH3SH concentrations in mixed fluids at Piccard, Von Damm, Rainbow, and 9°50′N are associated with excess
are typically produced simultaneously during hydrothermal alteration of organic matter (35, 36, 39, 44). High CH3SH concentrations in mixed fluids at Piccard, Von Damm, Rainbow, and 9°50′N are associated with excess  (Fig. 3C and Fig. S1) and, in some cases, low-molecular-weight hydrocarbon (methane, ethane, and propane) enrichments relative to conservative dilution of precursor endmember fluids. For example, the low-temperature Hot Chimlet #1 vent at Piccard is enriched by more than 25% in
(Fig. 3C and Fig. S1) and, in some cases, low-molecular-weight hydrocarbon (methane, ethane, and propane) enrichments relative to conservative dilution of precursor endmember fluids. For example, the low-temperature Hot Chimlet #1 vent at Piccard is enriched by more than 25% in  and CH4 relative to conservative dilution of high-temperature endmember concentrations, and the low-temperature Ecurie vent at Rainbow is also enriched in both species (Dataset S1). Ethane and propane are substantially enriched in mixed fluids at Piccard,* with concentrations as high as 90 nmol/kg ethane and 60 nmol/kg propane in the mixed fluids Hot Chimlet #1 and Hot Chimlet #2, whereas associated endmember fluids have much lower C2+ hydrocarbon concentrations [8–20 nmol/kg ethane and propane <10 nmol/kg (below detection)].
and CH4 relative to conservative dilution of high-temperature endmember concentrations, and the low-temperature Ecurie vent at Rainbow is also enriched in both species (Dataset S1). Ethane and propane are substantially enriched in mixed fluids at Piccard,* with concentrations as high as 90 nmol/kg ethane and 60 nmol/kg propane in the mixed fluids Hot Chimlet #1 and Hot Chimlet #2, whereas associated endmember fluids have much lower C2+ hydrocarbon concentrations [8–20 nmol/kg ethane and propane <10 nmol/kg (below detection)].
Abiotic N2 reduction is unlikely to be responsible for the  enrichment in mixed fluids, given that it is only thought to occur under high-temperature reaction zone conditions (42, 44). Admixing and abiotic reduction of seawater nitrate (NO3−) may be an alternate possible source of
enrichment in mixed fluids, given that it is only thought to occur under high-temperature reaction zone conditions (42, 44). Admixing and abiotic reduction of seawater nitrate (NO3−) may be an alternate possible source of  to mixed fluids because experiments suggest that metal catalysts (e.g., Fe–Ni alloy) could mediate such reduction at low temperatures (45, 46), but this does not appear to be an important process in low-temperature (∼150 °C) mixed fluids from other mafic-hosted hydrothermal systems based on N-isotope measurements (42). Thermal degradation of dissolved organic nitrogen (DON) from admixed ambient deep ocean seawater is also an unlikely source of
to mixed fluids because experiments suggest that metal catalysts (e.g., Fe–Ni alloy) could mediate such reduction at low temperatures (45, 46), but this does not appear to be an important process in low-temperature (∼150 °C) mixed fluids from other mafic-hosted hydrothermal systems based on N-isotope measurements (42). Thermal degradation of dissolved organic nitrogen (DON) from admixed ambient deep ocean seawater is also an unlikely source of  . Some mixed fluids are enriched in
. Some mixed fluids are enriched in  above conservative endmember dilution by up to 4 μM (Fig. 3C), exceeding what might reasonably be expected for entrainment and complete N release from typical deep-ocean DON concentrations [<3 μM (47)]. Although microbially mediated nitrogen transformations can increase the abundance of
above conservative endmember dilution by up to 4 μM (Fig. 3C), exceeding what might reasonably be expected for entrainment and complete N release from typical deep-ocean DON concentrations [<3 μM (47)]. Although microbially mediated nitrogen transformations can increase the abundance of  in very low-temperature/diffuse fluids (42), most large
in very low-temperature/diffuse fluids (42), most large  and CH3SH enrichments observed during this study (e.g., Hot Chimlet #1) are in mixed fluids hotter than the 122 °C limit for microbial life (48), suggesting that microbial activity is not responsible for production of either
and CH3SH enrichments observed during this study (e.g., Hot Chimlet #1) are in mixed fluids hotter than the 122 °C limit for microbial life (48), suggesting that microbial activity is not responsible for production of either  or CH3SH.
or CH3SH.
It is widely proposed that the permeable and porous upper oceanic crust (Layer 2A)—where mixing predominantly occurs—harbors microbial communities that constitute a deep biosphere (41–43). Given that CH3SH in low-temperature mixed fluids is consistently associated with thermogenic indicators (Fig. 3 and Fig. S1) in systems free of sedimentary influence, we propose that entrainment and thermal alteration of microbial biomass, and/or associated dissolved organic matter (DOM), are responsible for production of CH3SH within subsurface zones of mixing between high-temperature fluids and seawater. Previous observations of organic compounds derived from microbial biomass pyrolysis in low-temperature fluids support this possibility. Brault et al. (49) first described the presence of nonvolatile hydrocarbons in a diffuse (∼15 °C) and a high-temperature (>250 °C) vent from 13°N, East Pacific Rise, and found strong enrichments of microbial lipid residues in the cooler vent. More recent work also suggests that dissolved organic carbon (DOC) is enriched in low-temperature fluids relative to both endmember precursors and ambient seawater, for example, and correlates with microbial cell counts (50). Although these observations could reflect active microbial processes, our results suggest that higher-temperature portions of subsurface mixing zones generate dissolved organic compounds through abiotic thermal degradation. Fluids in the temperature range presented here (e.g., 126–191 °C) have rarely been reported but provide key insights into processes occurring within crustal hydrothermal aquifers beyond the known 122 °C (48) temperature limit of life.
The ubiquitous and uniformly low levels of CH3SH (∼10−8 M) in endmember fluids from unsedimented systems, despite the broad range of temperature, salinity, and H2 concentrations, may be a remnant of pyrolysis of trace organic matter at high temperatures. Brault et al. (49) noted minor quantities of thermally mature hopanes in a hot fluid (>250 °C) from 13°N, suggesting that entrainment and rapid pyrolysis of biomass to a high extent of maturity occurs during fluid venting at the seafloor. Endmember fluids are also known to be depleted in DOC relative to ambient seawater (50), implying thermal decomposition. A thermogenic carbon source would provide an explanation for the presence of CH3SH in fluids with no thermodynamic drive to create it from CO2. In a similar manner to the hotter endmember fluids at Guaymas Basin, consumption of CH3SH by reaction 2 in high-temperature fluids could therefore represent the high maturity stage of much more limited organic matter pyrolysis during hydrothermal circulation or venting that produces insignificant changes in the abundance of major species like CH4. Lost City endmember fluids, despite quite low vent temperatures (94–96 °C; Dataset S1), are also consistent with this explanation. Several lines of evidence from the inorganic (51) and organic (52) compositions of endmember fluids there point to substantial conductive cooling and much higher temperatures (likely in excess of 250 °C) in the subsurface reaction zone. Collectively, our data therefore imply that the distribution of methanethiol in seafloor hydrothermal fluids is largely controlled by thermal maturation of preexisting biological organic matter, with endmember and mixed vent fluids representing higher and lower thermal maturities, respectively.
Implications.
Despite the diversity of geologic settings and potential catalytic minerals present in hydrothermal reaction zones, our results show no evidence for abiotic methanethiol synthesis from the inorganic precursors CO2, H2, and H2S in modern hydrothermal fluids. This suggests that analogous hydrothermal systems on early Earth may not represent an abundant source of abiotic CH3SH necessary for thioester production (4, 8, 12). The production of thermogenic organic compounds in crustal mixing zones, however, has numerous interesting biogeochemical implications for modern seafloor hydrothermal systems. Not only does widespread pyrolysis of subsurface organic matter provide further indirect support for a putative deep biosphere in unsedimented hydrothermal aquifers, it implies that such carbon may be recycled and returned to cooler near-surface environments by subsurface mixing processes. Production of methylated organic compounds by subsurface pyrolysis raises diverse possibilities for microbial organotrophic metabolisms (e.g., methylotrophy) in hydrothermal systems traditionally considered to have limited available organic compounds. A predominance of thermogenic products in low-temperature fluids could support larger populations of organotrophic microbes in these mixing zones relative to those immediately surrounding high-temperature vent structures, for example. Given that thiol functional groups are hypothesized to play a significant role in the complexation and delivery of hydrothermal trace metals (e.g., Fe and Cu) to the deep ocean (17–19), thermogenic production of organosulfur compounds with a high affinity for metals may constitute a key mechanism for this process in unsedimented hydrothermal systems.
Materials and Methods
All fluid samples were collected using isobaric gas-tight (IGT) samplers (27) during cruises to the Mid-Atlantic Ridge, Guaymas Basin, and East Pacific Rise in 2008 and Mid-Cayman Rise in 2012, using either ROV Jason or HOV Alvin. In most cases a minimum of two IGT samples were taken from each vent. Reported vent temperatures are the maximum measured in real time during fluid collection (27).
Dissolved CH3SH concentrations were determined at sea upon sampler recovery by purge-and-trap gas chromatography (GC) with flame ionization detection (FID). FID, unlike sulfur-specific detection, is insensitive to the extremely high H2S concentrations in vent fluids. Gas-tight fluid aliquots (<4 mL) were acidified with ∼1 mL of 25 wt % phosphoric acid, and CH3SH was sparged with He gas (30 mL/min for 10 min) and cryofocused on an n-octane–coated silica trap (−78 °C), then thermally desorbed (145 °C) directly onto a Carbograph 1SC packed GC column (30 mL/min He, 40 °C isothermal). To limit potential losses of gaseous CH3SH during sparging (53, 54), deactivated glass and polytetrafluoroethylene tubing were used wherever possible in the purge-and-trap system. Sparging was assumed to be quantitative given the long sparge time and volatility of CH3SH, and resparging tests on samples revealed no significant evidence of incomplete removal. Before calibration and between samples, the trap was heated at >145 °C with He flowing (30 mL/min) for a minimum of 10 min to completely eliminate carryover of any residual CH3SH remaining in the trap (typically <1%) from previous analyses. In almost all cases, resulting CH3SH concentrations from separate discrete samples of the same vent yield mixing lines between bottom seawater (no detectable CH3SH) and a hydrothermal endmember or mixed fluid composition when plotted against Mg (see Endmember and Mixed Vent Fluid Compositions for further details). This indicates not only that methanethiol is conservative with respect to accidental seawater entrainment during sample collection but that any losses or additions of CH3SH due to the analytical method are not significant. Reported uncertainties (2s) for CH3SH (Dataset S1) are the larger of either the error of reproducibility or the uncertainty of the commercial gas standard used (±5%).
H2 and CO were analyzed at sea by a headspace extraction GC technique, using thermal conductivity and helium ionization detection, respectively (37, 39). H2S (total dissolved, ΣH2S) was determined either gravimetrically by precipitation as Ag2S (55) or at sea by electrochemical (28, 34) or iodometric titration. pH(25 °C) was determined at sea by electrode (28, 34, 55). Aliquots for dissolved inorganic carbon (ΣCO2, abbreviated as CO2), CH4, and C2+ hydrocarbons were stored in evacuated glass serum vials (poisoned with HgCl2) for headspace gas GC analysis (28, 55). Cl was determined by ion chromatography [IC, ±5% (55)] or electrochemical titration [±0.5% (28, 34)], and Mg was determined by either IC or inductively coupled plasma methods (28, 34, 55, 56). 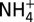 was determined by either flow injection analysis (57) for unsedimented systems or IC (Guaymas Basin), with reported errors representing the larger of either error of reproducibility (2s) or the typical ±5% reproducibility of prepared standards. Analytical uncertainties (2s) are ±0.05 for pH(25 °C); ±10% for H2S, CO, H2, and C2+ hydrocarbon concentrations; and ±5% for Mg, ΣCO2, and CH4 concentrations (28, 39, 55).
was determined by either flow injection analysis (57) for unsedimented systems or IC (Guaymas Basin), with reported errors representing the larger of either error of reproducibility (2s) or the typical ±5% reproducibility of prepared standards. Analytical uncertainties (2s) are ±0.05 for pH(25 °C); ±10% for H2S, CO, H2, and C2+ hydrocarbon concentrations; and ±5% for Mg, ΣCO2, and CH4 concentrations (28, 39, 55).
Supplementary Material
Acknowledgments
We thank the captains and crews of the R/V Atlantis and R/V Roger Revelle and the crews of the HOV Alvin and the ROV Jason for their indispensable skills in sample collection. We are grateful to N. Pester and W. Seyfried for providing Mg and H2S data for TAG and Lost City. This research was supported by National Science Foundation Grants OCE-0702677, OCE-0549829, and OCE-1061863, and NASA Grant NNX-327 09AB75G (to J.S.S.); and by funding from the Woods Hole Oceanographic Institution Deep Ocean Exploration Institute, InterRidge, and the Deutsche Forschungsgemeinschaft Research Center/Cluster of Excellence MARUM “The Ocean in the Earth System” (E.P.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
*McDermott JM, et al., AGU Fall Meeting, December 3–7, 2012, San Francisco, CA, abstr. OS22B-07.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1400643111/-/DCSupplemental.
References
- 1.Corliss JB, Baross JA, Hoffman SE. An hypothesis concerning the relationship between submarine hot springs and the origin of life on Earth. Oceanol Acta. 1981;4(Supplement):59–69. [Google Scholar]
- 2.Baross JA, Hoffman SE. Submarine hydrothermal vents and associated gradient environments as sites for the origin and evolution of life. Orig Life Evol Biosph. 1985;15(4):327–345. [Google Scholar]
- 3.Holm N. Why are hydrothermal systems proposed as plausible environments for the origin of life? Orig Life Evol Biosph. 1992;22(1-4):5–14. doi: 10.1007/BF01808024. [DOI] [PubMed] [Google Scholar]
- 4.Martin W, Baross J, Kelley D, Russell MJ. Hydrothermal vents and the origin of life. Nat Rev Microbiol. 2008;6(11):805–814. doi: 10.1038/nrmicro1991. [DOI] [PubMed] [Google Scholar]
- 5.Sousa FL, et al. Early bioenergetic evolution. Phil Trans R Soc B. 2013;368(1622):20130088. doi: 10.1098/rstb.2013.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelley DS, Baross JA, Delaney JR. Volcanoes, fluids, and life at mid-ocean ridge spreading centers. Annu Rev Earth Planet Sci. 2002;30:385–491. [Google Scholar]
- 7.McCollom TM, Seewald JS. Abiotic synthesis of organic compounds in deep-sea hydrothermal environments. Chem Rev. 2007;107(2):382–401. doi: 10.1021/cr0503660. [DOI] [PubMed] [Google Scholar]
- 8.Huber C, Wächtershäuser G. Activated acetic acid by carbon fixation on (Fe,Ni)S under primordial conditions. Science. 1997;276(5310):245–247. doi: 10.1126/science.276.5310.245. [DOI] [PubMed] [Google Scholar]
- 9.Huber C, Wächtershäuser G. Peptides by activation of amino acids with CO on (Ni,Fe)S surfaces: Implications for the origin of life. Science. 1998;281(5377):670–672. doi: 10.1126/science.281.5377.670. [DOI] [PubMed] [Google Scholar]
- 10.Cody GD, et al. Primordial carbonylated iron-sulfur compounds and the synthesis of pyruvate. Science. 2000;289(5483):1337–1340. doi: 10.1126/science.289.5483.1337. [DOI] [PubMed] [Google Scholar]
- 11.Huber C, Wächtershäuser G. α-Hydroxy and α-amino acids under possible Hadean, volcanic origin-of-life conditions. Science. 2006;314(5799):630–632. doi: 10.1126/science.1130895. [DOI] [PubMed] [Google Scholar]
- 12. Russell MJ, Hall AJ (2006) The onset and early evolution of life. Evolution of Early Earth's Atmosphere, Hydrosphere, and Biosphere - Constraints from Ore Deposits, Geological Society of America Memoir, eds Kesler SE, Ohmoto H, (Geol Soc of Am, Boulder, CO), Vol 198, pp 1–32.
- 13.Martin W, Russell MJ. Review: On the origin of biochemistry at an alkaline hydrothermal vent. Phil Trans R Soc B. 2007;362(1486):1887–1926. doi: 10.1098/rstb.2006.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novikov Y, Copley SD. Reactivity landscape of pyruvate under simulated hydrothermal vent conditions. Proc Natl Acad Sci USA. 2013;110(33):13283–13288. doi: 10.1073/pnas.1304923110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers KL, Schulte MD. Organic sulfur metabolisms in hydrothermal environments. Geobiology. 2012;10(4):320–332. doi: 10.1111/j.1472-4669.2012.00324.x. [DOI] [PubMed] [Google Scholar]
- 16.Lomans BP, et al. Formation of dimethyl sulfide and methanethiol in anoxic freshwater sediments. Appl Environ Microbiol. 1997;63(12):4741–4747. doi: 10.1128/aem.63.12.4741-4747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sander SG, Koschinsky A, Massoth G, Stott M, Hunter KA. Organic complexation of copper in deep-sea hydrothermal vent systems. Environ Chem. 2007;4(2):81–89. [Google Scholar]
- 18.Sander SG, Koschinsky A. Metal flux from hydrothermal vents increased by organic complexation. Nat Geosci. 2011;4(3):145–150. [Google Scholar]
- 19.Toner BM, et al. Preservation of iron(II) by carbon-rich matrices in a hydrothermal plume. Nat Geosci. 2009;2(3):197–201. [Google Scholar]
- 20.Heinen W, Lauwers AM. Organic sulfur compounds resulting from the interaction of iron sulfide, hydrogen sulfide and carbon dioxide in an anaerobic aqueous environment. Orig Life Evol Biosph. 1996;26(2):131–150. doi: 10.1007/BF01809852. [DOI] [PubMed] [Google Scholar]
- 21.Loison A, Dubant S, Adam P, Albrecht P. Elucidation of an iterative process of carbon-carbon bond formation of prebiotic significance. Astrobiology. 2010;10(10):973–988. doi: 10.1089/ast.2009.0441. [DOI] [PubMed] [Google Scholar]
- 22.Schulte MD, Rogers KL. Thiols in hydrothermal solution: Standard partial molal properties and their role in the organic geochemistry of hydrothermal environments. Geochim Cosmochim Acta. 2004;68(5):1087–1097. [Google Scholar]
- 23. Charlou JL, et al. (2010) High production and fluxes of H2 and CH4 and evidence of abiotic hydrocarbon synthesis by serpentinization in ultramafic-hosted hydrothermal systems on the Mid-Atlantic Ridge. Diversity of Hydrothermal Systems on Slow Spreading Ocean Ridges, Geophysical Monograph Series, eds Rona P, Devey CW, Dyment J, Murton BJ (Am Geophys Union, Washington, DC), Vol 188, pp 265–296.
- 24.Proskurowski G, et al. Abiogenic hydrocarbon production at Lost City hydrothermal field. Science. 2008;319(5863):604–607. doi: 10.1126/science.1151194. [DOI] [PubMed] [Google Scholar]
- 25.Lang SQ, Butterfield DA, Schulte M, Kelley DS, Lilley MD. Elevated concentrations of formate, acetate and dissolved organic carbon found at the Lost City hydrothermal field. Geochim Cosmochim Acta. 2010;74(3):941–952. [Google Scholar]
- 26. McCollom TM (2008) Observational, experimental, and theoretical constraints on carbon cycling in mid-ocean ridge hydrothermal systems. The Subseafloor Biosphere at Mid-Ocean Ridges, Geophysical Monograph Series, eds Wilcock WSD, DeLong EF, Kelley DS, Baross JA (Am Geophys Union, Washington, DC), Vol 144, pp 193–213.
- 27.Seewald JS, Doherty KW, Hammar TR, Liberatore SP. A new gas-tight isobaric sampler for hydrothermal fluids. Deep Sea Res Part I Oceanogr Res Pap. 2002;49(1):189–196. [Google Scholar]
- 28.Pester NJ, et al. Subseafloor phase equilibria in high-temperature hydrothermal fluids of the Lucky Strike Seamount (Mid-Atlantic Ridge, 37°17’N) Geochim Cosmochim Acta. 2012;90:303–322. [Google Scholar]
- 29.Foustoukos DI, Seyfried WE. Redox and pH constraints in the subseafloor root zone of the TAG hydrothermal system, 26°N Mid-Atlantic Ridge. Earth Planet Sci Lett. 2005;235(3-4):497–510. [Google Scholar]
- 30.Proskurowski G, Lilley MD, Olson EJ. Stable isotopic evidence in support of active microbial methane cycling in low-temperature diffuse flow vents at 9°50'N East Pacific Rise. Geochim Cosmochim Acta. 2008;72(8):2005–2023. [Google Scholar]
- 31.German CR, et al. Diverse styles of submarine venting on the ultraslow spreading Mid-Cayman Rise. Proc Natl Acad Sci USA. 2010;107(32):14020–14025. doi: 10.1073/pnas.1009205107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinsey JC, German CR. Sustained volcanically-hosted venting at ultraslow ridges: Piccard Hydrothermal Field, Mid-Cayman Rise. Earth Planet Sci Lett. 2013;380:162–168. [Google Scholar]
- 33.Connelly DP, et al. Hydrothermal vent fields and chemosynthetic biota on the world’s deepest seafloor spreading centre. Nat Commun. 2012;3:620. doi: 10.1038/ncomms1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seyfried WE, Pester NJ, Ding K, Rough M. Vent fluid chemistry of the Rainbow hydrothermal system (36°N, MAR): Phase equilibria and in situ pH controls on subseafloor alteration processes. Geochim Cosmochim Acta. 2011;75(6):1574–1593. [Google Scholar]
- 35.Von Damm KL, Edmond JM, Measures CI, Grant B. Chemistry of submarine hydrothermal solutions at Guaymas Basin, Gulf of California. Geochim Cosmochim Acta. 1985;49(11):2221–2237. [Google Scholar]
- 36.Welhan JA, Lupton J. Light hydrocarbon gases in Guaymas Basin hydrothermal fluids: Thermogenic versus abiogenic origin. AAPG Bull. 1987;71(2):215–223. [Google Scholar]
- 37.Seewald JS, Zolotov MY, McCollom T. Experimental investigation of single carbon compounds under hydrothermal conditions. Geochim Cosmochim Acta. 2006;70(2):446–460. [Google Scholar]
- 38.Kawka OE, Simoneit BRT. Survey of hydrothermally-generated petroleums from the Guaymas Basin spreading center. Org Geochem. 1987;11(4):311–328. [Google Scholar]
- 39.Cruse AM, Seewald JS. Geochemistry of low-molecular weight hydrocarbons in hydrothermal fluids from Middle Valley, northern Juan de Fuca Ridge. Geochim Cosmochim Acta. 2006;70(8):2073–2092. [Google Scholar]
- 40.Krein EB. Organic sulfur in the geosphere: Analysis, structures and chemical processes. In: Patai S, Rappoport Z, editors. Supplement S: The Chemistry of Sulphur-Containing Functional Groups. New York: Wiley; 1993. pp. 975–1032. [Google Scholar]
- 41.Summit M, Baross JA. A novel microbial habitat in the mid-ocean ridge subseafloor. Proc Natl Acad Sci USA. 2001;98(5):2158–2163. doi: 10.1073/pnas.051516098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bourbonnais A, Lehmann MF, Butterfield DA, Juniper SK. Subseafloor nitrogen transformations in diffuse hydrothermal vent fluids of the Juan de Fuca Ridge evidenced by the isotopic composition of nitrate and ammonium. Geochem Geophys Geosyst. 2012;13(1):Q02T01. [Google Scholar]
- 43.Orcutt BN, Sylvan JB, Knab NJ, Edwards KJ. Microbial ecology of the dark ocean above, at, and below the seafloor. Microbiol Mol Biol Rev. 2011;75(2):361–422. doi: 10.1128/MMBR.00039-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
44.Lilley MD, et al. Anomalous CH4 and
 concentrations at an unsedimented mid-ocean-ridge hydrothermal system. Nature. 1993;364(6432):45–47. [Google Scholar]
concentrations at an unsedimented mid-ocean-ridge hydrothermal system. Nature. 1993;364(6432):45–47. [Google Scholar] - 45.Brandes JA, Hazen RM, Yoder HS., Jr Inorganic nitrogen reduction and stability under simulated hydrothermal conditions. Astrobiology. 2008;8(6):1113–1126. doi: 10.1089/ast.2007.0187. [DOI] [PubMed] [Google Scholar]
- 46.Smirnov A, Hausner D, Laffers R, Strongin DR, Schoonen MAA. Abiotic ammonium formation in the presence of Ni-Fe metals and alloys and its implications for the Hadean nitrogen cycle. Geochem Trans. 2008;9(1):5. doi: 10.1186/1467-4866-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong CS, Yu Z, Waser NAD, Whitney FA, Johnson WK. Seasonal changes in the distribution of dissolved organic nitrogen in coastal and open-ocean waters in the North East Pacific: Sources and sinks. Deep Sea Res Part II Top Stud Oceanogr. 2002;49(24-25):5759–5773. [Google Scholar]
- 48.Takai K, et al. Cell proliferation at 122 °C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proc Natl Acad Sci USA. 2008;105(31):10949–10954. doi: 10.1073/pnas.0712334105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brault M, Simoneit BRT, Marty JC, Saliot A. Hydrocarbons in waters and particulate material from hydrothermal environments at the East Pacific Rise, 13°N. Org Geochem. 1988;12(3):209–219. [Google Scholar]
- 50.Lang SQ, Butterfield DA, Lilley MD, Johnson HP, Hedges JI. Dissolved organic carbon in ridge-axis and ridge-flank hydrothermal systems. Geochim Cosmochim Acta. 2006;70(15):3830–3842. [Google Scholar]
- 51.Foustoukos DI, Savov IP, Janecky DR. Chemical and isotopic constraints on water/rock interactions at the Lost City hydrothermal field, 30°N Mid-Atlantic Ridge. Geochim Cosmochim Acta. 2008;72(22):5457–5474. [Google Scholar]
- 52.Reeves EP, Seewald JS, Sylva SP. Hydrogen isotope exchange between n-alkanes and water under hydrothermal conditions. Geochim Cosmochim Acta. 2012;77:582–599. [Google Scholar]
- 53.Simó R. Trace chromatographic analysis of dimethyl sulfoxide and related methylated sulfur compounds in natural waters. J Chromatogr A. 1998;807(2):151–164. doi: 10.1016/s0021-9673(98)00086-7. [DOI] [PubMed] [Google Scholar]
- 54.Wardencki W. Problems with the determination of environmental sulphur compounds by gas chromatography. J Chromatogr A. 1998;793(1):1–19. [Google Scholar]
- 55.Reeves EP, et al. Geochemistry of hydrothermal fluids from the PACMANUS, Northeast Pual and Vienna Woods hydrothermal fields, Manus Basin, Papua New Guinea. Geochim Cosmochim Acta. 2011;75(4):1088–1123. [Google Scholar]
- 56.Craddock PR, et al. Rare earth element abundances in hydrothermal fluids from the Manus Basin, Papua New Guinea: Indicators of sub-seafloor hydrothermal processes in back-arc basins. Geochim Cosmochim Acta. 2010;74(19):5494–5513. [Google Scholar]
-
57.Hall PO, Aller RC. Rapid, small-volume, flow injection analysis for ΣCO2 and
 in marine and fresh-waters. Limnol Oceanogr. 1992;37(5):1113–1119. [Google Scholar]
in marine and fresh-waters. Limnol Oceanogr. 1992;37(5):1113–1119. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.