Abstract
Basic and clinical evidence suggests that omega-3 (n-3) polyunsaturated fatty acids (PUFAs) decrease fatal arrhythmias and infarct sizes. This study investigated if pericardial delivery of n-3 PUFAs would protect the myocardium from ischemic damages and arrhythmias. Acute myocardial infarctions were induced in 23 pigs with either 45 min balloon inflations or clamp occlusions of the left anterior descending coronary arteries and 180 min reperfusion. Docosahexaenoic acid (C22:6n-3, DHA, 45 mg), one of the main n-3 PUFAs in fish oil, was infused within the pericardial space only during the 40-min stabilizing phase, 45 min ischemia and initial 5 min reperfusion. Hemodynamics and cardiac functions were very similar between the DHA-treated and control groups. However, DHA therapy significantly reduced infarct sizes from 56.8 ± 4.9% for controls (n = 12) to 28.8 ± 7.9% (P < 0.01) for DHA-treated animals (n = 11). Compared with controls, DHA-treated animals significantly decreased heart rates and reduced ventricular arrhythmia scores during ischemia. Furthermore, three (25%) control animals experienced eight episodes of ventricular fibrillation (VF), and two died subsequent to unsuccessful defibrillation. In contrast, only 1 (9%) of 11 DHA-treated pigs elicited one episode of VF that was successfully converted via defibrillation to normal rhythm; thus, mortality was reduced from 17% in the controls to 0% in the DHA-treated animals. These data demonstrate that pericardial infusion of n-3 PUFA DHA can significantly reduce both malignant arrhythmias and infarct sizes in a porcine infarct model. Pericardial administration of n-3 PUFAs could represent a novel approach to treating or preventing myocardial infarctions.
ischemia-reperfusionpericardiumarrhythmiamyocardial infarctionn-3 polyunsaturated fatty acid since the original epidemiological studies were published three decades ago (2), dietary n-3 polyunsaturated fatty acids (PUFAs) have been correlated with a reduced risk of coronary heart disease. The primary sources of n-3 PUFAs in the human diet are mainly plant-derived α-linolenic acid (C18:3n-3) and marine-derived eicosapentaenoic acid (EPA, C20:5n-3) plus docosahexaenoic acid (DHA, C22:6n-3). Recently, increased n-3 PUFA intake has been shown to have reduction of both ischemia-induced myocardial damage (10, 20, 29) and arrhythmias (15, 22, 25). A major benefit of n-3 PUFAs is that, unlike several cardiac pharmaceuticals, they have only minor side effects (16, 19) and are on the Food and Drug Administration's Generally Accepted as Safe list. Although dietary supplementation is the most common administration of n-3 PUFAs in both clinical and animal studies, the resulting myocardial concentrations of free n-3 PUFAs depend on several phenotypic factors related to both the gastrointestinal system and blood. Although n-3 PUFAs have been intravenously administrated in experimental animals for cardiac arrhythmia prevention (3), and in humans for anti-inflammatory therapy (7), it has been reported that, in blood, albumins bind to n-3 PUFAs and greatly reduce their free form concentrations. Thus, in the present study, we hypothesize that a more focalized and perhaps better controlled approach to deliver n-3 PUFAs to the heart would be through the pericardial space. Compared with blood, pericardial fluid contains lower amounts of proteins and is only ~1% of the total blood volume. Therefore, pericardial infusion of n-3 PUFAs was considered to be an effective way to build up a high concentration around the heart. It was also considered that high concentrations of n-3 PUFAs should rapidly enhance their protective effects on myocardium, since they are highly lipid-soluble. Furthermore, previous studies with the pericardial administration of amiodarone and procainamide have shown that the active motion of the heart within the pericardium mixes and distributes the drugs throughout the epicardium and that deep penetrations can occur (32).
Importantly, both basic research studies and clinical trials have shown that n-3 PUFAs reduce the risk of acute myocardial infarction (10, 20, 22–25, 35, 38). Dietary supplementation of fish oil attenuated myocardial infarct size, cardiac dysfunction, and lethal ventricular arrhythmias in ischemia-reperfusion animal models (23, 24, 35, 38). However, the effects of n-3 PUFAs being directly infused to the heart on ischemic myocardium were not assessed in these studies. In the present study, we thus delivered DHA to the pericardial space in an acute ischemia-reperfusion porcine model. Our results indicate that pericardial delivery of the n-3 PUFA (DHA) reduced ischemia-induced ventricular arrhythmias, infarct sizes, and mortality. Thus this promising novel approach may have clinically relevant applications, since a number of new devices allow for less-invasive access to the pericardial space through subxyphoid or transvascular catheters (17).
METHODS AND MATERIALS
This study protocol was conducted in accordance with the Guide for the Care and Use of Laboratory Animals [Department of Health and Human Services Publication No. (NIH) 85-23, Revised 1996], and was approved by the Institutional Animal Care and Use Committee of the University of Minnesota. This ischemia- and reperfusion-induced myocardial infarction model was performed using male castrated Yorkshire swine. These animals were randomly assigned into two groups, control (n = 12) and DHA treated (n = 11).
Surgical procedures
Otherwise healthy swine with an averaged body weight of 42.3 ± 1.1 kg (mean ± SE, n = 23, P > 0.05 between control and DHA-treated groups) were initially sedated with midazolam intramuscularly (2 mg/kg), and subsequently anesthesia was maintained with intravenous pentobarbital sodium (20 mg/kg initial bolus, followed by a continuous infusion at 5–20 mg·kg−1·h−1). A er endotracheal intuba on, ventilation (2:1 air to oxygen mixture) was adjusted to maintain an arterial Pco2 of 40 ± 2 mmHg, and rectal temperatures were maintained at 38 ± 0.5°C using convective air warming as needed (Bair Hugger; Arizant Medical, Eden Prairie, MN). Two Mikro-Tip catheter transducers (5 Fr, model MPC-500; Millar Instruments, Houston, TX) were placed for ventricular pressure monitoring: 1) via the right jugular vein in the right ventricle and 2) the other via the right carotid artery in the left ventricle. A Swan-Ganz catheter was placed in the pulmonary artery via the right external jugular vein and connected up to a “Q2 Continuous Cardiac Output Computer” (Abbot Critical Care) to continuously monitor the cardiac output.
Two approaches were used to occlude the left anterior descending (LAD) coronary artery. In one group (n = 6), a guide catheter was inserted in the left coronary artery via the left carotid artery. A balloon (3.5 × 6 mm) catheter was advanced via a guide catheter to the site between the second and third branches of the LAD under fluoroscopic guidance. To ensure that vessels were completely occluded via the balloon method, a second LAD occlusion group (n = 17) was also used in our study. In these animals, following a medial sternotomy, a small incision was made in the anterior portion of the pericardial sac in each animal, and ~0.5 cm of the LAD between the second and third branches was dissected for placing a vessel clamp (Sklar Vascular Size 2 Single Clamp; Sklar Instruments, West Chester, PA). A femoral artery was cannulated for measurement of blood pressure and withdrawal of blood samples.
In all animals, a medial sternotomy was performed to expose the heart and pericardial sac. A soft infusion catheter was placed in the sac near the apex of the heart for perfusion of the control saline or DHA (45 mg, ~1 mg/kg) solutions. The animals were fully heparinized following surgical preparation and throughout the subsequent experimental protocol (300 IU/kg iv bolus of heparin followed by an infusion of 67 IU·kg–1·h–1). After 60 min stabilization following completion of surgery, the intra-arterial balloons were inflated, or the vascular clamps were placed to induce ischemia. To verify the effectiveness of these occlusions, four million microspheres (E-Z TRAC 15 μm diameter blue ultraspheres; Interactive Medical Technologies, Irvine, CA) were injected via the guide catheter in the left atria 30 min after occlusion of the coronary artery flow. The balloon was deflated, or the clamp was removed after 45 min ischemia. The autoreperfusion (physiological reperfusion) period lasted 180 min. DHA (Cayman Chemical, Ann Arbor, MI) was obtained as a stock solution of 250 mg DHA/ml ethanol and diluted to 3 mg/ml in 0.9% saline solution for subsequent pericardial infusion. The reason to choose DHA in our study is that DHA is present in all organs and is the most abundant n-3 PUFA in membranes, including in heart tissue (1). The control animals received the same volume of the saline solution plus the equivalent amount of the substrate vehicle (ethanol). Blood pressures, heart rates, left and right intraventricular pressures, dP/dt, and electrocardiograms were recorded during experiments to monitor hemodynamics, cardiac function, and rhythm. Data were acquired with SonoLAB software (Sonometrics, London, Ontario, Canada). Postacquisition analysis was performed using both CardioSoft software and data analysis software (EMKA Technologies, Falls Church, VA).
Measurements of infarct sizes and risk areas
At the end of each experiment, the LAD was reoccluded (i.e., the arterial balloon was reinflated or the clamp was repositioned), and patent blue dye was injected in the left atrial appendage to verify the ischemic area (area at risk), as previously described (9, 27). Subsequently, all animals were killed, and the hearts were removed and frozen at –20°C. Infarc on sizes were determined on the next experimental day. The hearts were sectioned to 4-mm transverse slices. The slices were then incubated with 1% triphenyltetrazolium chloride in phosphate buffer (pH 7.4) at 37°C for 10 min and fixed in 10% formalin for 1 min. Triphenyltetrazolium chloride forms a red formazan derivative when reacting with viable tissue, whereas necrotic tissue is pale white. A person blinded to the applied treatments photographed the slices and quantified the morphology of infarct size and area at risk of the left ventricles using Adobe Photoshop (CS version 8.0; Adobe Systems, San Jose, CA). In addition, microspheres disposed from homogenized infarct, area at risk, and nonrisk left ventricular tissues were counted under microscope according to the manufacturer's protocol (Ultraspheres Microsphere Extraction Protocol for Regional Blood Flow Measurement; Interactive Medical Technologies). The results were 0.8 ± 0.5, 1.0 ± 0.7, and 98.3 ± 31.0 microspheres/g myocardium for infarct, areas at risk, and nonrisk of left ventricular myocardium, respectively. These results indicate the high effectiveness of our LAD occlusions.
Arrhythmia assessments
Standard peripheral, five-lead electrocardiograms were used to monitor potential arrhythmias during each experiment (model 9030; SpaceLabs, Redmond, WA). Analyses using the Ponemah Physiology Platform version 3.1 software (Gould Instrument Systems, Valley View, OH) were completed by a person who was blinded to the given experimental protocols. A modified scoring system (9, 27) was used to quantify arrhythmias by this individual: 0 = ≤10 premature ventricular contractions (PVCs) in 9 min; 1 = 11–50 PVCs in 9 min; 2 = >50 PVCs in 9 min; 3 = 1 episode of ventricular fibrillation (VF) in 9 min; 4 = 2–5 episodes of VF in 9 min; and 5 = >5 episodes of VF in 9 min. When elicited, attempts were made to reverse VF using 30-J shocks administered via internal paddles with biphasic DC cardioversion (Lifepak 12; Medtronic, Minneapolis, MN), and, if unsuccessful, they were repeated until effective or success was considered impossible.
Fatty acid measurements
In each experiment, a 2-ml blood sample was drawn 30 min after ceasing pericardial perfusion. Also, pericardial fluid (1.5 ml) and right atrial tissue (5–10 g) in each animal were collected 180 min after termination of pericardial perfusion and before left atrial appendage injection of patent blue dye. These samples were stored at –80°C immediately a er collec on. Analysis was performed by a laboratory (Lipid Technologies, Austin, MN) in blinded fashion. Plasma, pericardial fluid, and atrial tissue lipids were extracted and analyzed according to methods described previously (18).
Data analysis
Data are presented as means ± SE. Unpaired Student's t-tests and Chi-squares test were applied accordingly. Statistical significance was considered if a P value was <0.05 between two means.
RESULTS
Following the surgical operation of each animal, they were allowed to stabilize for 20 min and another 40 min with pericardial perfusion (Fig. 1, A and B, inset). In each animal, a 2-min bolus injection of 6 ml saline solution contained either DHA for the treatment, or the same amount of vehicle (alcohol) for the control was delivered in the pericardial space. Subsequently, a pericardial infusion was started and lasted 90 min with a speed of 0.1 ml/min. Each DHA-treated animal received 45 mg DHA in 15 ml solution (3 mg/ml). Mean blood pressures in both groups averaged ~100 mmHg during the stabilization period and gradually decreased after initiation of ischemia and reperfusion (Fig. 1A). The recorded time courses showed that pericardial bolus infusions induced increases in heart rates in control animals but not in DHA-treated pigs (Fig. 1B). LAD occlusions further increased the heart rates in controls (Fig. 1B, P < 0.01). With the exception of the heart rates during ischemia, the maximal left ventricular systolic pressures, the left ventricular end diastolic pressures, the maximal rate of positive and negative left ventricular pressure changes (±dP/dt), the cardiac outputs, the maximal right ventricular systolic pressures, the right ventricular end diastolic pressures, and mean pulmonary artery pressures displayed no significant differences among DHA-treated and untreated animals during the entire experimental observation (Table 1).
Fig. 1.
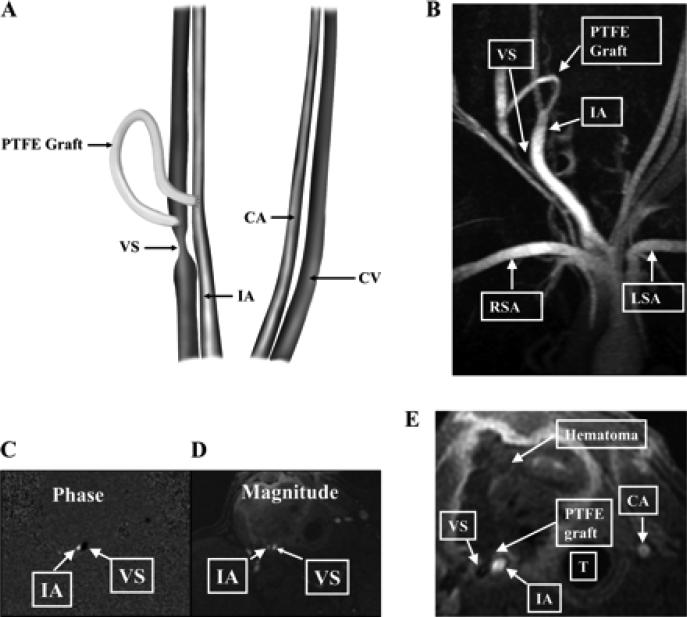
Placement of the polytetrafluoroethylene (PTFE) hemodialysis graft and representative MRI and phase-contrast magnetic resonance angiography (PC MRA) of the vein-to-graft anastomosis. A: placement of the PTFE hemodialysis graft. CA, control carotid artery; CV, control jugular vein; VS, venous stenosis, IA, inflow artery. B: MRI with PC MRA performed on day 14 that shows high grade VS formation. RSA, right subclavian artery; LSA, left subclavian artery. C: PC MRA showing the direction of blood vessels in the opposite direction as depicted by black in the VS and white in the IA. D: magnitude of the blood flow in the same vessels. E: a hematoma surrounding the PTFE graft. T, trachea.
The arrhythmia scores determined during 45 min of LAD occlusion and the first 9 min of reperfusion were markedly lower in DHA-treated pigs than in controls (Fig. 1C). The pericardial infusions of DHA significantly reduced the sum of accumulated arrhythmia scores during occlusion (Fig. 1D, P < 0.05) but not during the initial 45-min period of reperfusion (Fig. 1D, P > 0.05). Importantly, 3 out of 12 control animals elicited 8 episodes of VF during reperfusion. Applied electric defibrillation initially restored their cardiac function, but in two animals secondary episodes of VF occurred 2 h after reperfusion; aggressive defibrillation therapy failed to restore an effective rhythm (Fig. 2). In contrast, only 1 out of 11 DHA-treated pigs elicited an episode of VF during reperfusion. In this animal, electric defibrillation successfully restored the heart rhythm without further reoccurrence of VF (Fig. 2). Thus the overall morbidity from VF was decreased from 25% in the controls to 9% in the DHA-treated animals (Fig. 2C, P < 0.01); most of these VF episodes occurred within 15 min following reperfusion. The overall animal mortality was reduced from 17% in the controls to 0% in the DHA-treated pigs (Fig. 2D, P < 0.01).
Fig. 2.
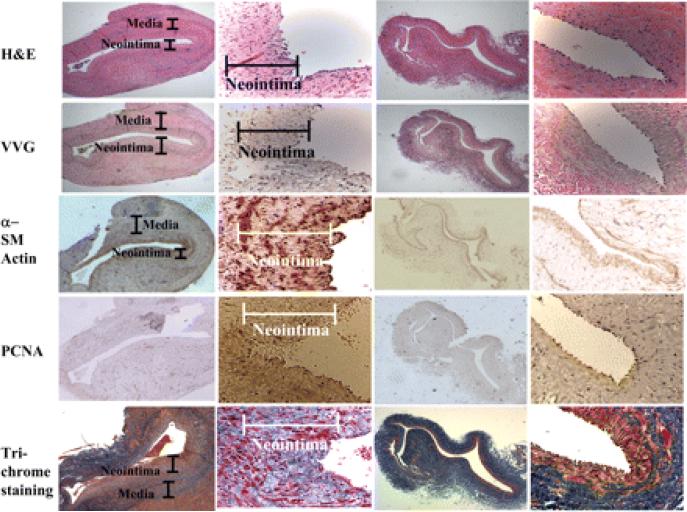
Morphological changes in the venous wall and the development of neointimal hyperplasia. Animals were killed at the three various time points, and representative sections from a day 14 animal are shown. The explanted vein-to-graft anastomosis and contralateral jugular vein were stained using hematoxyln and eosin (H&E), Verhoeff's van Gieson (VVG) stain, α-smooth muscle (SM) actin, PCNA, and Masson's trichrome. The results show that neointimal hyperplasia had developed by day 14 compared with the control vein. An analysis of the expression of α-SM actin at the vein-to-graft anastomosis and normal vein was performed. The control external jugular vein showed well-organized layers of SM cells expressing α-SM actin appearing red-brown. On day 14 after the graft placement, the neointima and media at the venous anastomosis stained more intensely than the control for α-SM actin. Cells positive for PCNA are brown. There was an increased number of cells staining brown in the neointima of the venous anastomosis by day 14 compared with the control vein. The control external jugular vein showed the most extracellular matrix (appearing blue by trichrome staining) in the media and adventitia. By day 14, more cells (appearing pink) accumulated in the media and neointima accompanied by prominent extracellular matrix deposition in the media and inner neointima.
The animals treated with pericardial DHA perfusion had significantly reduced infarct sizes compared with controls (Fig. 3). Importantly, the areas at risk for ischemia of the left ventricle for controls were 21.2 ± 2.3% (n = 12), which were not significantly different from that for DHA-treated animals that were actually slightly larger (24.2 ± 2.5%, n = 11, P > 0.05). Nevertheless, DHA treatment significantly reduced the resultant infarct sizes (Fig. 3, P < 0.01).
Fig. 3.
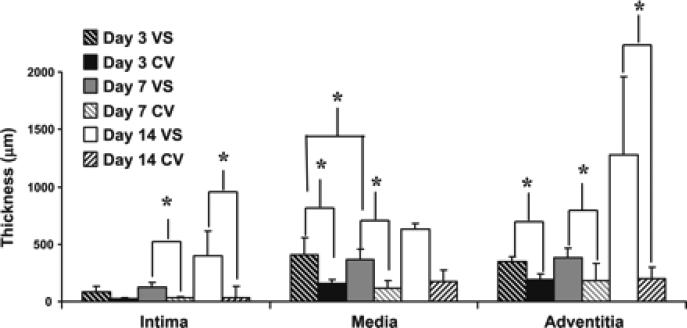
Thickness of the nointima, media, and adventitia at the vein-to-graft anastomosis (VS) and control veins (CV) over time. Specimens from explanted vein-to-graft anastomosis and control vessels were obtained from pigs killed on days 3, 7, and 14. The thickness of the neointima, media, and adventitia from the vein-to-graft anastomosis and control vessels were individually quantified using computer-assisted planimetry on VVG-stained slides and was pooled for the vein-to-graft anastomosis and control veins on days 3, 7, and 14. The average thickness of the intima from the vein-to-graft anastomosis increased over time compared with control vessels. By days 7 and 14, it was significantly increased compared with control vessels (P < 0.05). The mean thickness of the media and adventitial of the vein-to-graft anastomosis was higher than that of the control vein at all time points. By days 3 and 7, the average thickness of the media was significantly higher than the control veins (P < 0.05). At all time points, the average thickness of the adventitia of the vein-to-graft anastomosis was significantly higher than the control vein (P < 0.05). Data are means ± SD. *P < 0.05.
To determine the effects of pericardial perfusion of DHA on fatty acid components during ischemia and reperfusion, we collected blood, pericardial fluid, and atrial tissue at various time points. Table 2 summarizes the results of fatty acid components in the collected samples. Because we sampled atrial tissue and pericardial fluid 180 min after termination of pericardial perfusion in each animal, DHA measurements did not show significant differences between control and DHA-treated groups (P > 0.05). The contents of n-3 and n-6 PUFAs, as well as monounsaturated and saturated fatty acids for control samples, were also not significantly different from those for DHA-treated tissues (Table 2). These results suggest that the pericardium-infused DHA could be quickly diluted to other compartments or metabolized by the body.
DISCUSSION
Worldwide, millions of patients die annually from sudden cardiac death. More than half of such deaths occur within 1 h following an acute myocardial infarction, and this is typically associated with sustained ventricular arrhythmias. In the present study, we report that, compared with control animals, pericardial infusion of DHA at a time before and during ischemia significantly reduced infarct sizes and associated ventricular arrhythmias. However, reduction of myocardial injuries and/or induced arrhythmias has been reported for rats (35, 36), rabbits (24), and humans (6). In such reports, these benefits were associated with long-term chronic dietary intake of n-3 PUFAs. The major advantage of such a dietary approach is noninvasive, and n-3 PUFAs can be absorbed and transported to heart cells. It has been proposed that membrane-stored n-3 can then be released promptly to perform their antiarrhythmic action (21). However, the overall process of ingested n-3 PUFAs to providing cardioprotection is quite complicated compared with intravenous or pericardial perfusion (21). Many phenotypic factors may affect dietary PUFA absorption, transportation, membrane storage, and the subsequent release from heart cells. In addition, accumulation of these ingested compounds in heart cells to a cardioprotective level may take weeks or months (28, 37). Therefore, the ultimate myocardial accumulation of n-3 PUFAs to therapeutic levels with dietary approach can be quite variable and unpredictable in a given individual. Here, we report that a novel delivery approach with dosed DHA directly to the pericardial space had a dramatic cardioprotective effect.
Previously, the antiarrhythmic effects of intravenous infusion of DHA or EPA were assessed in an ischemic canine model (4). In that study, n-3 PUFAs had to be mixed with albumin before infusion, since infused DHA or EPA can cause hemolysis. Unfortunately, infused albumin would expand the intravascular volume acutely, which might in itself induce acute congestive heart failure, particularly in a subject with an already compromised cardiovascular system (4). Importantly, our described pericardial approach does not require additional albumin administration and thus would help avoid such potentially serious consequences as mentioned above. It is important to note that the amount of DHA employed in our present study (45 mg) is only 5.2% of the dose (860 mg) of that used in the aforementioned canine study (4). Similarly, this dose is only 1.2% of that used in a clinical study (3.8 g) in which it was reported that blood infusion of n-3 PUFAs resulted in reductions of sustained ventricular tachycardias in patients at high risk of sudden cardiac death (26). Thus the pericardial approach perhaps may also maximize the protective effects of n-3 PUFAs and at the same time minimize potential systemic side effects, such as hemolysis (4).
The reductions of the arrhythmia scores calculated for 9-min epochs during occlusion did not reach statistical significances except for the third 9-min epoch (Fig. 1C); yet the sum of the individual 9-min arrhythmia scores during occlusion was found to be significantly reduced for the DHA-treated animals (Fig. 1D). Interestingly, the sum of the arrhythmia scores during the initial 45-min reperfusion was not significant between two groups (Fig. 1D). The significant reduction of arrhythmia score only occurred during the first 9 min of reperfusion after cessation of DHA perfusion (Fig. 1C). These results may indicate that DHA effects quickly subsided after termination of DHA perfusion. Consistent with this finding, we observed that the heart rates were significantly lower in DHA-treated animals during occlusion. These results are also in agreement with the previous observations that intravenous infusion of n-3 fatty acids evoked significant reductions in heart rates both before and during the coronary occlusion (3). Because the blood samples were collected 30 min after cessation of DHA perfusion and the atrial tissues and pericardial fluids were sampled at the end of experiments, DHA levels in these samples were not found to be significantly different between control and DHA-treated groups (Table 2). Because the effects of pericardium-delivered DHA appeared to be quickly on and off, the fatty acid may act more likely as a pharmacological agent that may differ from its dietary supplement. A possible explanation is that, since DHA is highly lipophilic, the infused DHA in the pericardium might rapidly diffuse and dilute in myocardium, blood, and other intra- and extracellular fluids. Because the incidence of VF and mortality was significantly lower in DHA-treated animals (Fig. 2), the cardioprotective effect of DHA at certain levels was most likely extended to the whole reperfusion period. This is consistent with the dramatic reduction of infarct sizes in DHA-treated animals (Fig. 3). However, the arrhythmia scores during reperfusion were not significant between two experimental groups (Fig. 1). The underlying mechanism of such differences is unknown, but the parameters measured in this study may reflect their different sensitivities to cell damage during and after ischemia.
In the present study, we found that pericardial delivery of DHA significantly reduced ischemia- and reperfusion-induced infarct sizes but did not significantly improve ventricular functions. The possible explanation is that, in such a particular model, drastic myocardial stunning might prevent any potential acute beneficial hemodynamic effects from DHA, even in animals with little or no infarct. This porcine infarct model has been characterized in great detail in our earlier studies (8, 27). In these previous studies, we have instrumented the pigs with arrays of sonomicrometry crystals and left ventricular balloons to study global and regional function using pressure-volume loops, regional wall motion, etc.
We have learned from the regional functional data obtained via sonomicrometry that the ischemic areas were consistently and severely stunned in our model, 45 min of LAD occlusion throughout the 3-h reperfusion period, even in animals with minimal or no infarcted area (8, 27). Therefore, in DHA-treated animals with reduction of infarct sizes, severe stunning could prevent its acute beneficial functional improvement. Because the pericardial sac had to be kept with intact for pericardial delivery of DHA, we were unable to instrument these animals with epicardial sonomicrometry crystals to monitor regional myocardial contraction. However, it could be anticipated that stunning in these animals would eventually (hours-days-weeks) subside and that those DHA-treated animals with reduced infarcts would have better chronic cardiac function compared with the controls with larger infarcts.
Although the precise mechanisms how n-3 PUFAs protect the heart from ischemic damage need to be investigated further, in the present study, we have carefully looked at several mechanistic aspects of cardiac protection, i.e., hemodynamic function, infarct size, and arrhythmias in this model, aside from assessing pharmacokinetics of this novel delivery approach. More specifically, we have demonstrated very important and direct evidence for the cardioprotective effects of PUFAs delivered pericardially, i.e., infarct size reduction and arrhythmia reduction, while function was not improved acutely, which was consistent with our and other authors' previous work, as mentioned earlier (8, 27). One potential mechanism for cardiac protection other than the ones already discussed is the bradycardic effect of DHA, which could lower myocardial oxygen consumption during ischemia. In addition, previous cellular studies suggest that n-3 PUFAs could suppress inflammation (7, 33), reduce platelet aggregations (38), decrease resultant ischemic oxidative injury (13), and/or inhibit apoptotic signals (12). A recent study reported that, in rabbits, dietary-fed EPA reduced subsequent induced myocardial infarct sizes via the activation of Ca2+-activated K+ channels and nitric oxide-mediated mechanisms (24). It has been noted that inhibition of Ca2+ and Na+/Ca2+ exchanger currents by EPA or DHA prevents an increase in intracellular Ca2+ concentrations which when elevated are likely main cause of cell death during ischemia (31, 34). However, relative to this latter finding, the contractility of cardiomyocytes was not significantly affected in the presence of n-3 PUFAs (20). Nevertheless, reducing cell damage from free radicals generated during ischemia or hypoxia by the actions of n-3 PUFAs may also protect ischemic myocardium and has been reported in other studies as potential mechanisms (5, 30).
The direct actions of n-3 PUFAs on cardiac ion channels have been proposed for their antiarrhythmic effects (34). More specifically, n-3 PUFAs have been shown to inhibit voltage-gated Na+, K+, and Ca2+ channels, as well as Na+/Ca2+ exchangers (34) and Ca2+-activated K+ channels (24). Taken together, these combined effects would serve to stabilize the membrane excitability of cardiomyocytes (14). The cardiomyocytes in an ischemic border zone have relatively depolarized resting membrane potentials and possibly trigger VF due to easily being excited (20). Therefore, the elevation of n-3 PUFAs helps to stabilize the enhanced excitability of partially depolarized cells in ischemic myocardium (34) and thus prevent arrhythmias. This hypothesis is supported by the recent finding that dietary fish oil reduces the occurrence of early after depolarizations in porcine ventricular myocytes (11). Although there is no clinical report on pericardial delivery of n-3 PUFAs, we believe that such an approach could be potentially beneficial to an ischemic heart and may prevent sudden cardiac death in the event of an infarction. In summary, our current data demonstrate that pericardial infusion of the n-3 PUFA DHA significantly reduced infarct sizes and malignant arrhythmias (including VF) in an ischemia-reperfusion porcine model. However, the long-term effects of n-3 fatty acids on myocardial infarct sizes should be assessed and confirmed in future studies.
Fig. 4.
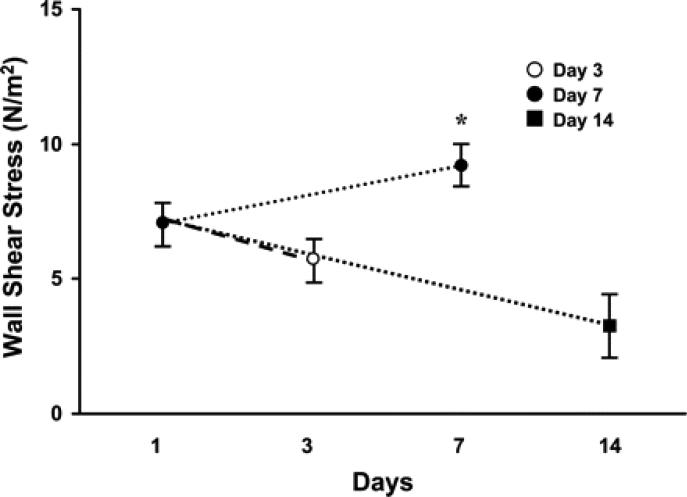
MRI measurements of the vein-to-graft anastomosis on days 1, 3, 7, and 14. The wall shear stress (WSS) was calculated from the MRI data. At all time points, the mean WSS of the VS was significantly higher (P < 0.05) than the control vein. By day 7, the WSS was significantly higher than on days 3 and 14 (P < 0.05). Data are means ± SD. *P < 0.05
Fig. 5.
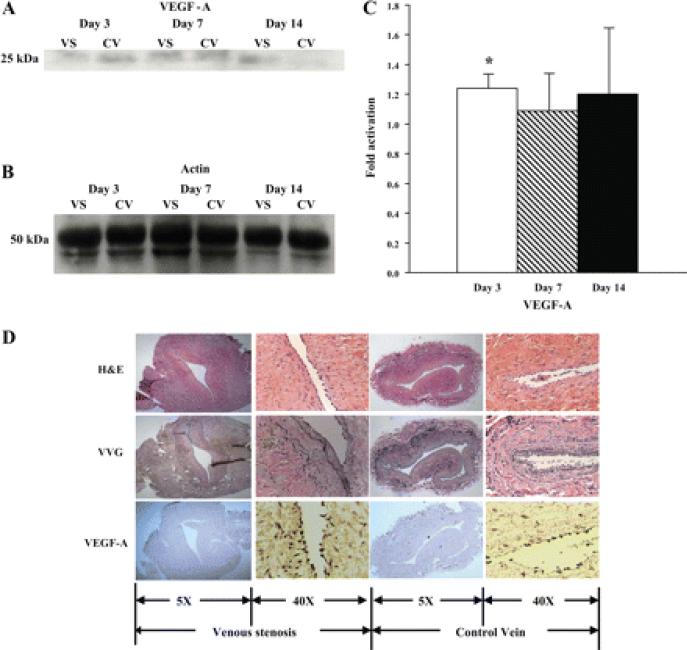
A: graph representing the appropriate protein band of VEGF-A from Western blot analysis. B: graph representing the appropriate band for β-actin from Western blot analysis for protein loading. C: semiquantitative analysis for VEGF-A performed on days 3, 7, and 14. The normalized density of VEGF-A was significantly higher at the vein-to-graft anastomosis compared with the control vein by day 3. *Significantly higher value (P < 0.05). Data are means ± SD. D: immunohistochemistry for VEGF-A for localization of expression on day 3. By immunohistochemistry, brown staining cells are positive for VEGF-A. Representative sections from a day 3 animal are shown. The left and middle left columns are from the vein-to-graft anastomosis, and middle right and right columns are from the control vein. The left and middle right columns are ×10 magnification, and the middle left and right columns are ×40 magnification. There were more cells staining brown located in the intima and media at the vein-to-graft anastomosis compared with controls
Fig. 6.
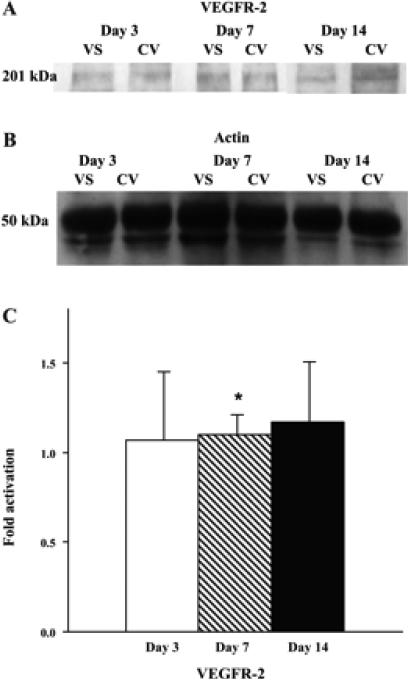
A: graph representing the appropriate protein band of VEGF receptor (VEGFR)-2 from Western blot analysis. B: graph representing the appropriate band for β-actin from Western blot analysis for protein loading. C: semiquantitative analysis performed on days 3, 7, and 14. The normalized density of VEGFR-2 was significantly higher on day 7 compared with day 3. *Significantly higher value (P < 0.05). Data are means ± SD
Fig. 7.
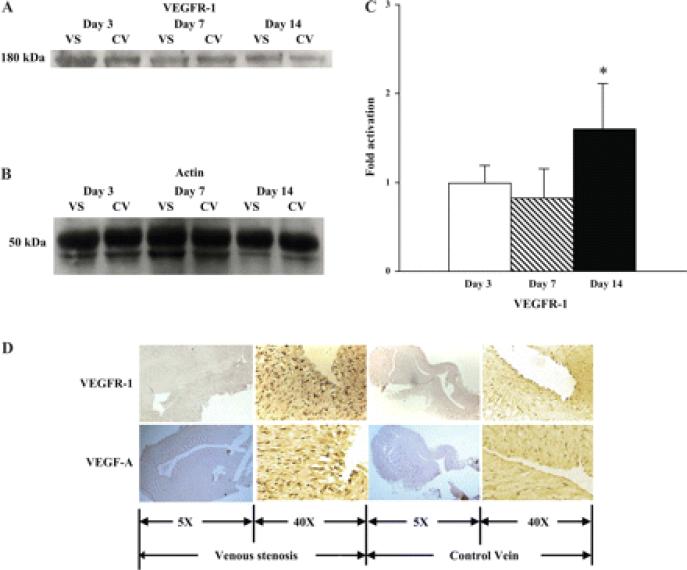
A: graph representing the appropriate protein band of VEGFR-1 from Western blot analysis. B: graph representing the appropriate band for β-actin from Western blot analysis for protein loading. C: semiquantitative analysis performed on days 3, 7, and 14. The normalized density of VEGFR-1 was significantly higher on day 14 compared with the control vein. *Significantly higher value (P < 0.05). Data are means ± SD. D: immunohistochemistry for VEGFR-1 for localization of expression on day 14. Representative sections from a day 14 animal are shown. The left and middle left columns are from the vein-to-graft anastomosis, and the middle right and right columns are from the control vein. The left and middle right columns are ×10 magnification, and the middle left and right columns are ×40 magnification. By immunohistochemistry, brown staining cells are positive for VEGF-A and VEGFR-1. There were more cells staining brown located in the intima and media at the vein-to-graft anastomosis compared with controls.
Fig. 8.
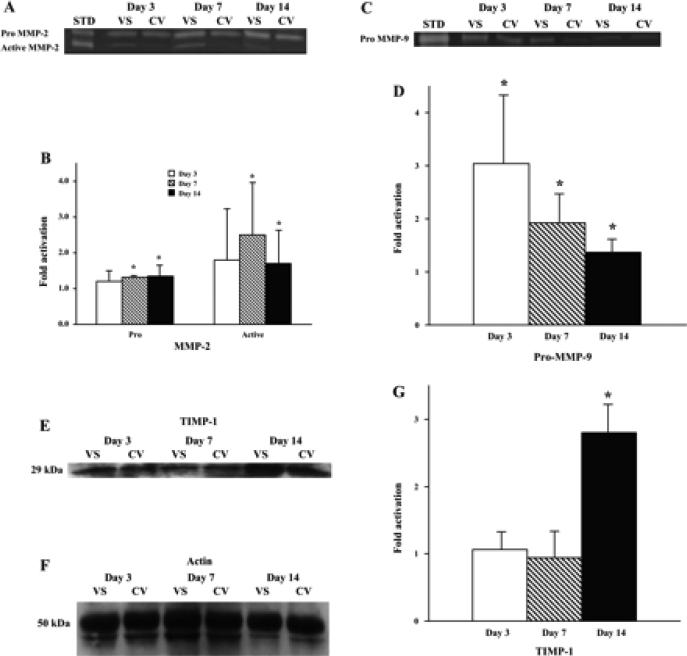
A: graph representing the appropriate protein bands of pro-matrix metalloproteinase (MMP)-2 and MMP-2 by zymography. STD, standard. B: semiquantitative analysis performed on days 3, 7, and 14. The normalized density of pro-MMP-2 and active MMP-2 at the vein-to-graft anastomosis was significantly higher than the control veins by days 7 and 14. *Significantly higher value (P < 0.05). Data are means ± SD. C: graph representing the appropriate protein band of pro-MMP-9 by zymography. D: semiquantitative analysis performed on days 3, 7, and 14. The normalized density of pro-MMP-9 was significantly higher (*) at all time points compared with the control vein. E: graph representing the appropriate protein band of tissue inhibitor of matrix metalloproteinase (TIMP)-1 by Western blot analysis. F: graph representing the appropriate band for β-actin from Western blot analysis for protein loading. G: semiquantitative analysis performed on days 3, 7, and 14. The normalized density of TIMP-1 was significantly higher on day 14 compared with the control vein. *Significantly higher value (P < 0.05).
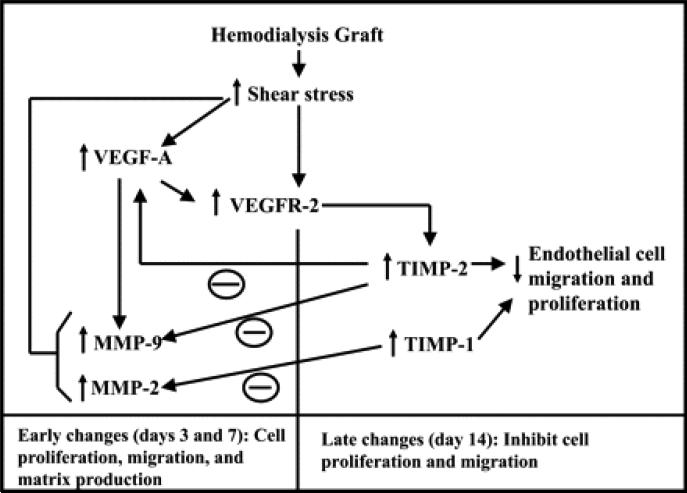
Fig. 9.
Schematic depicting potential pathways from the present study
Acknowledgments
We are grateful to the team members of the Visible Heart Laboratory at the University of Minnesota for technical help.
GRANTS
This study was supported in part by Medtronic, Inc., and the Institute for Engineering in Medicine at the University of Minnesota.
Footnotes
•The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83:1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. MedlineWeb of Science. [DOI] [PubMed] [Google Scholar]
- 2.Bang HO, Dyerberg J, Sinclair HM. The composition of the Eskimo food in north western Greenland. Am J Clin Nutr. 1980;33:2657–2661. doi: 10.1093/ajcn/33.12.2657. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 3.Billman GE, Hallaq H, Leaf A. Prevention of ischemia-induced ventricular fibrillation by omega 3 fatty acids. Proc Natl Acad Sci USA. 1994;91:4427–4430. doi: 10.1073/pnas.91.10.4427. Abstract/FREE Full Text. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billman GE, Kang JX, Leaf A. Prevention of sudden cardiac death by dietary pure omega-3 polyunsaturated fatty acids in dogs. Circulation. 1999;99:2452–2457. doi: 10.1161/01.cir.99.18.2452. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 5.Bordoni A, Angeloni C, Leoncini E, Danesi F, Maranesi M, Biagi PL, Hrelia S. Hypoxia/reoxygenation alters essential fatty acids metabolism in cultured rat cardiomyocytes: protection by antioxidants. Nutr Metab Cardiovasc Dis. 2005;15:166–173. doi: 10.1016/j.numecd.2004.04.003. CrossRefMedlineWeb of Science. [DOI] [PubMed] [Google Scholar]
- 6.Calo L, Bianconi L, Colivicchi F, Lamberti F, Loricchio ML, de Ruvo E, Meo A, Pandozi C, Staibano M, Santini M. N-3 Fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a randomized, controlled trial. J Am Coll Cardiol. 2005;45:1723–1728. doi: 10.1016/j.jacc.2005.02.079. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 7.Carpentier YA, Hacquebard M. Intravenous lipid emulsions to deliver omega 3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2006;75:145–148. doi: 10.1016/j.plefa.2006.05.004. CrossRefMedlineWeb of Science. [DOI] [PubMed] [Google Scholar]
- 8.Coles JA, Jr, Sigg DC, Iaizzo PA. Role of kappa-opioid receptor activation in pharmacological preconditioning of swine. Am J Physiol Heart Circ Physiol. 2003;284:H2091–H2099. doi: 10.1152/ajpheart.00843.2002. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 9.Curtis MJ, Walker MJ. Quantification of arrhythmias using scoring systems: an examination of seven scores in an in vivo model of regional myocardial ischaemia. Cardiovasc Res. 1988;22:656–665. doi: 10.1093/cvr/22.9.656. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 10.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–785. doi: 10.1161/01.cir.99.6.779. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 11.Den Ruijter HM, Verkerk AO, Berecki G, Bakker D, van Ginneken AC, Coronel R. Dietary fish oil reduces the occurrence of early afterdepolarizations in pig ventricular myocytes. J Mol Cell Cardiol. 2006;41:914–917. doi: 10.1016/j.yjmcc.2006.08.001. CrossRefMedlineWeb of Science. [DOI] [PubMed] [Google Scholar]
- 12.Engelbrecht AM, Engelbrecht P, Genade S, Niesler C, Page C, Smuts M, Lochner A. Long-chain polyunsaturated fatty acids protect the heart against ischemia/reperfusion-induced injury via a MAPK dependent pathway. J Mol Cell Cardiol. 2005;39:940–954. doi: 10.1016/j.yjmcc.2005.08.004. CrossRefMedlineWeb of Science. [DOI] [PubMed] [Google Scholar]
- 13.Grynberg A. Effectors of fatty acid oxidation reduction: promising new anti-ischaemic agents. Curr Pharm Des. 2005;11:489–509. doi: 10.2174/1381612053382061. CrossRefMedlineWeb of Science. [DOI] [PubMed] [Google Scholar]
- 14.Kang JX, Xiao YF, Leaf A. Free, long-chain, polyunsaturated fatty acids reduce membrane electrical excitability in neonatal rat cardiac myocytes. Proc Natl Acad Sci USA. 1995;92:3997–4001. doi: 10.1073/pnas.92.9.3997. Abstract/FREE Full Text. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kottke TE, Wu LA, Brekke LN, Brekke MJ, White RD. Preventing sudden death with n-3 (omega-3) fatty acids and defibrillators. Am J Prev Med. 2006;31:316–323. doi: 10.1016/j.amepre.2006.06.006. CrossRefMedlineWeb of Science. [DOI] [PubMed] [Google Scholar]
- 16.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 17.Laham RJ, Simons M, Hung D. Subxyphoid access of the normal pericardium: a novel drug delivery technique. Catheter Cardiovasc Interv. 1999;47:109–111. doi: 10.1002/(SICI)1522-726X(199905)47:1<109::AID-CCD24>3.0.CO;2-3. CrossRefMedlineWeb of Science. [DOI] [PubMed] [Google Scholar]
- 18.Lands WE, Libelt B, Morris A, Kramer NC, Prewitt TE, Bowen P, Schmeisser D, Davidson MH, Burns JH. Maintenance of lower proportions of (n - 6) eicosanoid precursors in phospholipids of human plasma in response to added dietary (n-3) fatty acids. Biochim Biophys Acta. 1992;1180:147–162. doi: 10.1016/0925-4439(92)90063-s. Medline. [DOI] [PubMed] [Google Scholar]
- 19.Leaf A, Jorgensen MB, Jacobs AK, Cote G, Schoenfeld DA, Scheer J, Weiner BH, Slack JD, Kellett MA, Raizner AE, Weber PC, Mahrer PR, Rossouw JE. Do fish oils prevent restenosis after coronary angioplasty? Circulation. 1994;90:2248–2257. doi: 10.1161/01.cir.90.5.2248. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 20.Leaf A, Kang JX, Xiao YF, Billman GE. Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation. 2003;107:2646–2652. doi: 10.1161/01.CIR.0000069566.78305.33. FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 21.Leaf A, Xiao YF, Kang JX, Billman GE. Prevention of sudden cardiac death by n-3 polyunsaturated fatty acids. Pharmacol Ther. 2003;98:355–377. doi: 10.1016/s0163-7258(03)00039-1. CrossRefMedlineWeb of Science. [DOI] [PubMed] [Google Scholar]
- 22.Marchioli R, Levantesi G, Macchia A, Maggioni AP, Marfisi RM, Silletta MG, Tavazzi L, Tognoni G, Valagussa F. Antiarrhythmic mechanisms of n-3 PUFA and the results of the GISSI-Prevenzione trial. J Membr Biol. 2005;206:117–128. doi: 10.1007/s00232-005-0788-x. CrossRefMedlineWeb of Science. [DOI] [PubMed] [Google Scholar]
- 23.Mehra MR, Lavie CJ, Ventura HO, Milani RV. Fish oils produce anti-inflammatory effects and improve body weight in severe heart failure. J Heart Lung Transplant. 2006;25:834–838. doi: 10.1016/j.healun.2006.03.005. CrossRefMedlineWeb of Science. [DOI] [PubMed] [Google Scholar]
- 24.Ogita H, Node K, Asanuma H, Sanada S, Takashima S, Minamino T, Soma M, Kim J, Hori M, Kitakaze M. Eicosapentaenoic acid reduces myocardial injury induced by ischemia and reperfusion in rabbit hearts. J Cardiovasc Pharmacol. 2003;41:964–969. doi: 10.1097/00005344-200306000-00020. CrossRefMedlineWeb of Science. [DOI] [PubMed] [Google Scholar]
- 25.Rauch B, Schiele R, Schneider S, Gohlke H, Diller F, Gottwik M, Steinbeck G, Heer T, Katus H, Zimmer R, Erdogan A, Pfafferott C, Senges J. For The Omega-Study Group. Highly Purified Omega-3 Fatty Acids for Secondary Prevention of Sudden Cardiac Death After Myocardial Infarction-Aims and Methods of the OMEGA-Study. Cardiovasc Drugs Ther. 2006;20:365–375. doi: 10.1007/s10557-006-0495-6. CrossRefMedlineWeb of Science. [DOI] [PubMed] [Google Scholar]
- 26.Schrepf R, Limmert T, Claus Weber P, Theisen K, Sellmayer A. Immediate effects of n-3 fatty acid infusion on the induction of sustained ventricular tachycardia. Lancet. 2004;363:1441–1442. doi: 10.1016/S0140-6736(04)16105-9. CrossRefMedlineWeb of Science. [DOI] [PubMed] [Google Scholar]
- 27.Sigg DC, Coles JA, Jr, Oeltgen PR, Iaizzo PA. Role of delta-opioid receptor agonists on infarct size reduction in swine. Am J Physiol Heart Circ Physiol. 2002;282:H1953–H1960. doi: 10.1152/ajpheart.01045.2001. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 28.Song JH, Fujimoto K, Miyazawa T. Polyunsaturated (n-3) fatty acids susceptible to peroxidation are increased in plasma and tissue lipids of rats fed docosahexaenoic acid-containing oils. J Nutr. 2000;130:3028–3033. doi: 10.1093/jn/130.12.3028. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 29.Stone NJ. The Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardio (GISSI)-Prevenzione Trial on fish oil and vitamin E supplementation in myocardial infarction survivors. Curr Cardiol Rep. 2000;2:445–451. doi: 10.1007/s11886-000-0059-5. Medline. [DOI] [PubMed] [Google Scholar]
- 30.Supari F, Ungerer T, Harrison DG, Williams JK. Fish oil treatment decreases superoxide anions in the myocardium and coronary arteries of atherosclerotic monkeys. Circulation. 1995;91:1123–1128. doi: 10.1161/01.cir.91.4.1123. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 31.ter Keurs HE, Zhang YM, Davidoff AW, Boyden PA, Wakayama Y, Miura M. Damage induced arrhythmias: mechanisms and implications. Can J Physiol Pharmacol. 2001;79:73–81. CrossRefMedlineWeb of Science. [PubMed] [Google Scholar]
- 32.Ujhelyi MR, Hadsall KZ, Euler DE, Mehra R. Intrapericardial therapeutics: a pharmacodynamic and pharmacokinetic comparison between pericardial and intravenous procainamide delivery. J Cardiovasc Electrophysiol. 2002;13:605–611. doi: 10.1046/j.1540-8167.2002.00605.x. CrossRefMedlineWeb of Science. [DOI] [PubMed] [Google Scholar]
- 33.Witte KK, Clark AL. Fish oils–adjuvant therapy in chronic heart failure? Eur J Cardiovasc Prev Rehabil. 2004;11:267–274. doi: 10.1097/01.hjr.0000136728.27524.f5. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 34.Xiao YF, Sigg DC, Leaf A. The antiarrhythmic effect of n-3 polyunsaturated fatty acids: modulation of cardiac ion channels as a potential mechanism. J Membr Biol. 2005;206:141–154. doi: 10.1007/s00232-005-0786-z. CrossRefMedlineWeb of Science. [DOI] [PubMed] [Google Scholar]
- 35.Yang B, Saldeen TG, Nichols WW, Mehta JL. Dietary fish oil supplementation attenuates myocardial dysfunction and injury caused by global ischemia and reperfusion in isolated rat hearts. J Nutr. 1993;123:2067–2074. doi: 10.1093/jn/123.12.2067. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 36.Zaloga GP, Harvey KA, Stillwell W, Siddiqui R. Trans fatty acids and coronary heart disease. Nutr Clin Pract. 2006;21:505–512. doi: 10.1177/0115426506021005505. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 37.Zaloga GP, Ruzmetov N, Harvey KA, Terry C, Patel N, Stillwell W, Siddiqui R. (N-3) long-chain polyunsaturated fatty acids prolong survival following myocardial infarction in rats. J Nutr. 2006;136:1874–1878. doi: 10.1093/jn/136.7.1874. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 38.Zhu BQ, Sievers RE, Sun YP, Morse-Fisher N, Parmley WW, Wolfe CL. Is the reduction of myocardial infarct size by dietary fish oil the result of altered platelet function? Am Heart J. 1994;127:744–755. doi: 10.1016/0002-8703(94)90540-1. CrossRefMedlineWeb of Science. [DOI] [PubMed] [Google Scholar]