Abstract
Background
Mechanical ventilation of preterm infants is associated with pulmonary inflammation. Intubated infants often develop bacterial tracheal colonization but little is known about endotoxin in tracheal aspirates (TAs) or the mobilization of innate immunity towards endotoxin, a potent stimulus that contributes to inflammatory disease. We characterized mobilization of endotoxin-directed innate immunity in TAs from an observational cohort of mechanically ventilated neonates.
Methods
TA supernatants (n=42; GA=23-40 weeks, postnatal age=1-71 days) were assayed for endotoxin (Limulus amoebocyte lysate assay) and endotoxin-modulating proteins: bactericidal/permeability-increasing protein (BPI), LPS-binding protein (LBP) and soluble CD14 (sCD14). TA cellular BPI was measured by ELISA, western blot, flow cytometry, and bactericidal assay. TA mRNAs encoding endotoxin-modulating proteins were measured by quantitative RT-PCR.
Results
Endotoxin in TA supernatants was proportional to both postnatal age and polymorphonuclear leukocytes (PMN). Neonatal TAs were rich in PMN containing BPI and expressed mRNAs encoding Toll-like receptor 4 (TLR4), CD14, and MD-2. Extracellular BPI was consistently detectable and correlated with TA PMN and GA. Both extracellular- and cellular-BPI increased during the first postnatal week. TA extracellular BPI, LBP, and sCD14 were positively correlated.
Conclusions
TAs from intubated neonates demonstrate endotoxin accumulation and mobilization of endotoxin-directed innate immunity, potentially contributing to pulmonary inflammation.
Introduction
Innate immunity of the newborn is functionally distinct from that of adults (1, 2) with respect to the expression of pattern recognition receptors, signaling adaptor proteins, and host defense effector molecules (3). Innate immunity of newborns to Gram-negative bacteria (GNB) and GNB-derived endotoxin or lipopolysaccharide (LPS) is of interest because: 1) GNB are common intestinal commensal flora, 2) GNB are life-threatening neonatal pathogens (4), and 3) multiple diseases of prematurity are associated with endotoxin including sepsis (5), bronchopulmonary dysplasia (6), necrotizing enterocolitis (7), and cerebral palsy due to intracranial white matter damage (8).
Humans are highly sensitive to very low concentrations (picograms/mL) of endotoxin. This reflects efficient and sequential humoral and cell-based protein-endotoxin and protein-protein interactions involving LBP, membrane and soluble forms of CD14, MD-2, and TLR4 (9, 10). Sensitivity to endotoxin can be dampened by changes in expression of these proteins (11) as competition with other LPS-binding proteins precludes transfer of LPS to CD14 and to the MD-2/TLR4 receptor complex on monocytes (12). Endotoxin induces tracheobronchial epithelial cell production of antimicrobial peptides (13) and cytokines that recruit and activate PMN (14).
PMN play key roles in innate immunity deploying both oxygen-dependent and - independent means to dispose of pathogens and neutralize their toxins (15). BPI is a cationic 55kDa protein of PMN primary granules that possesses anti-infective activities against GNB: endotoxin-neutralization based upon high affinity (nM) binding of BPI to the lipid A region of LPS, bactericidal activity via membrane permeabilization, and opsonic activity (16). Human newborn cord blood PMN are deficient in BPI: full term neonatal cord blood PMN contain ~25-33% of adult PMN BPI content (17) and preterm human cord blood demonstrates impaired stimulus-induced release of extracellular BPI (18).
Endotoxin could contribute to airway inflammation in mechanically ventilated human newborns by inducing tracheobronchial epithelial cell NF-κB activation (19, 22) and consequent production of cytokines/chemokines that recruit/activate PMN (14)(19-21). Accordingly, endotoxin instillation in the lungs of mechanically ventilated preterm lambs impairs gas exchange and induces systemic inflammation, mimicking aspects of severe lung disease in preterm humans (23).
Endogenous protein mediators of innate immunity are expressed in a gestational age (GA)-dependent manner (3, 24-26). Relative to adults, human newborns are deficient in BPI content of cord blood PMN (17, 18), and in serum concentrations of the liver-derived acute phase reactants LBP and sCD14 (3). However, neonatal expression of these LPS-modulating proteins at inflammatory sites, such as TAs has not been reported. We undertook an observational study to characterize the presence of endotoxin and mobilization of endotoxin-modulating proteins in TAs of critically ill human newborns. We demonstrate endotoxin and early mobilization of endotoxin-directed innate immunity in respiratory secretions of intubated newborns, suggesting a possible role for endotoxin in pulmonary inflammation.
Methods
Employing an IRB-approved protocol (Partners Human Research Committee), TA samples were collected from mechanically ventilated newborns (n=42) at the Brigham and Women’s Hospital Newborn Intensive Care Unit. GA ranged from 23-40 weeks (mean 28) and 70% were male. Preterm neonates were intubated for respiratory failure (surfactant deficiency; n=38), while full term neonates were intubated for surfactant deficiency (n=2), pneumonia (n=1) and persistent pulmonary hypertension (n=1). TAs were collected after endotracheal instillation of 0.5ml/kg sterile, pyrogen-free saline using sterile suction catheters and placed on ice for processing within 4 hours. Due to TA volume constraints, subsets of samples were analyzed for different markers, with n provided in each figure legend.
Collection of neonatal tracheal aspirates
After centrifugation (700g, 7 minutes at 4°C) and collection of TA supernatants, TA pellets were subjected to hypotonic lysis (sterile, pyrogen-free water; Baxter, Deerfield, IL) to remove erythrocytes (~45 sec at 4°C), 5% saline was added (final [NaCl] = 0.14M), prior to washing (Hanks’ Balanced Salt Solution without divalent cations). TA PMN were enumerated by microscopy of Wright's stained aliquots (Shandon Cytospin 3, Thermo Fisher Scientific, Waltham, MA), typically demonstrating ≥70% PMN. Cell viability (trypan blue exclusion) was ≥80%. TA pellets and supernatants were stored at −80°C prior to batch analysis.
Measurement of endotoxin
Endotoxin was measured using the Limulus amoebocyte lysate (LAL) assay according to the manufacturer's instructions (Charles River, Boston, MA).
PMN Isolation
PMN were isolated from adult whole blood by dextran sedimentation and Ficoll-hypaque gradient centrifugation, using pyrogen-free reagents as previously described (17) and enumerated by automated cell count and differential (≥85% pure; ADVIA 2120, Siemens Medical Solutions Diagnostics, Tarrytown, NY), prior to storage at −80°C.
Sulfuric acid extraction of PMN BPI
Cell extracts were prepared using sonication and sulfuric acid, as previously described (17).
Western blotting
PMN acid extracts (5 × 105 PMN equivalents/well) were fractionated by SDS-PAGE. Western blotting employed anti-BPI goat serum and I125 protein G as previously described (17), providing BPI detection in the 10-200 ng range. rBPI-50, a kind gift from XOMA (US) LLC, was used as BPI standard.
Measurement of soluble BPI, LBP and CD14
Enzyme-linked immunosorbent assays (ELISAs) for measurement of BPI and LBP (Hycult Biotechnology, The Netherlands) and sCD14 (R&D Systems, Minnesota) were used according to the manufacturer's instructions.
Total protein measurement
The Bradford protein assay (Bio-Rad Laboratories, Inc. Hercules, CA), was employed using human serum albumin as standard.
Antibacterial activity
Activity of PMN acid extracts against the serum-resistant BPI-sensitive bacteremic isolate E.coli K1/r, a K1-encapsulated rough LPS chemotype strain, was measured as previously described (17, 27) and expressed as %viability (ratio to input innoculum).
Isolation of RNA
RNA in TA pellets (3×105–106 TA cells) was purified according to the manufacturer's instructions (RNeasy Plus Mini Kit; Qiagen, Valencia, CA) either immediately or after storage (−80°C) in RNA Cell Protect Reagent (Qiagen, Valencia, CA).
Quantitative real-time PCR
cDNA was prepared using the RT2 First Strand Kit and treated with DNase (Superarray, Frederick, MD) prior to measurement of relative mRNA abundance by qRT-PCR (SuperArray), employing an ABI Prism 7000 Sequence Detector (Applied Biosystems). RT² SYBR Green/ROX qPCR Master Mix was added to cDNA according to the manufacturer's instructions (SuperArray). Expression levels were normalized to housekeeping genes using the ΔCt method.
Flow Cytometry
Fc binding sites on TA cells were blocked using 10% normal human serum (60 min RT) to prevent non-specific binding. For detecting granulocytes, cells were surface stained (20 min RT) with FITC-conjugated anti-CD66b mAb (mouse IgM, clone G10F5) at 5μL/5x105 cells, or the corresponding FITC-conjugated isotype control (mouse IgM, clone G155-228; BD Pharmingen). After washing (Stain Buffer; BD Pharmingen) and permeabilization (1x fluorescence activated cell sorter lysing solution with 1x permeabilization/wash buffer containing saponin; BD Pharmingen, 10 min RT), BPI was detected by staining (20 mins RT) with anti-BPI mAb (mouse IgG1(κ), clone HM2042; HyCult Biotechnology, Uden, Holland) or isotype control (mouse IgG1(κ), clone MOPC-21; BD Pharmingen), followed by incubation (20 min RT) with a secondary PE-conjugated mAb (rat anti-mouse IgG1(κ), clone A85-I; BD Pharmingen) at 5μL/5×105 cells. Cells were preserved overnight (1% formalin and Stain buffer, 4°C) prior to analysis by flow cytometry (Cytomation MoFlo flow cytometer) and analyzed with Summit Software (v. 4.3, Dako Colorado).
Fluorescent/confocal microscopy
Formalin-fixed cell preparations were imaged on a Nikon TE-2000 inverted microscope fitted with a video-rate confocal system: spinning disk confocal head (Yokogawa), Kr/Ar laser illumination source (Melles Griot), fluorescence filters, and an Orca ERG cooled CCD camera (Hamamatsu). Image capture and 3D rendering employed Slidebook software (Intelligent Imaging Innovations, Inc.). Confocal images were collected either as single 2D planes (paired with a differential interference contrast (DIC) view), or as 3D stacks (focal step size 0.3 microns).
Statistics
Statistical analyses employed Prism 4.0a for MacIntosh (Graphpad Software, Inc., San Diego, CA) and SAS 9.1 (SAS Institute, Inc., Cary, NC). Antibacterial activity of neonatal TA and adult peripheral blood PMN extracts was compared using the unpaired t-test. Correlations were drawn using the Pearson or the Spearman method based on normality of the data. The non-parametric Mann-Whitney test was used for unequal variances (28). P values ≤0.05 were considered significant.
Results
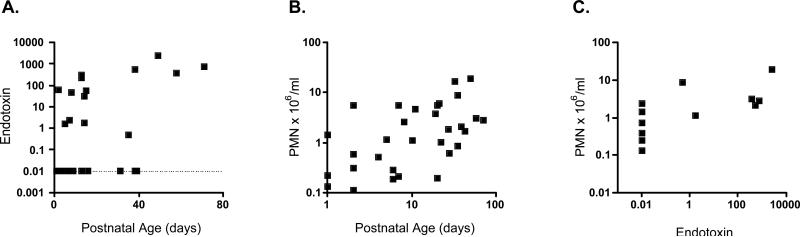
We initially assessed whether endotoxin was detectable in a subset of TA supernatants. Fifteen of 37 (38%) TAs tested positive for endotoxin. Endotoxin concentrations ranged from undetectable (<0.01) to 2542 endotoxin units per milliliter (EU/ml) and positively correlated with postnatal age (Figure 1A). TA PMN also increased with postnatal age (Figure 1B) and correlated with TA endotoxin concentration (Figure 1C).
1. Endotoxin and PMN in neonatal TAs as a function of postnatal age.
(A) Neonatal TA supernatants were assayed for endotoxin (endotoxin units/ml) using the limulus amoebocyte lysate assay and the data positively correlated with postnatal age both with respect to the total data set (n=34, Spearman r=0.38, p<0.05) and in a sub-analysis of endotoxin positive samples (n=15, Spearman r=0.59, p<0.05). (B) TA PMN (106/ml) also increased with postnatal age (n=35, Spearman r=0.46, p<0.01) and correlated with TA endotoxin concentration (n=12, Spearman r=0.79, p<0.01). The dotted line represents samples negative for endotoxin.
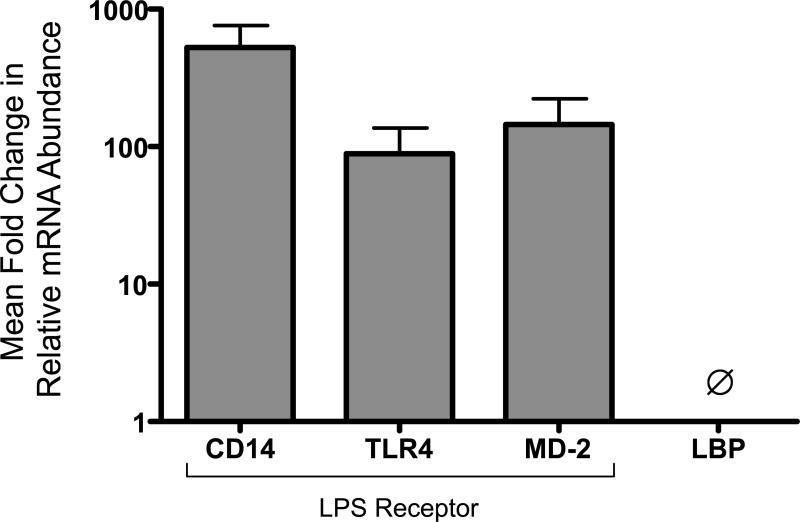
To determine whether genes encoding endotoxin-modulating proteins are activated in TAs, we measured expression of endotoxin receptor components by qRT PCR (Figure 2). mRNAs encoding TLR4, MD-2, and CD14 were consistently detected (n=6/6 TAs). In contrast, BPI mRNA was inconsistently (n=4/6) detected at low levels (<10-fold ratio to lower limit of detection; not shown) and mRNA encoding the liver-derived acute phase reactant LBP was not detected (n=0/6).
2. TA cells express endotoxin receptor components CD14, TLR4 and MD-2.
TA pellet RNA were analyzed by qRT-PCR. mRNA levels are expressed as mean mRNA abundance relative to housekeeping controls as a ratio to the lower limit of detection. mRNA for proteins encoding LPS receptor components TLR4, CD14, and MD-2 were consistently detected (N=6/6 TAs tested; mean GA=29 weeks). In contrast LBP mRNA was not detected (0/6 TAs tested).
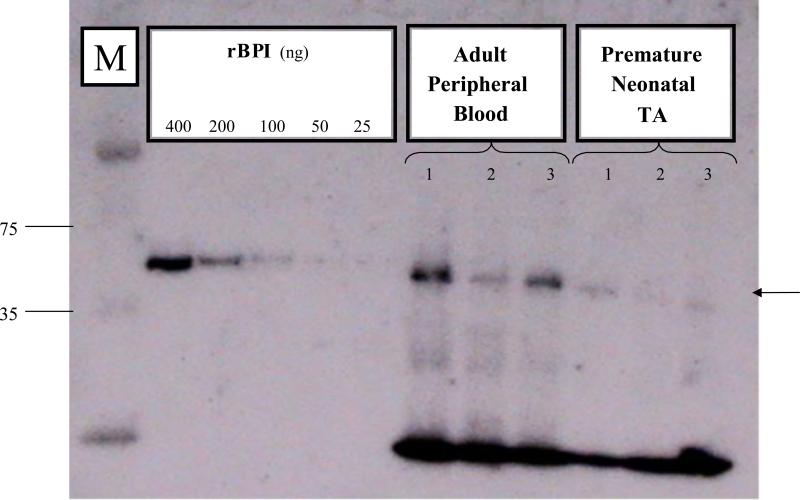
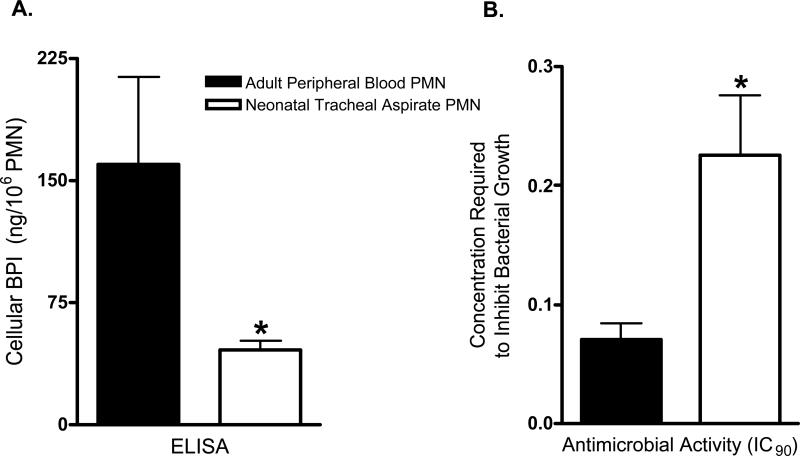
We next assessed whether BPI protein was detectable in TA pellets (Figure 3). Bands corresponding to native BPI in adult PMN extracts were noted in premature neonatal TAs (right-most lanes in Figure 3) indicating expression of BPI in these samples, albeit at relatively lower amounts. Measurement of TA cellular BPI by ELISA of acid extracts (Figure 4A) demonstrated mean cellular BPI concentrations of neonatal TA PMN nearly three-fold lower than those of adult peripheral blood PMN. Accordingly, neonatal TA acid extracts were ~three-fold less potent than adult extracts with respect to killing of the BPI-sensitive clinical isolate E.coli K1/r (Figure 4B).
3. Neonatal TA PMN express cellular BPI.
Cell pellets were solubilized and fractionated by SDS-PAGE (5 × 105 PMN equivalents per lane) for western blotting as described in Methods. In addition to molecular weight markers (M; sizes indicated in kDa), two-fold dilutions of pure recombinant holo-BPI (rBPI) were included as standard. In this representative example, PMN samples from 3 adults (peripheral blood PMN) and 3 preterm newborns (TA cells) were analyzed. Premature neonatal TAs demonstrate relatively weak expression of the ~55kDa protein (arrow).
4. Neonatal TA PMN contain lesser amounts of cellular BPI than adult peripheral blood PMN.
(A) BPI content of acid extracts of neonatal TA PMN (mean ± SEM of 46 ± 6 ng/106 PMN; n=8) and adult peripheral blood PMN (160 ± 54 ng/106 PMN; n=6) were determined by ELISA; (*) = p<0.05, unpaired t-test. (B) BPI-mediated killing activity of PMN acid extracts as measured by the concentration of PMN required to inhibit 90% of bacterial growth (IC90) was measured in vitro against the BPI-sensitive neonatal pathogen E. coli K1/r. Extracts of neonatal TA PMN had less antibacterial activity (mean ± SEM IC90=0.226% ± 0.050%, n=8) than extracts of adult PMN (IC90=0.070% ± 0.013%, n=6), (*) = p<0.05, unpaired t-test.
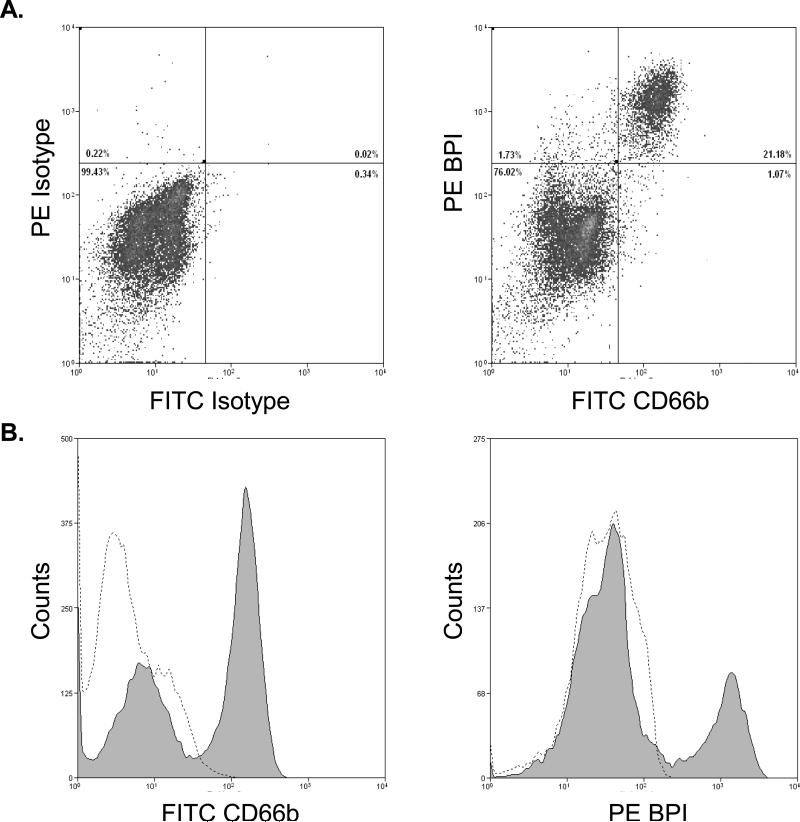
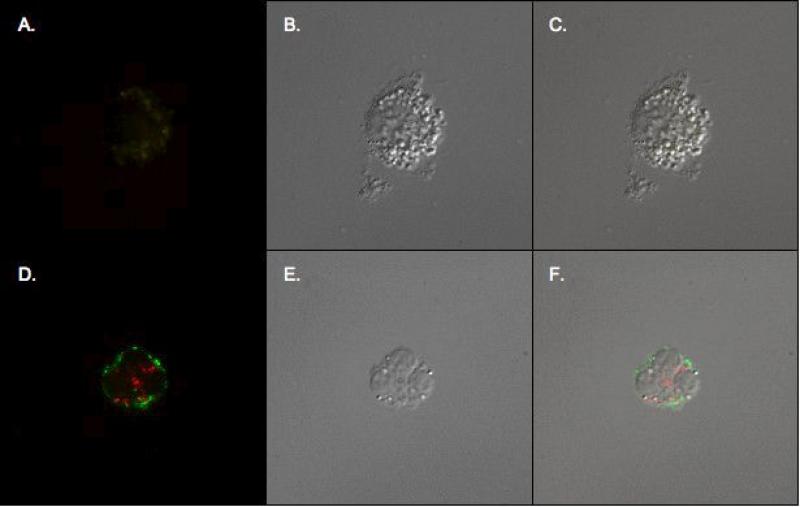
To verify the source of BPI, we stained TA cells for the PMN marker CD66b and for intracellular BPI (Figure 5). CD66b+/BPI+ cells were readily detected in TAs (Fig. 5A) and the vast majority (>95 %) of BPI positive cells were PMN. Confocal fluorescent and DIC imaging also demonstrated CD66b+BPI+ cells (Figure 6D & F).
5. PMN are the primary source of BPI in neonatal TAs.
Representative flow cytometry of neonatal TA cells (28 week gestational age male): (A) stained with both FITC- and PE-labeled isotypes, with background staining set at 1%. (B) staining with FITC-labeled granulocyte cell surface antigen CD66b and intracellular PE-labeled BPI. Most CD66b+ cells were also BPI+. Percentages of the total amount of cells are given within the charts for each quadrant. (C) The histograms depict a shift in median fluorescence for CD66b positive cells (shaded) versus isotype (dashed line) and (D) BPI positive cells (shaded) versus the isotype (dashed line).
6. Immunofluorescent staining of PMN.
Confocal fluorescent images (A, D), differential interference contrast (DIC) images (B, E), and DIC/confocal fluorescent image overlay (C, F) of TA cells stained with FITC isotype (A, B, C) or with extracellular FITC-labeled CD66b and intracellular PE-labeled BPI (D, E, F). Double positive immunofluorescence is seen in (D) and (F).
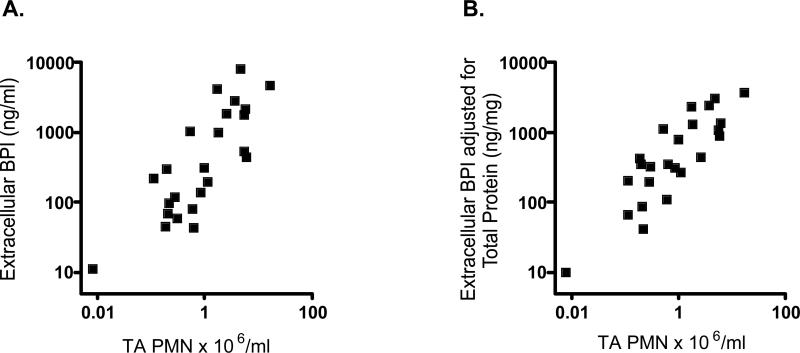
We next characterized extracellular BPI expression and its correlates. Extracellular BPI was detectable (>0.1 ng/mL) in >98% of TAs (range 4-7920 ng extracellular BPI/ml TA supernatant). Extracellular BPI positively correlated with TA PMN content (Figure 7A) even after adjustment for total protein (Figure 7B).
7. Extracellular BPI correlates with TA PMN content.
Extracellular BPI (ELISA of TA supernatants), correlated positively with TA PMN content (n=13 subjects) both by unadjusted measures (A; Spearman r=0.73, p<0.0001) and when normalized for total protein (B; Spearman r=0.79, p<0.0001).
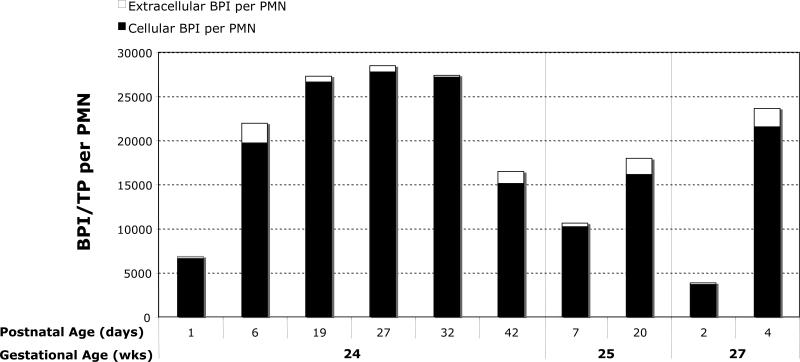
Under basal conditions the vast majority of PMN BPI is intracellular, degranulation of BPI can occur during inflammation (29). We assessed the relative expression of BPI in the cellular and extracellular fractions of neonatal TAs. Cellular BPI (TA pellet extracts) and extracellular BPI (TA supernatants) were measured by ELISA. Data were analyzed after adjusting for both total protein content and PMN count. Representative data from a subset of subjects in Figure 8 demonstrate that the majority (79-99%) of neonatal TA BPI remained cell-associated.
8. BPI in neonatal TAs is predominantly cell-associated.
Representative data (n=3 subjects) sampled at 2-6 postnatal time points, as indicated. BPI in PMN acid extracts of TA pellets (cellular BPI) and TA supernatants (extracellular BPI) was measured by ELISA and adjusted for total protein and PMN count. Most TA BPI is in the form of cellular BPI (filled black bars), with a lesser proportion of extracellular BPI (white extensions at top of bar graphs).
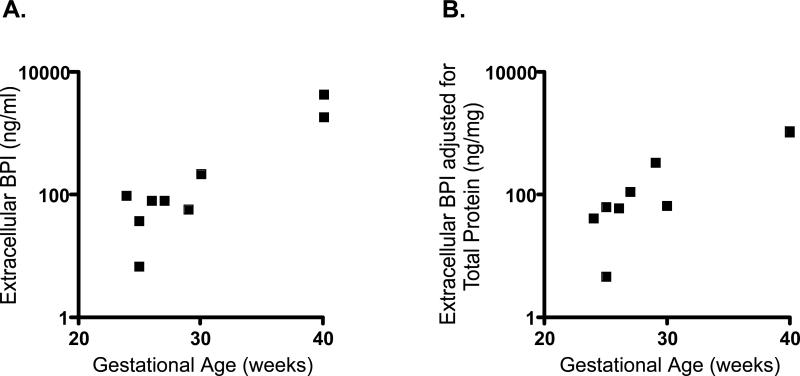
Extracellular BPI concentrations in TAs sampled during the first two postnatal days also correlated with GA (Figure 9A) even when adjusted for total protein (Figure 9B). TA PMN count and GA did not correlate (not shown).
9. Early postnatal extracellular BPI concentrations correlate with gestational age.
Extracellular BPI of TAs collected during the first postnatal week correlated positively with increasing GA both by unadjusted measures (A; n=9, Spearman r=0.69, p<0.05) and when normalized for total protein (B; Spearman r=0.89, p<0.005).
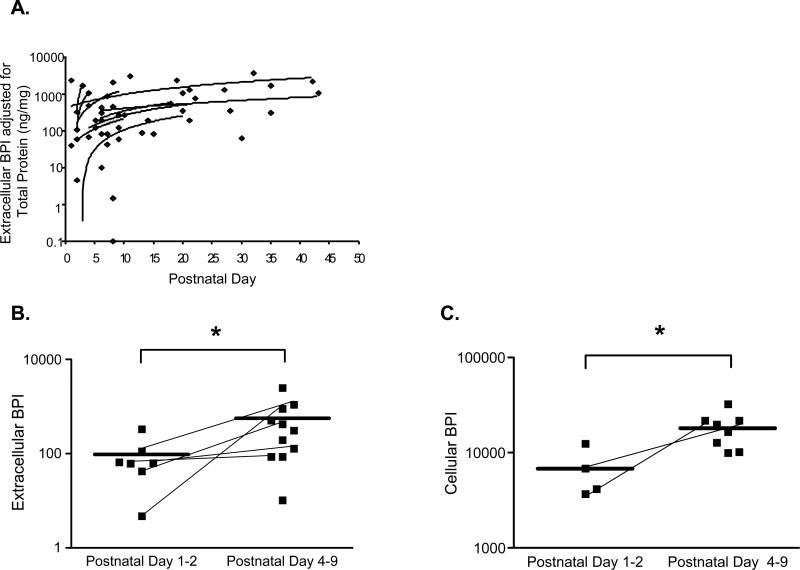
Extracellular BPI normalized for total protein also increased with postnatal age, independently of GA (Figure 10A). When the analysis was dichotomized by postnatal age, extracellular BPI adjusted for total protein increased significantly over the first postnatal week (Figure 10B). Indeed, cellular BPI adjusted for total protein and PMN count increased significantly in the first postnatal week (Figure 10C). Cellular- and extracellular-BPI did not correlate (not shown), suggesting that increased cellular BPI content of TA PMN during the first week of life was not secondary to reduced release of extracellular BPI.
10. BPI increases with postnatal age.
(A) Extracellular BPI adjusted for total protein is plotted as a function of postnatal age (days). Trend lines were fit to subjects for whom two or more samples were obtained. (B) Extracellular BPI adjusted for total protein rises significantly over the first postnatal week, from mean ± SEM of 97±41 (postnatal days 1-2; n=7) to 566±218 ng BPI/mg total protein (postnatal days 4-9; n=11); p<0.05, Mann-Whitney. (C) Cellular BPI, measured by ELISA of TA pellet extracts, rises from mean ± SEM 6794±2038 (postnatal days 1-2; n=4) to 18073±2642 ng cellular BPI/mg total protein /106 PMN (postnatal days 4-9; n=8; p<0.05, Mann-Whitney). Lines connect data points for subjects for whom there are data at both time ranges.
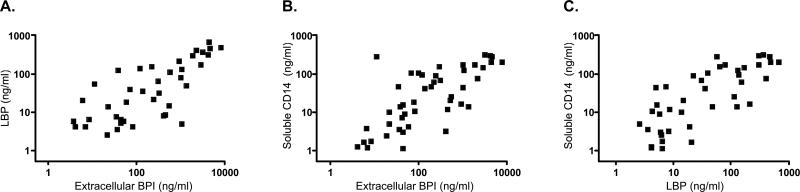
LBP and sCD14 were readily detected in neonatal TA supernatants (mean ± SEM LBP 106±25 ng/mL; sCD14 68±13 ng/mL) and correlated with extracellular BPI. Extracellular BPI positively correlated with LBP (Figure 11A) and sCD14 (Figure 11B). LBP also positively correlated with sCD14 (Figure 11C).
11. Positive correlation of extracellular BPI, LBP and sCD14 in TA supernatants.
ELISA measure of extracellular BPI, LBP, and sCD14 in TA supernatants reveals that: extracellular BPI is positively correlated with (A) LBP (n=13 subjects, Spearman r=0.72, p<0.0001), and (B) sCD14 (n=15 subjects, Spearman r=0.73, p<0.0001), and that (C) LBP is positively correlated with sCD14 (n=13 patients, Spearman r=0.75, p<0.0001).
Discussion
We have for the first time characterized the presence of endotoxin and the expression of key endotoxin-modulating proteins in TAs of an observational cohort of mechanically ventilated human infants. We show that endotoxin is present in tracheal secretions especially after prolonged intubation corresponding to early mobilization of endotoxin-directed innate immunity.
BPI is a key PMN-derived protein mobilized to tracheal secretions. Neonatal TA PMN express BPI antigen of identical molecular weight to that of adults, albeit at lower levels than adult peripheral blood PMN (Figures 3,4). TA BPI is predominately cell-associated (Figure 8), consistent with the sub-cellular localization of BPI to PMN primary granules which are less prone to extracellular degranulation (30). Nevertheless, neonatal TA extracellular BPI was consistently detectable and correlated with TA PMN content (Figure 7), suggesting that PMN are the predominant source of BPI in these upper respiratory secretions. TA BPI expression was both GA and postnatal age-dependent: a) extracellular BPI concentrations during the first 2 postnatal days correlated with GA (Figure 9), b) extracellular BPI adjusted for total protein increased with postnatal age (Figure 10A,B), and c) cellular BPI adjusted for total protein and PMN count specifically increased in the first postnatal week (Figure 10C). Postnatal increases in cellular BPI might reflect developmental increases in availability/recruitment of more mature PMN and/or potential transcriptional activation of mature PMN (31), that can up regulate gene expression (32).
In addition to substantial TA supernatant concentrations of extracellular BPI (mean ~1μg/mL), we also detected substantial amounts of extracellular LBP (0.3-680 ng/mL) and sCD14 (0.5-315 ng/mL), concentrations in the bioactive range (11). As TAs were obtained after endotracheal instillation of saline, they represent diluted samples and true local airway fluid concentrations of these host defense proteins are likely even higher. Extracellular BPI was very likely PMN-derived based on correlation with PMN count (Fig. 7), flow cytometry (Fig. 5), and confocal microscopy (Fig. 6). CD14 mRNA was abundant in TA pellets (Fig. 2), indicating possible local CD14 production, but we do not exclude contribution from a liver-derived acute phase response/plasma exudation (11, 33). LBP mRNA was not detected in TAs (Fig. 2) and its presence at the protein level may reflect respiratory epithelial cell production (34) and/or an acute phase response (11, 33). Positive correlations between extracellular BPI, LBP, and sCD14 in neonatal TA supernatants (Fig. 11) demonstrate coordinated mobilization of endotoxin-modulating proteins to the trachea of mechanically ventilated newborns.
Endotoxin (Fig. 1) introduced to the neonatal respiratory tract during peripartum infection and/or upon endotracheal tube colonization could induce mobilization of endotoxin-modulating proteins to respiratory secretions. TLR-mediated activation of NF-kB and subsequent cytokine production may contribute to the mobilization of BPI, LBP and sCD14 to the neonatal respiratory tract (3, 19). Maturation of pathways mediated by C/EBP epsilon, a transcription factor important for BPI expression (35), could contribute to the postnatal increase in BPI expression. In mice, endotracheal LPS induces an IL-6-mediated acute phase response, including LBP and sCD14 production (33). Moreover, during the first postnatal week, human newborns demonstrate increasing basal serum concentrations of IL-6 (36), a cytokine that is also detected in neonatal TAs (37). Consistent with such a mechanism of microbe-induced activation of innate immunity, our study documents that endotoxin is detected in TA supernatants, especially after prolonged intubation, and that multiple endotoxin-modulating proteins are up regulated after intubation.
Our findings may have potential clinical relevance. The magnitude and duration of exposure of the neonatal respiratory tract to endotoxin coupled with individual susceptibility to endotoxin-induced inflammation might contribute to susceptibility of newborns to a variety of endotoxin-associated conditions, as has been found in other settings (38-40). Indeed, the early presence of endotoxin likely contributes to recruitment of PMN to the trachea, which has been associated with subsequent chronic lung disease (41). Our characterization of endotoxin-directed innate immunity in this observational cohort sets the stage for testing such hypotheses in larger cohorts powered to correlate with clinical outcomes. To the extent that endotoxin plays a role in ventilator-associated lung inflammation and disease, potential prophylaxis and/or treatment might be forthcoming in the form of investigational endotoxin antagonists, including recombinant BPI congeners and lipid A antagonists (e.g., E5564), agents that safely reduce endotoxin-driven inflammation in human studies in vivo (42-44).
Acknowledgements
We thank Drs. Michael Wessels, Richard Malley, and Tobias Strunk for their intellectual contributions, Kevin Chi and Leighanne Gallington for their assistance with PMN isolation, and Jessica Wagner of the Harvard Digestive Diseases Center Imaging Core for her assistance with confocal microscopy.
Statement of financial support: This study was supported by the National Institutes of Health Specialized Center of Research (SCOR) Grant (HL72931), National Institutes of Health National Center for Research Resources K30 Grant (RR022292-07), and an unrestricted grant from XOMA (U.S.) L.L.C.
References
- 1.Lewis DB, Wilson CB. Developmental Immunology and Role of Host Defenses in Fetal and Neonatal Susceptibility to Infection. In: Remington J, Klein J, editors. Infectious Diseases of the Fetus and Newborn Infant. W.B. Saunders; Philadelphia: 2001. pp. 25–138. [Google Scholar]
- 2.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nature Reviews. Immunology. 2004;4(7):553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 3.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 4.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, Lemons JA, Donovan EF, Stark AR, Tyson JE, Oh W, Bauer CR, Korones SB, Shankaran S, Laptook AR, Stevenson DK, Papile LA, Poole WK. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants.[see comment]. New England Journal of Medicine. 2002;347:240–247. doi: 10.1056/NEJMoa012657. [DOI] [PubMed] [Google Scholar]
- 5.Opal SM. The host response to endotoxin, antilipopolysaccharide strategies, and the management of severe sepsis. Int J Med Microbiol. 2007;297:365–377. doi: 10.1016/j.ijmm.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Jobe AH. Antenatal associations with lung maturation and infection. J Perinatol 25 Suppl. 2005;2:S31–35. doi: 10.1038/sj.jp.7211317. [DOI] [PubMed] [Google Scholar]
- 7.Caplan MS, Simon D, Jilling T. The role of PAF, TLR, and the inflammatory response in neonatal necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:145–151. doi: 10.1053/j.sempedsurg.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Sherwin C, Fern R. Acute lipopolysaccharide-mediated injury in neonatal white matter glia: role of TNF-alpha, IL-1beta, and calcium. J Immunol. 2005;175:155–161. doi: 10.4049/jimmunol.175.1.155. [DOI] [PubMed] [Google Scholar]
- 9.Gioannini TL, Teghanemt A, Zhang D, Levis EN, Weiss JP. Monomeric endotoxin:protein complexes are essential for TLR4-dependent cell activation. J Endotoxin Res. 2005;11:117–123. doi: 10.1179/096805105X35198. [DOI] [PubMed] [Google Scholar]
- 10.Prohinar P, Re F, Widstrom R, Zhang D, Teghanemt A, Weiss JP, Gioannini TL. Specific high affinity interactions of monomeric endotoxin.protein complexes with Toll-like receptor 4 ectodomain. J Biol Chem. 2007;282:1010–1017. doi: 10.1074/jbc.M609400200. [DOI] [PubMed] [Google Scholar]
- 11.Kitchens RL, Thompson PA. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J Endotoxin Res. 2005;11:225–229. doi: 10.1179/096805105X46565. [DOI] [PubMed] [Google Scholar]
- 12.Levy O. A neutrophil-derived anti-infective molecule: bactericidal/permeability-increasing protein. Antimicrobial Agents & Chemotherapy. 2000;44:2925–2931. doi: 10.1128/aac.44.11.2925-2931.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganz T. Antimicrobial polypeptides in host defense of the respiratory tract. J Clin Invest. 2002;109:693–697. doi: 10.1172/JCI15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neff SB, Z'Graggen BR, Neff TA, Jamnicki-Abegg M, Suter D, Schimmer RC, Booy C, Joch H, Pasch T, Ward PA, Beck-Schimmer B. Inflammatory response of tracheobronchial epithelial cells to endotoxin. Am J Physiol Lung Cell Mol Physiol. 2006;290:L86–96. doi: 10.1152/ajplung.00391.2004. [DOI] [PubMed] [Google Scholar]
- 15.Levy O. Antimicrobial proteins and peptides: Anti-infective molecules of mammalian leukocytes. J Leuk Biol. 2004;76:909–925. doi: 10.1189/jlb.0604320. [DOI] [PubMed] [Google Scholar]
- 16.Schultz H, Weiss JP. The bactericidal/permeability-increasing protein (BPI) in infection and inflammatory disease. Clin Chim Acta. 2007;384:12–23. doi: 10.1016/j.cca.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy O, Martin S, Eichenwald E, Ganz T, Valore E, Carroll S, Lee K, Goldmann D, Thorne G. Impaired innate immunity in the newborn: newborn neutrophils are deficient in bactericidal/permeability-increasing protein (BPI). Pediatrics. 1999;104:1327–1333. doi: 10.1542/peds.104.6.1327. [DOI] [PubMed] [Google Scholar]
- 18.Nupponen I, Turunen R, Nevalainen T, Peuravuori H, Pohjavuori M, Repo H, Andersson S. Extracellular release of bactericidal/permeability-increasing protein in newborn infants. Pediatric Research. 2002;51:670–674. doi: 10.1203/00006450-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Shimotake TK, Izhar FM, Rumilla K, Li J, Tan A, Page K, Brasier AR, Schreiber MD, Hershenson MB. Interleukin (IL)-1 beta in tracheal aspirates from premature infants induces airway epithelial cell IL-8 expression via an NF-kappa B dependent pathway. Pediatr Res. 2004;56:907–913. doi: 10.1203/01.PDR.0000145274.47221.10. [DOI] [PubMed] [Google Scholar]
- 20.Harris MC, D'Angio CT, Gallagher PR, Kaufman D, Evans J, Kilpatrick L. Cytokine elaboration in critically ill infants with bacterial sepsis, necrotizing entercolitis, or sepsis syndrome: correlation with clinical parameters of inflammation and mortality. J Pediatr. 2005;147:462–468. doi: 10.1016/j.jpeds.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 21.De Dooy J, Colpaert C, Schuerwegh A, Bridts C, Van Der Planken M, Ieven M, De Clerck L, Stevens W, Mahieu L. Relationship between histologic chorioamnionitis and early inflammatory variables in blood, tracheal aspirates, and endotracheal colonization in preterm infants. Pediatr Res. 2003;54:113–119. doi: 10.1203/01.PDR.0000069702.25801.D1. [DOI] [PubMed] [Google Scholar]
- 22.Cheng DS, Han W, Chen SM, Sherrill TP, Chont M, Park GY, Sheller JR, Polosukhin VV, Christman JW, Yull FE, Blackwell TS. Airway epithelium controls lung inflammation and injury through the NF-kappa B pathway. J Immunol. 2007;178:6504–6513. doi: 10.4049/jimmunol.178.10.6504. [DOI] [PubMed] [Google Scholar]
- 23.Kramer BW, Ikegami M, Jobe AH. Intratracheal endotoxin causes systemic inflammation in ventilated preterm lambs. Am J Respir Crit Care Med. 2002;165:463–469. doi: 10.1164/ajrccm.165.4.2011118. [DOI] [PubMed] [Google Scholar]
- 24.Dorschner RA, Lin KH, Murakami M, Gallo RL. Neonatal skin in mice and humans expresses increased levels of antimicrobial peptides: innate immunity during development of the adaptive response. Pediatric Research. 2003;53:566–572. doi: 10.1203/01.PDR.0000057205.64451.B7. [DOI] [PubMed] [Google Scholar]
- 25.Kai-Larsen Y, Bergsson G, Gudmundsson GH, Printz G, Jornvall H, Marchini G, Agerberth B. Antimicrobial components of the neonatal gut affected upon colonization. Pediatr Res. 2007;61:530–536. doi: 10.1203/pdr.0b013e318045be83. [DOI] [PubMed] [Google Scholar]
- 26.Starner TD, Agerberth B, Gudmundsson GH, McCray PB., Jr. Expression and activity of beta-defensins and LL-37 in the developing human lung. J Immunol. 2005;174:1608–1615. doi: 10.4049/jimmunol.174.3.1608. [DOI] [PubMed] [Google Scholar]
- 27.Levy O, Sisson R, Kenyon J, Eichenwald E, Macone A, Goldmann D. Enhancement of neonatal innate defense: Effects of adding an N-terminal recombinant fragment of bactericidal/permeability-increasing protein (rBPI21) on growth and TNF-inducing activity of Gram-negative bacteria tested in neonatal cord blood ex vivo. Infect Immun. 2000;68:5120–5125. doi: 10.1128/iai.68.9.5120-5125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pagano M, Gauvreau K. Principles of Biostatistics. Duxbury Press; Belmont, CA.: 1993. [Google Scholar]
- 29.Opal SM, Palardy JE, Marra MN, Fisher CJ, Jr., McKelligon BM, Scott RW. Relative concentrations of endotoxin-binding proteins in body fluids during infection. Lancet. 1994;344:429–431. doi: 10.1016/s0140-6736(94)91767-1. [DOI] [PubMed] [Google Scholar]
- 30.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- 31.Lennartsson A, Vidovic K, Pass MB, Cowland JB, Gullberg U. All-trans retinoic acid-induced expression of bactericidal/permeability-increasing protein (BPI) in human myeloid cells correlates to binding of C/EBP{beta} and C/EBP{varepsilon} to the BPI promoter. J Leukoc Biol. 2006 doi: 10.1189/jlb.1205759. [DOI] [PubMed] [Google Scholar]
- 32.Borregaard N, Sorensen OE, Theilgaard-Monch K. Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 2007;28:340–345. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Vernooy JH, Reynaert N, Wolfs TG, Cloots RH, Haegens A, de Vries B, Dentener MA, Buurman WA, Wouters EM. Rapid pulmonary expression of acute-phase reactants after local lipopolysaccharide exposure in mice is followed by an interleukin-6 mediated systemic acute-phase response. Exp Lung Res. 2005;31:855–871. doi: 10.1080/01902140600611645. [DOI] [PubMed] [Google Scholar]
- 34.Dentener MA, Vreugdenhil AC, Hoet PH, Vernooy JH, Nieman FH, Heumann D, Janssen YM, Buurman WA, Wouters EF. Production of the acute-phase protein lipopolysaccharide-binding protein by respiratory type II epithelial cells: implications for local defense to bacterial endotoxins. American Journal of Respiratory Cell & Molecular Biology. 2000;23:146–153. doi: 10.1165/ajrcmb.23.2.3855. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka M, Gombart AF, Koeffler HP, Shiohara M. Expression of bactericidal/permeability-increasing protein requires C/EBP epsilon. Int J Hematol. 2007;85:304–311. doi: 10.1532/IJH97.05162. [DOI] [PubMed] [Google Scholar]
- 36.Angelone DF, Wessels MR, Coughlin M, Suter EE, Valentini P, Kalish LA, Levy O. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-alpha production in vitro and in vivo. Pediatr Res. 2006;60:205–209. doi: 10.1203/01.pdr.0000228319.10481.ea. [DOI] [PubMed] [Google Scholar]
- 37.Choi CW, Kim BI, Kim HS, Park JD, Choi JH, Son DW. Increase of interleukin-6 in tracheal aspirate at birth: a predictor of subsequent bronchopulmonary dysplasia in preterm infants. Acta Paediatr. 2006;95:38–43. doi: 10.1080/08035250500404085. [DOI] [PubMed] [Google Scholar]
- 38.Jones CA, Holloway JA, Popplewell EJ, Diaper ND, Holloway JW, Vance GH, Warner JA, Warner JO. Reduced soluble CD14 levels in amniotic fluid and breast milk are associated with the subsequent development of atopy, eczema, or both. Journal of Allergy & Clinical Immunology. 2002;109:858–866. doi: 10.1067/mai.2002.123535. [DOI] [PubMed] [Google Scholar]
- 39.Gubern C, Lopez-Bermejo A, Biarnes J, Vendrell J, Ricart W, Fernandez-Real JM. Natural antibiotics and insulin sensitivity: the role of bactericidal/permeability-increasing protein. Diabetes. 2006;55:216–224. [PubMed] [Google Scholar]
- 40.Chien JW, Zhao LP, Hansen JA, Fan WH, Parimon T, Clark JG. Genetic variation in bactericidal/permeability-increasing protein influences the risk of developing rapid airflow decline after hematopoietic cell transplantation. Blood. 2006;107:2200–2207. doi: 10.1182/blood-2005-06-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang HC, Tai FY, Wang FS, Liu CA, Hsu TY, Ou CY, Yang KD. Correlation of augmented IL-8 production to premature chronic lung disease: implication of posttranscriptional regulation. Pediatr Res. 2005;58:216–221. doi: 10.1203/01.PDR.0000175886.46201.D7. [DOI] [PubMed] [Google Scholar]
- 42.von der Mohlen M, van Deventer S, Levi M, et al. Inhibition of endotoxin-induced cytokine release and neutrophil activation in humans by use of recombinant bactericidal/permeability-increasing protein. J. Infect. Dis. 1995;172:144–151. doi: 10.1093/infdis/172.1.144. [DOI] [PubMed] [Google Scholar]
- 43.von der Mohlen MA, van der Poll T, Jansen J, Levi M, van Deventer SJ. Release of bactericidal/permeability-increasing protein in experimental endotoxemia and clinical sepsis. Role of tumor necrosis factor. Journal of Immunology. 1996;156:4969–4973. [PubMed] [Google Scholar]
- 44.Levin M, Quint PA, Goldstein B, Barton P, Bradley JS, Shemie SD, Yeh T, Kim SS, Cafaro DP, Scannon PJ, Giroir BP. Recombinant bactericidal/permeability-increasing protein (rBPI21) as adjunctive treatment for children with severe meningococcal sepsis: a randomised trial. Lancet. 2000;356:961–967. doi: 10.1016/s0140-6736(00)02712-4. [DOI] [PubMed] [Google Scholar]