Abstract
Three cases of acute flaccid paralysis (AFP) with preceding fever are described. One patient had a quadriparesis with a florid meningoencephalitic picture and the other two had asymmetric flaccid paralysis with fasciculations at the onset of illness. Magnetic resonance imaging in two cases showed prominent hyperintensitities in the spinal cord and brainstem with prominent involvement of the grey horn (polio-myelitis). Cerebrospinal fluid (CSF) polymerase chain reaction was positive for West Nile virus (WNV) in the index patient. All three cases had a positive WNV immunoglobulin M antibody in serum/CSF and significantly high titer of WNV neutralizing antibody in serum, clearly distinguishing the infection from other Flaviviridae such as Japanese encephalitis. WNV has been recognized in India for many decades; however, AFP has not been adequately described. WNV is a flavivirus that is spread by Culex mosquitoes while they take blood meals from humans and lineage 1 is capable of causing a devastating neuro-invasive disease with fatal consequences or severe morbidity. We describe the first three laboratory confirmed cases of WNV induced AFP from Kerala and briefly enumerate the salient features of this emerging threat.
Keywords: Acute flaccid paralysis, poliomyelitis, west nile associated poliomyelitis, west nile infection in India, west nile in Kerala, west nile meningoencephalitis, west nile paralysis, west nile poliomyelitis in India, west nile virus
Introduction
Polioviruses were the most common cause of asymmetric acute flaccid paralysis (AFP) in India. After mass vaccination campaigns, polio has declined from a hyperendemic to a near polio-free status with the last known case in 13 January 2011. It is hoped that by 2014 India will be free from wild polio virus transmission.[1] However, emerging threats such as West Nile virus (WNV) lurk and often affect adults unlike polio, which mainly afflicts children. WNV came into prominence after an outbreak in New York city in 1999.[2] Phylogenetically there are five lineages of WNV (1-5). The most important neuropathogen is lineage 1 (Clade 1a). Lineage 1 (Clade 1b) and lineage 2 cause self-limiting illnesses. The other lineages are less well characterized. It is noteworthy that WNV of genetic lineage 1 Clade 1a was isolated from an outbreak of encephalitis in Alappuzha, Kerala in 2013.[3] However, AFP due to WNV has not been adequately characterized. We describe the first cases of laboratory confirmed WNV associated AFP in India.
Case Reports
Case 1
A 55-year-old man developed fever of 1 week duration, followed by altered sensorium of 1 day duration. On admission, he had neck rigidity with intermittent opisthotonus. Computed tomography brain and routine blood investigations were normal. He developed hypotension after admission and required intubation and vasopressors. Cerebrospinal fluid (CSF) showed 700 cells/cmm with 43% polymorphs, 57% lymphocytes, normal sugar and elevated protein of 75 mg/dl. He was started on ceftazidime, vancomycin and acyclovir. After 2 days, his condition deteriorated. On examination, he was in a “locked in state” (conscious, opened eyes and blinked and tracked his eyes and protruded his tongue to command). He had a flaccid quadriplegia with grade 0 power in all limbs and global are flexia. Sensory examination was normal. Magnetic resonance imaging (MRI) spine showed hyperintensities in the cervical cord extending from C2 to C7 levels. Axial MRI sections showed intense hyperintensities predominantly involving the grey matter (polio-myelitis) [Figure 1]. MRI brain was essentially normal. His chest X-ray showed bilateral fluffy infiltrates. He then developed intermittent dysautonomia with intermittent tachycardia, tachypnea, blood pressure swings and hypersalivation. Nerve conduction (NCV) studies showed normal motor and sensory responses with absent F waves in all nerves. The initial diagnosis was AFP with polyradiculopathy due to a meningo-encephalitis. The differential diagnosis included paralytic rabies, Cytomegalovirus (CMV), human immunodeficiency virus (HIV) infection and leptomeningeal metastases [Table 1]. On day 5, he developed oliguric renal failure and persistent hypotension following which he became comatose. CSF polymerase chain reaction (PCR) panel was positive for WNV and negative for CMV, human herpes virus-6, rabies, John Cunningham virus, herpes simplex virus (HSV) 1 and 2, dengue, Japanese encephalitis (JE), nipah, chikungunya, chandipura, mumps, measles and enterovirus including poliovirus, fungi and bacteria including Myobacterium tuberculosis on day 7 (Xcyton acute encephalitis syndrome [AES], Bangalore). The serum and CSF samples were positive for both Japanese encephalitis virus (JEV) and WNV immunoglobulin M (IgM) antibodies by IgM capture enzyme-linked immunosorbent assay (ELISA) (JEV IgM ELISA, National Institute of Virology [NIV] Pune and WNV IgM ELISA, InBiosinc. USA). However, the serum sample on microneutralization assay showed a high titer of neutralizing antibodies to WNV (titer: 40) and undetectable neutralizing antibodies to JEV (titer <10). Hence, the WNV etiology was confirmed.[4] CSF was negative for malignant cells. He was offered sustained low efficiency dialysis (SLED) due to persistent hypotension. He underwent three sessions of SLED. On day 14, the relatives decided to withdraw treatment and he expired on day 15.
Figure 1.
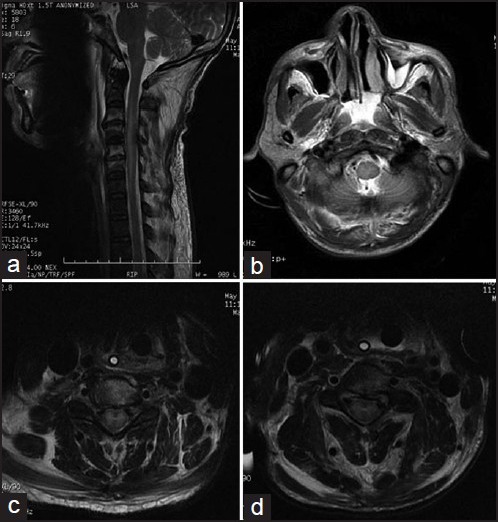
Panel a shows a long segment hyperintensity in the cervical cord on T2 weighted image (WI). Panels b-d show central cord hyperintensity. Panels c and d show intense grey matter hyperintensity on T2 WI
Table 1.
Differential diagnosis of an encephalitic illness with acute flaccid paralysis
Case 2
A 42-year-old man presented to us with buttock pain of 1 week duration. Two days prior to admission, he developed high grade fever and back pain followed the next day by weakness of the right leg and intense twitching of the right thigh. He had grade 0/5 power in the right leg, 4/5 power in the left leg with absent deep tendon reflexes bilaterally and a normal sensory exam. Fasciculations were noticed over both thighs. An MRI spine with contrast was normal. Routine blood investigations were normal. CSF showed 135 cells with 93% lymphocytes and 7% polymorphs and normal sugar and protein. PCR for bacteria, including Mycobacterium tuberculosis, Streptococcus pneumonia, Neisseria meningitidis and HSV, CMV and varicella zoster virus were negative. He was treated for presumptive viral myelitis with acyclovir, ceftazidime and vancomycin for 10 days. Three serum samples collected within 2 weeks after the onset of central nervous system (CNS) symptoms were positive for both JEV and WNV IgM antibodies by IgM capture ELISA (JEV IgM ELISA, NIV Pune and WNV IgM ELISA, InBiosinc., USA). However, the serum samples on microneutralization assay showed significantly high titre of neutralizing antibodies to WNV (titre: 320) and undetectable neutralizing antibodies to JEV (titer <10) confirming the WNV etiology. His fever subsided, but he had residual flaccid paralysis of the right leg, proximal > distal at follow up 1 month later.
Case 3
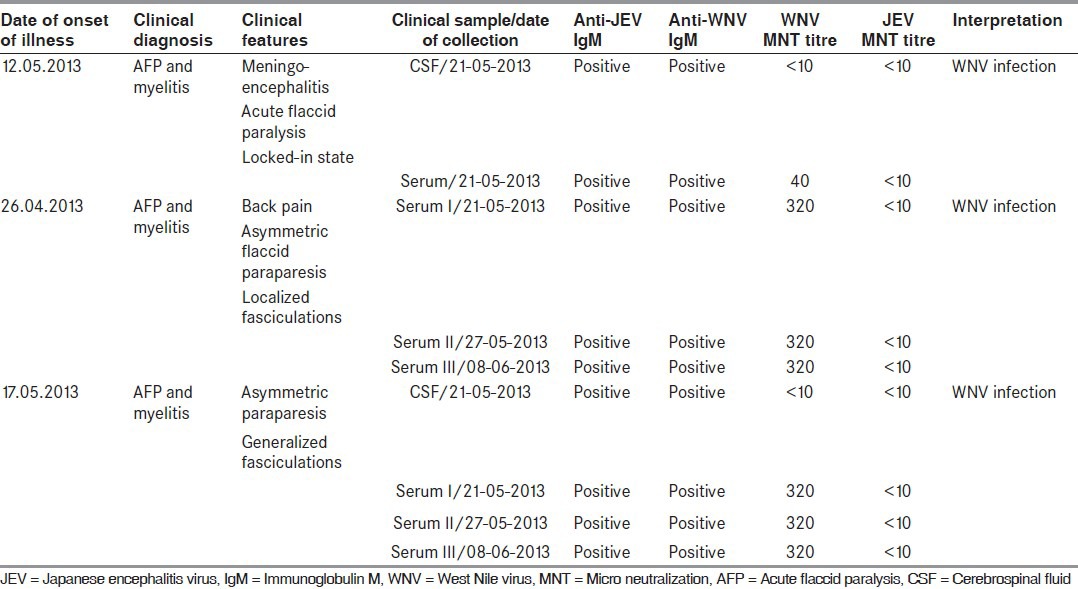
A 45-year-old man presented to us with high grade fever of 5 days duration, generalized weakness, twitching of both thighs and difficulty in walking of 1 day duration. He had grade 3 power in the right hip flexion and knee extension, absent deep tendon reflexes in both lower limbs and generalized fasciculations all over the body, including the facial muscles. Sensory examination was normal. MRI spine showed an ill-defined non-enhancing hyperintensity in the cervico-thoracic cord. CSF showed 246 cells, 80% polymorphs and 20% lymphocytes, with normal sugar and protein. CSF PCR panel for multiple organisms including WNV was negative. Routine blood investigations including erythrocyte sedimentation rate were normal. He was treated with acyclovir and antibiotics and made a near complete recovery. At follow up 3 weeks later, he complained with severe fatigue and occasional buckling at the right knee. The serum and CSF samples were positive for both JEV and WNV IgM antibodies by IgM capture ELISA (JEV IgM ELISA, NIV Pune and WNV IgM ELISA, InBiosinc. USA). However, the serum samples (three samples collected within 2 weeks of onset of CNS symptoms) on micro-neutralization assay showed significantly high titer of neutralizing antibodies to WNV (titer: 320) and undetectable neutralizing antibodies to JEV (titer <10) confirming the WNV etiology. Demographics are shown in [Table 2].
Table 2.
Results of investigations
Discussion
WNV is single stranded ribonucleic acid virus of the family Flaviviridae, related to JE, Murray valley and St. Louis encephalitis viruses. WNV infection is primarily maintained in nature in a cycle between birds and mosquitoes (usually Culex). WNV is transmitted during blood meals by mosquitoes to man and horses (dead end hosts) [Figure 2]. Cases of man-man transmission have been documented only after solid organ transplantation or blood transfusions.[5,6] WNV infections have been documented in India, as early as 1984, but AFP has not been described.[7,8] Akin to the US, where a recent introduction of WNV has resulted in 3 million infections and 780,000 illnesses in the last decade, India may be poised on the cusp of a WNV outbreak.[9]
Figure 2.
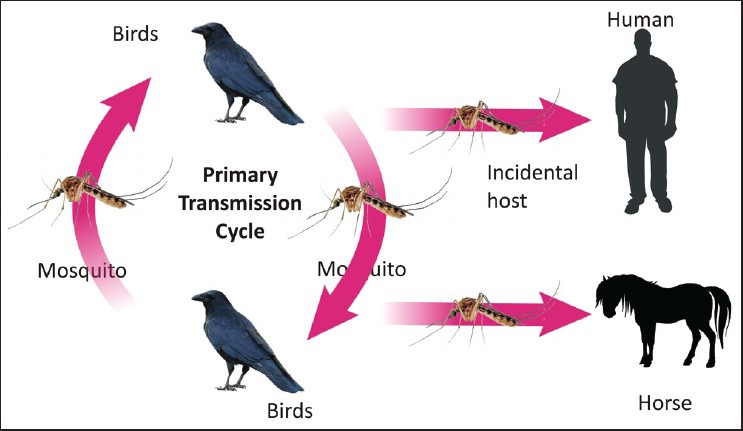
West nile virus transmission cycle
WNV infection is usually asymptomatic or results in a non-specific viral fever. One in 140 individuals go on to develop neuroinvasive WNV infection, often a non-specific menigoencephalitis.[10] A small percentage of patients develop AFP.[11] Clues to the anterior horn cell involvement include fasciculations at the onset in the involved muscles. WNV can produce AFP; monoplegia, diplegia, triplegia or quadriplegia with occasional brainstem involvement and respiratory failure. The sensory system is usually spared, although bowel and bladder function can be involved. Rare presentations include polyradiculitis (Guillain-Barre like syndrome [GBS]), brachial plexus (brachial plexitis) or dysautonomia due to involvement of the sympathetic neurons.[12] Recovery from AFP is usually incomplete.
MRI can show T2-weighted abnormalities in the cortex, subcortex, brainstem especially the midbrain (rhombencephalitis), basal ganglia, thalami or cerebellum. Four patients with AFP can show T2 hyperintensitiesin the cervical cord or spinal cord anterior horns with enhancement around the conus medullaris and cauda equina.[13] Contrast enhancement of the anterior roots can be seen in patients with polyradiculitis.[14]
On NCV studies, motor compound muscle action potential amplitudes are reduced and sensory nerve action potentials are spared. Electromyogram may show denervation. CSF usually shows >200 cells/cmm with normal sugars and increased protein levels. An initial neutrophilic leukocytosis is followed by lymphocytic predominance. The finding of CSF pleocytosis differentiates WNV polyradiculitis from GBS and should prompt consideration of WNV, CMV, HIV, sarcoidosis or leptomeningeal metastases.
Like JEV, WNV has a viremic phase of nearly a week following the mosquito bite but the virus is rapidly cleared from blood within 2 days of onset of illness. Hence, nucleic acid amplification based tests such as PCR and real-time PCR are positive only for 2-3 days after the onset of illness. Hence, the main stay of diagnosis of WNV encephalitis is serological tests. However, the high level of cross reactivity between WNV and JEV complex flaviviruses makes the differentiation by virus specific IgM capture ELISA difficult. The preferred method of confirmation would be the demonstration of significantly high level of virus specific neutralizing antibodies or fourfold rise in titer in the patient serum/CSF by micro-neuralization assay or plaque reduction neutralization test assay.[15]
Treatment for WNV is supportive. There are anecdotal reports of improvement with interferon alfa-2b at a dose of 3 million U S/C daily for up to 2 weeks. Four ribavirin has also been used.[16]
All our cases of WNV associated AFP reported in the month of May 2013 were spatially and temporally clustered and correlated with the pre-monsoon period of JEV/WNV seasonality in Kerala. In the wake of reports of neurotropic WNV lineage 1 (Clade 1a) from Kerala, it is important to differentiate JEV and WNV infections in AES cases. Further, in the post-polio eradication era it may be worthwhile testing AFP cases for WNV. A high level of clinical suspicion, active clinical case detection and appropriate laboratory confirmation is the way forward to increase the hospital based surveillance of WNV infection in India.
Acknowledgments
The virological part of the work was supported by the Indian Council of Medical Research grant of Dr. G. Arunkumar (File No. 5/8/7/15/2010/ECD-I and VIR/41/2010-ECD-I).
Footnotes
Source of Support: The virological part of the work was supported by the Indian Council of Medical Research grant of Dr. G. Arunkumar
Conflict of Interest: Nil
References
- 1.John TJ, Vashishtha VM. Eradicating poliomyelitis: India's journey from hyperendemic to polio-free status. Indian J Med Res. 2013;137:881–94. [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). Outbreak of West Nile-like viral encephalitis — New York, 1999. MMWR Morb Mortal Wkly Rep. 1999;48:845–9. [PubMed] [Google Scholar]
- 3.Balakrishnan A, Butte DK, Jadhav SM. Complete genome sequence of West Nile virus isolated from Alappuzha district, Kerala, India. Genome Announc. 2013;1:230–13. doi: 10.1128/genomeA.00230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis LE, DeBiasi R, Goade DE, Haaland KY, Harrington JA, Harnar JB, et al. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60:286–300. doi: 10.1002/ana.20959. [DOI] [PubMed] [Google Scholar]
- 5.Iwamoto M, Jernigan DB, Guasch A, Trepka MJ, Blackmore CG, Hellinger WC, et al. Transmission of West Nile virus from an organ donor to four transplant recipients. N Engl J Med. 2003;348:2196–203. doi: 10.1056/NEJMoa022987. [DOI] [PubMed] [Google Scholar]
- 6.Biggerstaff BJ, Petersen LR. Estimated risk of transmission of the West Nile virus through blood transfusion in the US, 2002. Transfusion. 2003;43:1007–17. doi: 10.1046/j.1537-2995.2003.00480.x. [DOI] [PubMed] [Google Scholar]
- 7.Paramasivan R, Mishra AC, Mourya DT. West Nile virus: The Indian scenario. Indian J Med Res. 2003;118:101–8. [PubMed] [Google Scholar]
- 8.George S, Gourie-Devi M, Rao JA, Prasad SR, Pavri KM. Isolation of West Nile virus from the brains of children who had died of encephalitis. Bull World Health Organ. 1984;62:879–82. [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen LR, Carson PJ, Biggerstaff BJ, Custer B, Borchardt SM, Busch MP. Estimated cumulative incidence of West Nile virus infection in US adults, 1999-2010. Epidemiol Infect. 2012;28:1–5. doi: 10.1017/S0950268812001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalil AC, Devetten MP, Singh S, Lesiak B, Poage DP, Bargenquast K, et al. Use of interferon-alpha in patients with West Nile encephalitis: Report of 2 cases. Clin Infect Dis. 2005;40:764–6. doi: 10.1086/427945. [DOI] [PubMed] [Google Scholar]
- 11.Jeha LE, Sila CA, Lederman RJ, Prayson RA, Isada CM, Gordon SM. West Nile virus infection: A new acute paralytic illness. Neurology. 2003;61:55–9. doi: 10.1212/01.wnl.0000073617.08185.0a. [DOI] [PubMed] [Google Scholar]
- 12.Leis AA, Stokic DS. Neuromuscular manifestations of West Nile virus infection. Front Neurol. 2012;3:37. doi: 10.3389/fneur.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali M, Safriel Y, Sohi J, Llave A, Weathers S. West Nile virus infection: MR imaging findings in the nervous system. AJNR Am J Neuroradiol. 2005;26:289–97. [PMC free article] [PubMed] [Google Scholar]
- 14.Park M, Hui JS, Bartt RE. Acute anterior radiculitis associated with West Nile virus infection. J Neurol Neurosurg Psychiatry. 2003;74:823–5. doi: 10.1136/jnnp.74.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Busch MP, Kleinman SH, Tobler LH, Kamel HT, Norris PJ, Walsh I, et al. Virus and antibody dynamics in acute West Nile virus infection. J Infect Dis. 2008;198:984–93. doi: 10.1086/591467. [DOI] [PubMed] [Google Scholar]
- 16.Anderson JF, Rahal JJ. Efficacy of interferon alpha-2b and ribavirin against West Nile virus in vitro. Emerg Infect Dis. 2002;8:107–8. doi: 10.3201/eid0801.010252. [DOI] [PMC free article] [PubMed] [Google Scholar]


