Abstract
The Kala-azar/visceral leishmaniasis (VL) turns epidemic form once in every 15 years in the endemic regions of Indian subcontinent. The goal of elimination of Kala-azar from India by 2010 was lost despite paramount efforts taken by the Government of India and World Health Organization and Regional Office for South East Asia. The main objective of this review was to elucidate the possible reason for the failure of Kala-azar elimination program and to suggest possible remedial measures to achieve the goal in future. The annual numbers of VL cases and deaths recorded in India since 1977 were plotted on a graph, to see if the temporal trends could be associated with changes in the vector control practices or co-infection with human immunodeficiency virus (HIV) or therapeutic modalities used against VL. The VL cases flares up whenever the effect of dichlorodiphenyltrichloroethane (DDT) diminished after the withdrawal of spray. The fading effectiveness was clearly correlated with an increasing number of VL cases. Therapeutic modalities were found to be highly correlating with VL mortality not with VL morbidity. The diminishing efficacy of first and second line drugs and the introduction of new drugs and drugs combination were responsible for ups and downs in the VL mortality. The VL mortality is constantly declining since 1993, but cases started increasing from 2003 to 2007 and then recently again from 2010 to 2011. This shows a serious lacuna in the vector control practices applied. The extent of HIV co-infection did not show any correlation with number/trend of VL cases or death over the study period. It is concluded that, by strict vector control practices, the VL cases can be reduced and by applying proper therapeutic strategies, the VL mortality can be reduced. HIV-VL co-infection does not seem to be in a worried stage.
Keywords: Co-infection, cyclic occurrence, elimination, human immunodeficiency virus, Kala-azar, therapeutic modalities, vector control practices, visceral leishmaniasis
INTRODUCTION
Leishmaniasis threatens about 350 million people in 88 countries and 12 million people are believed to be currently infected, with about 1-2 million estimated new cases occurring every year.[1] The natural transmission of leishmania parasite is carried out by an insect vector called sand-fly of the genus Phlebotomus (in Old World) or Lutzomyia (in New World). The disease is present in three different forms: (i) Visceral, (ii) cutaneous and (iii) mucosal leishmaniasis. The visceral form, also known as “black sickness” or “Kala-azar”, is the most severe form of all and if left untreated, is usually fatal.[2] Although confirmed cases of Kala-azar have been reported from 66 countries, 90% of the world's Kala-azar burden on the Indian subcontinent, Brazil and Sudan.[3] In India, the disease is endemic in 52 districts of Bihar, Uttar Pradesh, Jharkhand and West Bengal [Figure 1] and countries adjoining to this region such as Nepal (12 districts) and Bangladesh (45 districts).[4,5] The State Bihar alone captured almost 50% out of the total burden in the Indian sub-continent. Being a border State, Bihar seems to be the “hot spot” of visceral leishmaniasis (VL).[6] Bhutan has recently joined in the list of countries neighboring India (Bihar) affected by VL.[7] VL has a huge social and economic impact due to lost educational potential, reduced economic productivity due to missed days of work for adults, especially the family breadwinner and stigma. Therefore, VL not only occurs in the context of poverty, but through its adverse social impact may also promote poverty. VL leads to a loss of about 400,000 disability adjusted life years (DALYs) every year in this region. This amounts to a loss of approximately US $140 million annually (calculated at a loss of about US $350 per DALY lost which is average yearly income in the endemic countries of the region).[8]
Figure 1.
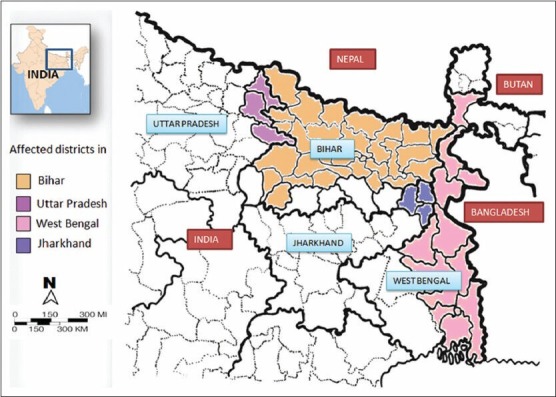
The visceral leishmaniasis hot zone of India
VL had re-emerged from near eradication in the Indian subcontinent.[9] In 1960s intensive vector control measures were adapted under the Malaria Eradication Program and VL had almost been eliminated from this part of the world, illustrating the vulnerability of the transmission cycle.[3] Before 1977-78, due to the absence of an effective surveillance system in Bihar and other affected areas, only limited data are available about cases of Kala-azar.[9] Until 1990, the Kala-azar control activities in India were carried out by the affected States, but concerned with increasing incidence of Kala-azar, the Government of India, launched a centrally sponsored “Kala-azar Control Program” during 1991.[10] The initial success of the program with a significant decline in morbidity could not be retained further by the affected States. Hence, an expert committee chaired by the director General of health services, reviewed the program in 2000 and recommended to include the elimination of Kala-azar from India in the National Health Policy.[11] Accordingly it was included in the National Health Policy 2002 which set a goal for the “elimination of Kala-azar by 2010”.[12] The elimination is defined an achievement of zero incidences in a defined territory. The Kala-azar control program was then merged with “National Rural Health Mission” in the year 2005 under the name of National Vector Borne Diseases Control Program.[13] Since VL mainly affects the border areas of India, Bangladesh and Nepal, it was realized that, the elimination has to be started in all the affected countries of the Indian subcontinent. As a result, a Tripartite MoU was signed by the three affected countries under the objective of reducing the incidence of Kala-azar and post Kala-azar dermal leishmaniasis (PKDL) to < 1/10,000 populations at the district level.[14] Several factors were thought to favor the elimination of VL in the Indian subcontinent; such as: the disease in this area is anthroponotic, with human beings are the only reservoir and Phlebotomous argentipes is the only known vector which in turn restricted to live in and around residential houses, the disease is endemic in only a limited number of districts, new and more effective drugs (such as miltefosine) and a rapid diagnostic test (rk39) are available that can be used even in the field and also there is a strong political commitment and inter-country collaboration.[15] Despite the efforts taken for the elimination of VL and favorable factors, the number of VL cases started to increase from 18,088 in 2003[16] and reached a peak of 45,508 in 2007 and then declined to 25,060 in 2010[17] [Figures 2 and 3].
Figure 2.
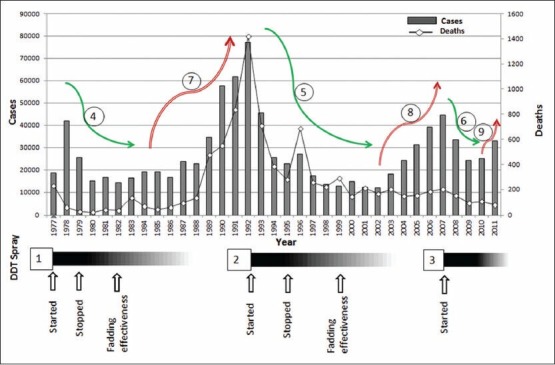
Kala-azar re-emerges whenever the effect of dichlorodiphenyltrichloroethane (DDT) diminishes. In the period under study (1977-2011), DDT spray was carried out three times (1, 2 and 3) when visceral leishmaniasis (VL) cases flared up. The first spray operation was carried out in 1977, 1978 and discontinued in 1979. The effect of DDT was persistent up to 1982.[18] During this period the VL cases declined (green arrow 4) from as high as 41,953 in 1978 to 14,388 in 1982.[21] When the cases peaked an ever high level of 77,099[16] the second spray operation was started, continued through 1992-1994 and discontinued in 1995 and the effect was persistent up to 2001.[18] In the corresponding period, the VL cases declined from 77,099 in 1992 to 12,176 in 2001[16] (green arrow 5). Third spray operation was carried out in the mid 2007 with corresponding declining [green arrow 6] of cases from 44,508 to 24,169 in 2009.[17] But this decline was only short lived and the cases started to resurge to 25,060 in 2010 and 33,108 in 2011[17] (red arrow 9). After the withdrawal of DDT spray and fading residual effect of sprayed DDT, the VL started to re-emerge as happened in 1983 and 2003 (red arrow 7 and 8). Although reports of improper[11] spray with insufficient concentration of DDT,[24,25] no data is presently available about withdrawal of spray that started in 2007. The declining of cases (green arrows) always correlates with corresponding DDT spray and its residual effect periods. The absence of DDT spray and no residual effect always correlates with the resurgence of cases. Surprisingly, the deaths did show any correlation with DDT spray operations
Figure 3.
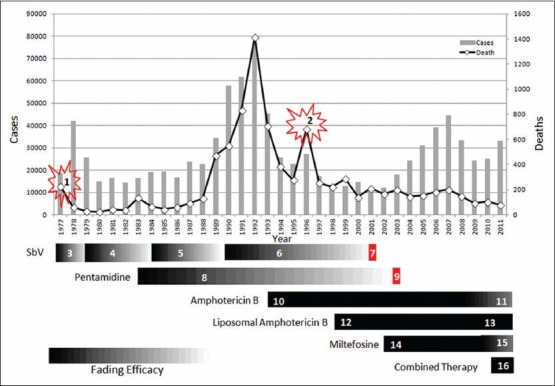
Visceral leishmaniasis (VL) mortality in response to changing therapeutic modalities. (1) The exacerbated mortality was due to an acute shortage of drugs necessary for treatment which was mainly due to local firms' decision to limit their production in India. Then it was restored by the intervention of World Health Organization (WHO) and Government of India.[37] (2) The unexpected rise in the mortality during 1996 was due to the usage of defective lot of sodium stibogluconate (SbV).[56] (3) In 1977, around 30% cases were unresponsive to SbV.[36] (4) An expert committee recommended to use 10 mg/kg for two 10 day courses and the cure rate was significantly improved initially,[38] but in 1983, the cure rate was declined to 86%.[39] (5) In 1984, WHO expert committee recommended to increase daily dose from 10 to 20 mg/kg and (6) in 1990 duration from 20 days to 40 days. This dose escalation policy did not prevent the emergence of drug resistance; ultimately the cure rate was declined to 35% in 2001.[45] (7) In 2001, the use of SbV was abandoned in the endemic areas.[45] (8) In 1983, pentamidine was introduced with cure rate of 99% and this brought down the mortality from 135 in 1983 to 67 in 1984. In early 1990s the efficacy of pentamidine had dwindled and the cure rate was declined to 70-75%.[47,48] The combined effect of unresponsive SbV and pentamidine was responsible for the paramount increase of deaths in 1992. The pentamidine was ultimately abandoned in 2003.[14,49] (10) In 1993, amphotericin B was beginning to be used with a cure rate of 99-100%[50] and it maintained its cure rate constantly from 97% to 100%.[52] (11) But recently it was shown that the efficacy of amphotericin B was declined to 93%.[53] (12) In 1998, liposomal amphotericin B was introduced with cure rate of 100%[51] and (13) maintains its cure rate at 98.8% in 2010.[55] (14) Miltefosine was introduced in India in 2002 with >95% cure rate.[15] (15) But the cure rate of miltefosine was also declined subsequently, in 2010, its cure rate was 90.3%. (16) A new concept of multidrug treatment which was introduced in 2011 holds much promises for future VL control.[53] The death due to VL has slowly reduced from 706 in 1993 to 80 in 2011 with the combined effect of amphotericin B, liposomal amphotericin B, miltefosine and after the introduction of amphotericin B multidrug treatments. At the same time number of VL cases increased from 12,066 in 2002 to 45,508 in 2007[16,17]
This was not the first experience; three attempts made in independent India to eliminate VL had failed.[9,11] The first attempt was done through National Malaria Eradication Program (NMEP) which initiated an intensive dichlorodiphenyltrichloroethane (DDT) spraying between 1953 and 1964. As a secondary effect, the number of VL cases decreased from 60,000 to almost nil during 1955-56.[18] The second attempt was done by “Kala-azar control program” which was launched by the affected States with the help of Government of India during the 1977 outbreak. DDT spraying was done for 3 years between 1977 and 1979 and discontinued at the end of 1979.[18] Third attempt was carried out during 1991-92 outbreak by centrally sponsored “Kala-azar control program”. The DDT spraying was started in 1992 and again discontinued in 1995.[18] This has strengthened the conventional belief that VL would occur in an epidemic form once in every 10-15 years[5,19] and the reason behind this to be studied. The main objective of this review was to correlate the vector control strategies adapted so far, co-infection with human immunodeficiency virus (HIV) and therapeutic modalities with the annual incidence of Kala-azar and mortality for a period of 34 years to find out possible reasons for the failure of the program “Elimination of Kala-azar by 2010” and to suggest the possible remedial measures to be undertaken to achieve the goal in future.
MATERIALS AND METHODS
Official reports of the annual numbers of VL cases recorded in India since 1977 were plotted on a graph, to see if the temporal trends could be associated with changes in the vector control practices or co-infection with HIV or therapeutic modalities used against VL. The data of annual incidence of Kala-azar for the period from 1977 to 2011 collected from the health information/health statistics of India/National Health Profile, published by Government of India and also through literature collections.[16,17,20] The incidence of VL and mortality data from the States such as Uttar Pradesh, Bihar, Jharkhand and West Bengal which are in the Kala-azar foci is alone taken into consideration while the negligible number of 1 or 2 cases reported from other places such as Delhi, Gujarat or Assam were omitted. Since the reported data is from Government Health Facilities, it may have limitations in terms of its completeness as Private Medical and Health Care Institutions still need to strengthen their reporting to their respective Government Health units.[17] Although under-reporting is a major problem in India and the actual number of cases would be several fold higher than the reported cases, the number of cases reported for each year in the study period is assumed to be a fairly stable proportion of the actual number of cases.[21] The information about vector control practices followed, therapeutic modalities adapted and incidence of HIV co-infection was collected from Govt. data source and literature survey.
VECTOR CONTROL PRACTICES
As a secondary effect of DDT spray done under the NMEP which started in the year 1953 and ended in 1966, VL had supposed to be eliminated from the State Bihar.[18] However, in 1977, Bihar experienced a resurgence of VL with 18,676 cases.[20] Immediately, the Government of India launched DDT spray, which continued until 1979 and the effect was persistent up to 1982[18] and this correlates with declining of VL cases from 41,953 in 1978 to 16,946 in 1980[20] and then reached more or less a stationary phase until 1982 [Figure 2]. Although, the number of VL cases had increased again from 1983, peaking at 77,099 cases in 1992,[16] no DDT spray was done till 1991. Then in 1992, the DDT spray was started again and discontinued in 1995 and its effect was persistent up to 2001.[18] This correlates with a drastic fall in the number of cases from 77,099 in 1992 to 12,176 in 2001.[16] No DDT spraying was conducted until 2006[22] which correlates with another increase in the number of cases from 12,066 in the year 2002[16] and reached its peak of 44,508 in the year 2007.[17] Then again, DDT spray was started in the mid of 2007 in the affected areas which correlated with another decreasing trend that continued until 2009.[18] However, this decline was short lived and the cases started to resurge to 25,060 in 2010 and 33,108 in 2011.[18] The analysis presented in the Figure 2 displays the possible reason for the cyclic occurrence of Kala-azar in India. The DDT spray was started whenever there was a flare-up of cases. Although the sand-fly vector, P. argentipes reappear in 9 months after a single DDT spraying,[23] the effect of DDT may persist from 3 years to as long as up to 7 years with the intensity and duration of spray[18] [Figure 2]. Whenever the effect of DDT had diminished, the cases of VL started to re-emerge. The DDT spray that started in 2007 was reported to be improper because the spray operation was not at all conducted in some of the villages located in the endemic area of north Bihar.[11] The spray operation was done only patchily and the DDT concentration was not sufficient.[24,25] Development of resistance in P. argentipes collected from most of the villages of the endemic area[26] was probably another reason for the resurgence of cases in 2010. However, no relation was observed between the vector control practices followed and the number of deaths due to VL.
The lessons learned from the failure of the Malaria Eradication Program[27] had not been taken into consideration and many of the same mistakes were repeated[11,24,27] in the Kala-azar elimination by 2010 program. There should be a sufficient number of supervisors to monitor the spray operation in comparably fewer houses for better performance to avoid patchy spray or insufficient concentration.[24,25] The inspectors and spray men have to be educated for the task they are undertaking and the responsibility of protecting people from the deadly disease, to increase the morale and to have respect on the job they are undertaking. Strong management and rigorous inspections are required to ensure that the sprayers are not missing any house or spraying several times in the same house by different spray-man. The common public has to be educated via mass media to understand the importance of insecticide spray to avoid the refusal and for allowing the spray-man inside their houses. Lack of accountability has to be rectified and the spray-man, inspector, etc., should be held responsible for the occurrence of VL cases in a house where spray operations had properly been carried out as on record. Adapting a system where the spray-men and other vector control staff are permanently employed and have some sort of reasonable at the same time performance-based pay structure could be a far more realistic way of ensuring that the teams perform their function better. Central and State governments have to be cooperative and have to interact efficiently and have to draw a time bound achievable goals in the elimination program.[11] The vital information gap between the areas affected by VL and the government authorities has to be rectified. As soon as the information is received, active interventions have to be implemented. The unplanned and inappropriate use of insecticide could be the reason for the development of resistance in sand-fly vector, which would further complicate the control strategies.[10] In those area where DDT resistance was reported, the alternative insecticide, which are still very effective in controlling P. argentipes such as deltamethrin[26] can be tried.
CO-INFECTION WITH HIV
Apart from having the largest number of VL cases, India is also known for having second largest HIV infected population. The possible overlap in the distribution of VL and HIV in countries like India may have grave consequences. Although, India contributes heavily to the global VL and HIV disease burdens, information about co-infection in the country is surprisingly sparse.[28] With close to 40-50% of VL cases worldwide and about 80-90% of all cases in India occurring in Bihar state, it lies at the heart of the VL problem regionally as well as globally.[29] Bihar is one of the least developed and most populous States in India, with poverty, malnutrition and poorly functioning health care systems, the people from Bihar travel for work to various metropolitan cities and economically strong southern States, where HIV infection become a serious problem among commercial sex workers (CSWs). Although away from their families, they engage in high-risk sexual activities with CSWs.[30] Hence it was expected that, the migrant population of Bihar may influence the incidence of VL and HIV co-infection. However surprisingly, the first case of co-infection of VL and HIV was diagnosed in the early 1999 not in Bihar, but in the Kumaon region of sub-Himalayas.[31] Since then, fluctuating rate of HIV co-infection had been reported from as low as 0.88, 2.18%[32] to as high as 6.3%[30] among the hospitalized VL patients in Bihar. The probability of death or treatment failure was estimated to be 69.8%[33] and 67%[28] after 2 year of completion of treatment. Although, the co-infection of VL and HIV is a serious public health problem and HIV infection dramatically increases the risk of progression from asymptomatic infection to VL disease and VL accelerates HIV disease progression,[33] the overall adult new infection of HIV per million people in India had declined from 27 in 2000 to 12 in 2009.[34] Thakur et al.[35] in their study have reported that the percentage of HIV infection was much less in VL patients (1.5%) comparing with age and sex matched controls (4.1%). Although the Indian subcontinent is suffering by high rate of resistance to antimonials and unresponsiveness to common antileishmanial drugs, the extent of overlap between VL and HIV is still very limited.[5] The HIV co-infection did not show any correlation with number of VL cases or death over the study period.
THERAPEUTIC MODALITIES
In 1977 an estimated 200,000 patients were affected in Bihar of which 30% cases were unresponsive to sodium stibogluconate (SbV).[36] The mortality rate was exacerbated by the acute shortage of drugs necessary for treatment, which was mainly due to local firms' decision to limit their production in India. During this crisis, the World Health Organization (WHO) played an important role in providing emergency supplies of medicine to the region and also involved along with the Government of India, in convincing pharmaceutical companies to restart the manufacture of anti-Kala-azar drugs, which allowed the dispensaries and hospitals to provide treatment to the infected.[37] This correlates with the sudden decline in the number of deaths [Figure 3] from 229 in 1977 to 62 in 1978 (but the number of cases increased from 18,676 to 41,953 in the same period).[20] The SbV was the first-line drug to treat VL in the affected areas. Response to relatively small daily doses of SbV (10 mg/kg; 600 mg maximum) for short duration (6 ± 10 days) had initially been excellent but later, during 1977, around 30% of cases were unresponsive to SbV. When reports of treatment failure and increasing unresponsiveness to SbV appeared, modifications in SbV treatment were suggested to overcome the drug failure.[36] Following this, in 1979 an expert committee revised recommendations to use SbV in two 10 day courses with an internal of 10 days and a significant improvement in cure rate (99%) was observed.[38] However a few years later in 1983 the cure rate was declined to 86% with 20 days of continuous treatment with this regiment.[39]
Hence, in 1984, the WHO expert committee recommended an increase in the daily dose from 10 to 20 mg/kg body weight[40] and in 1990, duration from 20 to 40 days.[41] However the dose escalation policy did not prevent further emergence of resistance, but rather selected resistant parasites.[42] 4 years later, in 1988 the regiment was evaluated by Thakur et al.[43] and reported that only 81% of patients was cured. In 1991 the cure rate was declined to 71% and in 1992 the cure rate was further declined to 64% even after extension for therapy for 30 days. This progressive erosion of efficacy of SbV clearly correlates with increasing number of deaths that reached its peak in 1992 [Figure 3]. In 1996, the cure rate was further fell down to 36% and in 2001 the cure rate was only 35%[44] hence it was recommended to abandon its usage in the endemic areas.[45]
To circumvent the problem of clinical resistance to SbV, pentamidine was tried in 1983 with initial cure rate 99%.[46] This correlates with the decrease in the number of deaths from 135 in 1983 to 67 in 1984. However, in early 1990s, the pentamidine also suffered the same fate of SbV as its efficacy had dwindled and the cure rate had decreased to 70-75%.[47,48] The cumulative effect of increased unresponsiveness of both first and second line drugs was probably responsible for the paramount number of deaths (1417) occurred in 1992. Hence, the use of pentamidine was ultimately abandoned in 2003 due to its decreased efficacy and increased toxicities.[14,49]
In 1993, amphotericin B was beginning to be used in India with a cure rate of 99-100%.[40] This highly correlates with the sudden fall in the number of deaths from 1417 in the year 1992 to 706 in 1993 and further successive decline in deaths. In late 1990s, liposomal amphotericin B was beginning to be used in India that had further reduced the number of deaths.[50,51] The cure rate of amphotericin B was seems to be constant over the decade around 97%, 100% and 98.8% during 2002, 2004 and 2007 respectively.[52] However surprisingly, recent reports showed a declining cure rate (93%) of amphotericin B[53] and development of resistance in a patient from Lucknow, Uttar Pradesh in 2011.[54] Unlike amphotericin B, liposomal amphotericin B still maintains its cure rate at 98.8%.[55] The unexpected sudden rise in the death during 1996 was reported to be due to the usage of a defective lot of SbV.[56]
Although, the first ever oral drug for leishmaniasis, the miltefosine with a cure rate of > 95% was registered in India in 2002, there was no improvement in the reduction of deaths until 2007. During this period, the drug was available only in the private market, at a retail cost of US $125-200 per treatment course[15] which was unaffordable for many in the affected area. By the cumulative effect of amphotericin B and liposomal amphotericin B, the number of deaths due to VL probably came almost to a stationary phase of 166 in 2002-202 in 2007 (despite there had been a steady increase in the number of Kala-azar cases reported annually from 2003 to 2007). From 2008 onwards, the miltefosine has been distributed free of cost through public sectors and government hospitals in addition to incentives to patients for loss of wages at Rs. 50 ($1)/day during the period of treatment, free dietary support to patients and one attendant and incentives to Kala-azar activists/health workers at Rs. 100 for referring a suspected case and ensuring the completion of treatment[57] which collectively seems to be responsible for the gradual fall in the VL deaths from 202 in 2007 to 93 in 2009. But in 2010, death due to VL had once again increased from 93 to 105. During the same period, a study conducted by Sundar et al.[58] revealed that, the cure rate of miltefosine also declined to 90.3%. This declining efficacy of miltefosine combined with declining efficacy of amphotericin B[54] could probably be the reason for increasing VL deaths during 2010.
In a phase 2 randomized, open-label, controlled trial of paromomycin (aminosidine) at different doses versus SbV for treating VL in North Bihar, India, paromomicin dosed at 16 mg/kg/day for 21 days cured 94% of cases at 6 months of follow-up.[59,60] Paromomycin was shown to be safe and effective for treatment of VL in a phase 3 clinical trial in India, with a final cure rate of 94.6%[52] and subsequently was registered for the treatment of VL in India in 2006 and was included in the WHO Essential Medicines List[61] and WHO Essential Medicine for Children.[62] The phase 4 study was conducted recently showed the final cure rate of 94.2%[29] and is expected to be available for general usage in public sectors shortly.
Drug combinations for the treatment of VL represent a promising and challenging chemotherapeutic strategy that has recently been implemented in different endemic areas. This approach has several advantages over single-drug therapies, including shortening of the treatment period and reduction of the probability of selecting drug-resistant parasites.[63] A recent study show that a cure rate of 93.0% was observed for amphotericin B alone, but the combination of liposomal amphotericin B and miltefosine gave 97.5% cure rate, liposomal amphotericin B with paromomycin gave 97.5% cure rate and the cure rate of miltefosine with paromomycin was 98.7%. However, this approach must be used with care given to the possibility that, if not applied in a controlled and regulated way, resistance could be induced in leishmania parasite, thus resulting in a rapid loss of efficacy of not one but two therapeutic options.[64] The lasting therapeutic efficacy of paromomycin and combination therapy on the trend of VL mortality could presently not possible to correlate.
There are pharmokinetic reasons as to why leishmania parasite develops easy resistance to the newer agents such as miltefosine and paromomycin. The anthroponotic foci in India provide a perfect ground for evolving primary drug resistance.[65] Although the general use of SbV is abandoned in the VL endemic area of India, the continued use of SbV in the interior part of endemic region,[15] could be the reason for the continued deaths [Figure 3]. I and my team have personally also observed the continued usage of SbV in the endemic areas of Bihar during outbreak investigations and survey carried out in 2010-12. Like pentamidine, which was abandoned, the SbV also must be strictly abandoned in the entire endemic regions and the efforts of Kala-azar elimination must reach the interior Bihar. A person has to be nominated in every village and held responsible to bring the patients with VL symptoms to the government hospitals. One medical officer has to be appointed at every primary health center (PHC) for VL control or existing medical officers have to be trained, and an in-charge has to be arranged at the district level.
CONCLUSION
It is concluded that, the vector control practices have direct impact on the incidence of VL while, the treatment modalities evolved in response to the development of drug resistance have direct impact on case fatality. The co-infection of VL and HIV, although considered to be an important health problem which needs much attention, it seems not playing any major role in determining the cyclic occurrence of VL. After recovery, some patients develop PKDL[66] or after treatment some 67-70% of the patients with VL and HIV co-infection may get relapse and may act as reservoir for leishmania parasite and would maintain the transmission continuously and facilitate its transmission during inter-outbreak periods. Those patients must adequately be isolated and cared in separate facilities to achieve the goal of elimination of VL from India. The success story of elimination of VL from the once badly affected State, Tamil Nadu has to be considered. Factors responsible for the elimination of Kala-azar from Tamil Nadu includes strong political commitment, DDT spray oriented to vector ecology of sandflies, increased access to medical care, anti-Kala-azar unit with strong infrastructure (1-2 trained entomologists for each district), active search every four months and well supervised implementation of the program.[48] A recent TDR study suggests that, indoor residual spray (IRS) and to a lesser extends environmental vector management and long lasting insecticidal nets significantly reduced sand-fly population in the study villages.[8] Even though, VL is a notifiable disease in India,[67] the case surveillance/reporting through active and passive case detection need to be strengthened. Early diagnosis by dipstick and complete treatment, effective vector control through integrated vector management with a focus on IRS, insecticide treated bed nets and environmental management, social mobilization of the population at risk and partnership are urgently required. Synthetic pyrethroids could be used in Nepal and Bangladesh where the use of DDT was either stopped or banned.[26] Because humans are the reservoir for VL in the Indian subcontinent, the infection could be eliminated with widespread treatment of patients and rigorous vector control (although elimination may not be possible in regions where zoonotic VL is prevalent, such as Brazil).[52] If all these problems are properly addressed, the re-fixed goal of elimination (=reduction below levels of public health importance) of Kala-azar from the Indian subcontinent by 2015[68] is not far from complete success. There are few possible limitations which cannot be ruled out, such as the analysis is based mainly on the number of reported cases in the government hospitals and treatment facilities, which is several fold less when comparing with actual number of cases. The increase in the number of reported cases in recent years may also due to intensive control efforts along with other reasons analyzed in this review.
ACKNOWLEDGMENTS
The help of Mr. V. Rajamannar, R. Sathis Babu, R. Gopal and Mr. J. Nagaraj during statistical analysis is thankfully remembered.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.WHO. Leishmaniasis home. 2011. [Last accessed on 2011 Jun 6]. Available from: http://www.who.int/leishmaniasis/en/
- 2.Cox FE, Kreier JP, Wakelin D. Parasitology. In: Collier L, Balows A, Sussman M, editors. Topley and Wilson's Microbiology and Microbial Infections. Vol. 5. 338, Euston Road, London NWI 3BH: Hodder Arnold; 1998. [Google Scholar]
- 3.Bhattacharya SK, Sur D, Sinha PK, Karbwang J. Elimination of leishmaniasis (kala-azar) from the Indian subcontinent is technically feasible and operationally achievable. Indian J Med Res. 2006;123:195–6. [PubMed] [Google Scholar]
- 4.Sundar S, Rai M. Laboratory diagnosis of visceral leishmaniasis. Clin Diagn Lab Immunol. 2002;9:951–8. doi: 10.1128/CDLI.9.5.951-958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvar J, Yactayo S, Bern C. Leishmaniasis and poverty. Trends Parasitol. 2006;22:552–7. doi: 10.1016/j.pt.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Singh SP, Reddy DC, Rai M, Sundar S. Serious underreporting of visceral leishmaniasis through passive case reporting in Bihar, India. Trop Med Int Health. 2006;11:899–905. doi: 10.1111/j.1365-3156.2006.01647.x. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharya SK, Rinzin N, Chusak P, Dash AP, Chowdhury R, Tobgay T, et al. Occurrence and significance of kala-azar in Bhutan. Indian J Med Res. 2010;132:337–8. [PubMed] [Google Scholar]
- 8.Joshi A, Narain JP, Prasittisuk C, Bhatia R, Hashim G, Jorge A, et al. Can visceral leishmaniasis be eliminated from Asia? J Vector Borne Dis. 2008;45:105–11. [PubMed] [Google Scholar]
- 9.Bora D. Epidemiology of visceral leishmaniasis in India. Natl Med J India. 1999;12:62–8. [PubMed] [Google Scholar]
- 10.Kishore K, Kumar V, Kesari S, Dinesh DS, Kumar AJ, Das P, et al. Vector control in leishmaniasis. Indian J Med Res. 2006;123:467–72. [PubMed] [Google Scholar]
- 11.Thakur CP, Meenakshi Thakur AK, Thakur S. Newer strategies for the kala-azar elimination programme in India. Indian J Med Res. 2009;129:102–4. [PubMed] [Google Scholar]
- 12.NVBDCP. National Vector Borne Disease Control Programme, Directorate General of Health Services, Ministry of Health and Family Welfare, Govt. of India 2008. Implementation Plan of National Vector Borne Disease Control Support Project, Under World Bank on Malaria Control and Kala-azar Elimination (2008-2013) [Last accessed on 2011 Aug 19]. Available from: http://nvbdcp.gov.in/Doc/PIP.pdf .
- 13.Singh RK, Pandey HP, Sundar S. Visceral leishmaniasis (kala-azar): Challenges ahead. Indian J Med Res. 2006;123:331–44. [PubMed] [Google Scholar]
- 14.Gupta A, Nagar M, Mishra SS, Lahariya C. Visceral leishmaniasis (Kala-azar) elimination from Indian sub-continent by 2015. Int J Trop Dis Health. 2013;3:73–81. [Google Scholar]
- 15.Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, et al. Visceral leishmaniasis: What are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5:873–82. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- 16.Health Information of India 13-28 (1986-2004) New Delhi: Controller of Publications, Department of Publications, Civil Lines; 2006. [Google Scholar]
- 17.National Health Profile (2005-2011), Central Bureau of Health Intelligence, Govt. of India. [Last accessed on 2012 Dec 20]. Available from: http://www.cbhidghs.nic.in/inde×1.asp?linkid=267 .
- 18.Thakur CP. A new strategy for elimination of kala-azar from rural Bihar. Indian J Med Res. 2007;126:447–51. [PubMed] [Google Scholar]
- 19.Napier LE. The Principles and Practice of Tropical Medicine. New York: The Macmillan Co; 1946. [Google Scholar]
- 20.Pocket Book of Health Statistics of India 4-7 (1977-1980) New Delhi: Controller of Publications, Department of Publications, Civil Lines; 1982. [Google Scholar]
- 21.Muniaraj M, Paramasivan R, Mariappan T, Arunachalam N, Sinha PK. The treatment of visceral leishmaniasis (kala-azar) in India: No obvious signs of long-term success. Trans R Soc Trop Med Hyg. 2012;106:770–2. doi: 10.1016/j.trstmh.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Mubayi A, Castillo-Chavez C, Chowell G, Kribs-Zaleta C, Ali Siddiqui N, Kumar N, et al. Transmission dynamics and underreporting of Kala-azar in the Indian state of Bihar. J Theor Biol. 2010;262:177–85. doi: 10.1016/j.jtbi.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Verghese T, Rehman SJ. Proceedings of workshop on Entomological and Vector Control Aspects of Kala-azar, 1991. Delhi: NICD; 1993. Critical appraisal of dynamics of kala-azar transmission visa. vis control measures in India; pp. 43–61. [Google Scholar]
- 24.Kumar V, Kesari S, Kumar AJ, Dinesh DS, Ranjan A, Prasad M, et al. Vector density and the control of kala-azar in Bihar, India. Mem Inst Oswaldo Cruz. 2009;104:1019–22. doi: 10.1590/s0074-02762009000700014. [DOI] [PubMed] [Google Scholar]
- 25.Kumar V, Kesari S, Dinesh DS, Tiwari AK, Kumar AJ, Kumar R, et al. A report on the indoor residual spraying (IRS) in the control of Phlebotomus argentipes, the vector of visceral leishmaniasis in Bihar (India): An initiative towards total elimination targeting 2015 (Series-1) J Vector Borne Dis. 2009;46:225–9. [PubMed] [Google Scholar]
- 26.Dinesh DS, Das ML, Picado A, Roy L, Rijal S, Singh SP, et al. Insecticide susceptibility of Phlebotomus argentipes in visceral leishmaniasis endemic districts in India and Nepal. PLoS Negl Trop Dis. 2010;4:e859. doi: 10.1371/journal.pntd.0000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tren R. Malaria Control and Climate Change. Delhi, India: Barun Mitra on Behalf of Library Institute; 2002. [Google Scholar]
- 28.Mathur P, Samantaray JC, Vajpayee M, Samanta P. Visceral leishmaniasis/human immunodeficiency virus co-infection in India: The focus of two epidemics. J Med Microbiol. 2006;55:919–22. doi: 10.1099/jmm.0.46574-0. [DOI] [PubMed] [Google Scholar]
- 29.Sinha PK, Jha TK, Thakur CP, Nath D, Mukherjee S, Aditya AK, et al. Phase 4 pharmacovigilance trial of paromomycin injection for the treatment of visceral leishmaniasis in India. J Trop Med. 2011;2011:645203. doi: 10.1155/2011/645203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinha PK, Rabidas VN, Pandey K, Verma N, Gupta AK, Ranjan A, et al. Visceral Leishmaniasis and HIV Coinfection in Bihar, India. J Acquir Immune Defic Syndr. 2003;32:115–6. doi: 10.1097/00126334-200301010-00017. [DOI] [PubMed] [Google Scholar]
- 31.Singh S, Biswas A, Wig N, Aggarwal P, Sood R, Wali JP. A new focus of visceral leishmaniasis in sub-Himalayan (Kumaon) region of northern India. J Commun Dis. 1999;31:73–7. [PubMed] [Google Scholar]
- 32.Alvar J, Aparicio P, Aseffa A, Den Boer M, Cañavate C, Dedet JP, et al. The relationship between leishmaniasis and AIDS: The second 10 years. Clin Microbiol Rev. 2008;21:334–59. doi: 10.1128/CMR.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinha PK, van Griensven J, Pandey K, Kumar N, Verma N, Mahajan R, et al. Liposomal amphotericin B for visceral leishmaniasis in human immunodeficiency virus-coinfected patients: 2-year treatment outcomes in Bihar, India. Clin Infect Dis. 2011;53:e91–8. doi: 10.1093/cid/cir521. [DOI] [PubMed] [Google Scholar]
- 34.Technical Report: India HIV Estimates ???: National Institute of Medical Sciences (Indian Council of Medical Research) and National AIDS Control Organization Department of AIDS Control, Government of India, New Delhi. 2010.
- 35.Thakur CP, Narayan S, Ranjan A. Kala-Azar (visceral leishmaniasis) and HIV coinfection in Bihar, India: Is this combination increasing? J Acquir Immune Defic Syndr. 2003;32:572–3. doi: 10.1097/00126334-200304150-00017. [DOI] [PubMed] [Google Scholar]
- 36.Peters W. The treatment of kala-azar-New approaches to an old problem. Indian J Med Res. 1981;73(Suppl):1–18. [PubMed] [Google Scholar]
- 37.Dutta AK. Fighting the fever, the return of kala-azar in India. Wellcome Hist. 2010;43:2–3. [Google Scholar]
- 38.Aikat BK, Sahaya S, Pathania AG, Bhattacharya PK, Desai N, Prasad LS, et al. Clinical profile of cases of kala-azar in Bihar. Indian J Med Res. 1979;70:563–70. [PubMed] [Google Scholar]
- 39.Thakur CP, Kumar M, Singh SK, Sharma D, Prasad US, Singh RS, et al. Comparison of regimens of treatment with sodium stibogluconate in kala-azar. Br Med J (Clin Res Ed) 1984;288:895–7. doi: 10.1136/bmj.288.6421.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The leishmaniases. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1984;701:1–140. [PubMed] [Google Scholar]
- 41.Control of the leishmaniases. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1990;793:1–158. [PubMed] [Google Scholar]
- 42.Sundar S, More DK, Singh MK, Singh VP, Sharma S, Makharia A, et al. Failure of pentavalent antimony in visceral leishmaniasis in India: Report from the center of the Indian epidemic. Clin Infect Dis. 2000;31:1104–7. doi: 10.1086/318121. [DOI] [PubMed] [Google Scholar]
- 43.Thakur CP, Kumar M, Pandey AK. Evaluation of efficacy of longer durations of therapy of fresh cases of kala-azar with sodium stibogluconate. Indian J Med Res. 1991;93:103–10. [PubMed] [Google Scholar]
- 44.Guerin PJ, Olliaro P, Sundar S, Boelaert M, Croft SL, Desjeux P, et al. Visceral leishmaniasis: Current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect Dis. 2002;2:494–501. doi: 10.1016/s1473-3099(02)00347-x. [DOI] [PubMed] [Google Scholar]
- 45.Sundar S. Drug resistance in Indian visceral leishmaniasis. Trop Med Int Health. 2001;6:849–54. doi: 10.1046/j.1365-3156.2001.00778.x. [DOI] [PubMed] [Google Scholar]
- 46.Jha TK. Evaluation of diamidine compound (pentamidine isethionate) in the treatment resistant cases of kala-azar occurring in North Bihar, India. Trans R Soc Trop Med Hyg. 1983;77:167–70. doi: 10.1016/0035-9203(83)90058-5. [DOI] [PubMed] [Google Scholar]
- 47.Berman JD. Human leishmaniasis: Clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis. 1997;24:684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 48.WHO-SEARO. Elimination of visceral leishmaniases: Report of an inter-country informal consultative meeting Varanasi, India, 10-14 November 2003. 2004. [Last accessed on 2011 May 15]. Available from: http://209.61.208.233/LinkFiles/Kala_azar_VBC.84.pdf .
- 49.Jha TK. Drug unresponsiveness and combination therapy for kala-azar. Indian J Med Res. 2006;123:389–98. [PubMed] [Google Scholar]
- 50.Thakur CP, Sinha GP, Pandey AK, Barat D, Sinha PK. Amphotericin B in resistant kala-azar in Bihar. Natl Med J India. 1993;6:57–60. [PubMed] [Google Scholar]
- 51.Berman JD, Badaro R, Thakur CP, Wasunna KM, Behbehani K, Davidson R, et al. Efficacy and safety of liposomal amphotericin B (AmBisome) for visceral leishmaniasis in endemic developing countries. Bull World Health Organ. 1998;76:25–32. [PMC free article] [PubMed] [Google Scholar]
- 52.Sundar S, Jha TK, Thakur CP, Sinha PK, Bhattacharya SK. Injectable paromomycin for Visceral leishmaniasis in India. N Engl J Med. 2007;356:2571–81. doi: 10.1056/NEJMoa066536. [DOI] [PubMed] [Google Scholar]
- 53.Sundar S, Sinha PK, Rai M, Verma DK, Nawin K, Alam S, et al. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: An open-label, non-inferiority, randomised controlled trial. Lancet. 2011;377:477–86. doi: 10.1016/S0140-6736(10)62050-8. [DOI] [PubMed] [Google Scholar]
- 54.Srivastava P, Prajapati VK, Rai M, Sundar S. Unusual case of resistance to amphotericin B in visceral leishmaniasis in a region in India where leishmaniasis is not endemic. J Clin Microbiol. 2011;49:3088–91. doi: 10.1128/JCM.00173-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinha PK, Roddy P, Palma PP, Kociejowski A, Lima MA, Rabi Das VN, et al. Effectiveness and safety of liposomal amphotericin B for visceral leishmaniasis under routine program conditions in Bihar, India. Am J Trop Med Hyg. 2010;83:357–64. doi: 10.4269/ajtmh.2010.10-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sundar S, Sinha PR, Agrawal NK, Srivastava R, Rainey PM, Berman JD, et al. A cluster of cases of severe cardiotoxicity among kala-azar patients treated with a high-osmolarity lot of sodium antimony gluconate. Am J Trop Med Hyg. 1998;59:139–43. doi: 10.4269/ajtmh.1998.59.139. [DOI] [PubMed] [Google Scholar]
- 57.WHO-SEARO. Programme Managers' Meeting on Elimination of Kala-azar in the South-East Asia Region Faridabad, Haryana, India, February 17-19, 2009. New Delhi: World Health Organization; 2010. [Last accessed on 2011 May 22]. Available from: http://203.90.70.117/PDS_DOCS/B4518.pdf. [Google Scholar]
- 58.Sundar S, Singh A, Rai M, Prajapati VK, Singh AK, Ostyn B, et al. Efficacy of miltefosine in the treatment of visceral leishmaniasis in India after a decade of use. Clin Infect Dis. 2012;55:543–50. doi: 10.1093/cid/cis474. [DOI] [PubMed] [Google Scholar]
- 59.Jha TK, Olliaro P, Thakur CP, Kanyok TP, Singhania BL, Singh IJ, et al. Randomised controlled trial of aminosidine (paromomycin) v sodium stibogluconate for treating visceral leishmaniasis in North Bihar, India. BMJ. 1998;316:1200–5. doi: 10.1136/bmj.316.7139.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thakur CP, Kanyok TP, Pandey AK, Sinha GP, Zaniewski AE, Houlihan HH, et al. A prospective randomized, comparative, open-label trial of the safety and efficacy of paromomycin (aminosidine) plus sodium stibogluconate versus sodium stibogluconate alone for the treatment of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2000;94:429–31. doi: 10.1016/s0035-9203(00)90130-5. [DOI] [PubMed] [Google Scholar]
- 61.WHO. Model List of Essential Medicines. 15th ed. Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- 62.WHO. Model List of Essential Medicines for Children. 2nd ed. Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- 63.Van Griensven J, Balasegaram M, Meheus F, Alvar J, Lynen L, Boelaert M. Combination therapy for visceral leishmaniasis. Lancet Infect Dis. 2010;10:184–94. doi: 10.1016/S1473-3099(10)70011-6. [DOI] [PubMed] [Google Scholar]
- 64.García-Hernández R, Manzano JI, Castanys S, Gamarro F. Leishmania donovani develops resistance to drug combinations. PLoS Negl Trop Dis. 2012;6:e1974. doi: 10.1371/journal.pntd.0001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moore EM, Lockwood DN. Treatment of visceral leishmaniasis. J Glob Infect Dis. 2010;2:151–8. doi: 10.4103/0974-777X.62883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh R, Das RK, Sharma SK. Resistance of sandflies to DDT in Kala-azar endemic districts of Bihar, India. Bull World Health Organ. 2001;79:793. [PMC free article] [PubMed] [Google Scholar]
- 67.WHO-SEARO. Regional Technical Advisory Group on Kala-azar Elimination Manesar, Haryana, 20-23 December 2004. World Health Organization; 2005. [Last accessed on 2011 Jun 22]. Available from: http://209.61.208.233/LinkFiles/Kala_azar_VBC.88.pdf . [Google Scholar]
- 68.WHO-SEARO. Technical Consultation with Partners for Elimination of Kala-azar in Endemic Countries of WHO South-East Asia Region. Behror, Rajasthan, India, 29-31 August 2005. World Health Organization; 2006. [Last accessed on 2011 Apr 20]. Available from: http://203.90.70.117/PDS_ DOCS/B0304.pdf. [Google Scholar]


