Abstract
This study investigated cognitive control of social and nonsocial information in autism using functional magnetic resonance imaging. Individuals with autism spectrum disorders (ASDs) and a neurotypical control group completed an oddball target detection task where target stimuli were either faces or nonsocial objects previously shown to be related to circumscribed interests in autism. The ASD group demonstrated relatively increased activation to social targets in right insular cortex and in left superior frontal gyrus and relatively decreased activation to nonsocial targets related to circumscribed interests in multiple frontostriatal brain regions. Findings suggest that frontostriatal recruitment during cognitive control in ASD is contingent on stimulus type, with increased activation for social stimuli and decreased activation for nonsocial stimuli related to circumscribed interests.
Keywords: Autism, functional magnetic resonance imaging, cognitive control, repetitive behaviors, frontostriatal
Introduction
Functional magnetic resonance imaging (fMRI) studies of individuals with autism spectrum disorders (ASDs) have revealed anomalous patterns of frontostriatal brain activation during cognitive control tasks (for a review, see Dichter, 2012), including hyperactivation in inferior and orbital frontal gyri during motor and cognitive interference-inhibition (Schmitz, Rubia, Daly, Smith, Williams et al., 2006; Dichter & Belger, 2007), hyperactivation in rostral anterior cingulate cortex during an antisaccade task (Thakkar, Polli, Joseph, Tuch, Hadjikhani et al., 2008), hypoactivation in anterior prefrontal cortex during a task requiring overcoming prepotent response tendencies (Solomon, Ozonoff, Ursu, Ravizza, Cummings et al., 2009), and hyperactivation in dorsomedial prefrontal cortex during social target detection (Dichter, Felder, & Bodfish, 2009). These findings have been interpreted to reflect deficits in behavioral inhibition and/or generation of adaptive behaviors linked to the expression of symptoms of restricted and repetitive behaviors and interests (e.g., South, Ozonoff, & McMahon, 2007; Lopez, Lincoln, Ozonoff, & Lai, 2005). Although the direction of effects has varied across studies (i.e., frontostriatal hyperactivation vs hypoactivation), likely due to differing task demands and analysis methods, anomalous frontostriatal activation during tasks requiring cognitive control has been a consistent finding.
In nonclinical contexts, detection of oddball target events evokes activity within frontostriatal regions, including the striatum, superior, middle, and inferior frontal gyri, and dorsal medial prefrontal cortex (Kirino, Belger, Goldman-Rakic, & McCarthy, 2000a, 2000b; Huettel, 2004); Kirino et al., 2000). Oddball tasks measure specific aspects of cognitive control, a construct that subsumes working memory, inhibition, and mental flexibility abilities that share the purpose of engaging, disengaging, and reengaging with the environment to guide behavior (Lezak, 1995). In the context of oddball tasks, prefrontal activation to target events is thought to reflect the context-dependent strategic control of behavior (Huettel, Misiurek, Jurkowski, & McCarthy, 2004; Casey, Forman, Franzen, Berkowitz, Braver et al., 2001), dynamic changes in behavioral response strategies (Huettel & McCarthy, 2004), as well as set shifting and inhibitory control (Rubia, Russell, Overmeyer, Brammer, Bullmore et al., 2001; Konishi, Nakajima, Uchida, Kikyo, Kameyama et al., 1999; Rogers, Andrews, Grasby, Brooks, & Robbins, 2000), whereas striatal (i.e., caudate nucleus and putamen) activation has been implicated in planning and the execution of self-generated novel actions (Monchi, Petrides, Strafella, Worsley, & Doyon, 2006).
Our research group has conducted a series of studies examining frontostriatal brain function during oddball tasks in individuals with ASDs. We demonstrated that individuals with ASDs were characterized by frontostriatal hypoactivation to geometric shape targets in a manner that predicted the severity of restricted and repetitive behaviors and interests (Shafritz, Dichter, Baranek, & Belger, 2008). In a follow-up study, we reported dorsomedial prefrontal cortex hyperactivation in ASD to oddball targets that were images of faces, and that activation in dorsal anterior cingulate cortex was inversely correlated with social symptom severity (Dichter et al., 2009). We interpreted this pattern of frontostriatal hyperactivation to reflect compensatory mechanisms reflective of cortical inefficiency to respond flexibly to social targets in ASD (see also Schmitz et al., 2006). This account is consistent with patterns of increased brain activation in other forms of psychopathology during tasks requiring cognitive control (e.g., Wagner, Sinsel, Sobanski, Kohler, Marinou et al., 2006; Buchsbaum, Buchsbaum, Hazlett, Haznedar, Newmark et al., 2007; Manoach, 2003).
The purpose of the present study was to extend this line of research to examine frontostriatal responses in individuals with ASDs to oddball stimuli selected to be related to restricted and repetitive behaviors and interests. This symptom domain is not a unitary construct, and factor analytic studies have indicated three or more factors, where a factor related to circumscribed interests has consistently emerged (Lam & Aman, 2007; Honey, McConachie, Randle, Shearer, & Couteur, 2006; Tadevosyan-Leyfer, Dowd, Mankoski, Winklosky, Putnam et al., 2003; Lam, Bodfish, & Piven, 2008). This factor reflects the types of unusual and intense interests, preoccupations, and attachments commonly seen in individuals with ASD (Kanner, 1968; Turner-Brown, Lam, Holtzclaw, Dichter, & Bodfish, 2011). To date, there has been very little mechanistic research on this unique aspect of autism, despite the fact that previous phenomenological studies have pointed out that parents report that this feature of autism is among the most difficult aspects of autism to manage on a day-today basis (South, Ozonoff, & McMahon, 2005).
Our research group has created a set of 34 images conceptually and empirically related to circumscribed interests in ASDs. These images, which include trains, electronics, and vehicles, contain no social content, elicit greater visual attention from individuals with ASDs (Sasson, Elison, Turner-Brown, Dichter, & Bodfish, 2011; Sasson, Turner-Brown, Holtzclaw, Lam, & Bodfish, 2008), are more subjectively pleasing to individuals with ASDs relative to images of other objects and images of people (Sasson, Dichter, & Bodfish, 2012), and have been shown to differentially activate reward circuitry in individuals with ASDs (Dichter, Felder, Green, Rittenberg, Sasson et al., 2012). Taken together, these eyetracking, behavioral, and brain imaging data suggest that these images, referred to here as “High Autism Interest” (HAI) images, are disproportionately salient and rewarding for individuals with ASDs.
In the present study, we compared neural responses both to faces and HAI images within the context of an oddball target detection task. Based on our previous findings (Dichter et al., 2009), we hypothesized that the ASD group would be characterized by relative frontostriatal hyperactivation to face targets, reflecting processing inefficiency while responding flexibly to these social stimuli. Conversely, because HAI images were selected to be salient and rewarding for individuals with ASDs, we hypothesized that the ASD group would be characterized by relative frontostriatal hypoactivation to these non-social targets, reflecting relatively decreased “cognitive effort” to respond flexibly to these stimuli. Finally, we evaluated relations between neural responses to both classes of target stimuli and autism symptom severity, and predicted that the magnitude of frontostriatal activation to social and non-social targets would predict the severity of clinical manifestations of autism within the autism group.
Methods
Participants
Participants included fifteen individuals with ASDs (thirteen males; mean age (SD): 26.3 (9.4); range: 16.9–45.3, fourteen right handed) and seventeen neurotypical controls (twelve males; mean age (SD): 24.3 (3.7); range: 20.1–33.3, all right handed). Groups did not differ in age, t(30)= .80; p>.20, or gender distribution, χ2 (1) = 2.05, p>.10; however, groups did differ significantly on full-scale IQ as measured by the Wechsler Abbreviated Scale of Intelligence, t(30)=3.59; p<.01, and thus full-scale IQ was included as a covariate in imaging analyses. The ASD group (two diagnosed with Asperger’s syndrome and thirteen with high functioning autism) were recruited via the Autism Subject Registry maintained through the Carolina Institute for Developmental Disabilities. Exclusion criteria included a prior history of gestational age <34 weeks, birth weight <2000 g, intraventricular hemorrhage, history of known medical conditions associated with autism including Fragile X Syndrome, tuberous sclerosis, neurofibromatosis, phenylketonuria, epilepsy and gross brain injury, full scale intelligence score ≤ 75 or MRI contradictions (e.g. presence of metal in body) as assessed by MRI safety questionnaire. The control group was recruited from lists maintained by the Duke-UNC Brain Imaging and Analysis Center.
Autism spectrum diagnoses were based on a history of clinical diagnosis informed by scores on the Autism Diagnostic Observation Schedule (ADOS-G; Lord, Risi, Lambrecht, Cook, Leventhal et al., 2000) administered by a research reliable assessor and using standard cutoffs. All participants consented to protocols approved by the Human Investigations Committees at both UNC-Chapel Hill and Duke University Medical Centers and were paid $40 for completing the imaging portion of the study. All participants had normal or corrected-to-normal vision and had either participated in fMRI studies in the past or completed a mock scan session prior to the fMRI session to acclimate to the scanner environment.
fMRI task
A visual oddball target detection task similar to that described previously (Dichter et al., 2009) was used and is illustrated in Figure 1. Briefly, each of 8 runs contained 160 stimuli presented centrally for 500 ms with an interstimulus interval (ISI) that was jittered between 1000 ms and 2500 ms, during which a fixation cross was presented. Each run lasted 5 min 4 sec, and thus acquisition time for all eight runs was 40 min 32 sec. There were three stimulus categories, circles of various colors and sizes, pictures of faces, and HAI images. At the start of each run, participants were instructed both verbally and via an instruction screen (e.g., “Targets = Faces”) which stimulus category would be targets on that particular run. Each run included frequent ‘standard’ stimuli (circles) that occurred on 90% of trials, infrequent ‘novel’ stimuli that occurred on 5% of trials, and infrequent ‘target’ stimuli that occurred on 5% of trials. On alternating runs, either face or HAI images were targets with the other category serving as novel stimuli. Participants responded via a right-hand button box to every stimulus as quickly and as accurately as possible and pressed one button for all non-target stimuli and an alternate button for all target stimuli. The run type presented first (i.e., face target or HAI target) was counterbalanced across participants. Stimuli were presented using CIGAL presentation software (Voyvodic, 1999) and displayed in the scanner through magnet-compatible goggles (Resonance Technology, Inc., Northridge, CA, USA).
Figure 1.
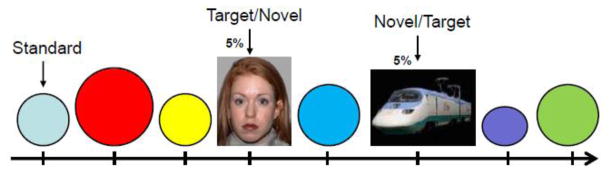
The Target Detection Oddball task. Runs alternated between images of faces and High Autism Interest (“HAI”) images as targets.
Face and High Autism Interest (HAI) Stimuli
Face stimuli were neutral closed-mouth images from the NimStim set of facial expressions (Tottenham, Tanaka, Leon, McCarry, Nurse et al., 2009). As described previously (Dichter, Felder et al., 2012), the non-social images were systematically derived by our research group in the following manner. First, a large number of potential nonsocial images was selected based on response profiles from semi-structured parent-report interviews about circumscribed interests in ASDs (e.g., machines, mechanical systems, trains and electronic devices; Turner-Brown et al., 2011; South et al., 2005; Klin, Danovitch, Merz, & Volkmar, 2007). Next, the visual salience of these images was evaluated via passive-viewing visual exploration eyetracking studies of individuals with and without ASDs (Sasson et al., 2011; Sasson et al., 2008). These eyetracking studies identified 34 images without social content that garnered relatively greater visual attention (i.e., number of fixations and duration of fixations) in ASD samples. Finally, 56 adults with self-identified ASDs provided significantly higher valence ratings of these images relative to 213 adults without ASD (Sasson et al., 2012). These 34 nonsocial images were used in the present study and are depicted in the Appendix of Dichter et al (2012).
Imaging Methods
Scanning was performed on a General Electric Health Technologies, 3 Tesla Signa Excite HD scanner system with 50-mT/m gradients (General Electric, Waukesha, Wisconsin, USA). An eight-channel head coil was used for parallel imaging. Head movement was restricted using foam cushions and Velcro straps. Sixty-eight high resolution images were acquired using a 3D fast SPGR pulse sequence (TR = 500 ms; TE = 20 ms; FOV = 24 cm; image matrix = 2562; voxel size = 0.9375 0.09375 1.9 mm3) and used for coregistration with the functional data. These structural images were aligned in the near axial plane defined by the anterior and posterior commissures. Whole brain functional images consisted of 34 slices parallel to the AC-PC plane using a BOLD-sensitive gradient-echo sequence with spiral-in k-space sampling and SENSE encoding to take advantage of the 8-channel coil, at TR of 1500 ms (TE= 27 ms; FOV: 25.6 cm; isotropic voxel size: 4.00; SENSE factor= 2). Runs began with 4 discarded RF excitations to allow for steady state equilibrium.
Imaging Data Analysis
Functional data were preprocessed using FSL version 4.1.4 (Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB), Oxford University, U.K.). Preprocessing was applied in the following steps: (i) non-brain removal using BET (Smith, Jenkinson, Woolrich, Beckmann, Behrens et al., 2004), (ii) motion correction using MCFLIRT (Smith, 2002), (iii) spatial smoothing using a Gaussian kernel of FWHM 5 mm, (iv) mean-based intensity normalization of all volumes by the same factor, and (v) high-pass filtering (Jenkinson, Bannister, Brady, & Smith, 2002). Functional images of each participant were co-registered to structural images in native space, and structural images were normalized into a standard stereotaxic space (Montreal Neurological Institute) for intersubject comparison. The same transformation matrices used for structural-to-standard transformations were then used for functional-to-standard space transformations of co-registered functional images. All registrations were carried out using an intermodal registration tool (Jenkinson et al., 2002; Smith et al., 2004). Voxel-wise temporal autocorrelation was estimated and corrected using FMRIB’s Improved Linear Model (Jenkinson & Smith, 2001).
Onset times of stimulus presentation were used to model a signal response containing a regressor for each response type which was convolved with a double-γ function to model the hemodynamic response. Model fitting generated whole brain images of parameter estimates representing average signal change from baseline. Group-wise activation images were calculated by a mixed effects higher level analysis using Bayesian estimation techniques, FMRIB Local Analysis of Mixed Effects (FILM, Woolrich, Ripley, Brady, & Smith, 2001). Consistent with guidelines of Lieberman and Cunningham (2009) for clinical studies where a balance of Types I and II error probabilities is sought, clusters of ten or more voxels with Z-values >2.58 (p < 0.005) (FLAME 1+2, Beckmann, Jenkinson, & Smith, 2003) were classified as significant. We also report whether central findings were significant with a more conservative FWE-corrected p<.05 significance threshold by using a small volume correction consisting of the striatum (i.e., caudate nucleus, putamen, and nucleus accumbens), defined on the basis of the Harvard-Oxford subcortical probabilistic atlas (Desikan, Segonne, Fischl, Quinn, Dickerson et al., 2006), and the frontal lobes, defined on the basis of the MNI structural probabilistic atlas (Mazziotta, Toga, Evans, Fox, Lancaster et al., 2001) thresholded at 25%, binarized, and then combined via fslmaths. The cluster size for uncorrected statistical thresholds of p<.005 to reflect cluster-corrected p<.05 significance were determined by 1000 Monte Carlo simulations using AlphaSim (Ward, 2000) to be 38.5 voxels (308 mm3) using this frontostriatal small volume correction.
Results
In-scanner participant motion
In-scanner participant motion was extracted with MCFLIRT (FMRIB). Participants did not differ in deviation of center of mass (in mm), p’s>.15: ASD means (SD): x: 0.024 (0.044); y: 0.019 (0.089); z: 0.050 (0.081); Control means (SD): x: 0.026 (0.016); y: 0.011 (0.026); z: 0.015 (0.046).
In-scanner behavior
A series of 2 (Group: ASD, Control) × 5 (Category: Face Target, HAI Target, Face Novel, Object Novel, Standard) repeated measures ANOVAs were conducted separately for accuracy (i.e. percent correct) and latency (i.e. reaction time) data, followed by within-group and within-condition t-tests.
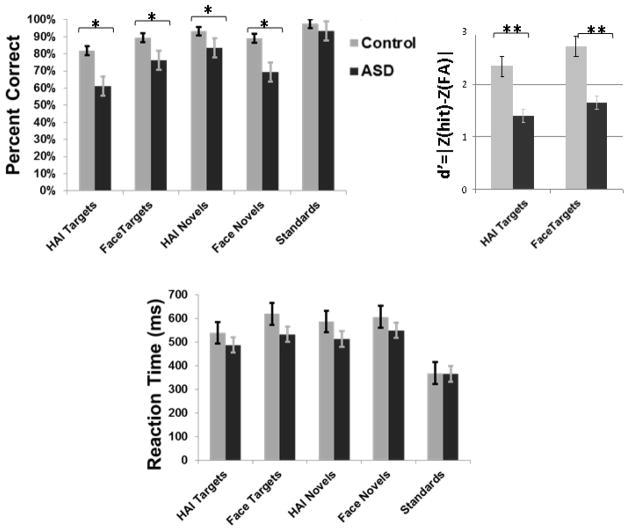
Accuracy analyses revealed a main effect of Category, multivariate F(4, 120) = 25.25, p<.001, a main effect of Group, F(1, 30) = 10.55, p<.003, and a Group x Category interaction, multivariate F(4, 120) = 3.68, p<.007 (see the top left of Figure 2). Between-groups t-tests revealed that the ASD group was relatively less accurate in response to all stimulus categories other than standard stimuli, p’s<.05. Within the control group, paired t-tests indicated greater accuracy to standard stimuli versus other categories, p’s<.01, to face targets versus HAI targets, p<.02, and to both HAI novels and face novels versus HAI targets, p’s<.04. Paired t-tests within the ASD group indicated greater accuracy to standard stimuli versus other categories, p’s<.005. The ASD group was more accurate to face targets versus HAI targets, p<.005, as well as HAI novels versus HAI targets, p<.001 and face novels, p<.01. We also compared groups on target discriminability via d′, calculated as |ZHits−ZFalse Alarms|, with hits reflecting correct responses to targets and the false alarms reflecting incorrect responses to standards or novels. The top right of Figure 2 illustrates that the ASD group was characterized by poorer discriminability to face and HAI targets, p’s<.0001. In summary, the ASD group was relatively less accurate overall and demonstrated decreased accuracy to both face and HAI stimuli.
Figure 2.
In-scanner accuracy (top left), d′ (top right), and reaction time (bottom). Errors bars represent group standard errors of the mean. * p<.05; ** p<.001.
Latency analyses revealed a main effect of Category, multivariate F(4, 120)= 48.35, p<.0001, but not of Group, multivariate F(1, 30)= 3.53, p>.05, or Group x Category interaction, F(4, 120)= 2.43, p>.05 (see the bottom of Figure 2). Between-groups t-tests revealed that groups did not differ in latency across all stimulus categories, p’s>.05. The control group had shorter reaction times to standard stimuli versus all other categories, p’s<.0001 and longer reaction times to face targets compared with all other categories, p’s<. 01. The ASD group had shorter reaction times to standard stimuli versus all other categories, p’s<.005. In summary, groups did not differ in reaction times across all stimulus categories, and both groups had quicker responses to standards than other categories.
Imaging Data
Analyses of functional imaging data included all trials and included accuracy, reaction times for condition-specific responses, and full-scale IQ as covariates. Analyses without these covariates yielded highly similar results (see Supplementary Figure 1). Primary analyses included models that directly compared groups (ASD>Control, Control>ASD) within each target type, followed by results of whole-brain Group (ASD, Control) × Target Type (Face Target, HAI Target) interaction analyses.
Group Contrasts to Face Targets
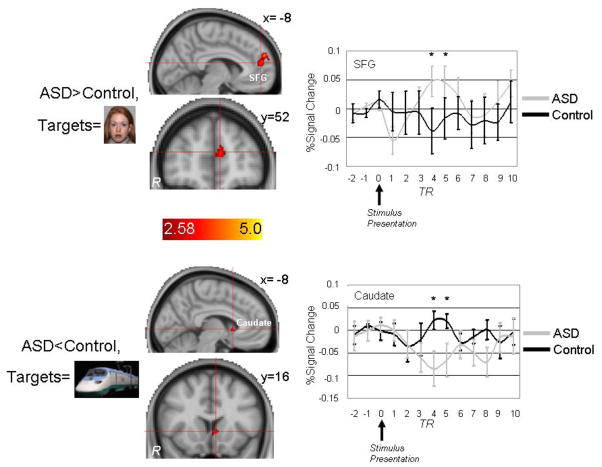
The top left of Figure 3 and the top of Table 2 illustrate brain areas showing relatively greater activation in the ASD group than the control group to face targets (there were no brain areas with relatively decreased activation to face targets in the ASD group). Brain areas with relatively increased activation to face targets in the ASD group included clusters within left superior frontal gyrus and the right insular cortex. Average hemodynamic responses across subjects in the SFG are presented in the top right of Figure 3 and indicate greater BOLD signal change in the ASD group 6 and 7.5 seconds after face target presentation. The sizes of these clusters (104–128 mm3) were not large enough to survive more conservative cluster-correction (>308 mm3).
Figure 3.
Top: Brain areas showing increased activation to face targets in ASD participants relative to controls included two superior frontal gyrus (SFG) clusters and a cluster within right insular cortex (not shown). Bottom: Brain areas showing decreased activation to High Autism Interest (“HAI”) targets in ASD participants relative to controls included the left caudate nucleus. p<.05; Coordinates are in MNI space.
Table 2.
Clusters showing significant group differences to Face and HAI targets.
| Region | Side | Brodmann Area | Size (mm3) | Z Max | MNI | MNI | MNI |
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Face Targets | |||||||
| ASD > Control | |||||||
| Insular Cortex | Right | 112 | 3.31 | 40 | 8 | −8 | |
| Superior Frontal Gyrus | Left | 9 | 104 | 2.85 | −6 | 52 | 18 |
| Superior Frontal Gyrus | Left | 128 | 3.23 | −6 | 56 | 28 |
| Region | Side | Brodmann Area | Size (mm3) | Z Max | MNI | MNI | MNI |
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| HAI Targets | |||||||
| ASD < Control | |||||||
| Amygdala | Left | 96 | 2.94 | −26 | −2 | −20 | |
| Caudate | Left | 104 | 2.84 | −6 | 14 | 0 | |
| Central Opercular Cortex | Left | 160 | 3.03 | −48 | −10 | 8 | |
| Inferior Frontal Gyrus, pars opercularis | Left | 44 | 192 | 3.42 | −58 | 10 | 14 |
| Lateral Occipital Cortex (superior) | Left | 248 | 3.35 | −34 | −76 | 24 | |
| Anterior Cingulate Gyrus | Midline | 96 | 2.82 | 2 | 6 | 30 | |
| Central Opercular Cortex | Right | 42 | 248 | 3.23 | 62 | −8 | 10 |
| Lingual Gyrus | Right | 18 | 328 | 3.16 | 10 | −82 | −4 |
| Middle Frontal Gyrus | Right | 240 | 2.99 | 26 | 20 | 42 | |
| Occipital Fusiform Gyrus | Right | 392 | 3.14 | 28 | −66 | −18 | |
| Precentral Gyrus | Right | 88 | 2.75 | 50 | 2 | 26 | |
| Temporal Occipital Fusiform | Right | 344 | 3.23 | 30 | −56 | −12 |
Group Contrasts to HAI Targets
The bottom left of Figure 3 and the bottom of Table 2 illustrate brain areas showing relatively decreased activation in the ASD group than the control group to HAI targets (there were no brain areas with relatively increased activation to HAI targets in the ASD group). Brain areas with relatively decreased activation to HAI targets in the ASD group included a cluster in the left caudate nucleus as well as clusters within left inferior frontal gyrus, anterior cingulate gyrus, right middle frontal gyrus, and the left amygdala. Average hemodynamic responses across subjects in the left caudate nucleus cluster are presented in the bottom right of Figure 3 and indicate decreased BOLD signal change in the ASD group 6 and 7.5 seconds after HAI target presentation. The size of this caudate cluster (104 mm3) was not large enough to survive more conservative cluster-correction (>308 mm3)
Group × Target Type Interaction
Figure 4 illustrates results of a Group (ASD, Control) × Target Type (Face Target, HAI Target) fMRI model. This analysis yielded a midline caudate nucleus cluster which was further queried by analyzing subject- and condition-specific signal intensities via a mixed repeated measures ANOVA. This analysis revealed a significant Group x Target Type interaction, multivariate F (1,30)=4.41, p<.05, but no significant effects of Target Type or Group, p’s>.39. Consistent with findings above, a follow-up ttest indicated that responses in this caudate cluster to HAI targets were significantly less in the ASD group, p<.05. The size of this caudate nucleus cluster (648 mm3) was large to survive more conservative cluster-correction (>308 mm3).
Figure 4.
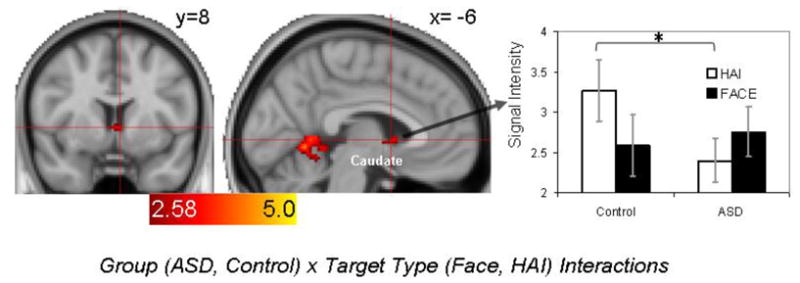
Results of the Group (ASD, Neurotypical) × Target Type (Face Target, HAI Target) fMRI model (left) and subject- and condition-specific signal intensities extracted from the significant midline caudate nucleus cluster. p<.05; Coordinates are in MNI space.
Relations to Symptoms
We evaluated whether the magnitude of brain activation in frontostriatal clusters that differentiated groups in response to social (i.e., left superior frontal gyrus and right insular cortex) and HAI (left caudate, left inferior frontal gyrus, anterior cingulate gyrus, and right middle frontal gyrus) targets as well as the caudate nucleus cluster yielded by the Group × Target Type Interaction model predicted symptom severity measured by the SRS-SR and the RBS-R within the ASD group. These analyses revealed that higher RBS-R scores were correlated with decreased left inferior frontal gyrus activation (r= −0.53, p<.03) and decreased right middle frontal gyrus activation (r= −0.65, p<.007) to social targets in the ASD group.
Discussion
Previous research has demonstrated that ASD is characterized by aberrant frontostriatal activation during tasks that require cognitive control. These findings represent a possible neural mechanism of restricted and repetitive behaviors and interests that are a core feature of the disorder (Dichter et al., 2009; Schmitz et al., 2006; Thakkar et al., 2008; Solomon et al., 2009; Shafritz et al., 2008). The aim of the present study was to extend this line of research to investigate neural correlates of cognitive control of both social stimuli and nonsocial stimuli related to circumscribed interests in ASD via an oddball target detection task. This task requires flexible responding and inhibition of prepotent responses and has been shown to recruit frontostriatal brain regions, including the striatum, superior, middle, and inferior frontal gyri, and dorsal medial prefrontal cortex (Huettel & McCarthy, 2004; Kirino et al., 2000). Faces were used as social stimuli given their centrality to social functioning, and nonsocial images of objects related to circumscribed interests known to be salient and rewarding to individuals with ASDs were used as nonsocial targets (Dichter, Felder et al., 2012; Sasson et al., 2011; Sasson et al., 2008).
We found that the ASD group was characterized by relatively increased prefrontal activation to social targets and by relatively decreased activation to HAI targets in the caudate nucleus and multiple prefrontal brain regions. Although the localization of these clusters at uncorrected thresholds suggested to be appropriate in smaller-scale clinical studies (Lieberman & Cunningham, 2009) are consistent with hypotheses and previous fMRI research addressing the neural correlates of cognitive control in autism, only the caudate nucleus cluster yielded by the Group × Target Type interaction model was significant at a more conservative cluster-corrected threshold.
Findings in the present study of hyperactivation in a medial aspect of superior frontal gyrus in the ASD group to face targets are consistent with previous results given the central role the superior frontal gyrus plays in executive tasks (Fan, McCandliss, Fossella, Flombaum, & Posner, 2005; MacDonald, Cohen, Stenger, & Carter, 2000). The insular cortex, and the inferior frontal gyrus more broadly, mediates strategic planning in oddball tasks (Huettel, 2004; Kirino et al., 2000b) and modulates arousal to facilitate selective attention, particularly in the context of conflict (Eckert, Menon, Walczak, Ahlstrom, Denslow et al., 2009). Thus, localization of hyperactivation in the superior frontal gyrus and insular cortex to face targets implicates prefrontal brain areas that mediate flexible patterns of behavioral responding. Because hyperactivation in prefrontal regions during tasks requiring cognitive control may reflect compensatory neural mechanisms (Dichter et al, 2009), the ASD group may have required greater neural resources to respond flexibly to social stimuli requiring cognitive control. This interpretation is consistent with studies in control samples indicating that dorsal prefrontal cortical regions play a key role in regulating response selection, goal maintenance and recall of task-relevant information (Milham, Banich, Claus, & Cohen, 2003; Woodward, Metzak, Meier, & Holroyd, 2008).
The novel finding in the present study was that the ASD group was characterized by relatively decreased activation to HAI oddball targets in multiple frontostriatal brain regions that mediate cognitive control, including the caudate nucleus, left inferior frontal gyrus, anterior cingulate gyrus, and right middle frontal gyrus, (Fan et al., 2005; Kirino et al., 2000b; Huettel, 2004; Kirino et al., 2000a). We have demonstrated previously with multiple methodologies (i.e., behavioral ratings (Sasson et al., 2012), eye-tracking (Sasson et al., 2008; Sasson et al., 2011), and functional brain imaging (Dichter, Felder et al., 2012; Dichter, Richey, Rittenberg, Sabatino, & Bodfish, 2012; Richey, Rittenberg, Hughes, Damiano, Sabatino et al., 2013)) that social and HAI stimuli have different motivational value for individuals with autism. Cognitive control is impacted by the motivational value of the information being processed (Padmala & Pessoa, 2011, 2010; Krebs, Boehler, Appelbaum, & Woldorff, 2013). Thus, we interpret the present findings to suggest that cognitive control is not a pervasive deficit in ASD, but rather that the degree of deficit is likely impacted by the nature of the information being processed, and that the increased motivational value associated with processing HAI information may diminish the cognitive control deficits in ASD.
In-scanner behavioral performance indicated that both diagnostic groups were slower and less accurate to target stimuli relative to novel and standard images, confirming that target responses required greater cognitive control. Additionally, the ASD group made slower and less accurate responses across stimulus categories and were slower and less accurate to both target categories. This domain-general pattern of impaired performance stands in contrast to functional brain imaging results indicating activation patterns that were moderated by target type in the ASD group. Individuals with ASDs have been consistently found to demonstrate slower reaction times in a range of cognitive control tasks (Geurts, Corbett, & Solomon, 2009; Hill, 2004). As reviewed above, the social and HAI stimulus categories hold differential motivational value for individuals with ASDs, and motivational properties would be expected to impact behavioral performance in a conflict paradigm. Thus, we interpret the present behavioral results to reflect general response slowing characteristic of ASD rather than the established motivational differences between these two stimulus categories. Of central importance, however, is that behavioral responses in the ASD group were apparently produced via differential patterns of neural activation within the cognitive control network. This differential pattern of behavioral versus neural results has been found previously in studies of cognitive control (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Rushworth, Walton, Kennerley, & Bannerman, 2004), and particularly in autism studies (Agam, Joseph, Barton, & Manoach, 2010; Dichter et al., 2009). Conflict-related neural hyperactivation in the context of poorer behavioral performance has been interpreted to reflect neural inefficiency, differential strategies, and/or overactive performance monitoring, whereas conflict-related neural hypoactivation may be evident in contexts of relatively decreased conflict processing or task engagement (Melcher, Falkai, & Gruber, 2008). Thus, it may be the case that the presence of social versus HAI conflict stimuli exposed different neural correlates of cognitive control deficits in ASD.
Analyses of relations between neural response and symptom profiles within the ASD group revealed that activation in two prefrontal clusters to social targets predicted the severity of repetitive behaviors and restricted interests in the ASD group. This finding provides further evidence that responses during cognitive control tasks are related to the severity of repetitive behavior symptoms (e.g., Agam et al., 2010), and in particular during a task that requires cognitive control of social information, the stimulus condition that would be most likely to tap cognitive deficits in autism (Ozonoff, 1995).
Limitations of the present study should be addressed in future research. First, all participants viewed the same set of HAI images. Although this approach provided experimental internal validity, circumscribed interests in ASD are idiosyncratic and person-specific. In this regard, HAI images were not used as a proxy for person-specific interests but rather as a ‘press’ to investigate differences in activation patterns to social and salient non-social images across both groups. The use of standardized object images is likely a conservative estimate of patterns of brain activation to person-specific interests, but future research with person-specific images and other object images not associated with circumscribed interests will be necessary to address this. Additionally, given that social stimuli were faces with neutral expressions and that face expression moderates brain activation patterns in ASD (Kleinhans, Richards, Weaver, Johnson, Greenson et al., 2010), future research should address the potential moderating effect of face expression on cognitive control in autism. We also note that social and nonsocial stimuli were not equated with respect to perceptual properties, and future research that parametrically varies these stimulus properties may evaluate to what extent these features effect brain activation.
Despite these limitations, the present study extends the extant literature on the neural mechanisms of cognitive control in ASD and suggests that functioning of cognitive control systems in ASD is critically dependent on the type of stimulus processed. Specifically, individuals with ASDs appear to be characterized by frontostriatal hyper- and hypoactivation to social and nonsocial stimuli related to circumscribed interest, respectively. The present findings indicate a potential novel neural correlate of circumscribed interests in individuals with ASDs.
Supplementary Material
Table 1.
Means (SDs) of demographic data and symptom profiles.
| Autism (n=15) | Control (n=17) | t (p) | |
|---|---|---|---|
| Age | 26.3 (9.4) | 24.3 (3.7) | .24 (.81) |
|
| |||
| ADOS
| |||
| Comm | 5.8 (5.3) | ||
| SI | 8.7 (2.2) | ||
| SBRI | 2.25 (1.8) | ||
| WASI †
| |||
| Verbal | 108.1 (24.9) | 128.5 (7.2) | −2.68 (.015) |
| Performance | 109.1 (14.1) | 122.2 (7.5) | −3.26 (.0039) |
| Full | 109.9 (20.3) | 127.0 (8.1) | −3.08 (.0066) |
|
| |||
| AQ | 24.7 (13.1) | 12.4 (5.3) | 3.55 (.002) |
|
| |||
| RBS-R | 20.8 (24.8) | 3.6 (4.7) | 4.44 (.0004) |
|
| |||
| SRS-SR (raw scores) | 70.7 (34.3) | 33.7 (18.5) | 3.89 (0.0008) |
WASI missing from 1 autism participant with Leiter IQ score of 121
Abbreviations:
WASI: Wechsler Abbreviated Scale of Intelligence (Weschler, 1999);
ADOS: Autism Diagnostic Observation Scale (Lord et al., 2000); Comm: Communication; SI: Reciprocal Social Interaction; SBRI: Stereotyped Behaviors and Restricted Interests;
AQ: Autism Spectrum Quotient (Baron-Cohen, Wheelwright, Skinner, Martin, & Clubley, 2001); a threshold of 32 or higher suggests cause for clinical concern in community samples.
RBS-R: Repetitive Behavior Scale-Revised (Bodfish, Symons, & Lewis, 1999).
Table 3.
Clusters showing significant Group (ASD, Control) × Target Type (Face Target, HAI Target) interactions.
| Region | Side | Size (mm3) | Z Max | MNI | MNI | MNI |
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Lingual Gyrus | L | 328 | 4.14 | −16 | −60 | −6 |
| Lingual Gyrus | R | 240 | 3.98 | 22 | −50 | −2 |
| Posterior Cingulate Gyrus | 1880 | 3.96 | 0 | −50 | 4 | |
| Caudate Nucleus | B | 648 | 3.41 | 0 | 0 | −4 |
| Precentral Gyrus | R | 104 | 3.18 | 26 | −2 | 48 |
Acknowledgments
This research was supported by R01 MH073402 (JWB and GSD), K23 MH081285 (GSD), and H325D070011 (AS). Assistance for this study was provided by the Participant Registry Core of the Carolina Institute for Developmental Disabilities (P30 HD03110). The authors thank Josh Bizzell, Chris Petty, Zoe Englander, and Todd Harshbarger for assistance with image analysis, MRI technologists Susan Music, Natalie Goutkin, and Luke Poole for assistance with data acquisition, and BIAC Director Dr. Allen Song for assistance with various aspects of this project. Finally, we gratefully acknowledge the participation of individuals with autism in this research.
References
- Agam Y, Joseph RM, Barton JJ, Manoach DS. Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. Neuroimage. 2010;52(1):336–347. doi: 10.1016/j.neuroimage.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. 2001;31(1):5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Symons FW, Lewis MH. Western Carolina Center Research Reports. 1999. The Repetitive Behavior Scale-Revised. [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Buchsbaum BR, Hazlett EA, Haznedar MM, Newmark R, Tang CY, Hof PR. Relative glucose metabolic rate higher in white matter in patients with schizophrenia. Am J Psychiatry. 2007;164(7):1072–1081. doi: 10.1176/ajp.2007.164.7.1072. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Forman SD, Franzen P, Berkowitz A, Braver TS, Nystrom LE, Thomas KM, Noll DC. Sensitivity of prefrontal cortex to changes in target probability: a functional MRI study. Hum Brain Mapp. 2001;13(1):26–33. doi: 10.1002/hbm.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dichter GS. Functional magnetic resonance imaging of autism spectrum disorders. Dialogues Clin Neurosci. 2012;14(3):319–351. doi: 10.31887/DCNS.2012.14.3/gdichter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Belger A. Social stimuli interfere with cognitive control in autism. Neuroimage. 2007;35(3):1219–1230. doi: 10.1016/j.neuroimage.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Bodfish JW. Autism is characterized by dorsal anterior cingulate hyperactivation during social target detection. Soc Cogn Affect Neurosci. 2009;4(3):215–226. doi: 10.1093/scan/nsp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, Bodfish JW. Reward circuitry function in autism spectrum disorders. Soc Cogn Affect Neurosci. 2012;7:160–172. doi: 10.1093/scan/nsq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Richey JA, Rittenberg AM, Sabatino A, Bodfish JW. Reward circuitry function in autism during face anticipation and outcomes. J Autism Dev Disord. 2012;42(2):147–160. doi: 10.1007/s10803-011-1221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Menon V, Walczak A, Ahlstrom J, Denslow S, Horwitz A, Dubno JR. At the heart of the ventral attention system: the right anterior insula. Hum Brain Mapp. 2009;30(8):2530–2541. doi: 10.1002/hbm.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26(2):471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Corbett B, Solomon M. The paradox of cognitive flexibility in autism. Trends Cogn Sci. 2009;13(2):74–82. doi: 10.1016/j.tics.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends Cogn Sci. 2004;8(1):26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Honey E, McConachie H, Randle V, Shearer H, Couteur AS. One-year Change in Repetitive Behaviours in Young Children with Communication Disorders Including Autism. J Autism Dev Disord. 2006 doi: 10.1007/s10803-006-0191-1. [DOI] [PubMed] [Google Scholar]
- Huettel SA. Non-linearities in the blood-oxygenation-level dependent (BOLD) response measured by functional magnetic resonance imaging (fMRI) Conf Proc IEEE Eng Med Biol Soc. 2004;6:4413–4416. doi: 10.1109/IEMBS.2004.1404227. [DOI] [PubMed] [Google Scholar]
- Huettel SA, McCarthy G. What is odd in the oddball task? Prefrontal cortex is activated by dynamic changes in response strategy. Neuropsychologia. 2004;42(3):379–386. doi: 10.1016/j.neuropsychologia.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Misiurek J, Jurkowski AJ, McCarthy G. Dynamic and strategic aspects of executive processing. Brain Res. 2004;1000(1–2):78–84. doi: 10.1016/j.brainres.2003.11.041. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Acta Paedopsychiatr. 1968;35(4):100–136. [PubMed] [Google Scholar]
- Kirino E, Belger A, Goldman-Rakic P, McCarthy G. Prefrontal activation evoked by infrequent target and novel stimuli in a visual target detection task: an event-related functional magnetic resonance imaging study. J Neurosci. 2000a;20(17):6612–6618. doi: 10.1523/JNEUROSCI.20-17-06612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino E, Belger A, Goldman-Rakic P, McCarthy G. Prefrontal activation evoked by infrequent target and novel stimuli in a visual target detection task: an event-related functional magnetic resonance imaging study. Journal of Neuroscience. 2000b;20(17):6612–6618. doi: 10.1523/JNEUROSCI.20-17-06612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Weaver K, Johnson LC, Greenson J, Dawson G, Aylward E. Association between amygdala response to emotional faces and social anxiety in autism spectrum disorders. Neuropsychologia. 2010;48(12):3665–3670. doi: 10.1016/j.neuropsychologia.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Danovitch JH, Merz AB, Volkmar F. Circumscribed Interests in Higher Functioning Individuals with Austim Spectrum Disorders: An Exploratory Study. Research and Practice for Persons with Severe Disabilities. 2007;32(2):89–100. [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122(Pt 5):981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- Krebs RM, Boehler CN, Appelbaum LG, Woldorff MG. Reward associations reduce behavioral interference by changing the temporal dynamics of conflict processing. PLoS One. 2013;8(1):e53894. doi: 10.1371/journal.pone.0053894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KS, Aman MG. The Repetitive Behavior Scale-Revised: independent validation in individuals with autism spectrum disorders. J Autism Dev Disord. 2007;37(5):855–866. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- Lam KS, Bodfish JW, Piven J. Evidence for three subtypes of repetitive behavior in autism that differ in familiality and association with other symptoms. J Child Psychol Psychiatry. 2008;49(11):1193–1200. doi: 10.1111/j.1469-7610.2008.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. 3. New York: Oxford University Press; 1995. [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4(4):423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of Autistic Disorder. J Autism Dev Disord. 2005;35(4):445–460. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism & Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;60(2–3):285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, Holmes C, Collins L, Thompson P, MacDonald D, Iacoboni M, Schormann T, Amunts K, Palomero-Gallagher N, Geyer S, Parsons L, Narr K, Kabani N, Le Goualher G, Boomsma D, Cannon T, Kawashima R, Mazoyer B. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philos Trans R Soc Lond B Biol Sci. 2001;356(1412):1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher T, Falkai P, Gruber O. Functional brain abnormalities in psychiatric disorders: neural mechanisms to detect and resolve cognitive conflict and interference. Brain Res Rev. 2008;59(1):96–124. doi: 10.1016/j.brainresrev.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Claus ED, Cohen NJ. Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. Neuroimage. 2003;18(2):483–493. doi: 10.1016/s1053-8119(02)00050-2. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Strafella AP, Worsley KJ, Doyon J. Functional role of the basal ganglia in the planning and execution of actions. Ann Neurol. 2006;59(2):257–264. doi: 10.1002/ana.20742. [DOI] [PubMed] [Google Scholar]
- Ozonoff S. Reliability and validity of the Wisconsin Card Sorting Test in studies of autism. Neuropsychology. 1995;9(4):491–500. [Google Scholar]
- Padmala S, Pessoa L. Interactions between cognition and motivation during response inhibition. Neuropsychologia. 2010;48(2):558–565. doi: 10.1016/j.neuropsychologia.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmala S, Pessoa L. Reward reduces conflict by enhancing attentional control and biasing visual cortical processing. J Cogn Neurosci. 2011;23(11):3419–3432. doi: 10.1162/jocn_a_00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richey JA, Rittenberg A, Hughes L, Damiano CR, Sabatino A, Miller S, Hanna E, Bodfish JW, Dichter GS. Common and distinct neural features of social and non-social reward processing in autism and social anxiety disorder. Soc Cogn Affect Neurosci. 2013 doi: 10.1093/scan/nss146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. J Cogn Neurosci. 2000;12(1):142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SC, Giampietro V, Andrew CM, Taylor E. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13(2):250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8(9):410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Sasson NJ, Dichter GS, Bodfish JW. Affective responses by adults with autism are reduced to social images but elevated to images related to circumscribed interests. PLoS One. 2012;7(8):e42457. doi: 10.1371/journal.pone.0042457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson NJ, Elison JT, Turner-Brown LM, Dichter GS, Bodfish JW. Brief report: Circumscribed attention in young children with autism. J Autism Dev Disord. 2011;41(2):242–247. doi: 10.1007/s10803-010-1038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson NJ, Turner-Brown L, Holtzclaw T, Lam KS, Bodfish J. Children with Autism Demonstrate Circumscribed Attention during Passive Viewing of Complex Social and Nonsocial Picture Arrays. Autism Research. 2008;1:31–42. doi: 10.1002/aur.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz N, Rubia K, Daly E, Smith A, Williams S, Murphy DG. Neural correlates of executive function in autistic spectrum disorders. Biol Psychiatry. 2006;59(1):7–16. doi: 10.1016/j.biopsych.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Dichter GS, Baranek GT, Belger A. The neural circuitry mediating shifts in behavioral response and cognitive set in autism. Biological Psychiatry. 2008;63(10):974–980. doi: 10.1016/j.biopsych.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Solomon M, Ozonoff S, Ursu S, Ravizza S, Cummings N, Ly S, Carter C. The Neural Substrates of Cognitive Control Deficits in Autism Spectrum Disorders. Neuropsychologia. 2009 doi: 10.1016/j.neuropsychologia.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South M, Ozonoff S, McMahon WM. Repetitive behavior profiles in Asperger syndrome and high-functioning autism. J Autism Dev Disord. 2005;35(2):145–158. doi: 10.1007/s10803-004-1992-8. [DOI] [PubMed] [Google Scholar]
- South M, Ozonoff S, McMahon WM. The relationship between executive functioning, central coherence, and repetitive behaviors in the high-functioning autism spectrum. Autism. 2007;11(5):437–451. doi: 10.1177/1362361307079606. [DOI] [PubMed] [Google Scholar]
- Tadevosyan-Leyfer O, Dowd M, Mankoski R, Winklosky B, Putnam S, McGrath L, Tager-Flusberg H, Folstein SE. A principal components analysis of the Autism Diagnostic Interview-Revised. J Am Acad Child Adolesc Psychiatry. 2003;42(7):864–872. doi: 10.1097/01.CHI.0000046870.56865.90. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Polli FE, Joseph RM, Tuch DS, Hadjikhani N, Barton JJ, Manoach DS. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD) Brain. 2008;131(Pt 9):2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner-Brown LM, Lam KS, Holtzclaw TN, Dichter GS, Bodfish JW. Phenomenology and measurement of circumscribed interests in autism spectrum disorders. Autism. 2011;15(4):437–456. doi: 10.1177/1362361310386507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyvodic JT. Real-time fMRI paradigm control, physiology, and behavior combined with near real-time statistical analysis. Neuroimage. 1999;10(2):91–106. doi: 10.1006/nimg.1999.0457. [DOI] [PubMed] [Google Scholar]
- Wagner G, Sinsel E, Sobanski T, Kohler S, Marinou V, Mentzel HJ, Sauer H, Schlosser RG. Cortical inefficiency in patients with unipolar depression: an event-related FMRI study with the Stroop task. Biol Psychiatry. 2006;59(10):958–965. doi: 10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. Medical College of Wisconsin; Milwaukee: Biophysics Research Institute; 2000. [Google Scholar]
- Weschler D. Weschler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- Woodward TS, Metzak PD, Meier B, Holroyd CB. Anterior cingulate cortex signals the requirement to break inertia when switching tasks: a study of the bivalency effect. Neuroimage. 2008;40(3):1311–1318. doi: 10.1016/j.neuroimage.2007.12.049. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.