Abstract
Plasmodium knowlesi is the fifth species of Plasmodium recently identified to cause human malaria. Infections with P. knowlesi are currently being reported from South-East Asian countries and the incidence is on the rise with a possibility of spread to the geographically contiguous countries. P. knowlesi infections can result in a high degree of parasitemia causing severe malaria in a larger proportion of infected individuals. If detected early and treated with appropriate antimicrobials, these infections show a significant clinical improvement. The widely used microscopic methods usually misidentify P. knowlesi as the less pathogenic Plasmodium malariae leading to inadequate therapy and adverse clinical outcomes. The currently popular rapid immuno-chromatographic card tests have a very low sensitivity in diagnosing knowlesi malaria and can erroneously report P. knowlesi as other Plasmodia and vice-versa. At present molecular methods are the most efficacious in diagnosing P. knowlesi infections, but these tests can produce a false positive report in Plasmodium vivax infections and require expensive equipment and trained personnel. An ideal diagnostic test for P. knowlesi infections, which is potent, cost-effective and practically feasible in the resource limited setting is yet to be developed.
Keywords: Microscopy, Plasmodium knowlesi, rapid diagnostic test, real time polymerase chain reaction, severe malaria
INTRODUCTION
Until recently, naturally occurring human malaria was long believed to be caused by any of the four species of Plasmodium namely; Plasmodium vivax, Plasmodium falciparum, Plasmodium ovale and Plasmodium malariae. It was only about a decade ago it was observed that Plasmodium knowlesi, a common malarial parasite of macaques could naturally infect humans. The discovery of this fifth species of Plasmodium infecting humans was made based on the discrepant observations between microscopy and molecular techniques.[1]
Although first reported in 2004, retrospective analysis of molecular and epidemiological data indicate that P. knowlesi is not a newly emergent human parasite, but a well-known animal parasite whose zoonotic potential was failed to be correctly identified.[2] P. knowlesi infections are potentially fatal, but readily treatable if detected early enough. Despite the availability of effective antimalarials, failure to make the correct diagnosis has resulted in inappropriate therapy leading to deaths due to P. knowlesi infections.[3] These mishaps can be readily avoided with an accurate, timely diagnosis followed by prompt treatment with appropriate antimalarial agents.[4] In this context, this review attempts to clarify the challenges faced by the different diagnostic modalities and sheds light on the recent advances in the diagnosis of P. knowlesi.
CLINICAL SUSPICION AND LABORATORY MARKERS
P. knowlesi has a shorter life cycle than its other counterparts causing human malaria with its erythrocytic cycle lasting for only 24 h. The erythrocytic development of the parasite is also asynchronous. Hence, the infection presents typically with a quotidian pattern of febrile illness characterized by daily fever and chills. Similar to other human malaria, the quotidian fever is usually accompanied by constitutional symptoms such as headache, rigors, malaise, myalgia, abdominal pain and breathlessness.[5]
Similar to vivax and falciparum malaria, knowlesi malaria also causes thrombocytopenia. Low platelet count early in the course of the disease is a characteristic feature of knowlesi malaria. Thrombocytopenia occurs at a much common frequency in knowlesi malaria than in vivax and falciparum malaria. It has been documented that almost all patients of knowlesi malaria are thrombocytopenic on admission and do not show features of coagulopathy even when platelet counts become very low; whereas the incidence of thrombocytopenia ranges from 20% to 90% in vivax and falciparum malaria and is associated with mild to severe bleeding tendencies.[6,7,8]
The parasitemia during presentation is usually low. However due to the short multiplication time and the ability to infect young and old red blood cells (RBCs), hyper parasitemia can develop very rapidly if treatment is not initiated promptly. Nearly, 7.5-10% of the P. knowlesi infections progress to severe malaria.[9] In endemic regions, severe malaria due to P. knowlesi infections are more common than that caused by P. falciparum.[4] Acute respiratory distress syndrome is the most common presentation of severe knowlesi malaria followed by hepato-renal dysfunction. Other manifestations include hypotension and hyponatremia. Cerebral involvement which occurs in complicated falciparum malaria and occasionally in severe vivax malaria has not yet been reported in knowlesi malaria.[6]
High parasitemia and low platelet counts during admission are independent markers of severity and mortality in knowlesi malaria. Although, the World Health Organization provides cut off values for these indicators in falciparum malaria, owing to biological differences of the two species, these values could not be extrapolated to knowlesi malaria. Willmann et al., through their detailed study have worked out these values for knowlesi malaria. The authors suggest that a parasitemia of ≥ 35,000/μl or > 1% of infected RBCs and a platelet count ≤ 45,000/μl in any adult patient infected with P. knowlesi should be considered as severe disease and treated accordingly. The authors also indicate that increase in the pigment containing neutrophils is a more accurate predictor of severe knowlesi malaria.[9]
LIMITATIONS OF MICROSCOPIC METHODS
Microscopy of stained blood smears is considered as the gold standard investigation and can establish the diagnosis in parasitemia of >50 parasites/μl (Anthony moody rapid malaria). The morphology of the different erythrocytic forms can be clearly observed in thin films enabling the identification of the species [Table 1]. However, it is well-established that microscopy alone cannot reliably distinguish P. knowlesi from P. falciparum, P. malariae and P. vivax.[5,10,11]
Table 1.
Description of morphological features of various erythrocytic stages of Plasmodium knowlesi
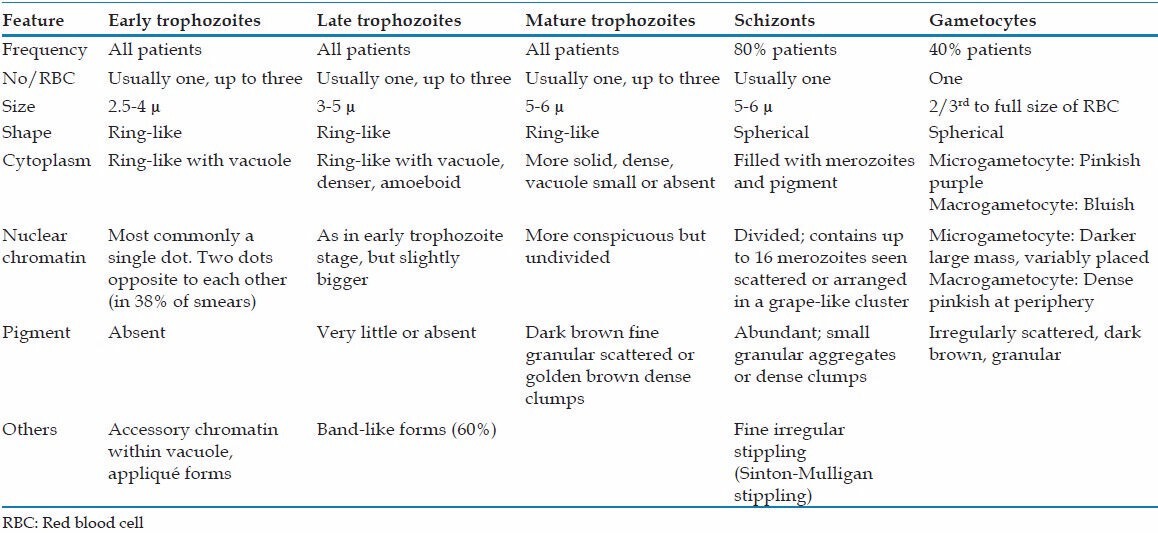
Early trophozoites of P. knowlesi may be mistaken for that of P. falciparum due to similar features such as the presence of double chromatin dots, multiply-infected erythrocytes and appliqué forms.[11,12] Late and mature trophozoites, schizonts and mature gametocytes of P. knowlesi are generally indistinguishable from those of P. malariae. Presence of a very amoeboid cytoplasm of the late trophozoites of P. knowlesi can be the only differentiating feature from those of P. malariae but are only observed occasionally and are difficult to be identified even by a trained observer. “Band form trophozoites,” a characteristic feature of P. malariae is also seen in P. knowlesi infection.[11]
In an attempt to evaluate the efficacy of microscopy in the diagnosis of knowlesi malaria, Barber et al. through their study demonstrated that microscopy could misdiagnose P. knowlesi commonly as both P. vivax and P. falciparum and vice versa. The authors observed that only 72% of polymerase chain reaction (PCR)-confirmed P. knowlesi could be identified correctly on microscopy and 30% of P. vivax was wrongly identified as P. malariae/P. knowlesi.[10]
INADEQUACY OF ANTIGEN DETECTION TESTS
Rapid diagnostic immunochromatographic tests (RDT) detecting malarial antigens are being widely used in most countries as an alternative to microscopic differentiation of the Plasmodium species. Due to the predominance of vivax and falciparum malaria, almost all of these RDTs are designed to detect either or both of these antigens in a single kit. RDTs with monoclonal antibodies against the P. vivax specific lactate dehydrogenase (pvLDH) exclusively identify P. vivax while those against histidine rich protein-2 or P. falciparum specific lactate dehydrogenase (pfLDH) specifically detect P. falciparum. In addition to this, certain RDTs also detect but not differentiate the antigens of other Plasmodium species using monoclonal antibodies against pan-malarial markers such as the pan-Plasmodium lactate dehydrogenase (pLDH) or Plasmodium aldolase (pALD).[13] Introduction of RDTs have revolutionized the diagnosis of human malaria with the advantages of being rapid, user friendly, versatile in point-of-care testing and cost-effectiveness. The RDTs have the capability to detect parasitemia of >100 parasites/μl of the four well-known Plasmodium species.[14]
Until date, P. knowlesi specific RDTs are not commercially available and the existing formats of RDTs are found to perform erroneously when used in P. knowlesi endemic regions.[15] The sensitivity of the pan malarial marker pALD, has been found to be the lowest while detecting P. knowlesi antigens when compared with the detection of P. falciparum and P. vivax antigens. The overall sensitivity of pALD for detecting P. knowlesi is reported to be as low as 23%. Furthermore, the sensitivity of pALD has been observed to be only 45% even with a heavy parasitemia of >10,000 parasites/μl making it unfit for the detection of P. knowlesi mono infections.[16] The inadequate performance of pALD as a diagnostic marker can be attributed to genetic variations in the aldolase gene, producing heterogenous molecules of varying affinity to the monoclonal antibody used in the detection system.[17]
The sensitivity of the other pan malarial marker pLDH in detecting P. knowlesi is estimated to be as low as 25% in parasite counts <1,000 parasites/μl and increased to 97% in parasite counts >1,000 parasites/μl. An interesting observation made is the direct correlation of the intensity of pLDH band to the amount of P. knowlesi parasitemia. With respect to these observations, it has been suggested that pLDH can only be used as a surrogate marker to predict the risk of severe disease, but not for detecting early uncomplicated knowlesi malaria.[16]
P. knowlesi infections also pose a hindrance in the specific diagnosis of P. vivax and P. falciparum using RDTs. The protein expression and characterization of the P. knowlesi lactate dehydrogenase (pkLDH) has demonstrated 96.8% homology to pvLDH and over 90% homology to pfLDH.[18] This antigenic similarity is held responsible for the cross reactions observed in the P. vivax and P. falciparum specific RDTs designed to detect the parasite specific LDH.[15] Due to the cross reactions in these RDTs, knowlesi malaria could be wrongly diagnosed as vivax or falciparum malaria leading to inappropriate treatment and false projection the epidemiological data.[16] McCutchan et al. suggest that the cross reaction of P. knowlesi antigens with the monoclonal antibodies of pvLDH and pfLDH can actually be used for the detection of P. knowlesi in endemic regions. However, the interpretation can be erroneous due to the non-specific nature and a mixed infection with more than one species can be overlooked.[19]
In view of the current situation, numerous candidate antigens for specific identification P. knowlesi are being studied. Using recombinant deoxyribonucleic acid (DNA) technology, Singh et al. in their study have developed a 34 kDa pkLDH and have proposed its role in RDTs for the specific diagnosis of P. knowlesi.[18] However, due to the cross reactivity exhibited among the LDH antigens of the different Plasmodia, its potential use is questionable. In this context, novel antigenic targets with increased specificity in species discrimination are being evaluated. Some of the targets studied include the P. knowlesi merozoite surface protein (pkMSP1-[33]), P. knowlesi merozoite surface protein-142 (pkMSP-142), P. knowlesi 1-Cys peroxiredoxin and the P. knowlesi surface protein containing an altered thrombospondin repeat domain.[20,21,22,23] However, these molecules too are not devoid of inter-species cross reactivity and an ideal antigenic target is yet to be identified.
TOOLS FOR MOLECULAR DETECTION
Considering the severe lacunae in microscopy and antigen detection tests, molecular methods are currently the only fail safe option for the definitive diagnosis of P. knowlesi malaria. In fact the very discovery of human knowlesi malaria was possible only due to molecular techniques.[1] The 18S small-subunit rRNA (ssu-rRNA), the circumsporozoite surface protein gene, a nuclear gene encoding a cysteine protease and the cytochrome b gene are some of the targets studied and exploited by the molecular detection tests.[24,25] Among the aforementioned targets, the genetic variations in the 18S ssu-rRNA are the most studied and widely used for the species discrimination of the different Plasmodia.[26] These tests can detect the parasitic DNA extracted from samples such as whole blood with anti-coagulants, dried blood spots on filter papers and scrapings from stained blood films on glass slides following microscopic analysis.[27] Newer assays also have an additional feature to detect human housekeeping genes such as the β-hemoglobin or β-actin which serve as the internal positive control to assess the success of DNA extraction procedure and to exclude the presence of DNA polymerase inhibitors.[28,29]
The initial molecular test deployed for the detection of P. knowlesi was the conventional nested multiplex PCR targeting the 18 S ssu-rRNA gene developed by Singh et al. In this technique, the target gene is amplified first using genus-specific primers (rPLU1 and rPLU5) and the resulting amplicons are subjected to second round of amplification using species-specific primers to identify the species; P. falciparum (rFAL1 and rFAL2), P. vivax (rVIV1 and rVIV2), P. ovale (rOVA1 and rOVA4), P. malariae (rMAL1 and rMAL2) and P. knowlesi (Pmk8 and Pmkr9).[1] Subsequently, it was observed that the Pmk8-Pmkr9 primers also amplified a proportion of P. vivax DNA, falsely identifying as P. knowlesi. Stochastic cross reactivity has been attributed to the spurious amplification of P. vivax DNA by P. knowlesi specific primers. To overcome this drawback, Imwong et al. recommend the use of the more specific PkF1150-PkR15560 primers in the second round of amplification instead of the erroneous Pmk8-Pmkr9 primers.[30] Alternatively, Van Hong et al. report that the specificity of the nested PCR can be increased by using the PkR4 primer to amplify the P. knowlesi DNA alone in a separate third round and not along with the other species amplified in the second round.[31]
The conventional nested PCR assays are very sensitive and are capable of detecting P. knowlesi even when the parasitemia is <10 parasites/μl.[30] Although, nested PCR is considered the “molecular gold standard” in the diagnosis of malaria, it has various drawbacks. As the technique involves five to six separate PCR reactions to detect the five Plasmodium species, it requires a large number of reagents and disposable consumables involving more handling and cross contamination. It is also cumbersome, time-consuming and labor-intensive.[28]
Having the advantages of rapidity, direct quantification without post amplification analysis and minimal cross contamination, real time multiplex PCR based methods have superseded the conventional ones. Divis et al. report a TaqMan assay which initially amplifies the DNA of all the five species of human Plasmodia using a common primer set, from which the individual species are identified by using species-specific hydrolysis probes. Although, the authors report a high degree of sensitivity and specificity in mono infections, they mention that there might be a compromise of the sensitivity in detecting the lesser parasite in mixed infections due to competition for primers by the DNA of the more abundant species.[32] In a subsequent study, Shokoples et al., addresses this issue with minor modifications in the primer and probe sequences thereby increasing the sensitivity of the real time PCR assay in the discrimination of mixed infections.[33] In the real time PCR assay employed by Babady et al., the authors faced the problem of overlapping melt curves of P. knowlesi and P. vivax, hindering differentiation of these two species. To overcome this, the authors emphasize the need of separate fluorophores for P. vivax and P. knowlesi probes or the use of unique primers specific for P. knowlesi.[34] Real time PCR assays are highly sensitive and can detect even very low parasitemia of 1-2 parasites/μl.[32] As such, real time PCR is one of the most advanced molecular tests available for the combined detection of P. knowlesi along with the other human Plasmodia. It can be introduced in any of the existing platforms in previously equipped laboratories. However, expensive reagents and high installation costs are the major drawbacks faced by introduction of these tests in laboratories with resource constraints.[28]
Considering the drawbacks faced by conventional nested PCR and the real time PCR assays, Chew et al. have developed a straightforward single-step hexaplex PCR system targeting the 18S ssu-rRNA of the five human Plasmodia along with a built-in internal control. The high degree of specificity of the species-specific primers eliminates the need for a nested procedure. With the ability to detect parasitemia of 0.25 parasites/μl, this assay is reported to have a higher sensitivity than any of the other PCR assays currently available for the detection of P. knowlesi. The capability to discriminate at least two Plasmodium species in mixed infections, reflect the high specificity of the assay. Abolishing the need of time-consuming multiple rounds of amplification as in nested multiplex PCR and the necessity of expensive equipment and reagents as with real time PCR, this novel hexaplex PCR proves to be more practically applicable.[28] This hexaplex assay marketed as PlasmoNex™ and can be run on existing conventional thermal cyclers followed by agarose gel documentation.[35] Likewise, Lucchi et al. have designed a simple single target conventional PCR employing the Pkr140-5 set of primers targeting the “species-specific and multi-copy sequences” in the genomic DNA of P. knowlesi. The authors suggest the novel target to be extremely specific to P. knowlesi with no cross reactivity to any of the human and simian Plasmodia. The authors indicate the potential of this assay as a simple molecular confirmatory tool in P. knowlesi endemic regions.[36]
Loop mediated isothermal amplification (LAMP) is one of the molecular methods which rapidly amplifies nucleic acids at a constant temperature. The technique possesses certain inherent advantages over the PCR such as rapidity, minimal user training and lower installation cost as it does not require the expensive thermal cycler.[37] Currently, two different LAMP assays have been independently developed and studied for their utility in the diagnosis of knowlesi malaria. The LAMP assay designed by Iseki et al. detects the P. knowlesi specific β-tubulin gene using three sets of primers while that of Lau et al. targets the P. knowlesi apical membrane antigen-1 gene with six primers. These assays are capable of detecting extremely low level parasitemia and are claimed to be 10-100 times more sensitive than the currently available PCR formats. Owing to the superior sensitivity, the LAMP assay can be performed directly on the blood sample bypassing the cumbersome and time-consuming extraction procedures.[38,39] Though possessing obvious advantages, the LAMP assays have failed to stir an interest as a diagnostic modality due the popularity of the currently preferred PCR systems.
CONCLUSIONS
Currently, human P. knowlesi infections have been limited to the South-East Asian countries with the bulk of cases being reported from Malaysia, Myanmar and Thailand.[5] In these endemic regions, microscopy forms the mainstay of malaria diagnosis. The laboratory detection is seldom missed but the infection is often misdiagnosed. In most occasions the misdiagnosis of knowlesi malaria as other forms of human malaria does not cause obvious harm, as a large proportion of these infections are usually mild and respond well to oral antimalarials.[40] However, a misdiagnosis in the heavy infections may delay the parenteral therapy leading to fatal consequences.[3] As knowlesi malaria has a three-fold greater risk in development of complications than falciparum malaria, an accurate and timely diagnosis can be life-saving.[16]
Numerous studies have reported the erroneous performance of microscopy demanding the need for a better diagnostic test for P. knowlesi infections. Although, state of the art molecular diagnostic techniques are available, their widespread application is limited owing to the resource constraints faced by most diagnostic facilities in the endemic regions. The cost-effective RDTs which are widely being used in malaria diagnosis, fail desperately in the detection of P. knowlesi. Genomic and protein expression studies to identify a specific antigenic target which can be effectively translated to a suitable RDT format are the need of the hour.
In this context, a high degree of clinical suspicion and careful correlation of cues derived from other laboratory markers can rectify a misidentified microscopy report to a certain extent. As the incidence of knowlesi malaria is on the rise, travelers returning from endemic countries presenting with malaria must be strongly suspected for P. knowlesi infection. With the presence of suitable animal hosts and vectors in geographically contiguous locations such as western India, the health care personnel in these regions should be aware of this challenging malarial parasite and be vigilant enough to make a timely diagnosis.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Singh B, Kim Sung L, Matusop A, Radhakrishnan A, Shamsul SS, Cox-Singh J, et al. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet. 2004;363:1017–24. doi: 10.1016/S0140-6736(04)15836-4. [DOI] [PubMed] [Google Scholar]
- 2.Singh B, Daneshvar C. Human infections and detection of Plasmodium knowlesi. Clin Microbiol Rev. 2013;26:165–84. doi: 10.1128/CMR.00079-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajahram GS, Barber BE, William T, Menon J, Anstey NM, Yeo TW. Deaths due to Plasmodium knowlesi malaria in Sabah, Malaysia: Association with reporting as Plasmodium malariae and delayed parenteral artesunate. Malar J. 2012;11:284. doi: 10.1186/1475-2875-11-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber BE, William T, Grigg MJ, Menon J, Auburn S, Marfurt J, et al. A prospective comparative study of knowlesi, falciparum, and vivax malaria in Sabah, Malaysia: High proportion with severe disease from Plasmodium knowlesi and Plasmodium vivax but no mortality with early referral and artesunate therapy. Clin Infect Dis. 2013;56:383–97. doi: 10.1093/cid/cis902. [DOI] [PubMed] [Google Scholar]
- 5.Kantele A, Jokiranta TS. Review of cases with the emerging fifth human malaria parasite, Plasmodium knowlesi. Clin Infect Dis. 2011;52:1356–62. doi: 10.1093/cid/cir180. [DOI] [PubMed] [Google Scholar]
- 6.Daneshvar C, Davis TM, Cox-Singh J, Rafa'ee MZ, Zakaria SK, Divis PC, et al. Clinical and laboratory features of human Plasmodium knowlesi infection. Clin Infect Dis. 2009;49:852–60. doi: 10.1086/605439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacerda MV, Mourão MP, Coelho HC, Santos JB. Thrombocytopenia in malaria: Who cares? Mem Inst Oswaldo Cruz. 2011;106(Suppl 1):52–63. doi: 10.1590/s0074-02762011000900007. [DOI] [PubMed] [Google Scholar]
- 8.Mohapatra S, Samantaray JC, Arulselvi S, Ghosh A. Disseminated intravascular coagulation following malaria due to Plasmodium vivax: A thromboelastography-based study. Malar J. 2013;12:336. doi: 10.1186/1475-2875-12-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willmann M, Ahmed A, Siner A, Wong IT, Woon LC, Singh B, et al. Laboratory markers of disease severity in Plasmodium knowlesi infection: A case control study. Malar J. 2012;11:363. doi: 10.1186/1475-2875-11-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barber BE, William T, Grigg MJ, Yeo TW, Anstey NM. Limitations of microscopy to differentiate Plasmodium species in a region co-endemic for Plasmodium falciparum, Plasmodium vivax and Plasmodium knowlesi. Malar J. 2013;12:8. doi: 10.1186/1475-2875-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee KS, Cox-Singh J, Singh B. Morphological features and differential counts of Plasmodium knowlesi parasites in naturally acquired human infections. Malar J. 2009;8:73. doi: 10.1186/1475-2875-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coatney GR, Collins WE, Warren MW, Contacos PG. The Primate Malarias. 1st ed. Washington: US Government Printing Office; 1971. [Google Scholar]
- 13.Wilson ML. Malaria rapid diagnostic tests. Clin Infect Dis. 2012;54:1637–41. doi: 10.1093/cid/cis228. [DOI] [PubMed] [Google Scholar]
- 14.Moody A. Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev. 2002;15:66–78. doi: 10.1128/CMR.15.1.66-78.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawai S, Hirai M, Haruki K, Tanabe K, Chigusa Y. Cross-reactivity in rapid diagnostic tests between human malaria and zoonotic simian malaria parasite Plasmodium knowlesi infections. Parasitol Int. 2009;58:300–2. doi: 10.1016/j.parint.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Barber BE, William T, Grigg MJ, Piera K, Yeo TW, Anstey NM. Evaluation of the sensitivity of a pLDH-based and an aldolase-based rapid diagnostic test for diagnosis of uncomplicated and severe malaria caused by PCR-confirmed Plasmodium knowlesi, Plasmodium falciparum, and Plasmodium vivax. J Clin Microbiol. 2013;51:1118–23. doi: 10.1128/JCM.03285-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JY, Kim HH, Shin HL, Sohn Y, Kim H, Lee SW, et al. Genetic variation of aldolase from Korean isolates of Plasmodium vivax and its usefulness in serodiagnosis. Malar J. 2012;11:159. doi: 10.1186/1475-2875-11-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh V, Kaushal DC, Rathaur S, Kumar N, Kaushal NA. Cloning, overexpression, purification and characterization of Plasmodium knowlesi lactate dehydrogenase. Protein Expr Purif. 2012;84:195–203. doi: 10.1016/j.pep.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 19.McCutchan TF, Piper RC, Makler MT. Use of malaria rapid diagnostic test to identify Plasmodium knowlesi infection. Emerg Infect Dis. 2008;14:1750–2. doi: 10.3201/eid1411.080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheong FW, Lau YL, Fong MY, Mahmud R. Evaluation of recombinant Plasmodium knowlesi merozoite surface protein-1 (33) for detection of human malaria. Am J Trop Med Hyg. 2013;88:835–40. doi: 10.4269/ajtmh.12-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheong FW, Fong MY, Lau YL, Mahmud R. Immunogenicity of bacterial-expressed recombinant Plasmodium knowlesi merozoite surface protein-142 (MSP-142) Malar J. 2013;12:454. doi: 10.1186/1475-2875-12-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hakimi H, Nguyen TT, Suganuma K, Masuda-Suganuma H, Angeles JM, Inoue N, et al. Development of monoclonal antibodies that target 1-Cys peroxiredoxin and differentiate Plasmodium falciparum from P. vivax and P. knowlesi. Trop Med Health. 2013;41:55–9. doi: 10.2149/tmh.2012-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palaeya V, Lau YL, Mahmud R, Chen Y, Fong MY. Cloning, expression, and immunocharacterization of surface protein containing an altered thrombospondin repeat domain (SPATR) from Plasmodium knowlesi. Malar J. 2013;12:182. doi: 10.1186/1475-2875-12-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conway DJ. Molecular epidemiology of malaria. Clin Microbiol Rev. 2007;20:188–204. doi: 10.1128/CMR.00021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vythilingam I, Noorazian YM, Huat TC, Jiram AI, Yusri YM, Azahari AH, et al. Plasmodium knowlesi in humans, macaques and mosquitoes in peninsular Malaysia. Parasit Vectors. 2008;1:26. doi: 10.1186/1756-3305-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucchi NW, Oberstaller J, Kissinger JC, Udhayakumar V. Malaria diagnostics and surveillance in the post-genomic era. Public Health Genomics. 2013;16:37–43. doi: 10.1159/000345607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox-Singh J, Davis TM, Lee KS, Shamsul SS, Matusop A, Ratnam S, et al. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis. 2008;46:165–71. doi: 10.1086/524888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chew CH, Lim YA, Lee PC, Mahmud R, Chua KH. Hexaplex PCR detection system for identification of five human Plasmodium species with an internal control. J Clin Microbiol. 2012;50:4012–9. doi: 10.1128/JCM.06454-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calderaro A, Piccolo G, Gorrini C, Rossi S, Montecchini S, Dell'Anna ML, et al. Accurate identification of the six human Plasmodium spp. causing imported malaria, including Plasmodium ovale wallikeri and Plasmodium knowlesi. Malar J. 2013;12:321. doi: 10.1186/1475-2875-12-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imwong M, Tanomsing N, Pukrittayakamee S, Day NP, White NJ, Snounou G. Spurious amplification of a Plasmodium vivax small-subunit RNA gene by use of primers currently used to detect P. knowlesi. J Clin Microbiol. 2009;47:4173–5. doi: 10.1128/JCM.00811-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Hong N, van den Eede P, Van Overmeir C, Vythilingham I, Rosanas-Urgell A, Vinh Thanh P, et al. A modified semi-nested multiplex malaria PCR (SnM-PCR) for the identification of the five human Plasmodium species occurring in Southeast Asia. Am J Trop Med Hyg. 2013;89:721–3. doi: 10.4269/ajtmh.13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Divis PC, Shokoples SE, Singh B, Yanow SK. A TaqMan real-time PCR assay for the detection and quantitation of Plasmodium knowlesi. Malar J. 2010;9:344. doi: 10.1186/1475-2875-9-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shokoples SE, Ndao M, Kowalewska-Grochowska K, Yanow SK. Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. J Clin Microbiol. 2009;47:975–80. doi: 10.1128/JCM.01858-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babady NE, Sloan LM, Rosenblatt JE, Pritt BS. Detection of Plasmodium knowlesi by real-time polymerase chain reaction. Am J Trop Med Hyg. 2009;81:516–8. [PubMed] [Google Scholar]
- 35.Goh XT, Lim YA, Vythilingam I, Chew CH, Lee PC, Ngui R, et al. Increased detection of Plasmodium knowlesi in Sandakan division, Sabah as revealed by PlasmoNex™. Malar J. 2013;12:264. doi: 10.1186/1475-2875-12-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucchi NW, Poorak M, Oberstaller J, DeBarry J, Srinivasamoorthy G, Goldman I, et al. A new single-step PCR assay for the detection of the zoonotic malaria parasite Plasmodium knowlesi. PLoS One. 2012;7:e31848. doi: 10.1371/journal.pone.0031848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:E63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iseki H, Kawai S, Takahashi N, Hirai M, Tanabe K, Yokoyama N, et al. Evaluation of a loop-mediated isothermal amplification method as a tool for diagnosis of infection by the zoonotic simian malaria parasite Plasmodium knowlesi. J Clin Microbiol. 2010;48:2509–14. doi: 10.1128/JCM.00331-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lau YL, Fong MY, Mahmud R, Chang PY, Palaeya V, Cheong FW, et al. Specific, sensitive and rapid detection of human Plasmodium knowlesi infection by loop-mediated isothermal amplification (LAMP) in blood samples. Malar J. 2011;10:197. doi: 10.1186/1475-2875-10-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilairatanal P, Krudsood S, Tangpukdee N. Management of Plasmodium knowlesi malaria without PCR confirmation. Southeast Asian J Trop Med Public Health. 2010;41:19–21. [PubMed] [Google Scholar]


