Abstract
BACKGROUND
Vitamin D deficiency is the most common nutritional deficiency in the United States. It is seldom measured or recognized, and rarely is treated, particularly in critically ill patients. The purpose of this study was to investigate the prevalence and impact of vitamin D deficiency in surgical intensive care unit patients. We hypothesized that severe vitamin D deficiency increases the length of stay, mortality rate, and cost in critically ill patients admitted to surgical intensive care units.
METHODS
We performed a prospective observational study of vitamin D status on 258 consecutive patients admitted to the Surgical Intensive Care Unit at Grady Memorial Hospital between August 2009 and January 2010. Vitamin D levels (25 [OH]2 vitamin-D3) were measured by high-pressure liquid chromatography and tandem mass spectrometry. Vitamin D deficiency was defined as follows: severe deficiency was categorized as less than 13 ng/mL; moderate deficiency was categorized as 14 to 26 ng/mL; mild deficiency was categorized as 27 to 39 ng/mL; and normal levels were categorized as greater than 40 ng/mL.
RESULTS
Of the 258 patients evaluated, 70.2% (181) were men, and 29.8% (77) were women; 57.6% (148) were African American and 32.4% (109) were Caucasian. A total of 138 (53.5%) patients had severe vitamin D deficiency, 96 (37.2%) had moderate deficiency, 18 (7.0%) had mild deficiency, and 3 (1.2%) of the patients had normal vitamin D levels. The mean length of stay in the Surgical Intensive Care Unit for the severe vitamin D–deficient group was 13.33 ± 19.5 days versus 7.29 ± 15.3 days and 5.17 ± 6.5 days for the moderate and mild vitamin D-deficient groups, respectively, which was clinically significant (P = .002). The mean treatment cost during the patient stay in the surgical intensive care unit was $51,413.33 ± $75,123.00 for the severe vitamin D–deficient group, $28,123.65 ± $59,752.00 for the moderate group, and $20,414.11 ± $25,714.30 for the mild vitamin D–deficient group, which also was clinically significant (P = .027). More importantly, the mortality rate for the severe vitamin D–deficient group was 17 (12.3%) versus 11 (11.5%) in the moderate group (P = .125). Because no deaths occurred in the mildly or normal vitamin D–deficient groups, we compared the mortality rate between severe/moderate and mild/normal vitamin D groups (P = .047).
CONCLUSIONS
In univariate analysis, severe and moderate vitamin D deficiency was related inversely to the length of stay in the surgical intensive care unit (r = .194; P = .001), related inversely to surgical intensive care unit treatment cost (r = .194; P = .001) and mortality (r = .125; P = .023), compared with the mild vitamin D–deficient group, after adjusting for age, sex, race, and comorbidities (myocardial infarctions, acute renal failure, and pneumonia); the length of stay, surgical intensive care unit cost, and mortality remained significantly associated with vitamin D deficiency.
Keywords: Vitamin D, Deficiency, Surgical intensive care unit, Severe
Vitamin D deficiency is the most common nutritional deficiency in the United States. Vitamin D deficiency affects more than 70% of the US general population (210 million people).1–3 Common causes of vitamin D deficiency include poor vitamin D content in foods (very few foods are fortified with vitamin D); sunscreen use, which blocks 95% of vitamin D production by the skin; melanin pigment in dark-skinned people (African Americans) who need 3 to 6 times longer sun exposure than Caucasians to produce the same amount of vitamin D; elderly, aging skin that reduces vitamin D production by 70% at age 70; obesity, which causes vitamin D to be stored in the fat cells instead of the liver in normal-sized people; chronic kidney disease; liver failure; people who stay indoors (nursing home patients); lack of, or inadequate, vitamin D supplementation (current recommended daily allowance is too low for optimal health); and seasonal variations, latitude, and time of day.1 In the United States, people living above the 32nd latitude (eg, Atlanta, GA) cannot produce vitamin D from November to March. This is because the sun hits the northern hemisphere at a 45° angle, instead of the 90° angle in the summer that produces ultraviolet-band sunlight of wavelengths from 290 to 315 nm, which is required for vitamin D production from 10 AM until 3 PM.4 Ultraviolet-band sunlight of these wavelengths are strongest during these hours.
Although vitamin D typically is classified as a fat-soluble vitamin that plays an important role in bone metabolism, it is actually also a steroid hormone with pleiotrophic effects.2 Vitamin D is derived from the skin and diet, and is metabolized in the liver to its major circulating form, 25-hydroxyvitamin D, which is the form used to determine a person’s vitamin D status.1 25-(OH)D3 is metabolized in the kidney to its biologically active form, 1,25 (OH)2 vitamin D3, by the enzyme 25-hydroxyvitamin D-1,25-α-hydroxylase.1
Recent studies have shown that vitamin D receptors are present in almost all tissues and cells in the human body. In addition, several studies have revealed that vitamin D is important in immunomodulation, regulation of inflammation and cytokines, cell proliferation, cell differentiation, apoptosis, angiogenesis, muscle strength, and muscle contraction, in addition to calcium, magnesium, phosphate homeostasis, and bone formation.1,2 Thus, vitamin D is a very powerful hormone affecting more than 2,000 genes in the human body, which suggests it may play a critical role in the maintenance of optimal health.
Several epidemiologic studies have suggested that vitamin D deficiency is associated with the development of cardiovascular diseases; autoimmune disorders; and 17 different types of cancer, including breast, prostate, lung, colon, and renal, with associated increased mortality rates.1,5–7 A meta-analysis of 18 randomized clinical trials on vitamin D supplementation found that randomization to vitamin D was associated with lower all-cause mortality rates in the general population.6 Although it is well known that the combination of vitamin D and calcium is necessary to maintain bone density as people age, vitamin D deficiency also may be an independent risk factor for falls and fractures among the elderly. Another study showed that vitamin D levels less than 17.8 ng/mL increase the risk of death by 26% from all causes in the general population.5,8 Unfortunately, little evidence guides clinicians when to screen for vitamin D deficiency or effective treatment options, particularly as it relates to trauma and critical ill surgical patients admitted to the intensive care unit.9,10
Despite these suggested associations, we found no published studies evaluating the relationship between the severity of vitamin D deficiency and mortality risk in surgical intensive care unit patients. We hypothesized that vitamin D deficiency is an independent risk factor for length of stay, surgical intensive care cost, and mortality in critically ill surgical patients.
Methods
We conducted a prospective observational study on the vitamin D status of 258 trauma, vascular, and general surgery patients admitted to the surgical intensive care unit at Grady Memorial Hospital between August 2009 and January 2011 to assess the prevalence of vitamin D deficiency and its impact on adverse outcomes in this patient population. The research protocol was approved by the Morehouse School of Medicine’s Institutional Review Board. All patients admitted to the surgical intensive care unit were included in the study. There was no exclusion criterion. Vitamin D levels were drawn on all patients within 24 hours of admission to the surgical intensive care unit. Vitamin D levels were measured using high-pressure liquid chromatography and tandem mass spectrometry (Quest Diagnostics Lab, Valencia, CA). All patients in the study were started on vitamin D 50,000 IU/wk once they were able to tolerate internal nutritional support. Follow-up levels were not drawn routinely in this population because it takes up to 3 months of high-dose weekly therapy to achieve adequate steady-state levels based on the currently suggested management of vitamin D deficiency by Dr. Mike Hollick, an expert on vitamin D metabolism.1 As a result, our treatment strategy did not initially influence our outcomes or confound our results. It is part of our standard of care to aggressively treat all vitamin and mineral deficiencies in our Surgical Intensive Care Unit. Based on current data, we believe that all deficiencies should be corrected for stressed, critically ill patients to achieve optimal health and recovery. Because vitamin D deficiency has become a worldwide epidemic, and appropriate replacement of identified nutritional deficiencies has become a routine part of our patient care regimen for this high-risk population, consent for standard treatment of vitamin deficiencies was not deemed necessary. In our future analysis, we will evaluate whether more aggressive treatment of vitamin D deficiency will affect outcome, and if vitamin D deficiency is more than simply a risk marker of poor outcome. There were no adverse reactions in any of the 258 patients treated in this study (no episodes of hypercalcemia).
A vitamin D–deficiency severity scale (Matthews/Danner/Ahmed scale) was created and defined as follows: severe deficiency was categorized as less than 13 ng/mL; moderate deficiency was categorized as 14 to 26 ng/mL; mild deficiency was categorized as 27 to 39 ng/mL; and a normal level was categorized as greater than 40 ng/mL (Table 1). Most of the recent knowledge on vitamin D has been acquired over the past 10 years. Based on the clinical and physiological aspects of vitamin D, we believed that a new vitamin D–deficiency scale was needed. Clinically, we noticed that most deaths occurred at vitamin D levels less than 13 ng/mL (our first inflection point). Next, we noticed that no deaths occurred at vitamin D levels greater than 26 ng/mL (our second inflection point) (Figure 1).
Table 1.
Matthews-Danner-Ahmed Vitamin D scale
| Vitamin D deficiency scale | Lower limit, ng/mL | Upper limit, ng/mL |
|---|---|---|
| Normal | ≥40 | ≤70 |
| Mild | 27 | 39 |
| Moderate | 14 | 26 |
| Severe | ≤4 | ≤13 |
Figure 1.
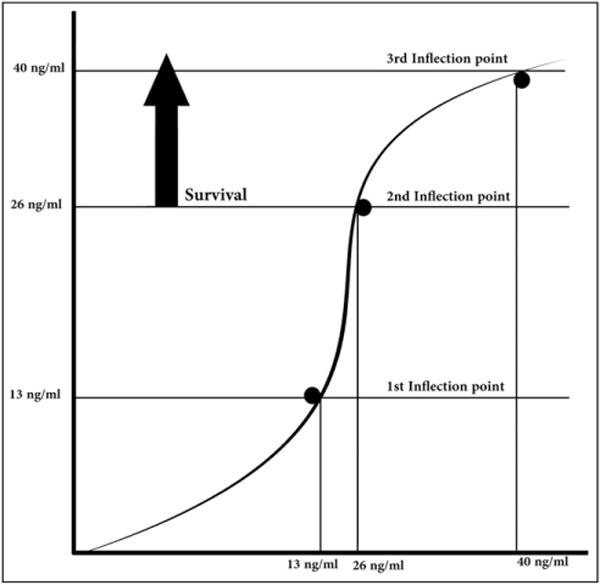
Displays serum vitamin D level (x-axis) versus survival (y-axis).
Furthermore, recent research has shown that muscle strength, performance speed, and maximum bone density increased until vitamin D levels reached 40 ng/mL or more.1,11 In addition, vitamin D levels tend to decrease by 20% to 30% during the winter months in temperate climates. Thus, using a baseline vitamin D level of 30 ng/mL or less is inadequate to confer optimal immunoprotection during these vulnerable periods of relative vitamin D deficiency.
We examined baseline patient demographics (age, race, sex) and nutritional status using the baseline levels of the biochemical markers calcium, albumin, and prealbumin. To examine the potential impact of comorbidities, the presence of the following conditions was evaluated: cardiovascular disease, pneumonia, urinary tract infections, pulmonary embolus, cancer, and trauma injuries.
The primary outcome assessed in our study was surgical intensive care unit length of stay and surgical intensive care unit cost. The secondary outcome was mortality rate among vitamin D–deficient groups. Statistical analysis was performed using SPSS 16.0 software (SPSS, Chicago, IL). Data were summarized as means plus (+) or minus (−) standard deviation and proportion (percentages) for continuous and qualitative data, respectively. Comparisons between groups were performed using the Student t test and the chi-square test for quantitative and qualitative data, respectively. A P value of less than .05 was considered statistically significant.
Results
Of the 258 patients evaluated, 70.2% (181) were men, and 29.8% (77) were women; 57.6% (148) were African American and 32.4% (109) were Caucasian. A total of 138 (53.5%) of the patients had severe vitamin D deficiency, 99 (38.4%) had moderate deficiency, 18 (6.9%) had mild deficiency, and 3 (1.2%) of the patients had normal vitamin D levels. Eighty percent (206) were trauma patients, 20.2% (52) developed pneumonia during the intensive care unit (ICU) stay, and 40.0% (85) of the patients were intubated. The baseline characteristics of patients are presented in Table 2.
Table 2.
Baseline population characteristics
| Characteristics | Frequency, n | Percentage |
|---|---|---|
| Men | 181 | 70.2 |
| Women | 77 | 29.8 |
| African American | 148 | 57.6 |
| Non–African American | 109 | 42.4 |
| Severe vitamin D deficiency (≤13 ng/mL) | 138 | 53.5 |
| Moderate vitamin D deficiency (14–26 ng/mL) | 96 | 37.2 |
| Mild vitamin D deficiency (27–39 ng/mL) | 18 | 7.0 |
| Normal vitamin D level (≥40 ng/mL) | 3 | 1.2 |
| Trauma | 206 | 79.8 |
| Intubated | 85 | 32.9 |
| Pneumonia | 52 | 20.2 |
| Comorbidity | 44 | 17.1 |
| Mortality | 28 | 10.9 |
The mean age of the severely deficient group was 46.3 ± 18.1 years, 44.3 ± 17.0 years for the moderately deficient group, and 57.22 ± 19 years for the mildly deficient group. The mean vitamin D level of the severely deficient group was 8.25 ng/mL, whereas the means for the moderately and mildly deficient groups were 18.74 and 30.5 ng/mL, respectively. The mean length of stay in the surgical intensive care unit for the severe vitamin D–deficient group was 13.44 + 20 days versus 6.66 ± 15.8 days and 5.2 ± 6.7 days for the moderate and mild vitamin D–deficient groups, respectively (Table 3).
Table 3.
Vitamin D–deficient group description
| Variable | Severe vitamin D–deficiency group N (mean ± SD) | Moderate vitamin D deficiency group N (mean ± SD) | Mild vitamin D deficiency group N (mean ± SD) |
|---|---|---|---|
| Age, y | 137 (46.3 ± 18.1) | 95 (44.3 ± 17.0) | 18 (57.22 ± 19.1) |
| Calcium level, mg/dL | 124 (8.54 ± .87) | 87 (8.77 ± .68) | 17 (9.07 ± .69) |
| Pre-albumin level, mg/dL | 110 (13.92 ± 6.8) | 87 (19.9 ± 7.5) | 17 (20.19 ± 7.2) |
| Albumin level, mg/dL | 124 (3.25 ± .84) | 87 (3.61 ± .53) | 17 (3.74 ± .61) |
| Vitamin D level, ng/mL | 138 (8.25 ± 3.07) | 96 (18.74 ± 3.52) | 18 (31.0 ± 3.06) |
| Length of ICU stay, d | 138 (13.33 ± 19.5) | 94 (7.29 ± 15.3) | 18 (5.17 ± 6.5) |
| ICU treatment cost, US $ | 138 (51,413.3 ± 75,123.0) | 92 (28,123.6 ± 59,752.0) | 17 (20,414.1 ± 25,714.3) |
SD = standard deviation.
Patients with normal and mild vitamin D deficiency had a similar stay in the surgical intensive care unit as those with moderate vitamin D deficiency. The mean length of stay in the surgical intensive care unit for the severe vitamin D–deficient group was 13 ± 19.4 days versus 7.2 ± 15.3 days and 5.17 ± 6.5 days for the moderate and mild moderate and mild vitamin D-deficient groups, respectively, which was clinically significant (P = .002) (Figure 2).
Figure 2.
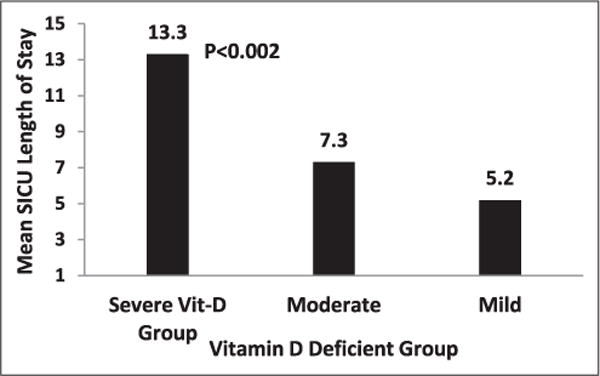
Displays the means length of stay in surgical intensive care units.
The mean treatment cost for staying in the surgical intensive care unit was $51,413.33 for the severe vitamin D–deficient group, $28,123.65 for the moderate group, and $20,414.11 for the mild vitamin D–deficient group, which also was clinically significant (P = .030). The estimated costs were calculated by multiplying the standard hospital ICU charge of $3,856 per day by the mean surgical intensive care unit length of stay. These estimates do not include the cost of any surgical interventions (Figure 3).
Figure 3.
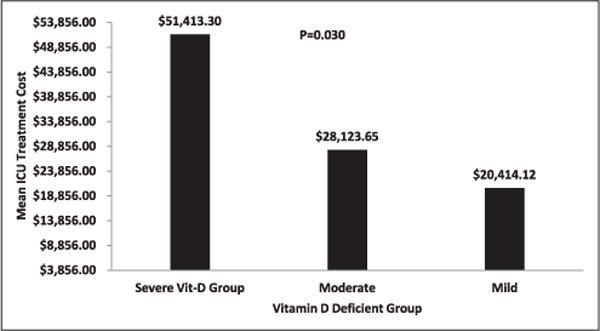
The mean treatment cost during surgical ICU stay.
The mortality rate for the severe vitamin D–deficient group was 12.3% versus 11.5% in the moderately deficient group (P < .199). Because no deaths occurred in the mildly or normal vitamin D–deficient groups, we compared the mortality rate between severe/moderate and mild/normal vitamin D groups (P = .047) (Figure 4).
Figure 4.
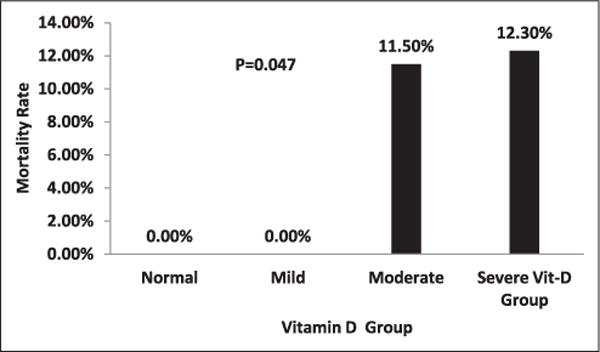
Displays the mortality rate in normal/mild and moderate/severe vitamin D–deficient groups.
In univariate analysis, severe and moderate vitamin D deficiency was related inversely to the length of stay in the surgical intensive care unit (r = −.199; P = .001), was related inversely to the surgical intensive care unit treatment cost (r = −.202; P = .001), and was related inversely to mortality (r = −.067; P = .142), compared with the mild vitamin D–deficient group. In multivariable analysis after adjusting for age, sex, race, and comorbidities (myocardial infarction, acute renal failure, and pneumonia), the length of stay in the ICU and the surgical intensive care unit treatment cost remained significantly associated with vitamin D–deficiency levels, indicating that severe vitamin D deficiency increases the length of stay and cost in critically ill patients admitted to surgical intensive care units.
Discussion
Vitamin D deficiency has been found to be very prevalent in modern society.1 Association between vitamin D deficiency and increased mortality in the general population is now fairly well established.5,8–10 According to many experts, normal vitamin D status seems to reduce the risk of almost every chronic disease of aging.6,7,12,13 In fact, a recent study showed that people with deficient vitamin D levels were twice as likely to die over a 7-year period as individuals with a normal serum vitamin D status.5
It has been suggested that vitamin D deficiency is present in at least 50% of critically ill patients admitted to intensive care units.9,10 However, the manner in which vitamin D deficiency was defined in previous studies may have grossly underestimated the true prevalence and extent of vitamin D deficiency and associated increased relative risk of an adverse outcome in this fragile and critical patient population. The goal of our study was first to define the prevalence of vitamin D deficiency in patients admitted to the surgical intensive care unit for all causes.
There has been a poor consensus in defining normal, insufficient, and deficient levels of vitamin 25(OH)D.7,12,14,15 It traditionally has been recognized that vitamin 25(OH)D serum levels of less than 5 to 7 ng/mL induce rickets with resultant osteomalacia, serum levels of less than 10 to 12 ng/mL induce secondary hyperparathyroidism and osteoporosis, and serum levels greater than 18 to 20 ng/mL were considered normal or adequate.1,12 In one study, serum levels of 25-(OH)-D3 were related directly to bone mineral density in white, black, and Hispanic American men and women.6,13,16,17 A maximum bone density was achieved only when the 25-hydroxyvitamin D level actually reached 40 ng/mL or more.1,14,18 When the levels were 30 ng per mL or less, there was a significant decrease in intestinal calcium absorption.18 Based on prior studies that showed that vitamin D levels of 40 ng/mL were necessary to suppress parathyroid hormone levels and achieve maximal bone mass density in the hips and lumbar spine, we selected a serum 25-hydroxyvitamin D at this level (40 ng/mL) as the lower limit of normal.1,14,19,20 These new cut-off levels suggest that, in the past, we may have been using the wrong statistical approach for defining “normal serum vitamin 25(OH)D levels,” if our main objective was to maintain optimal health.15,21,22
Secondarily, our aim was to stratify the patients into quartiles based on the severity of their vitamin D deficiency and assess their clinical performance and outcome, based on the degree of severity of their vitamin D levels. Blood samples were sent to an outside laboratory with a 1- to 2-week delay in attaining results, thus, no serum 25-(OH) vitamin D levels were available initially to direct therapy or influence our management strategy. The association between vitamin D deficiency and chronic illness has been well documented. It is likely that vitamin D levels progressively decline during surgical intensive care unit stay owing to lack of sunlight plus dietary depletion, which may help to explain the increased risk of mortality in critically ill patients in the intensive care setting.9,10 Patients admitted to the surgical intensive care unit tend to have a higher incidence of hospital-acquired infections, adult respiratory distress syndrome, and multisystem organ failure.
Traditionally, the increased risk of an unfavorable outcome has been attributed to infectious causes; and poor nutritional status as measured by a low serum albumin level, prealbumin level before admission; 10% weight loss; and pre-existing chronic medical illnesses. However, we postulate that the increased risk of ICU-related mortality directly correlates with the presence and severity of vitamin D deficiency. It now has been well established by multiple investigators including Liu et al,23 Jeng et al,24 Baeke et al,25,26 and Bikle27 that vitamin D has a pleiotrophic immunomodulatory effect and, in fact, may be the major regulatory hormone of the immune system.
Recent evidence suggests vitamin D may enhance the innate immune response by induction of cathelicidin (cathelicidin), an endogenous antimicrobial peptide produced by macrophages and neutrophils,1,23–25 as well as β-defensins, a novel class of endogenous antimicrobial peptides.1,25,26 Furthermore, local production of bioactive 1, 25-dihydroxyvitamin D has been shown to down-regulate proinflammatory cytokines such as interleukin (IL)-1, IL-2, interferon-γ, tumor necrosis factor-α, IL-6, IL-8, and IL-12, as well as type-1 T-helper cells and B lymphocytes in the adaptive immune system.1,25,26 In addition, activation of the vitamin D receptor by bioactive vitamin D up-regulates anti-inflammatory cytokine IL-10 and IL-4 production, and promotes expression of the T-suppressor cell lineage, which turns off the adaptive immune response.1,23 For optimal local production of 1, 25-(OH) 2-vitamin D, the serum 25-(OH)-vitamin D concentration needs to be at least 30 to 40 ng/mL, which is equivalent to greater than 75 to 100 nmol/L.1,14,19,20 Therefore, it seems plausible that patients with a lower serum vitamin D level would be less capable of mounting a sufficient response to insult, injury, or infection. This would provide a reasonable explanation as to why the patients who had more severe vitamin D deficiency have a much higher mortality rate than the moderate and mildly deficient patients.
There is increasing evidence for health benefits accomplished by activated vitamin D through interaction with the vitamin D receptor that go beyond calcium and bone homeostasis and regulation of parathyroid hormone secretion.1,2,28 It has been suggested there are biological functions of vitamin D that are independent of its interaction with the parathyroid glands. Available data indicate that these patients may enter a vicious cycle of low calcitriol, leading to increased inflammation markers, and renal impairment, which may be difficult to escape by simple vitamin D supplementation.29,30 These finding may help explain the reason that vitamin D deficiency may be linked to excess mortality in the general population and the intensive care unit setting.
Because of the inability of the patients in the severe vitamin D deficiency group to mount a satisfactory response to tissue trauma and infection, they tended to have an increased length of stay in the intensive care unit resulting from an ongoing, systemic, inflammatory response syndrome, and were likely to have increased ventilator days. Although the increased number of days in the surgical intensive care unit in the severe group versus the mild and moderate groups did not meet statistical significance, the extra 7 to 8 mean days did reach clinical and fiscal significance. In fact, the added time spent in the surgical intensive care unit resulted in a doubling cost of hospital stay for this subpopulation of patients because each ICU day cost 2 to 3 times more than a day on the general ward or in the intermediate care unit.
It also has been noted in several reviewed observational studies, as well as a meta-analysis of randomized controlled trials, that there may be a mortality benefit associated with higher serum vitamin 25(OH)D concentrations or vitamin D(2) ergocalciferol or D(3) cholecalciferol supplementation (mean dose, 528 IU/d).1 Because many trauma victims are community-dwelling adults, it has become increasingly important that vitamin D deficiency be monitored and recognized as a significant risk factor for increased morbidity and mortality in the setting of critical illness requiring admission to the surgical intensive care unit. Consequently, vitamin D deficiency needs to be treated in both the outpatient and inpatient settings. The role of vitamin D extends well beyond that of regulating calcium homeostasis. One of these areas is immune function, which can be both adaptive and innate. Vitamin D signaling is operative in both.30 As noted by Bikle,27 both adaptive and innate immune processes may be a double-edged sword with beneficial and harmful effects.25,26,30
Although suppression of adaptive immunity may be beneficial in a number of self-destructive diseases, such suppression may predispose to infection, which is not uncommon in the ICU setting. Enhancement of innate immunity is clearly beneficial in diseases such as tuberculosis, but potentiation of proinflammatory processes can increase tissue destruction as in bone loss caused by pneumonia, sepsis, and prolonged bed rest, which are not infrequently associated with severe injury and being critically ill.31 The balance favors adequate vitamin D nutrition in host defense against infection.1,11,27,31,32 Although vitamin D deficiency is known to result in abnormal mineralization of bones, other activities of vitamin D as it relates to defense of microbial infections, such as tuberculosis, prevention of cancer, contractility of muscle cells, and counteraction of congestive heart failure, have been shown by Mertens and Müller.33 Notably, their novel findings convincingly indicate that vitamin D exerts anti-inflammatory effects, which may reduce the inflammatory response typically associated with bacterial sepsis in surgical intensive care unit patients.31
The inability of patients with severe vitamin D deficiency to down-regulate the adaptive immune response, along with dysregulation of innate immunity, may be a plausible explanation for the increased mortality rates observed in the moderate and severe vitamin D deficiency groups in our study. Based on this observation, it is plausible to recommend more aggressive primary prevention in the outpatient setting and secondary intervention during hospital and ICU admission to minimize the consequences of suboptimal levels of vitamin D.14
Vitamin D deficiency has been shown to be a common condition in patients admitted to the ICU.1,9,10 The implications of vitamin D deficiency in patients admitted to the intensive care unit for infectious and noninfectious critical illness are just beginning to be understood. How various levels of vitamin D impact outcome in different disease states and conditions requiring admission to the ICU remains to be elucidated. In a recent study from France, it was confirmed that vitamin D deficiency was a common condition in patients at the time of ICU admission, and these findings identified a target population at risk for potential modifiable risk factors of low levels of active vitamin D precursor.34 The prevalence of vitamin D deficiency and its significance in the ICU are starting to be understood. The causes of hypovitaminosis D are multifactorial. Although limited exposure to sunlight before and during chronic illness is probably an important factor, decreased vitamin D intake and altered vitamin D and parathyroid metabolism during critical illness are important contributors to the condition. Vigilance is needed to identify subclinical vitamin D deficiency in these high-risk patients, and early initiation of vitamin D supplementation should be instituted.
Conclusions
Moderate to severe vitamin D deficiency increases length of stay in surgical intensive care unit patients, ICU treatment cost, and mortality rate. Therefore, vitamin D status should be screened in all surgical intensive care unit patients at admission, and potentially treated even when a serum level of less than 40 ng/mL is identified. Further study is needed to determine the optimal treatment for this potential risk factor in surgical intensive care unit patients.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Hamada Y, Fukagawa M. The pleiotropic effects of vitamin D on kidney disease. Clin Calcium. 2007;17:712–7. [PubMed] [Google Scholar]
- 3.Ginde AA, Liu MC, Camargo CA. Demographic differences and trends of vitamin D insufficiency in the US general population, 1988–2004. Arch Intern Med. 2009;169:626–32. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandeira F, Griz L, Dreyer P, et al. Vitamin D deficiency: a global perspective. Arq Bras Endocrinol Metab. 2006;50:640–6. doi: 10.1590/s0004-27302006000400009. [DOI] [PubMed] [Google Scholar]
- 5.Dobnig H, Pilz S, Scharnagi H, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–9. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 6.Melamed ML, Michos ED, Post W, et al. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–37. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–73. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 8.Zittermann A, Gummert JF, Börgermann J. Vitamin D deficiency and mortality. Curr Opin Clin Nutr Metab Care. 2009;12:634–9. doi: 10.1097/MCO.0b013e3283310767. [DOI] [PubMed] [Google Scholar]
- 9.Lee P, Eisman JA, Center JR. Vitamin D deficiency in the intensive care unit: an invisible accomplice to morbidity and mortality. Intensive Care Med. 2009;35:2028–32. doi: 10.1007/s00134-009-1642-x. [DOI] [PubMed] [Google Scholar]
- 10.Lee P, Eisman JA, Center JR. Vitamin D deficiency in critically ill patients. N Engl J Med. 2009;360:1912–4. doi: 10.1056/NEJMc0809996. [DOI] [PubMed] [Google Scholar]
- 11.Thomas KK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–83. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 12.Gomez-Alonzo C, Naves-Díaz N, Rodriguez-Garcia M, et al. Review of the concept of vitamin D “insufficiency and deficiency”. Nefrologia. 2003;23(Suppl 2):73–7. [PubMed] [Google Scholar]
- 13.Cranney A, Weiler HA, O’Donnell S, et al. Summary of evidence-based review on vitamin D efficacy and safety in relation to bone health. Am J Clin Nutr. 2008;88:513S–9S. doi: 10.1093/ajcn/88.2.513S. [DOI] [PubMed] [Google Scholar]
- 14.Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes (erratum appears in Am J Clin Nutr 2006;84:1253) Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 15.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin insufficiency. Lancet. 1998;351:805–6. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 16.Chapuy MC, Preziosi P, Maamer P, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–43. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 17.Holick MF, Siris ES, Binkley N, et al. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–24. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 18.Heaney RP, Dowell MS, Hale CA, et al. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22:142–6. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 19.Dawson-Hughes B, Heaney RP, Holick MF, et al. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–6. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 20.Kinyamu HK, Gallagher JC, Rafferty KA, et al. Dietary calcium and vitamin D intake in elderly women: effect on serum parathyroid hormone and vitamin D metabolites. Am J Clin Nutr. 1998;67:342–8. doi: 10.1093/ajcn/67.2.342. [DOI] [PubMed] [Google Scholar]
- 21.Binkley N, Krueger D, Cowgill CS, et al. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab. 2004;89:3152–7. doi: 10.1210/jc.2003-031979. [DOI] [PubMed] [Google Scholar]
- 22.Hollis BW. Editorial: the determination of circulating 25-hydroxyvitamin D: no easy task. J Clin Endocrinol Metab. 2004;89:3149–51. doi: 10.1210/jc.2004-0682. [DOI] [PubMed] [Google Scholar]
- 23.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 24.Jeng L, Yamshchikov AV, Judd SE, et al. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med. 2009;7:28. doi: 10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baeke F, Takiishi T, Korf H, et al. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–96. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Baeke F, Gysemans C, Korf H, et al. Vitamin D insufficiency: implications for the immune system. Pediatr Nephrol. 2010;25:1597–606. doi: 10.1007/s00467-010-1452-y. [DOI] [PubMed] [Google Scholar]
- 27.Bikle DD. Vitamin D and the immune system: role in protection against bacterial infection. Curr Opin Nephrol Hypertens. 2008;17:348–52. doi: 10.1097/MNH.0b013e3282ff64a3. [DOI] [PubMed] [Google Scholar]
- 28.Kulie T, Groff A, Redmer J, et al. Vitamin D: an evidence-based review. J Am Board Fam Med. 2009;22:698–706. doi: 10.3122/jabfm.2009.06.090037. [DOI] [PubMed] [Google Scholar]
- 29.Zittermann A, Gummert JF, Borgemann J. Vitamin D deficiency and mortality. Curr Opin Clin Nutr Metab Care. 2009;12:634–9. doi: 10.1097/MCO.0b013e3283310767. [DOI] [PubMed] [Google Scholar]
- 30.Baeke F, van Etten E, Gysemans C, et al. Vitamin D signaling in immune-mediated disorders: evolving insights and therapeutic opportunities. Mol Aspects Med. 2008;29:376–87. doi: 10.1016/j.mam.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Baeke F, Etten EV, Overbergh L, et al. Vitamin D3 and the immune system: maintaining the balance in health and disease. Nutr Res Rev. 2007;20:106–18. doi: 10.1017/S0954422407742713. [DOI] [PubMed] [Google Scholar]
- 32.Kinyamu HK, Gallagher JC, Prahl JM, et al. Association between intestinal vitamin D receptor, calcium absorption, and serum 1,25-dihydroxyvitamin D in normal young and elderly women. J Bone Miner Res. 1997;12:922–8. doi: 10.1359/jbmr.1997.12.6.922. [DOI] [PubMed] [Google Scholar]
- 33.Mertens PR, Müller R. Vitamin D and cardiovascular risk. Int Urol Nephrol. 2010;42:165–71. doi: 10.1007/s11255-009-9685-z. [DOI] [PubMed] [Google Scholar]
- 34.Lucidarme O, Messai E, Mazzoni T, et al. Incidence and risk factors of vitamin D deficiency in critically ill patients: results from a prospective observational study. Intensive Care Med. 2010;36:1609–11. doi: 10.1007/s00134-010-1875-8. [DOI] [PubMed] [Google Scholar]


