Abstract
Purpose
Primary androgen-deprivation therapy (PADT) is often used to treat clinically localized prostate cancer, but its effects on cause-specific and overall mortality have not been established. Given the widespread use of PADT and the potential risks of serious adverse effects, accurate mortality data are needed to inform treatment decisions.
Methods
We conducted a retrospective cohort study using comprehensive utilization and cancer registry data from three integrated health plans. All men were newly diagnosed with clinically localized prostate cancer. Men who were diagnosed between 1995 and 2008, were not treated with curative intent therapy, and received follow-up through December 2010 were included in the study (n = 15,170). We examined all-cause and prostate cancer-specific mortality as our main outcomes. We used Cox proportional hazards models with and without propensity score analysis.
Results
Overall, PADT was associated with neither a risk of all-cause mortality (hazard ratio [HR], 1.04; 95% CI, 0.97 to 1.11) nor prostate-cancer–specific mortality (HR, 1.03; 95% CI, 0.89 to 1.19) after adjusting for all sociodemographic and clinical characteristics. PADT was associated with decreased risk of all-cause mortality but not prostate-cancer–specific mortality. PADT was associated with decreased risk of all-cause mortality only among the subgroup of men with a high risk of cancer progression (HR, 0.88; 95% CI, 0.78 to 0.97).
Conclusion
We found no mortality benefit from PADT compared with no PADT for most men with clinically localized prostate cancer who did not receive curative intent therapy. Men with higher-risk disease may derive a small clinical benefit from PADT. Our study provides the best available contemporary evidence on the lack of survival benefit from PADT for most men with clinically localized prostate cancer.
INTRODUCTION
More than 200,000 men are diagnosed annually with prostate cancer (PCa) and there are more than 2 million survivors.1,2 Androgen-deprivation therapy (ADT) is effective palliative treatment for metastatic prostate cancer3 and improves survival rates in certain clinical settings. These clinical settings include adjuvant ADT for lymph node–positive disease treated with prostatectomy and pelvic lymphadenectomy4 or intermediate- or high-risk PCa undergoing radiation therapy.5,6 However, ADT use has increased as primary monotherapy in localized disease for men who do not undergo prostatectomy or radiation and for biochemical recurrence after potentially curative treatment.7–10 Although there is no evidence that primary ADT (PADT) improves survival rates,7–9 at least 40% of men older than 65 years who have clinically localized PCa that was initially managed without surgery or radiation received PADT monotherapy between 1998 and 2002.11,12 By the early 2000s, PADT was the second most common treatment after radiotherapy for clinically localized PCa among older men.11,12 ADT remains widely used despite some decline in use for lower-risk disease after 2004.13–15 A recent study reported that one in eight men ages 65 and older who had prostate cancer received PADT, which is discordant with recommended guidelines and costs Medicare an estimated $42 million per year.16
Some of the declines reported in the use of PADT may be because of mounting evidence that it can have substantial long-term adverse consequences on the quality and quantity of life. These adverse effects include impaired cognitive function, loss of muscle strength, anemia,17,18 bone loss or fractures,19,20 coronary heart disease,21–24 insulin sensitivity,25 and diabetes mellitus.22,24,26 In 2010, the US Food and Drug Administration notified manufacturers of ADT-injectable agents to add new warnings to their products regarding the potential risks of coronary heart disease and diabetes.27 Given the aging American population, it is imperative to determine whether these risks outweigh any mortality benefit from PADT.
Three prior observational studies that used cancer registry data linked with Medicare claims (Surveillance, Epidemiology, and End Results [SEER] –Medicare data28) attempted to assess mortality among men who received PADT but not curative intent therapy. These studies showed PADT to have no benefit,11 potential harm,29 or possible benefit.30 However, these studies focused on older men, were unable to account for key clinical prognostic variables likely to confound mortality-risk estimates, or used analytic methods that may not be informative for clinical decision-making.
We assessed the association of PADT with mortality in a diverse cohort of 15,170 men who were diagnosed with clinically localized PCa between 1995 and 2008 and received follow-up through 2010. We selected all-cause mortality as our primary end point because of the possibility of adverse effects of PADT on noncancer mortality. We also conducted a subgroup analysis to discern whether a clinical benefit exists in subgroups of men defined by age at diagnosis or risk of recurrence.
METHODS
Data Sources
We conducted a retrospective cohort study of men who were newly diagnosed with clinically localized PCa and were enrolled in one of three integrated healthcare delivery systems within the HMO Cancer Research Network31: Kaiser Permanente Northern California, Kaiser Permanente Southern California, or Henry Ford Health System in Detroit, MI. These health plans have comprehensive information from inpatient and outpatient diagnoses, clinical encounters, laboratory test values (including prostate-specific antigen [PSA] values), pharmacy data, and tumor-registry data.
Study Participants
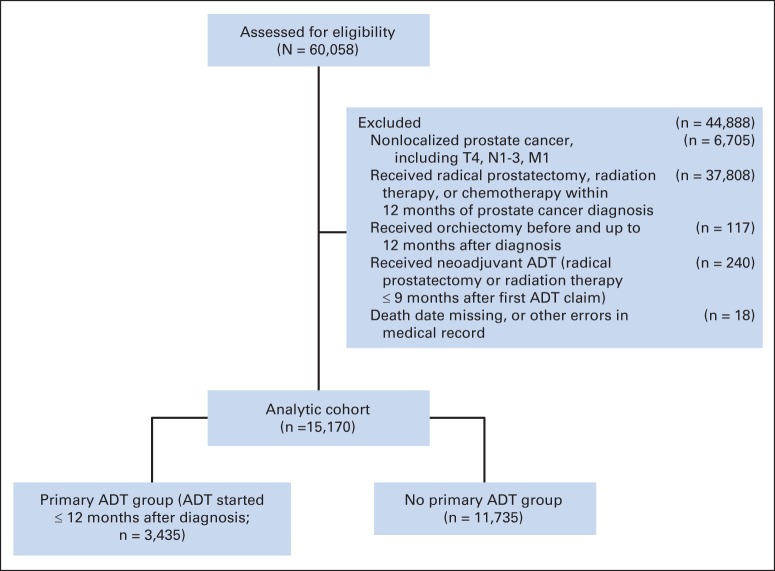
A total of 60,058 men diagnosed with PCa (per tumor registry data) were assessed for eligibility. Men were excluded in the following order: if they had nonlocalized PCa (defined as disease at clinical stage T4, with any nodal involvement, or with any distant metastasis) or were diagnosed after 2008 (n = 6,705); if they received radiation, radical prostatectomy, or chemotherapy within 1 year after PCa diagnosis (n = 37,808); if they received orchiectomy within 1 year after diagnosis (n = 117); if they received neoadjuvant ADT (radical prostatectomy or radiation therapy within 9 months of first ADT claim; n = 240); or if their records were missing date of death or had other data errors (n = 18). These exclusions resulted in a final cohort of 15,170 men (Appendix Fig A1, online only). All patients received follow-up through December 2010 or until censoring because of death or disenrollment (median follow-up, 61 months).
Primary Androgen-Deprivation Therapy
ADT was defined as either a gonadotropin-releasing hormone analog (eg, leuprolide, goserelin, or triporelin) or gonadotropin-releasing hormone antagonists (eg, abarelix or degarelix), with or without an oral antiandrogen (flutamide, bicalutamide, or nilutamide) for combined androgen blockade. We defined PADT based on receipt of medical ADT for localized PCa within the first 12 months after initial diagnosis without receipt of radiation or radical prostatectomy. We excluded 117 men who received orchiectomy to focus our comparison on medical ADT, that is, the standard method of ADT delivery in current clinical practice. Among the 15,170 men, 3,435 received PADT and 11,735 received no PADT within the first 12 months. Of the men in the latter group, 2,036 men (17%) who received ADT after 12 months were kept in the cohort to adhere to the principles of intent-to-treat analysis.
Mortality Outcomes
We used International Classification of Diseases 10th revision (ICD-10) codes to measure four outcomes: all-cause mortality, prostate cancer–specific mortality (C619, C61 185), any cancer mortality (C00-C97, D37-D48, 185), and cardiovascular mortality (I05-I99). Information on date and cause of death for health plan members was derived from a combination of clinical databases, linkages with California and Michigan death certificate records, and linkages with Social Security Administration data to ascertain deaths that may have occurred outside California or Michigan.
Independent Variables
We obtained from registry data age at diagnosis, race-ethnicity, year of diagnosis, and diagnosis of prior or subsequent primary cancers other than prostate cancer. We included the key clinical variables that determine PADT use and PCa-related mortality: serum PSA, Gleason score, and clinical T stage. All clinical variables were derived from the health plan tumor registries that operate similarly to the National Cancer Institute SEER registries and are the primary sources for data transmitted to the SEER program.
Staging for prostate cancer followed SEER conventions by using the tumor-node-metastasis system of the American Joint Committee on Cancer. We included the total serum PSA level (ng/mL) at baseline, which was defined as the closest value within 6 months before diagnosis. We obtained the two-value summed Gleason score from the first biopsy leading to the PCa diagnosis. Using these three variables, we computed the American Urological Association (AUA) risk groups, which are categorized as low, intermediate, or high.32 We ascertained the presence of 34 individual health conditions diagnosed between 2 years before PCa diagnosis to up to 3 months after PCa diagnosis (Appendix). For each condition, we required an inpatient diagnosis and/or at least two outpatient diagnoses codes at least 30 days apart to minimize false-positives. We used the 34 conditions when computing the propensity score. We also computed a simpler measure of comorbidity, the Elixhauser comorbidity index, 2 years before the PCa diagnosis date using the same strategy to avoid rule-out diagnoses.33
Statistical Analysis
We used the Cox proportional hazards regression model and fit four separate models to estimate the associations between PADT and each mortality outcome. For each model, follow-up time began on the date of diagnosis with death as the outcome event, and censoring corresponded to loss to follow-up (disenrollment) or end of the study period (December 31, 2010), whichever occurred first. For each outcome, we adjusted for patient sociodemographic and clinical prognostic factors and the 34 individual comorbidities in the multivariable models.
Because receipt of PADT was strongly associated with patient characteristics, we used a propensity score analysis to better balance covariates for the two PADT groups (Appendix Fig A2, online only). We repeated the Cox proportional hazards model analyses with propensity score weighting (and standardized mortality ratio [SMR] weights) and propensity score matching approaches using separate models to assess the mortality risk associated with PADT versus no-PADT for each of the four mortality outcomes.
Subgroup Analysis
We conducted stratified analyses by AUA risk groups and age groups to assess whether the association of PADT and mortality differed among clinical subgroups. AUA risk groups were defined as: low (pretreatment PSA level, ≤ 10 ng/mL; Gleason score, ≤ 6; clinical stage, ≤ T2a); intermediate (PSA, 10 to ≤ 20 ng/mL; Gleason score, 7; clinical stage, T2b); or high (PSA, > 20; Gleason score, 8 to 10; clinical stage, T2c-T3a). We classified age at PCa diagnosis into three age groups, younger than 65 years, 65 to 74 years, and ≥ 75 years. Separate models were created to estimate the adjusted risk of all-cause and cause-specific mortality for each subgroup, adjusting for propensity score SMR weights. We describe methods for handling missing data in the Appendix.
RESULTS
Population Characteristics
The cohort included 15,170 men who were newly diagnosed with localized PCa and did not receive curative intent therapy; 23% of the men had PADT initiated within the first year after diagnosis. Men who received PADT had worse prognostic factors (Table 1). The PADT group had higher PSA levels (42% v 10% for PSA > 20) and higher Gleason scores (26% v 7% for Gleason score ≥ 8) than the no-PADT group. Thus, 58% of men receiving PADT were in the AUA-defined high-risk group, versus just 18% of the no-PADT group who were categorized as high risk. The PADT group had more comorbidities (per Elixhauser index) than the no-PADT group (31% v 24% for at least three major comorbidities). After adjusting for propensity score, differences in all of the sociodemographic and clinical characteristics achieved balance (Appendix).
Table 1.
Demographic and Clinical Characteristics of 15,170 Men Initially Diagnosed With Clinically Localized Prostate Cancer in Three Health Plans From 1995-2008 Who Did Not Receive Curative Intent Therapy Within 12 Months After Diagnosis
| Characteristic | Primary ADT* (n = 3,435) |
No Primary ADT (n = 11,735) |
P† | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Age at diagnosis, years | < .001 | ||||
| 35-64 | 460 | 13.4 | 3,875 | 33.0 | |
| 65-69 | 419 | 12.2 | 2,159 | 18.4 | |
| 70-74 | 605 | 17.6 | 2,076 | 17.7 | |
| 75-79 | 835 | 24.3 | 2,031 | 17.3 | |
| ≥ 80 | 1,116 | 32.5 | 1,594 | 13.6 | |
| Median | 76 | 69 | |||
| Race/ethnicity | .05 | ||||
| Non-Hispanic white | 2,302 | 67.0 | 7,701 | 65.6 | |
| Hispanic | 340 | 9.9 | 1,342 | 11.4 | |
| Non-Hispanic black | 536 | 15.6 | 1,758 | 15.0 | |
| All others or unknown | 257 | 7.5 | 934 | 8.0 | |
| Year of diagnosis | < .001 | ||||
| 1995-2000 | 1,183 | 34.4 | 2,991 | 25.5 | |
| 2001-2005 | 1,204 | 35.1 | 4,847 | 41.3 | |
| 2006-2011 | 1,048 | 30.5 | 3,897 | 33.2 | |
| Baseline PSA level, ng/mL | < .001 | ||||
| ≤ 4 | 146 | 4.3 | 1,837 | 15.7 | |
| 4-10 | 857 | 25.0 | 6,046 | 51.5 | |
| 10-20 | 851 | 24.8 | 1,966 | 16.8 | |
| > 20 | 1,430 | 41.6 | 1,194 | 10.2 | |
| Unknown/missing | 151 | 4.4 | 692 | 5.9 | |
| Gleason score at first biopsy | < .001 | ||||
| ≤ 6 | 1,043 | 30.4 | 7,313 | 62.3 | |
| 7 | 1,196 | 34.8 | 2,312 | 19.7 | |
| 8 | 463 | 13.5 | 427 | 3.6 | |
| 9-10 | 421 | 12.3 | 368 | 3.1 | |
| Unknown/missing | 312 | 9.1 | 1,315 | 11.2 | |
| Tumor stage, extent | < .001 | ||||
| ≤ T2a | 1,592 | 46.4 | 8,273 | 70.5 | |
| T2b | 375 | 10.9 | 650 | 5.5 | |
| ≥ T2c | 523 | 15.2 | 588 | 5.0 | |
| Unknown/missing | 945 | 27.5 | 2,224 | 19.0 | |
| AUA risk group‡ | < .001 | ||||
| Low | 306 | 8.9 | 4,339 | 37.0 | |
| Intermediate | 957 | 27.9 | 3,182 | 27.1 | |
| High | 1,990 | 57.9 | 2,054 | 17.5 | |
| Unknown/missing | 182 | 5.3 | 2,160 | 18.4 | |
| Sequence of prostate cancer | < .001 | ||||
| Single primary | 2,748 | 80.0 | 9,926 | 84.6 | |
| Subsequent primary | 329 | 9.6 | 864 | 7.4 | |
| Prior primary | 358 | 10.4 | 945 | 8.1 | |
| Comorbidity count (Elixhauser index, 2 years before diagnosis date) | < .001 | ||||
| 0 | 741 | 21.6 | 3,023 | 25.8 | |
| 1 | 821 | 23.9 | 2,899 | 24.7 | |
| 2 | 581 | 16.9 | 1,906 | 16.2 | |
| ≥ 3 | 1,047 | 30.5 | 2,811 | 24.0 | |
| Unknown/missing | 245 | 7.1 | 1,096 | 9.3 | |
Abbreviations: ADT, androgen-deprivation therapy; AUA, American Urological Association; PSA, prostate-specific antigen; T, tumor.
Received ADT within 12 months of prostate cancer diagnosis.
P values were calculated using Pearson's χ2 test.
Risk group (after imputation) is defined as low (pre-treatment PSA level ≤ 10 ng/mL, Gleason score ≤ 6, and a clinical tumor stage of ≤ T2a), intermediate (10 ng/mL < PSA ≤ 20 ng/mL, Gleason score of 7, or T2b), or high (PSA > 20 ng/mL, Gleason score 8-10, or T2c-T3a).
Analysis of Mortality Outcomes
There were 4,921 deaths in the cohort, of which 1,049 deaths (32%) were related to prostate cancer (Table 2). The median follow-up time was 54 and 64 months in the PADT and no-PADT groups, respectively. Men who received PADT versus men who did not experienced a nearly two-fold increase in all-cause mortality (49% v 28%; hazard ratio [HR], 1.96; 95% CI, 1.85 to 2.08) and a nearly three-fold increase in prostate cancer–specific mortality (13% v 5%; HR, 2.91; 95% CI, 2.57 to 3.28), without adjustments for other variables. Using a Cox proportional hazards model without propensity score adjustment, PADT was not associated with the risk of all-cause mortality (HR, 1.04; 95% CI, 0.97 to 1.11) nor the risk of PCa mortality (HR, 1.03; 95% CI, 0.89 to 1.19), after adjusting for all other covariates. Propensity score adjustment did not materially alter the risk estimates from the conventional models (with all HRs closer to 1.0; Table 2). Because we included men diagnosed with prior or subsequent cancers other than prostate cancer, we looked at deaths from any cancer (Table 2) and observed no difference in mortality between the PADT and no-PADT groups. We observed an increased risk of cardiovascular deaths in the PADT group (13% v 8%; unadjusted HR, 1.81; 95% CI, 1.61 to 2.02), but this difference decreased after adjustment and was not statistically significant (HR, 1.11; 95% CI, 0.95 to 1.27). Adjusted results were similar in analyses of men who received prostatectomy after 12 months (n = 295; presumably as delayed, curative intent therapy) in censored observations (not shown). Using sensitivity analyses, we found that these results did not materially differ from the subset of complete cases, that is, those men whose records included complete information on baseline PSA, Gleason score, and stage.
Table 2.
Mortality Risk of Primary ADT Versus No Primary ADT Among Men Diagnosed With Clinically Localized Prostate Cancer Not Receiving Curative Intent Therapy Within 12 Months After Diagnosis
| Mortality | Total No. of Deaths (n = 15,170) |
Deaths According to Receipt of Primary ADT* |
Unadjusted Risk Estimates |
Conventional Cox Model Adjusted Risk Estimates† |
Propensity Score Adjusted Estimates Using Standardized Mortality Ratio Weighting |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes (n = 3,435) |
No (n = 11,735) |
||||||||||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| All-cause mortality | 4,921 | 32 | 1,672 | 49 | 3,249 | 28 | 1.96 | 1.85 to 2.08 | < .001 | 1.04 | 0.97 to 1.11 | .33 | 0.98 | 0.91 to 1.06 | .59 |
| Prostate cancer-specific mortality | 1,049 | 7 | 452 | 13 | 597 | 5 | 2.91 | 2.57 to 3.28 | < .001 | 1.03 | 0.89 to 1.19 | .67 | 1.01 | 0.86 to 1.16 | .93 |
| Any cancer mortality | 1,932 | 13 | 731 | 21 | 1,201 | 10 | 2.32 | 2.12 to 2.54 | < .001 | 1.09 | 0.97 to 1.22 | .11 | 1.02 | 0.90 to 1.14 | .76 |
| Cardiovascular mortality | 1,384 | 9 | 445 | 13 | 939 | 8 | 1.81 | 1.61 to 2.02 | < .001 | 1.11 | 0.95 to 1.27 | .12 | 1.04 | 0.88 to 1.20 | .57 |
Abbreviations: ADT, androgen-deprivation therapy; HR, hazard ratio; PSA, prostate-specific antigen.
Received ADT monotherapy within 12 months of prostate cancer diagnosis.
Multivariable analysis using a Cox proportional hazards model and imputed data for PSA, Gleason, and T stage. Median follow-up time was 61 months (54 months in primary-ADT group; 64 months in the no-primary-ADT group). HRs are adjusted for age, race-ethnicity, baseline PSA, Gleason score, T stage, sequence of prostate cancer, health plan, and 34 individual baseline comorbid conditions (yes/no) existing up to 2 years before diagnosis (see Appendix for list of these conditions).
Table 3 lists results from the conventional Cox proportional hazards model for our two primary outcomes. Other risk factors associated with all-cause and PCa death included older age, advanced stage, higher baseline PSA, higher Gleason score, and advanced tumor stage.
Table 3.
Risk of Mortality by Selected Covariates (N = 15,170)
| Covariate | All-Cause Mortality (No. of events = 4,921) |
Prostate Cancer–Specific Mortality (No. of events = 1,049) |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Receipt of primary ADT* | 1.04 | 0.97 to 1.11 | .33 | 1.03 | 0.89 to 1.19 | .67 |
| Age at diagnosis, years | ||||||
| 35-64 | 1.00 | |||||
| 65-69 | 1.67 | 1.48 to 1.88 | < .001 | 1.17 | 0.89 to 1.52 | .26 |
| 70-74 | 2.06 | 1.84 to 2.31 | < .001 | 1.71 | 1.35 to 2.16 | < .001 |
| 75-79 | 2.76 | 2.48 to 3.08 | < .001 | 2.3 | 1.83 to 2.88 | < .001 |
| ≥ 80 | 4.22 | 3.78 to 4.72 | < .001 | 3.22 | 2.57 to 4.03 | < .001 |
| Race/ethnicity | ||||||
| Non-Hispanic white | 1.00 | |||||
| Hispanic | 0.81 | 0.73 to 0.90 | < .001 | 0.85 | 0.69 to 1.05 | .13 |
| Non-Hispanic black | 1.08 | 1.00 to 1.17 | .06 | 1.11 | 0.94 to 1.31 | .23 |
| All others or unknown | 0.75 | 0.66 to 0.86 | < .001 | 0.6 | 0.45 to 0.81 | .001 |
| Baseline PSA level, ng/mL | ||||||
| ≤ 4 | 1.00 | |||||
| 4 to ≤ 10 | 1.01 | 0.91 to 1.13 | .83 | 1.05 | 0.77 to 1.44 | .76 |
| 11 to ≤ 20 | 1.17 | 1.04 to 1.32 | .008 | 1.6 | 1.16 to 2.21 | .005 |
| > 20 | 1.48 | 1.30 to 1.68 | < .001 | 2.74 | 2.01 to 3.74 | < .001 |
| Gleason score at first biopsy | ||||||
| ≤ 6 | 1.00 | |||||
| 7 | 1.22 | 1.14 to 1.32 | < .001 | 2.17 | 1.82 to 2.59 | < .001 |
| 8 | 1.39 | 1.24 to 1.56 | < .001 | 3.42 | 2.74 to 4.26 | < .001 |
| 9-10 | 1.72 | 1.54 to 1.93 | < .001 | 5.23 | 4.23 to 6.46 | < .001 |
| Tumor stage, extent | ||||||
| ≤ T2a | 1.00 | |||||
| T2b | 1.22 | 1.12 to 1.33 | < .001 | 1.41 | 1.19 to 1.67 | < .001 |
| ≥ T2c | 1.36 | 1.22 to 1.53 | < .001 | 1.78 | 1.46 to 2.17 | < .001 |
| Sequence of prostate cancer | ||||||
| Single primary only | 1.00 | |||||
| First of multiple primaries | 1.91 | 1.75 to 2.07 | < .001 | 0.82 | 0.65 to 1.03 | .09 |
| Prior other cancer | 1.53 | 1.40 to 1.66 | < .001 | 1.06 | 0.85 to 1.31 | .62 |
NOTE. Multivariable analysis using a Cox proportional hazards model and imputed data for PSA, Gleason, and T-stage. Median follow-up length was 61 months (54 months in Primary ADT group; 64 months in the no Primary ADT group). All estimates shown are also adjusted for health plan and 34 individual baseline comorbid conditions (yes/no) existing up to 2 years prior to diagnosis (not shown).
Abbreviations: ADT, androgen-deprivation therapy; HR, hazard ratio; PSA, prostate-specific antigen.
Received ADT within 12 months of prostate cancer diagnosis.
In the subgroup analyses (Table 4), we found no differential effects by age on the association of PADT with either all-cause or cause-specific mortality. However, we observed that the AUA risk group modified the relationship between PADT and the risk of all-cause mortality. Using Cox proportional hazards models with propensity score SMR weighting for each subgroup, PADT was associated with a decreased risk of all-cause mortality in men with AUA high-risk PCa (HR, 0.88; 95% CI, 0.78 to 0.97) but with an increased risk of death in men with low-risk PCa (HR, 1.41; 95% CI, 0.99 to 1.82) and no difference in risk for men with intermediate-risk PCa (HR, 1.12; 95% CI, 0.92 to 1.32). There were no differences in risk of prostate cancer mortality by AUA risk group category.
Table 4.
Subgroup Analyses of Mortality Risk by Age and Risk Groups
| Characteristic | All-Cause Mortality |
Prostate Cancer–Specific Mortality |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age at diagnosis, years | ||||||
| ≤ 64 | 1.14 | 0.77 to 1.51 | .40 | 1.20 | 0.50 to 1.89 | .52 |
| 65-74 | 1.00 | 0.84 to 1.16 | .99 | 0.95 | 0.65 to 1.24 | .71 |
| ≥ 75 | 0.93 | 0.85 to 1.02 | .13 | 0.95 | 0.77 to 1.13 | .58 |
| AUA risk group | ||||||
| Low | 1.41 | 0.99 to 1.82 | .02 | 1.14 | −0.22 to 2.50 | .82 |
| Intermediate | 1.12 | 0.92 to 1.32 | .18 | 1.07 | 0.59 to 1.55 | .76 |
| High | 0.88 | 0.78 to 0.97 | .02 | 0.95 | 0.78 to 1.12 | .53 |
NOTE. Using Cox proportional hazards model, propensity standardized mortality ratio weighting, and imputed data.
Abbreviations: AUA, American Urological Association; HR, hazard ratio; PSA, prostate-specific antigen; T, tumor.
Risk is defined as low (pre-treatment PSA level ≤ 10 ng/mL, Gleason score ≤ 6, and a clinical tumor stage of ≤ T2a), intermediate (PSA 10 to ≤ 20 ng/mL, Gleason score of 7, or tumor stage T2b), or high (PSA > 20 ng/mL, or Gleason score 8-10, or tumor stage T2c-T3a).
DISCUSSION
Using PADT to treat clinically localized prostate cancer has not been proven effective in any subgroup of men; it neither to reduces risk of PCa progression nor mortality, which is typically preceded by disease progression to symptomatic, castration-resistant metastatic disease. Given the uncertain risk-benefit ratio for PADT and the fact that no trials are ongoing or planned to address this gap, we designed a retrospective cohort study to compare the mortality risk for PADT versus no PADT among men with clinically localized prostate cancer.
We found no significant difference in the risk of all-cause mortality, prostate cancer–specific mortality, cancer mortality, or cardiovascular mortality between the PADT and no-PADT groups. Our results used conventional modeling approaches and paralleled the results observed when using the propensity score–weighting and propensity score–matching methods and, therefore, do not appear sensitive to the modeling approach.
Our main conclusion is that PADT does not seem to be an effective strategy as an alternative to no therapy among men diagnosed with clinically localized PCa who are not receiving curative-intent therapy. The risks of serious adverse events and the high costs associated with its use16 mitigate against any clinical or policy rationale for PADT use in these men. Although we did not evaluate the risk of disease progression associated with PADT, a prior study using SEER-Medicare data reported that early PADT treatment of low-risk localized PCa did not delay the receipt of subsequent secondary therapies and actually increased the use of subsequent chemotherapy for castration-resistant disease.34
Interestingly, our subgroup analysis revealed a slightly reduced risk of all-cause mortality in the high-risk subgroup treated with PADT, a slightly elevated risk among the low-risk men receiving PADT, and no difference among intermediate-risk men receiving PADT. Our point estimate for the cause-specific mortality reduction associated with PADT (HR, 0.95) was not statistically significant but was consistent with the small protective and significant effect we observed for all-cause mortality. The nonsignificance of this result may be in part because of limited power, as only 7% of the cohort died from PCa, and in part because of possible misclassification of cause of death. However, the decision to prescribe PADT in high-risk men based on this result should be carefully weighed against the possible harms.
Reports on the risk of cardiovascular morbidity and mortality related to ADT are mixed.35 We assessed whether there was an increased risk of cardiovascular mortality associated with PADT, but found no significantly increased risk in the PADT group versus the no-PADT group after adjustment for all other covariates, including pre-existing cardiovascular conditions. Further in-depth analysis of the risk of nonfatal cardiovascular events is ongoing using this cohort and will be reported separately.
Our results are consistent with three previous studies of PADT that used the SEER-Medicare–linked database. Two studies found that, in general, PADT was not associated with improved survival rates in early-stage prostate cancer in men diagnosed from 1992 to 2002 who received follow-up through 2005 to 2006.11,30 Another study found an increased risk of death associated with PADT.29 However, these studies examined patients older than 65 years and did not consider baseline PSA values or Gleason scores.
Two of these studies used an instrumental variables analysis (IVA) approach to account for unmeasured confounding.11,30 Some subgroup findings differed; one study showed an increased risk of cause-specific death among the low-risk men11 and another showed a protective effect of PADT on PCa-specific mortality in the high-risk men.30 This latter finding is consistent with our finding in high-risk men who experienced some benefit in all-cause mortality after receiving PADT, although we did not observe any benefit in PCa-specific mortality rates. The use of IVA as the primary analytic strategy is limited because the results estimate the average effect only in those patients who vary regarding the instrument selected. This makes IVA less useful for clinical decisions because it does not provide an estimate of the effects of treatment in the treated versus not treated groups and, thus, is more suitable for informing policy rather than clinical decisions.
Our study has several limitations that should be considered while interpreting our results. The most significant concern in treatment-outcome studies is the possibility of residual confounding, particularly for factors that have implications for treatment choice and are related to the outcome. For example, we were unable to account for potentially important risk factors that were unavailable in our data, such as prediagnosis PSA doubling time or the presence of undetected distant metastases because most men in our cohort did not receive imaging tests as part of their initial diagnostic work-up. Statistical adjustments cannot fully account for clinical judgments that incorporate information on these and other unmeasured variables used to drive clinical decisions. We did not conduct a secondary analysis evaluating the effects of delayed ADT in our no-primary-ADT group because we lacked key information regarding the clinical reasons for implementing delayed ADT in our cohort. A second limitation is that our finding of a reduced risk of all-cause mortality in the high-risk subgroup may be spurious, because we did not adjust for multiple comparisons in our evaluation of two outcomes in 12 subgroups. Finally, our study may have limited generalizability because our study was limited to three large, integrated health plans. However, these health plans include a socioeconomically and racially diverse community population.
Our study's strength is its data richness, particularly with respect to clinical prognostic factors in contrast with prior SEER-Medicare studies. Our propensity score analysis used information from all measured covariates to balance observed factors between treatment groups. As long as there are relationships between unobservable and measured factors, propensity score analysis can also reduce the bias associated with unobserved factors.36 Nevertheless, there is likely to be some unmeasured confounding that we are unable to control for.
In summary, we found that most men diagnosed with clinically localized PCa who do not receive curative-intent therapy receive no apparent mortality benefit from PADT compared with receiving no therapy. We did, however, find a small and statistically significant overall mortality benefit associated with PADT use in the subgroup of men with high-risk PCa. The observed benefit was relatively small and should not be taken as definitive, given the limitations of our data and the possibility of a spurious finding. Any actual benefit must be weighed against other evidence suggesting an increased risk of serious adverse effects of PADT. Because no randomized trials will likely ever definitively assess the utility of PADT, our study provides the best contemporary evidence available on the lack of survival benefit of PADT for most men with clinically localized prostate cancer.
Glossary Terms
- Cox proportional hazards regression model:
a statistical model for regression analysis of censored survival data, examining the relationship of censored survival distribution to one or more covariates. This model produces a baseline survival curve, covariate coefficient estimates with their standard errors, risk ratios, 95% CIs, and significance levels.
- Gleason score:
A pathologic description of prostate cancer grade based on the degree of abnormality in the glandular architecture. Gleason patterns 3, 4, and 5 denote low, intermediate, and high levels of histologic abnormality and tumor aggressiveness, respectively. The score assigns primary and secondary numbers based on the most common and second most common patterns identified.
- sensitivity analyses:
analyses that evaluate the impact of missing data and possible differences in interval assessments.
Appendix
Description of propensity score analysis.
The propensity score, defined in our study as the predicted probability of receiving primary androgen-deprivation therapy (PADT) based on observed patient characteristics, summarizes all covariates into a single measure. The distribution of covariates are the same for the treatment and comparison groups when conditioning on a propensity score in a large sample, under certain assumptions.
To calculate the propensity score, we used a logistic regression model with receipt of PADT as the dependent binary variable that included all of the sociodemographic and clinical factors as covariates. We examined the extent of overlapping on the distributions of the propensity score (p) between the PADT group and non-PADT group. Patients at the two extremes of the propensity score were trimmed because of poor overlap on covariates. The number of patients trimmed off the common support ranges from 225 to 236 across five imputations (1.5%). We used two alternative propensity score approaches to evaluate the robustness of our results: poststratification weighting and propensity score matching. For poststratification, we employed a standardized mortality ratio weighting method that assigns a weight of 1 for PADT cases and a weight of [p/(1-p)] for non-PADT cases.34,35 This approach gives additional weight to the non-PADT patients who most resemble the PADT patients on the covariates, so that the weighted distribution of characteristics in the two PADT groups is well balanced and equal to that of the original PADT cohort.
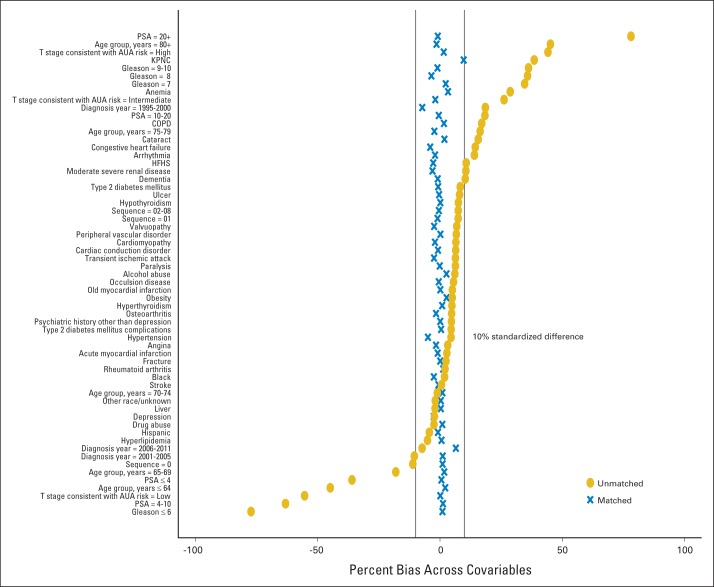
We also used a one-to-one matching approach on the propensity score within each health plan, without replacement. We assessed the covariate balance between the two groups after adjusting for the propensity score by examining for all covariates whether the standardized differences in proportions (for binary variables) or means (for continuous variables) between the two treatment groups for all covariates were less than 10% (Appendix Fig A2).
Handling of missing data.
A substantial proportion of cases (23%) lacked census tract or address information at the time of diagnosis. We evaluated but did not find any association between contextual US Census socioeconomic status variables with any of the four mortality outcomes among those with available information. Therefore, we removed US Census socioeconomic status variables from all further analyses.
A substantial proportion of cases (20%) had at least one or more of the key clinical prognostic variables (clinical stage, Gleason score, or baseline PSA) missing. We performed multiple imputations using all other covariates to predict values for these variables. We constructed five imputed data sets, each having estimates for the missing values for PSA, Gleason score, and T-stage. We then pooled the estimates and corresponding SEs across the five imputations using Rubin's method (Rubin DB: Multiple Imputation for Nonresponse in Surveys. New York, NY, J. Wiley & Sons, 1987). All model results used these imputed datasets; multivariable models using only the complete cases did not show any significant deviations from the results shown.
Fig. A1.
Selection criteria for study cohort. ADT, androgen-deprivation therapy.
Fig. A2.
Checking covariate balance after using propensity score as standardized mortality ratio (SMR) weights. This plot shows the standardized difference in the proportions (for binary variables) or means (for continuous variables) between the primary androgen-deprivation therapy (PADT) group and non-PADT group for each covariate, before matching (blue) and after adjusting (gold) for propensity score SMR weights, using one imputed data set. This is used to evaluate whether the standardized difference between groups on each variable is less than 10%, a conventional threshold for determining a meaningful difference. Before weighting, there are many variables with differences greater than 10%; after adjusting for propensity score weights, the differences by treatment group are all less than 10%, indicating balance has been achieved by use of these weights. AUA, American Urological Association; COPD, chronic obstructive pulmonary disease; HFHS, Henry Ford Health System; KPNC, Kaiser Permanente Northern California; PSA, prostate-specific antigen.
Footnotes
See accompanying article on page 1295
Supported by Grants No. R01CA142934, RC1CA146238, and P30CA051008 from the National Cancer Institute.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Terms in blue are defined in the glossary found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Arnold L. Potosky, Reina Haque, Marianne Ulcickas Yood, Huei-Ting Tsai, Stephen K. Van Den Eeden
Administrative support: Reina Haque, Stephen K. Van Den Eeden
Collection and assembly of data: Arnold L. Potosky, Reina Haque, Andrea E. Cassidy-Bushrow, Marianne Ulcickas Yood, Stephen K. Van Den Eeden
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. Prostate Cancer. http://www.cancer.gov/cancertopics/types/prostate.
- 3.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 4.Messing EM, Manola J, Sarosdy M, et al. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999;341:1781–1788. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]
- 5.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365:107–118. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
- 6.Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337:295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 7.Cooperberg MR, Grossfeld GD, Lubeck DP, et al. National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst. 2003;95:981–989. doi: 10.1093/jnci/95.13.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawakami J, Cowan JE, Elkin EP, et al. Androgen-deprivation therapy as primary treatment for localized prostate cancer: Data from Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) Cancer. 2006;106:1708–1714. doi: 10.1002/cncr.21799. [DOI] [PubMed] [Google Scholar]
- 9.Keating NL, O'Malley AJ, McNaughton-Collins M, et al. Use of androgen deprivation therapy for metastatic prostate cancer in older men. BJU Int. 2008;101:1077–1083. doi: 10.1111/j.1464-410X.2007.07405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeliadt SB, Etzioni R, Ramsey SD, et al. Trends in treatment costs for localized prostate cancer: The healthy screenee effect. Med Care. 2007;45:154–159. doi: 10.1097/01.mlr.0000241044.09778.3f. [DOI] [PubMed] [Google Scholar]
- 11.Lu-Yao GL, Albertsen PC, Moore DF, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300:173–181. doi: 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahinian VB, Kuo YF, Freeman JL, et al. Determinants of androgen deprivation therapy use for prostate cancer: Role of the urologist. J Natl Cancer Inst. 2006;98:839–845. doi: 10.1093/jnci/djj230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahinian VB, Kuo YF, Gilbert SM. Reimbursement policy and androgen-deprivation therapy for prostate cancer. N Engl J Med. 2010;363:1822–1832. doi: 10.1056/NEJMsa0910784. [DOI] [PubMed] [Google Scholar]
- 14.Weight CJ, Klein EA, Jones JS. Androgen deprivation falls as orchiectomy rates rise after changes in reimbursement in the U.S. Medicare population. Cancer. 2008;112:2195–2201. doi: 10.1002/cncr.23421. [DOI] [PubMed] [Google Scholar]
- 15.Krahn M, Bremner KE, Tomlinson G, et al. Androgen deprivation therapy in prostate cancer: Are rising concerns leading to falling use? BJU Int. 2011;108:1588–1596. doi: 10.1111/j.1464-410X.2011.10127.x. [DOI] [PubMed] [Google Scholar]
- 16.Kuykendal AR, Hendrix LH, Salloum RG, et al. Guideline-discordant androgen deprivation therapy in localized prostate cancer: Patterns of use in the Medicare population and cost implications. Ann Oncol. 2013;24:1338–1343. doi: 10.1093/annonc/mds618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barry MJ, Delorenzo MA, Walker-Corkery ES, et al. The rising prevalence of androgen deprivation among older American men since the advent of prostate-specific antigen testing: A population-based cohort study. BJU Int. 2006;98:973–978. doi: 10.1111/j.1464-410X.2006.06416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moul JW, Banez LL, Freedland SJ. Rising PSA in nonmetastatic prostate cancer. Oncology (Williston Park) 2007;21:1436–1445. [PubMed] [Google Scholar]
- 19.Shahinian VB, Kuo YF, Freeman JL, et al. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 20.Wei JT, Gross M, Jaffe CA, et al. Androgen deprivation therapy for prostate cancer results in significant loss of bone density. Urology. 1999;54:607–611. doi: 10.1016/s0090-4295(99)00301-5. [DOI] [PubMed] [Google Scholar]
- 21.D'Amico AV, Denham JW, Crook J, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007;25:2420–2425. doi: 10.1200/JCO.2006.09.3369. [DOI] [PubMed] [Google Scholar]
- 22.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 23.Tsai HK, D'Amico AV, Sadetsky N, et al. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99:1516–1524. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- 24.Keating NL, O'Malley AJ, Freedland SJ, et al. Diabetes and cardiovascular disease during androgen deprivation therapy: Observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006;91:1305–1308. doi: 10.1210/jc.2005-2507. [DOI] [PubMed] [Google Scholar]
- 26.Alibhai SM, Duong-Hua M, Sutradhar R, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. 2009;27:3452–3458. doi: 10.1200/JCO.2008.20.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US Food and Drug Administration. FDA Drug Safety Communication: Update to Ongoing Safety Review of GnRH Agonists and Notification to Manufacturers of GnRH Agonists to Add New Safety Information to Labeling Regarding Increased Risk of Diabetes and Certain Cardiovascular Diseases. 2011. www.fda.gov/Drugs/DrugSafety/ucm229986.htm.
- 28.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(suppl 8) doi: 10.1097/01.MLR.0000020942.47004.03. IV-3-18. [DOI] [PubMed] [Google Scholar]
- 29.Wong YN, Freedland SJ, Egleston B, et al. The role of primary androgen deprivation therapy in localized prostate cancer. Eur Urol. 2009;56:609–616. doi: 10.1016/j.eururo.2009.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuo YF, Montie JE, Shahinian VB. Reducing bias in the assessment of treatment effectiveness: Androgen deprivation therapy for prostate cancer. Med Care. 2012;50:374–380. doi: 10.1097/MLR.0b013e318245a086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner EH, Greene SM, Hart G, et al. Building a research consortium of large health systems: The Cancer Research Network. J Natl Cancer Inst Monogr. 2005;35:3–11. doi: 10.1093/jncimonographs/lgi032. [DOI] [PubMed] [Google Scholar]
- 32.Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Lu-Yao GL, Albertsen PC, Li H, et al. Does primary androgen-deprivation therapy delay the receipt of secondary cancer therapy for localized prostate cancer? Eur Urol. 2012;62:966–972. doi: 10.1016/j.eururo.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine GN, D'Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: A science advisory from the American Heart Association, American Cancer Society, and American Urological Association—Endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833–840. doi: 10.1161/CIRCULATIONAHA.109.192695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–524. [Google Scholar]