Abstract
The polyamines norspermidine and spermidine are among the environmental signals that regulate Vibrio cholerae biofilm formation. The effects of these polyamines are mediated by NspS, a member of the bacterial periplasmic solute binding protein superfamily. Almost all members of this superfamily characterized to date are components of ATP-binding cassette-type transporters involved in nutrient uptake. Consequently, in the current annotation of the V. cholerae genome, NspS has been assigned a function in transport. The objective of this study was to further characterize NspS and investigate its potential role in transport. Our results support a role for NspS in signal transduction in response to norspermidine and spermidine, but not their transport. In addition, we provide evidence that these polyamine signals are processed by c-di-GMP signalling networks in the cell. Furthermore, we present comparative genomics analyses which reveal the presence of NspS-like proteins in a variety of bacteria, suggesting that periplasmic ligand binding proteins may be widely utilized for sensory transduction.
Introduction
Periplasmic solute binding proteins (PBPs) comprise a large family of proteins found in the periplasmic space of Gram-negative bacteria and are generally involved in nutrient import as components of ATP-binding cassette (ABC) transporters. These proteins bind a variety of ligands with high affinity, including polyamines, sugars, amino acids, oligopeptides, metals, iron–siderophore complexes and vitamins (Davidson et al., 2008). In most cases, binding of the ligand leads to association of the PBPs with the permease complexes of the ABC transporter located in the cytoplasmic membrane. The ligand is then released and transported into the cytoplasm in a process driven by ATP hydrolysis catalysed by the ATPase component of the transporter (Davidson et al., 2008).
We have previously reported the initial characterization of NspS, a protein in the PBP family that is an activator of biofilm formation in the pathogenic bacterium Vibrio cholerae (Karatan et al., 2005). NspS belongs to the bacterial extracellular solute-binding protein family 1, which includes PBPs associated with ABC transporters for polyamines (http://www.ebi.ac.uk/Tools/InterProScan/). Polyamines are short hydrocarbon chains containing two or more amine groups that are positively charged at physiological pH. They are found in virtually all cells in millimolar quantities and are essential for normal growth of most prokaryotes and eukaryotes (Tabor & Tabor, 1984). In addition to their role in maintaining normal cell growth the polyamines putrescine, spermidine, norspermidine and spermine have been reported to influence biofilm formation in a number of bacteria (Burrell et al., 2010; Goytia et al., 2013; Karatan et al., 2005; Kolodkin-Gal et al., 2012; Lee et al., 2009; McGinnis et al., 2009; Patel et al., 2006).
NspS is a homologue of PotD and PotF, the PBPs of the well-characterized spermidine and putrescine uptake systems, respectively, in Escherichia coli. Due to its similarity to PotD and PotF, NspS was initially annotated as a putative spermidine/putrescine ABC transporter binding protein. In recently sequenced genomes, NspS homologues continue to be annotated as having a function in transport in various genome databases. Despite its similarity to the PBPs of transport systems, we hypothesize that NspS is not involved in transport but rather in signal transduction for the following reasons. In many cases, genes encoding components of ABC transporters are found adjacent to each other in an operon. For example, the V. cholerae genome contains two nspS homologues, potD1 and potD2, found adjacent to the potA, potB and potC genes, encoding the ATPase and permease components of the ABC transporter. PotD1 is responsible for spermidine import while the role of PotD2 has not yet been established (McGinnis et al., 2009). In contrast, the nspS gene is adjacent to and has overlapping reading frames with the mbaA gene, which encodes a protein that is likely to be involved in signal transduction. MbaA is a putative integral membrane protein containing GGDEF and EAL domains, which are found in enzymes that synthesize or degrade the ubiquitous bacterial secondary messenger cyclic-di-guanylate monophosphate (c-di-GMP). MbaA is also a repressor of V. cholerae biofilm formation (Bomchil et al., 2003; Karatan et al., 2005). Therefore, NspS and MbaA have opposite effects on V. cholerae biofilms. In addition, the polyamine norspermidine significantly enhances biofilm formation and expression of the vps genes, which encode proteins responsible for synthesis of the biofilm polysaccharide in V. cholerae (Karatan, et al., 2005). This effect is mediated by the protein NspS, as ΔnspS mutants do not respond to norspermidine addition. ΔmbaA mutants also do not respond to norspermidine, suggesting that MbaA plays a role in detecting and processing the norspermidine signal in the environment as well. Additionally, spermidine, a polyamine that is one methylene group longer than norspermidine, inhibits V. cholerae biofilm formation also in an NspS- and MbaA-dependent manner (McGinnis et al., 2009).
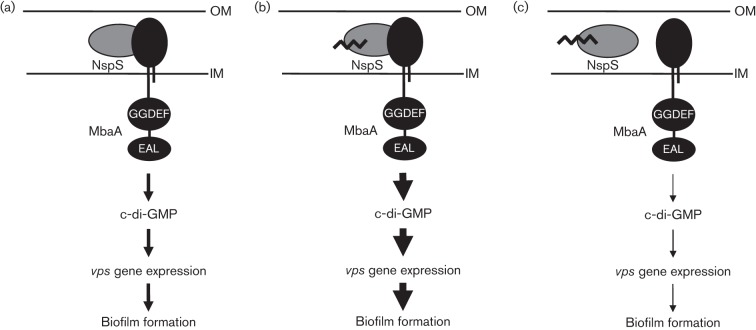
The proximity of the nspS and mbaA genes, their predicted cellular locations and their effect on biofilm formation has led to the hypothesis that NspS and MbaA make up a signalling complex that regulates V. cholerae biofilm formation in response to the presence of norspermidine and spermidine in the environment. Our current working model for this signalling system is depicted in Fig. 1. In the absence of norspermidine or spermidine, NspS interacts with MbaA and downregulates its enzymic activity, allowing for intermediate c-di-GMP levels, vps gene expression and propensity to form biofilms (Fig. 1a). Binding of norspermidine to NspS increases the inhibitory effect of NspS on MbaA, leading to an increase in biofilm formation (Fig. 1b). In contrast, binding of spermidine to NspS inhibits its interaction with MbaA, allowing maximal MbaA activity, which decreases c-di-GMP levels and hinders biofilm formation (Fig. 1c).
Fig. 1.
Working model of the NspS–MbaA signalling complex. Environmental inputs are communicated to the interior of the cell as a change in the enzymic activity of MbaA. This change is reflected in the c-di-GMP levels, which in turn influence vps gene expression and biofilm formation. (a) In the absence of a ligand. (b) With norspermidine. (c) With spermidine. Thin, thicker and thickest arrows correlate with low, medium and high c-di-GMP levels, vps gene expression, and biofilm formation. Short and long zigzag lines bound to NspS represent norspermidine and spermidine, respectively. IM, inner membrane; OM, outer membrane.
In this study, our objective was to provide support for our hypothesis that NspS is a signalling protein that communicates polyamine signals to the cell without mediating their transport and to determine whether NspS and MbaA serve as a new paradigm in c-di-GMP signalling.
Methods
Bacterial strains, plasmids and media.
The V. cholerae strain used was O139 MO10; more information on the bacterial strains and plasmids used in this study can be found in Table 1. Primers are listed in Table S1 (available in the online Supplementary Material). All experiments were done in Luria–Bertani broth (LB). Primer synthesis and DNA sequencing were performed by Eurofins MWG Operon and the Biotechnology Resource Center at Cornell University, respectively.
Table 1. Bacterial strains and plasmids.
| Strain/plasmid | Genotype | Reference/source |
| E. coli | ||
| SM10λpir | thi thr leu tonA lacY supE recA : : RP4-2-Tc : : MuλpirR6K;Kmr | Miller & Mekalanos (1988) |
| SHuffle T7 Express | fhuA2 lacZ : : T7 gene1 [lon] ompT ahpC gal λatt : : pNEB3-r1-cDsbC (SpecR, lacIq) ΔtrxB sulA11 R(mcr-73 : : miniTn10–TetS)2 [dcm] R(zgb-210 : : Tn10–TetS) endA1 Δgor Δ(mcrC-mrr)114 : : IS10 | New England Biolabs |
| NEB express | fhuA2 [lon] ompT gal sulA11 R(mcr-73 : : miniTn10–TetS)2 [dcm] R(zgb-210 : : Tn10–TetS) endA1 Δ(mcrC-mrr)114 : : IS10 | New England Biolabs |
| V. cholerae | ||
| PW249 | MO10, clinical isolate of V. cholerae O139 from India, SmR | Waldor & Mekalanos (1994) |
| PW357 | MO10 lacZ : : vpsLp→lacZ, SmR | Haugo & Watnick (2002) |
| AK314 | MO10 nspC : : kan, KanR, SmR | This study |
| AK317 | MO10 nspC : : kan, ΔpotD1, KanR, SmR | This study |
| AK160 | MO10 lacZ : : vpsLp→lacZ, ΔpotD1, SmR | This study |
| Plasmid | ||
| pWM91 | oriR6KmobRP4 lacI pTac tnp miniTn10Km; KmR, ApR | Metcalf et al. (1996) |
| pET28b | KanR | Novagen |
| pMAL-c5x | AmpR | New England Biolabs |
| pAR17 | pWM91 carrying an internal 981 bp fragment of nspC replaced with kanamycin acetyltransferase gene | This study |
| pRC1 | pMAL-c5x : : mbaA | This study |
| pRC2 | pMAL-c5x : : mbaAE553A | This study |
| pNP37 | pET28b : : nspS | This study |
Construction of the nspS expression vector and purification of NspS.
The signal peptide in NspS was predicted to be amino acids 1–33 with the Signal 4.1 Server (http://www.cbs.dtu.dk/services/SignalP/) using the default D cut-off values. The portion of the nspS gene downstream of this sequence was amplified from chromosomal DNA in two separate reactions using primer pairs P228/P231 (reaction 1) and P229/P230 (reaction 2). The amplified products were denatured at 98 °C for 5 min, combined and left to anneal by slow cooling to room temperature to yield sticky ends, as described by Ulijasz et al. (1996). These were cloned into NcoI/XhoI-digested cytoplasmic expression vector pET28b, in-frame with the plasmid encoded C terminus 6× histine tag yielding pNP37. Correct construction and sequence was verified by restriction digests followed by sequencing of several clones. pNP37 was transformed into Shuffle T7 Express cells (New England Biolabs), which are optimized for cytoplasmic production of periplasmic proteins. For protein production, cells were induced at mid-exponential phase with 0.1 mM IPTG and incubated for an additional 18 h at 30 °C. NspS was purified using metal affinity chromatography with a cobalt resin (Thermo Scientific).
Construction of the mbaA expression vector and mbaAE553A point mutant, and purification of MbaA.
To avoid complications resulting from solubility issues with membrane proteins, we chose to assess the enzymic activity of the cytoplasmic portion of MbaA containing the GGDEF and EAL domains (C-terminal 509 residues). The portion of the mbaA gene encoding the predicted cytoplasmic residues was amplified from chromosomal DNA using primers PA211 and PA212 which added 5′ NdeI and 3′ BamHI sites to the fragment, respectively. The amplified gene fragment was digested with NdeI and BamHI and cloned into NdeI/BamHI-digested pMAL-c5x (New England Biolabs) downstream of a gene encoding the maltose binding protein (MBP), generating pRC1. Presence of the fusion partner substantially increased the solubility of MbaA as expression of the same fragment using pET28b previously had resulted in the majority of the protein accumulating in inclusion bodies. pRC1 was transformed into NEB express cells (New England Biolabs), and correct construction and sequence was verified by colony PCR followed by sequencing of several clones. To produce the MBP–MbaA fusion protein, 1 litre cultures grown in LB with 0.2 % glucose were induced at mid-exponential phase with 0.3 mM IPTG and incubated for an additional 18 h at 30 °C. MBP–MbaA was affinity purified using an amylose resin (New England Biolabs). To generate a mutant in which the putative catalytic glutamate was changed to alanine, mbaA was first amplified in two fragments: the ‘up’ fragment was amplified using PA211 and PA216 that coded for an A to C nucleotide substitution; the ‘down’ fragment was amplified using PA215, which was complementary to PA216, and PA212. The up and down fragments were spliced together using overlap extension PCR (splicing by overlap extension) (Ho et al., 1989) and cloned into pMAL-c5x to generate pRC2, which was sequenced to verify the presence of the intended mutation and absence of other mutations.
Thermal shift assay.
To determine the binding ability of NspS to various ligands, a thermal shift assay (TSA) was performed essentially as previously described (Giuliani et al., 2008; Niesen et al., 2007). TSA relies on the principle that a protein bound to a ligand has a higher thermal stability than the ligand-free protein. The extent of denaturation is measured using the fluorescent protein-binding dye SYPRO Orange, the fluorescence of which is quenched in aqueous environments. As the protein denatures, more SYPRO Orange binds the protein, resulting in increased fluorescence. This assay has previously been used to identify ligands for ABC-type transporters (Giuliani et al., 2008; Tan et al., 2013). The TSA reaction mixtures contained 20 µM NspS alone or with polyamines in TSA buffer (100 mM HEPES, 150 mM NaCl, pH 7.5). SYPRO Orange (Invitrogen) was used at a concentration of 5×. The reactions were transferred to an Optical 96-well reaction plate, covered with an optical adhesive cover, and analysed in an Applied Biosystems 7300 real-time PCR system using the detection filter for the TAMRA dye. The instrument was set to increase the temperature from 25 to 95 °C in increments of 1 °C min−1. The binding assays were performed in triplicate with multiple biological replicates. Negative controls contained SYPRO Orange, assay buffer and polyamines to ensure no reactions were occurring between the polyamines and SYPRO Orange indicator. Data were analysed in SigmaPlot, where the first derivative of the raw fluorescence values was taken and plotted graphically using Microsoft Excel. The peak is the maximal rate of change of the fluorescence intensity, which is referred to as the melting point (Tm) and is a measure of the thermal stability of the protein. Therefore, an upward shift in Tm shows increased thermal stability and indicates a binding event.
Phosphodiesterase assay.
MBP–MbaA or MBP–MbaAE553A (2.5 µM) was mixed with 100 µM c-di-GMP or c-di-AMP (Biolog) and 2 mM MnCl2 in 50 mM Tris, pH 8.5, at a final volume of 100 µl and incubated for 2.5 h at 37 °C. After incubation, reactions were boiled for 5 min and centrifuged through a Nanosep 10 kDa Omega filter for 2 min at 14 000 g. The reaction products were separated using a SUPELCOSIL LC-18 column with a Waters 1525 Binary HPLC pump and were analysed using a Waters 2487 dual λ absorbance detector as described by Ryjenkov et al. (2005).
Construction of nspC : : kan, nspC : : kanΔpotD1 and ΔnspSΔpotD1 mutants.
Multiple attempts to make a markerless in-frame deletion in nspC, which encodes the last enzyme in the norspermidine biosynthesis pathway, failed. Therefore, we generated an nspC mutant by replacing 981 bp of the 1164 bp coding sequence with a kanamycin resistance cassette. The kanamycin aceytltransferase gene was amplified from pKD4 (Datsenko & Wanner, 2000) using primers PA207 and PA208. A 371 bp fragment containing a short 5′ portion of nspC and the upstream sequence was amplified using primers P328 and P329. Similarly, a 468 bp fragment containing a short 3′ portion of nspC and the downstream sequence was amplified using primers P330 and P331. Primers P329 and P330 contained complementary regions to PA207 and PA208, respectively. The three PCR products were then joined together by splicing by overlap extension. After adding adenines, this product was cloned into pCR2.1-TOPO, and the sequence was verified. This insert was then excised using XhoI and SpeI, ligated into pWM91 linearized with the same enzymes generating pAR17, and transformed into E. coli SM10λpir. E. coli SM10λpir containing pAR17 or pMM9 (McGinnis et al., 2009) were mated with wild-type V. cholerae, the ΔpotD1 mutant or the ΔnspS mutant to generate nspC : : kan, nspC : : kanΔpotD1 and ΔnspSΔpotD1 mutants, respectively, via double homologous recombination with sucrose selection as described by Metcalf et al. (1996).
Extraction, benzoylation and detection of polyamines.
Bacteria were grown at 27 °C to mid-exponential phase, pelleted, washed with 1× PBS and resuspended in 10 µl water per milligram wet cell weight. Then, 250 μl of the cell suspension corresponding to 25 mg of cells was lysed using sonication and the cell debris was removed by centrifugation. Cellular proteins were precipitated with 50 % (w/v) trichloroacetic acid and centrifuged, leaving the supernatant containing the polyamines. This supernatant was removed and benzoylated as described previously (McGinnis et al., 2009). Briefly, samples were extracted twice with chloroform, evaporated to dryness and dissolved in 100 µl of 60 % methanol in water. A standard mix containing 0.1 mM of each polyamine was also prepared and benzoylated each time. The resulting set of benzoylated polyamine samples were separated using a Phenomenex Sphereclone ODS column (5 µm, 250×4.6 mm) that was fitted with a 4.0×3.0 mm guard cartridge with the system described above. The runs were performed using a gradient of 45–60 % methanol in water for 30 min with a 10 min isocratic equilibration of 45 % methanol in water.
Biofilm assays.
Bacteria were diluted in 0.3 ml LB at an OD595 of 0.02 taken using a Bio-Rad MicroPlate Reader model 680 and incubated in borosilicate test tubes for 18 h at 27 °C. After 18 h, planktonic cells were removed. The biofilm was washed once with 0.3 ml of 1× PBS, mechanically disrupted in 0.3 ml of 1× PBS by vortexing with glass beads, and the cell density was measured at OD595. All experiments were performed in triplicate and repeated multiple times to confirm reproducibility.
RNA extraction, cDNA synthesis and RT-PCR.
Total RNA was extracted from 5 ml of cells grown to mid-exponential phase using the Ambion RiboPure-Bacteria kit and treated with DNase I for 2 h at 37 °C. One microgram of this RNA was reverse transcribed with random primers using the Protoscript First Strand cDNA Synthesis kit (New England Biolabs). Negative controls were also performed without reverse transcriptase to ensure a lack of genomic DNA contamination. The cDNA was then used in a PCR with gene-specific primers designed to amplify approximately 300 bp regions.
Bioinformatics.
To determine if nspS and mbaA genes were conserved in other members of the genus Vibrio, we utilized the Pathosystems Resource Integration Center (PATRIC) website (http://patricbrc.org/portal/portal/patric/Home; last accessed 4 October 2013) (Gillespie et al., 2011). To determine if nspS-like/mbaA-like gene pairs were present in genomes of other bacteria in a different genomic context, we performed a preliminary genome region comparison analysis using the 672 bacterial genomes available in the Comprehensive Microbial Resource (J. Craig Venter Institute; http://cmr.jcvi.org/cgi-bin/CMR/CmrHomePage.cgi; last accessed 24 September 2013).
Results
NspS interacts with norspermidine and spermidine
To determine if norspermidine and spermidine mediate their effect on V. cholerae biofilms by directly interacting with NspS, we evaluated the ability of NspS to bind these polyamines using a TSA. This assay operates on the principle that a ligand binding protein will be more thermally stable when bound to its ligand and thus will have a higher Tm. In the ligand-free state, NspS had a Tm of approximately 47 °C (Fig. 2). Presence of norspermidine and spermidine increased the melting point of NspS by approximately 10 °C, indicating a binding event (Fig. 2). The NspS homologue PotD has been shown to bind the diamine putrescine in addition to the triamine spermidine, albeit with less affinity (Kashiwagi et al., 1990, 1996). To determine if NspS is also capable of interacting with putrescine, we performed the binding assay with this polyamine as well as another diamine, cadaverine. Putrescine and cadaverine did not increase the thermal stability of NspS when used at the same concentration, indicating the increase in Tm was polyamine specific (Fig. 2). These results indicate that the triamines norspermidine and spermidine can interact with NspS whereas the diamines putrescine and cadaverine cannot.
Fig. 2.
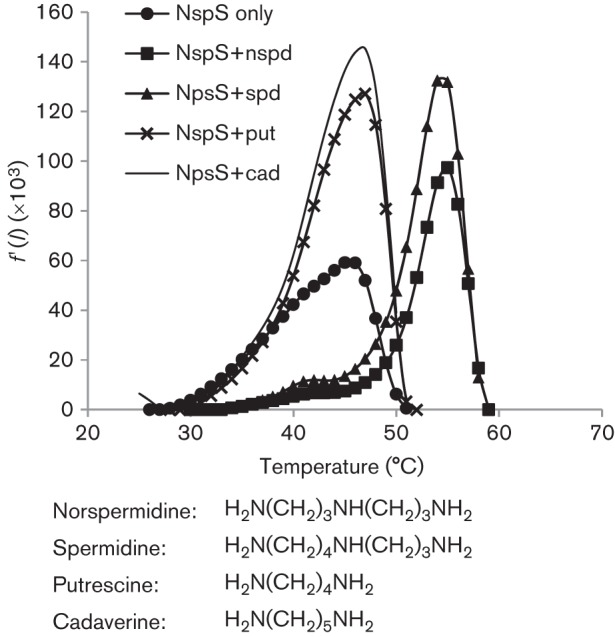
Binding of polyamines to NspS. TSAs were conducted in the presence or absence of polyamines. Denaturation profile of NspS without polyamines (NspS only) and with norspermidine (nspd), spermidine (spd), putrescine (put) and cadaverine (cad) are shown. The y-axis f′(I) is the rate of change of total fluorescence intensity (I). Peaks of the curve represent Tm, melting temperature, which was used to compare the stability of NspS under the assay conditions. Assays were performed in triplicate and means were plotted. Assays were repeated at least twice with protein purified from different cultures to ensure reproducibility; a representative graph is shown. Chemical formulas of the polyamines used are presented below the graph.
The presence of norspermidine and spermidine in the culture medium leads to enhanced and hindered accumulation in biofilms, respectively, as previously reported (Fig. S1) (Karatan et al., 2005; McGinnis et al., 2009). The results of the binding assays suggested that putrescine and cadaverine should not affect V. cholerae biofilm formation. To test this prediction, biofilm assays were conducted in the presence of putrescine and cadaverine. Consistent with the results of the TSA, the presence of these two polyamines did not affect biofilm formation (Fig. S1). These results support the hypothesis that the effect of norspermidine and spermidine on biofilms is the result of direct binding to NspS.
The nspS and mbaA genes are in an operon
In bacteria, genes functioning in the same pathway are often found in operons. Therefore, we wanted to determine whether nspS and mbaA are co-transcribed to add weight to our hypothesis that NspS is a sensor and MbaA is a signal transducer, which interact to regulate biofilm formation. We reverse-transcribed the RNA extracted from wild-type V. cholerae cells and amplified the junctions between nspS/mbaA and mbaA/VC0702 using primers that annealed to adjacent genes (Fig. 3). We have previously shown that the deletion of VC0702, the third gene in the predicted operon, does not affect biofilm formation under the conditions where deletion of nspS or mbaA has pronounced effects on biofilms (Karatan et al., 2005). The protein encoded by VC0702 was later shown to be an NTPase capable of cleaving dITP and dUTP (Ni et al., 2006). However, the role of VC0702 in V. cholerae physiology remains unknown. We detected products for all of the junctions, indicating that nspS, mbaA and VC0702 reside in an operon. Because proteins encoded by genes in an operon interact and/or work together in the same pathway, this result lends support to the hypothesis that NspS and MbaA work together to regulate biofilm formation.
Fig. 3.
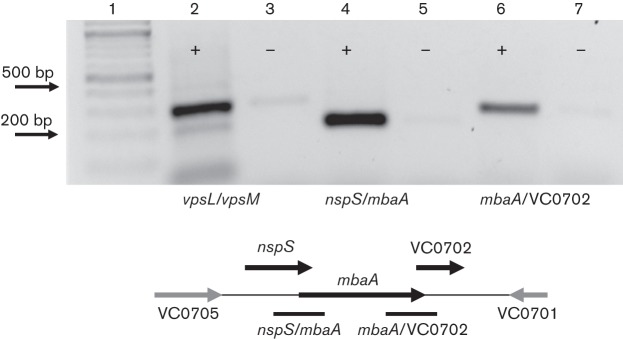
nspS/mbaA/VC0702 are co-transcribed: gene junctions between nspS/mbaA, mbaA/VC0702 and vpsL/vpsM (control) were amplified from cDNA reverse-transcribed from V. cholerae total RNA. The vpsL and vpsM genes are the first two genes of the vpsII (or vpsL) operon encoding proteins responsible for synthesis and transport of the biofilm exopolysaccharide. Lane 1, ladder; lanes 2 and 3, intergenic region between vpsL/vpsM amplified using PA108 and PA109; lanes 4 and 5, intergenic region between nspS/mbaA amplified using PA 112 and PA113; lanes 6 and 7, intergenic region between mbaA/VC0702 amplified with PA110 and PA111.+/−, Presence or absence, respectively, of reverse transcriptase in the cDNA synthesis reactions. Amplified regions are shown as black bars under the cartoon of the chromosomal region. The vpsL/vpsM region is not shown. The image was taken using Alpha Imager and reversed with the program software for better resolution.
MbaA is a c-di-GMP phoshpodiesterase
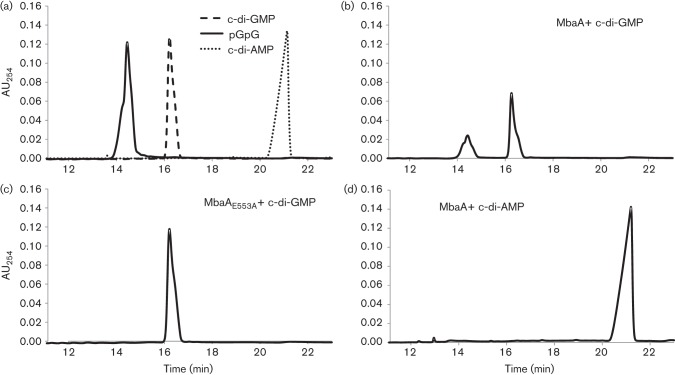
Our working model predicts that NspS and MbaA work together to regulate c-di-GMP levels in V. cholerae. MbaA is predicted to be a phosphodiesterase because the canonical GGDEF motif in this protein is altered to SGDEF, suggesting the GGDEF domain is not likely to have diguanylate cyclase activity. In addition, the increased propensity of mbaA mutants to accumulate in a biofilm is consistent with phenotypes of phosphodiesterase mutants, which have local or global increases in c-di-GMP levels (Römling et al., 2013). To confirm that MbaA could indeed contribute to c-di-GMP signalling by hydrolysing this molecule, we performed c-di-GMP phosphodiesterase assays with purified MbaA. We separated the reaction products by HPLC and compared the HPLC traces with c-di-GMP and pGpG standards to identify peaks (Fig. 4a). MbaA was able to break down c-di-GMP to pGpG, confirming that it is a phosphodiesterase (Fig. 4b). To ensure the phosphodiesterase activity was associated with the EAL domain, we constructed a mutant, MbaAE553A, in which the catalytic glutamate residue was changed to an alanine. As expected, this altered protein was incapable of degrading c-di-GMP to pGpG (Fig. 4c). We also tested the activity of MbaA against c-di-AMP, another cyclic dinucleotide utilized by bacteria. MbaA was not able to break down c-di-AMP, indicating it is a c-di-GMP-specific phosphodiesterase (Fig. 4d).
Fig. 4.
MbaA is a c-di-GMP phosphodiesterase. Phosphodiestrase assays were conducted as described in Methods and the reaction products were separated by HPLC. (a) c-di-GMP, pGpG and c-di-AMP standards. (b) MbaA with c-di-GMP. (c) MbaAE553A with c-di-GMP. (d) MbaA with c-di-AMP. Graphs shown are representative of data from different experiments using protein purified from at least three separate cultures. AU254, absorbance units at 254 nm.
NspS is not involved in spermidine transport
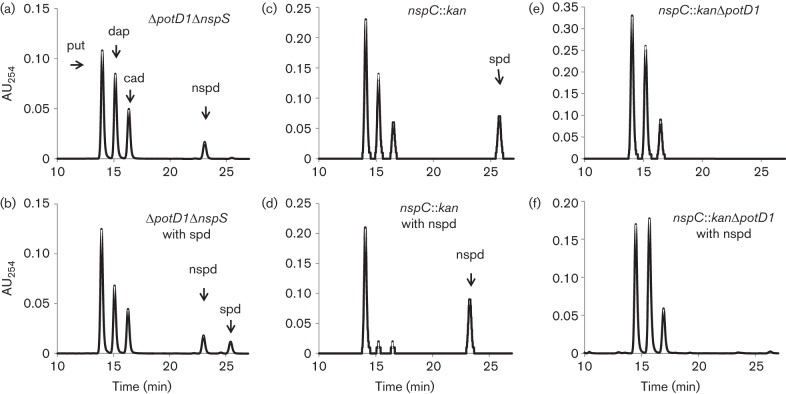
If NspS is a sensor that communicates extracellular levels of polyamines to MbaA, then it may not be involved in transport. Alternatively, it may be a bi-functional protein involved in both signalling and transport. To distinguish between these two possibilities, we investigated the role of NspS in polyamine transport, starting with spermidine uptake. We have previously shown that PotD1, a protein that has 66 % amino acid identity to E. coli PotD, is responsible for spermidine import in V. cholerae (McGinnis et al., 2009). In the absence of PotD1, NspS cannot support spermidine uptake at low concentrations of spermidine (10–40 µM in different batches of LB broth used), indicating that it does not play a role in high-affinity spermidine transport. However, these results do not preclude the possibility that NspS is part of a low-affinity transport system for spermidine. To test this hypothesis, we analysed the polyamine content of the ΔpotD1 strain grown in high concentrations of spermidine (1 mM). The presence of 1 mM spermidine in the culture medium resulted in accumulation of a small amount of spermidine in the cell, suggesting that NspS could indeed be a low-affinity binding protein utilized for spermidine import (data not shown). To test this hypothesis, we constructed a ΔnspSΔpotD1 double mutant and assessed its ability to uptake spermidine. In the absence of additional spermidine in the growth medium, this mutant contained putrescine, diaminopropane, cadaverine and norspermidine (Fig. 5a). All of these polyamines can be produced by V. cholerae, but spermidine has to be imported from the culture medium under the conditions of this experiment. (Lee et al., 2009; McGinnis et al., 2009). Growing this mutant in the presence of 1 mM spermidine still led to accumulation of spermidine in the cell (Fig. 5b). These results indicate that NspS is not responsible for spermidine transport. It is possible, however, that V. cholerae has a second protein in addition to PotD1 that is capable of mediating spermidine transport at high concentrations of this molecule.
Fig. 5.
The role of NspS in spermidine and norspermidine transport. Polyamines were extracted, derivatized by benzoylation and analysed by HPLC as described in Methods. Only data obtained between 10 and 27 min of a 40 min run are plotted for clarity. (a) ΔnspSΔpotD1 mutant. (b) ΔnspSΔpotD1 mutant with 1 mM spermidine. (c) nspC : : kan mutant. (d) nspC : : kan mutant with 1 mM norspermidine. (e) nspC : : kanΔpotD1 mutant. (f) nspC : : kanΔpotD1 mutant with 1 mM norspermidine. Peaks labelled in the wild-type chromatogram correspond to putrescine (put), diaminopropane (dap), cadaverine (cad), norspermidine (nspd) and spermidine (spd). AU254, absorbance units at 254 nm. At least three biological replicates were performed; data from one representative experiment are shown.
PotD1, but not NspS, is responsible for norspermidine import
Next, to rule out the involvement of NspS in norspermidine transport, we first eliminated norspermidine synthesis in the cell. We disrupted the nspC gene, which codes for the enzyme that catalyses the last step of norspermidine synthesis. As expected, this mutant was not able to produce norspermidine (Fig. 5c). Addition of norspermidine to the growth medium of this mutant restored the presence of norspermidine in the cell, indicating that the norspermidine uptake system was intact (Fig. 5d). Exogenous norspermidine also eliminated spermidine uptake, corroborating the results previously reported by us and others (Lee et al., 2009; McGinnis et al., 2009). The competition between spermidine and norspermidine transport suggested that PotD1 may be responsible for norspermidine uptake as well. To investigate this possibility, we constructed a double mutant, nspC : : kanΔpotD1, which synthesized putrescine, diaminopropane and cadaverine and was unable to import spermidine (Fig. 5e). The addition of norspermidine to the growth medium did not restore norspermidine levels in the cell, indicating that this mutant cannot import norspermidine (Fig. 5f). This result shows that PotD1 is the sole protein responsible for norspermidine uptake under the conditions of our experiment. In addition, because the nspS gene remains intact in this mutant our results also confirm that NspS does not play a role in norspermidine import.
The nspS and mbaA genes are conserved in a subset of vibrios
The presence of norspermidine signal detection systems have not been reported in other microbes. To determine if norspermidine-responsive signal transduction systems might be utilized by other members of the genus Vibrio, we searched for nspS and mbaA in the genomes of other vibrios. We found that nspS and mbaA were conserved in almost all V. cholerae isolates as well as many other members of this genus (Fig. S2). In these genomes, the gene identity and gene order upstream and downstream of the nspS and mbaA gene pair were also completely conserved. The gene upstream of nspS is annotated as chorismate mutase/prephenate dehydratase; the genes downstream of mbaA are annotated as inosine/xanthosine triphosphatase (VC0702), putative trp operon repressor (VC0701) and soluble lytic murein transglycosylase (VC0700), respectively. Several species contained the mbaA gene but not the nspS gene while retaining the synteny downstream and upstream of mbaA. Others did not harbour either nspS or mbaA; however, again the gene identity and gene order upstream and downstream of this region were completely conserved. Our results indicate that nspS and mbaA are not conserved in all members of the genus Vibrio; however, approximately 50 % of species of the genus Vibrio whose genomes have been sequenced should have the ability to respond to norspermidine using the NspS/MbaA system. Conversely, absence of nspS and mbaA from a significant number of vibrios suggests that these organisms may not have a need for detecting these polyamines in their environment.
nspS-like/mbaA-like gene pairs are present in the genomes of diverse Proteobacteria
Conservation of the nspS/mbaA gene pair in a number of Vibrio genomes prompted us to investigate whether potential signalling systems composed of periplasmic solute binding proteins and integral membrane proteins containing GGDEF/EAL domains could be a paradigm in bacterial signalling. Genome region comparisons were performed using the sequenced bacterial genomes available in the Comprehensive Microbial Resource database (http://cmr.jcvi.org/cgi-bin/CMR/CmrHomePage.cgi). This preliminary analysis produced 13 different species belonging to Proteobacteria that contain genes encoding PBPs adjacent to predicted integral membrane proteins containing GGDEF/EAL domains (Table 2). The predicted ligands for the PBPs as determined by the annotations available on the genome pages included putrescine, spermidine, phosphate, phosphonate, nitrate, sulphonate and bicarbonate for more detailed annotations as well as anions, amino acids, peptides, and amines for less detailed annotations. In all cases, the gene encoding the PBP was followed by another gene encoding a GGDEF/EAL domain protein that either had overlapping reading frames with the first gene or was only a few base pairs (3–13) downstream of this gene, an arrangement consistent with an operon structure.
Table 2. nspS/mbaA-like gene pairs in other bacteria.
| Organism | PBP | Predicted ligand | GGDEF/EAL | Proximity | ||||
| Vibrio cholerae | nspS | Norspermidine/spermidine | mbaA | Overlap | ||||
| Psychromonas ingrahamii | Ping_1238 | Spermidine/putrescine | Ping_1239 | 8 | ||||
| Hahella chejuensis | HCH_06688 | Spermidine/putrescine | HCH_06689 | 4 | ||||
| Shewanella sediminis | Ssed_2394 | Spermidine/putrescine | Ssed_2393 | Overlap | ||||
| Pseudomonas stutzeri | PST_0371 | Spermidine/putrescine | PST_0370 | 13 | ||||
| Sinorhizobium meliloti | SMc_00991 | Putrescine | SMc_00991 | 3 | ||||
| Magnetospirillum magneticum | amb_1105 | Phosphate/phosphonate | amb_1104 | 3 | ||||
| Nitratiruptor sp. SB155-21 | NIS_1757 | Nitrate/sulphonate/bicarbonate | NIS_1758 | Overlap | ||||
| Thiomicrospira crunogena | Tcr_1221 | Phosphonate | Tcr_1222 | Overlap | ||||
| Vibrio parahaemolyticus | VPA_1753 | Alkylphosphonate | VPA_1754 | Overlap | ||||
| Vibrio parahaemolyticus | VPA_1512 (ScrB) | S-signal | VPA_1511 (ScrC) | Overlap | ||||
| Shewanella paeleanna | Spea_3650 | ESBF-3 | Spea_3649 | 13 | ||||
| Xanthomonas oryzae pv. oryzae | X000RF_2004 | Phosphate | X000RF_2004 | Overlap | ||||
| Xanthomonas campestris pv. campestris | xcc-b100_1903 | Anions | xcc-b100_1904 | 3 | ||||
Discussion
The second messenger c-di-GMP is extensively utilized in bacteria to regulate cellular physiology in response to external or internal signals. The V. cholerae genome encodes more than 60 proteins that potentially contribute to the c-di-GMP pools in the cell. Yet, the signals detected directly or indirectly by these proteins remain largely unknown. Our previous work had shown that norspermidine and spermidine are two exogenous signals that are likely to influence cellular c-di-GMP levels in V. cholerae as a result of their interaction with the putative NspS/MbaA signalling system (Karatan et al., 2005; McGinnis et al., 2009). In this study, we demonstrate that both norspermidine and spermidine bind NspS. Other spermidine-binding PBPs have been reported; however, to our knowledge NspS is the first norspermidine-binding PBP that has been identified. Norspermidine greatly stimulates biofilm formation whereas spermidine has the opposite effect; therefore, these polyamines are antagonistic signals both of which are detected by NspS. We have also shown that the nspS and mbaA genes constitute an operon, providing evidence for the hypothesis that NspS and MbaA work together to process these signals. How NspS mediates the response to norspermidine and spermidine and whether this involves a direct interaction with MbaA is currently under investigation in our laboratory.
The ability of V. cholerae to both synthesize and detect norspermidine leads to the intriguing possibility that this molecule could be used in quorum sensing. This would require the cells to export norspermidine into the environment. We have previously investigated this possibility; however, we did not detect norspermidine in the spent culture media of cells grown in a variety of conditions (Parker et al., 2012). Therefore, norspermidine does not appear to be exported by V. cholerae in its unmodified form. In many cases, polyamines are modified by acetylation prior to being exported (Igarashi & Kashiwagi, 2010). Polyamine acetylation has not been studied in V. cholerae; however, the presence of acetyl-norspermidine has been reported in Vibrio parahaemolyticus (Yamamoto et al., 1989). It is possible that acetyl-norspermidine is indeed synthesized and exported by V. cholerae and detected by NspS to serve as a measure of cell density.
In this study, we have also shown that PotD1 is responsible for norspermidine import in V. cholerae. To our knowledge, this is the first identification of a PBP responsible for norspermidine import in bacteria. The ability to synthesize norspermidine de novo as well as to import it from the environment implies that this polyamine plays an important role in V. cholerae physiology. Norspermidine forms the backbone of the V. cholerae siderophore vibriobactin, implicating its importance especially in iron-limited environments (Keating et al., 2000). In addition, deletion of the nspC gene reduces the growth rate in this organism, indicating norspermidine synthesis is required for normal growth (Lee et al., 2009). Whether this molecule plays other roles in V. cholerae physiology in addition to its effect on growth, biofilm formation and iron acquisition remains to be studied.
The nspS and mbaA genes are conserved in a number of species in the genus Vibrio, indicating they should have the ability to respond to norspermidine and spermidine using the NspS/MbaA system. Being able to detect these polyamines is likely to be important for these organisms and may give insight into their interactions with other organisms in their environment. Norspermidine and spermidine are both produced by prokaryotes and eukaryotes. Norspermidine is made by many Vibrio species as well as other bacteria and archaea (Christensen et al., 2012; Hamana, 1997; Hamana & Itoh, 2001; Hamana et al., 2001). It is also the major polyamine found in aquatic invertebrates such as sea urchins, sea cucumbers and bivalves, as well as some aquatic plants and algae (Hamana et al., 1991, 1998, 2004). Spermidine is a common polyamine synthesized by many different bacteria and almost all eukaryotes (Tabor & Tabor, 1984). Therefore, norspermidine and spermidine may mediate interactions of some members of the genus Vibrio with other organisms containing these polyamines.
Because of its similarity to PotD and PotF, the PBPs of the E. coli ABC transporters for spermidine and putrescine, NspS has been annotated as having a function in polyamine transport. We have conclusively demonstrated that NspS does not play a role in the import of norspermidine or spermidine. Hence, NspS appears to be primarily used in signal transduction. Our results also underline the importance of consideration of genomic context in addition to sequence similarity to provide more accurate annotations for genes whose functions have not yet been studied.
Although rare, examples of PBPs involved in signal processing have been reported. A number of PBPs that are part of ABC transporters also play a role in sensory transduction by directly associating with membrane-bound components of signalling complexes. In E. coli, maltose, ribose and glucose binding proteins, which are involved in the transport of these sugars, also bind distinct chemotaxis receptors when complexed with their respective sugars (Eym et al., 1996; Gardina et al., 1992; Shilton et al., 1996). In Agrobacterium tumefaciens, ChvE, the sugar binding protein of the MmsAB transporter, also regulates virulence by interacting with the sensor kinase VirA (Hu et al., 2013; Zhao & Binns, 2011). Thus, these bi-functional PBPs coordinate nutrient uptake with cellular responses such as chemotaxis and virulence. In Vibrio harveyi, the PBP LuxP directly associates with the membrane-bound two-component sensor kinase LuxQ to communicate information about cell density (Neiditch et al., 2005). Binding of autoinducer-2 to LuxP affects its interaction with LuxQ, which in turn changes from a kinase to a phosphatase. In this case, LuxP is involved in sensing of autoinducer-2, but not its transport.
Our genomic analyses identified additional periplasmic binding proteins that are likely to be involved in signal transduction. The genes encoding these proteins were always adjacent to mbaA-like genes, suggesting that they are likely to be co-regulated. One of these gene pairs encode ScrB and ScrC of the V. parahaemolyticus ScrABC system that has been recently characterized (Trimble & McCarter, 2011). In this system, ScrA, a putative periplasmic enzyme, is thought to process an autoinducer signal termed the S-signal, which can interact with the periplasmic solute binding protein of the system, ScrB. ScrB is then thought to interact with ScrC, a membrane-bound GGDEF/EAL protein, to regulate c-di-GMP levels and swarming in response to cell density. NspS, ScrB and the other periplasmic solute binding proteins may constitute a subfamily of PBPs that are either utilized in signal transduction only or are bi-functional proteins utilized in signalling as well as transport. Distinguishing between these possibilities will have to await more in-depth characterization of these proteins.
Our results suggest that a variety of environmental signals can be processed by NspS/MbaA-like sensor/transducer pairs. The resulting information can feed into c-di-GMP regulatory networks to influence c-di-GMP-regulated phenotypes in diverse bacteria (Fig. 6). We propose these NspS-like sensors and MbaA-like transducers constitute a new paradigm in bacterial signalling.
Fig. 6.
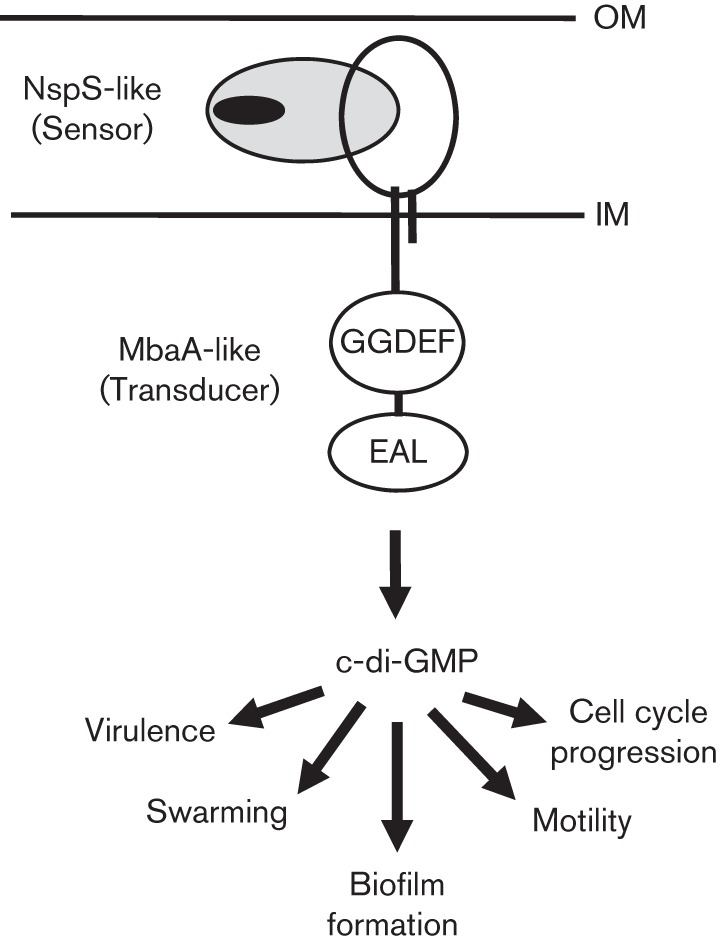
Model of c-di-GMP signalling complexes made up of NspS-like sensors and MbaA-like transducers. Extracellular ligands (black oval) bind NspS-like sensors that are involved in signalling, which in turn changes the interactions between these and their cognate MbaA-like transducers, leading to changes in their enzymic activities. This results in alteration of local or global c-di-GMP levels and affects a variety of responses. IM, inner membrane; OM, outer membrane.
Acknowledgements
We are grateful to Dr Paula Watnick for critical reading of and suggestions on the manuscript. We also thank Drs Tony Michael, Nathan Blow, Mary Connell, Ted Zerucha, Annkatrin Rose, Sue Edwards and Maryam Ahmed for helpful discussions, Drs Suzanna Brauer and Matt Estep for their suggestions on genomic analyses, and Dr Mary Connell for critical reading of the manuscript. We also thank Richard Sobe and Will Brennan for their suggestions on the manuscript and Blake Sanders also for his critical reading of the manuscript and for performing growth curves. This work was funded by the following sources: Appalachian State University Department of Biology, the Office of Student Research and the Graduate Student Association Senate at Appalachian State University, University Research Council grants (to E. K.) and by National Institute of Allergy and Infectious Disease (grant number AI096358 to E. K.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Disease or the National Institute of Health.
Abbreviations:
- ABC transporter
ATP-binding cassette transporter
- MBP
maltose binding protein
- PBP
periplasmic binding protein
- TSA
thermal shift assay
Footnotes
One supplementary table and two supplementary figures are available with the online version of this paper.
References
- Bomchil N., Watnick P., Kolter R. (2003). Identification and characterization of a Vibrio cholerae gene, mbaA, involved in maintenance of biofilm architecture. J Bacteriol 185, 1384–1390. 10.1128/JB.185.4.1384-1390.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell M., Hanfrey C. C., Murray E. J., Stanley-Wall N. R., Michael A. J. (2010). Evolution and multiplicity of arginine decarboxylases in polyamine biosynthesis and essential role in Bacillus subtilis biofilm formation. J Biol Chem 285, 39224–39238. 10.1074/jbc.M110.163154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H., Bertelsen M. F., Bojesen A. M., Bisgaard M. (2012). Classification of Pasteurella species B as Pasteurella oralis sp. nov. Int J Syst Evol Microbiol 62, 1396–1401. 10.1099/ijs.0.035246-0 [DOI] [PubMed] [Google Scholar]
- Datsenko K. A., Wanner B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97, 6640–6645. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A. L., Dassa E., Orelle C., Chen J. (2008). Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol Mol Biol Rev 72, 317–364. 10.1128/MMBR.00031-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eym Y., Park Y., Park C. (1996). Genetically probing the regions of ribose-binding protein involved in permease interaction. Mol Microbiol 21, 695–702. 10.1046/j.1365-2958.1996.261389.x [DOI] [PubMed] [Google Scholar]
- Gardina P., Conway C., Kossman M., Manson M. (1992). Aspartate and maltose-binding protein interact with adjacent sites in the Tar chemotactic signal transducer of Escherichia coli. J Bacteriol 174, 1528–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. J., Wattam A. R., Cammer S. A., Gabbard J. L., Shukla M. P., Dalay O., Driscoll T., Hix D., Mane S. P. & other authors (2011). PATRIC: the comprehensive bacterial bioinformatics resource with a focus on human pathogenic species. Infect Immun 79, 4286–4298. 10.1128/IAI.00207-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani S. E., Frank A. M., Collart F. R. (2008). Functional assignment of solute-binding proteins of ABC transporters using a fluorescence-based thermal shift assay. Biochemistry 47, 13974–13984. 10.1021/bi801648r [DOI] [PubMed] [Google Scholar]
- Goytia M., Dhulipala V. L., Shafer W. M. (2013). Spermine impairs biofilm formation by Neisseria gonorrhoeae. FEMS Microbiol Lett 343, 64–69. 10.1111/1574-6968.12130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamana K. (1997). Polyamine distribution patterns within the families Aeromonadaceae, Vibrionaceae, Pasteurellaceae, and Halomonadaceae, and related genera of the gamma subclass of the Proteobacteria. J Gen Appl Microbiol 43, 49–59. 10.2323/jgam.43.49 [DOI] [PubMed] [Google Scholar]
- Hamana K., Itoh T. (2001). Polyamines of the hyperthermophilic archaebacteria belonging to the genera Thermococcus and Methanothermus and two new genera Caldivirga and Palaeococcus. Microbios 104, 105–114. [PubMed] [Google Scholar]
- Hamana K., Niitsu M., Samejima K., Matsuzaki S. (1991). Novel tetraamines, pentaamines and hexaamines in sea-urchin, sea-cucumber, sea squirt and bivalves. Comp Biochem Physiol B 100, 59–62. [Google Scholar]
- Hamana K., Niitsu M., Samejima K. (1998). Unusual polyamines in aquatic plants: the occurrence of homospermidine, norspermidine, thermospermine, norspermine, aminopropylhomospermidine, bis(aminopropyl)ethanediamine, and methylspermidine. Can J Bot 76, 130–133. [Google Scholar]
- Hamana K., Niitsu M., Samejima K., Itoh T. (2001). Polyamines of the thermophilic eubacteria belonging to the genera Thermosipho, Thermaerobacter and Caldicellulosiruptor. Microbios 104, 177–185. [PubMed] [Google Scholar]
- Hamana K., Aizaki T., Arai E., Saito A., Uchikata K., Ohnishi H. (2004). Distribution of norspermidine as a cellular polyamine within micro green algae including non-photosynthetic achlorophyllous Polytoma, Polytomella, Prototheca and Helicosporidium. J Gen Appl Microbiol 50, 289–295. 10.2323/jgam.50.289 [DOI] [PubMed] [Google Scholar]
- Haugo A. J., Watnick P. I. (2002). Vibrio cholerae CytR is a repressor of biofilm development. Mol Microbiol 45, 471–483. 10.1046/j.1365-2958.2002.03023.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. (1989). Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77, 51–59. 10.1016/0378-1119(89)90358-2 [DOI] [PubMed] [Google Scholar]
- Hu X., Zhao J., DeGrado W. F., Binns A. N. (2013). Agrobacterium tumefaciens recognizes its host environment using ChvE to bind diverse plant sugars as virulence signals. Proc Natl Acad Sci U S A 110, 678–683. 10.1073/pnas.1215033110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K., Kashiwagi K. (2010). Modulation of cellular function by polyamines. Int J Biochem Cell Biol 42, 39–51. 10.1016/j.biocel.2009.07.009 [DOI] [PubMed] [Google Scholar]
- Karatan E., Duncan T. R., Watnick P. I. (2005). NspS, a predicted polyamine sensor, mediates activation of Vibrio cholerae biofilm formation by norspermidine. J Bacteriol 187, 7434–7443. 10.1128/JB.187.21.7434-7443.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi K., Hosokawa N., Furuchi T., Kobayashi H., Sasakawa C., Yoshikawa M., Igarashi K. (1990). Isolation of polyamine transport-deficient mutants of Escherichia coli and cloning of the genes for polyamine transport proteins. J Biol Chem 265, 20893–20897. [PubMed] [Google Scholar]
- Kashiwagi K., Pistocchi R., Shibuya S., Sugiyama S., Morikawa K., Igarashi K. (1996). Spermidine-preferential uptake system in Escherichia coli. Identification of amino acids involved in polyamine binding in PotD protein. J Biol Chem 271, 12205–12208. 10.1074/jbc.271.21.12205 [DOI] [PubMed] [Google Scholar]
- Keating T. A., Marshall C. G., Walsh C. T. (2000). Vibriobactin biosynthesis in Vibrio cholerae: VibH is an amide synthase homologous to nonribosomal peptide synthetase condensation domains. Biochemistry 39, 15513–15521. 10.1021/bi001651a [DOI] [PubMed] [Google Scholar]
- Kolodkin-Gal I., Cao S., Chai L., Böttcher T., Kolter R., Clardy J., Losick R. (2012). A self-produced trigger for biofilm disassembly that targets exopolysaccharide. Cell 149, 684–692. 10.1016/j.cell.2012.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lee J., Sperandio V., Frantz D. E., Longgood J., Camilli A., Phillips M. A., Michael A. J. (2009). An alternative polyamine biosynthetic pathway is widespread in bacteria and essential for biofilm formation in Vibrio cholerae. J Biol Chem 284, 9899–9907. 10.1074/jbc.M900110200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis M. W., Parker Z. M., Walter N. E., Rutkovsky A. C., Cartaya-Marin C., Karatan E. (2009). Spermidine regulates Vibrio cholerae biofilm formation via transport and signaling pathways. FEMS Microbiol Lett 299, 166–174. 10.1111/j.1574-6968.2009.01744.x [DOI] [PubMed] [Google Scholar]
- Metcalf W. W., Jiang W., Daniels L. L., Kim S. K., Haldimann A., Wanner B. L. (1996). Conditionally replicative and conjugative plasmids carrying lacZα for cloning, mutagenesis, and allele replacement in bacteria. Plasmid 35, 1–13. 10.1006/plas.1996.0001 [DOI] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. (1988). A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol 170, 2575–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiditch M. B., Federle M. J., Miller S. T., Bassler B. L., Hughson F. M. (2005). Regulation of LuxPQ receptor activity by the quorum-sensing signal autoinducer-2. Mol Cell 18, 507–518. 10.1016/j.molcel.2005.04.020 [DOI] [PubMed] [Google Scholar]
- Ni S., Forouhar F., Bussiere D. E., Robinson H., Kennedy M. A. (2006). Crystal structure of VC0702 at 2.0 Å: conserved hypothetical protein from Vibrio cholerae. Proteins 63, 733–741. 10.1002/prot.20919 [DOI] [PubMed] [Google Scholar]
- Niesen F. H., Berglund H., Vedadi M. (2007). The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat Protoc 2, 2212–2221. 10.1038/nprot.2007.321 [DOI] [PubMed] [Google Scholar]
- Parker Z. M., Pendergraft S. S., Sobieraj J., McGinnis M. M., Karatan E. (2012). Elevated levels of the norspermidine synthesis enzyme NspC enhance Vibrio cholerae biofilm formation without affecting intracellular norspermidine concentrations. FEMS Microbiol Lett 329, 18–27. 10.1111/j.1574-6968.2012.02498.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel C. N., Wortham B. W., Lines J. L., Fetherston J. D., Perry R. D., Oliveira M. A. (2006). Polyamines are essential for the formation of plague biofilm. J Bacteriol 188, 2355–2363. 10.1128/JB.188.7.2355-2363.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römling U., Galperin M. Y., Gomelsky M. (2013). Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev 77, 1–52. 10.1128/MMBR.00043-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryjenkov D. A., Tarutina M., Moskvin O. V., Gomelsky M. (2005). Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. J Bacteriol 187, 1792–1798. 10.1128/JB.187.5.1792-1798.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilton B. H., Flocco M. M., Nilsson M., Mowbray S. L. (1996). Conformational changes of three periplasmic receptors for bacterial chemotaxis and transport: the maltose-, glucose/galactose- and ribose-binding proteins. J Mol Biol 264, 350–363. 10.1006/jmbi.1996.0645 [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. (1984). Polyamines. Annu Rev Biochem 53, 749–790. 10.1146/annurev.bi.53.070184.003533 [DOI] [PubMed] [Google Scholar]
- Tan K., Chang C., Cuff M., Osipiuk J., Landorf E., Mack J. C., Zerbs S., Joachimiak A., Collart F. R. (2013). Structural and functional characterization of solute binding proteins for aromatic compounds derived from lignin: p-coumaric acid and related aromatic acids. Proteins 81, 1709–1726. 10.1002/prot.24305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble M. J., McCarter L. L. (2011). Bis-(3′-5′)-cyclic dimeric GMP-linked quorum sensing controls swarming in Vibrio parahaemolyticus. Proc Natl Acad Sci U S A 108, 18079–18084. 10.1073/pnas.1113790108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulijasz A. T., Grenader A., Weisblum B. (1996). A vancomycin-inducible lacZ reporter system in Bacillus subtilis: induction by antibiotics that inhibit cell wall synthesis and by lysozyme. J Bacteriol 178, 6305–6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldor M. K., Mekalanos J. J. (1994). Emergence of a new cholera pandemic: molecular analysis of virulence determinants in Vibrio cholerae O139 and development of a live vaccine prototype. J Infect Dis 170, 278–283. 10.1093/infdis/170.2.278 [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Nakao H., Koumoto Y., Shinoda S. (1989). Identification of N1-acetylnorspermidine in Vibrio parahaemolyticus and an enzyme activity responsible for its formation. FEMS Microbiol Lett 61, 225–230. 10.1111/j.1574-6968.1989.tb03583.x [DOI] [PubMed] [Google Scholar]
- Zhao J., Binns A. N. (2011). Characterization of the mmsAB-araD1 (gguABC) genes of Agrobacterium tumefaciens. J Bacteriol 193, 6586–6596. 10.1128/JB.05790-11 [DOI] [PMC free article] [PubMed] [Google Scholar]